Abstract
Physiologically appropriate levels of matrix metalloproteinases (MMPs) are likely important to varied aspects of CNS function. In particular, these enzymes may contribute to neuronal activity dependent synaptic plasticity and to cell mobility in processes including stem cell migration and immune surveillance. Levels of MMPs may, however, be substantially increased in the setting of HIV infection with methamphetamine abuse. Elevated MMP levels might in turn influence integrity of the blood brain barrier, as has been demonstrated in published work. Herein we suggest that elevated levels of MMPs can also contribute to microglial activation as well as neuronal and synaptic injury through a mechanism that involves cleavage of specific cell and synaptic adhesion molecules.
Keywords: Matrix metalloproteinase (MMP), synapse, neuron, microglial cell, adhesion, cell adhesion molecule (CAM), methamphetamine
I. INTRODUCTION
Matrix metalloproteinases (MMPs) are a family of zinc dependent endoproteases with 24 expressed in humans [1, 2]. The majority are released from cells in a regulated manner. These soluble MMPs typically contain a pro- domain, a catalytic domain and a hemopexin-like domain which can function in binding interactions. Chromosomal loci vary though a cluster is localized to chromosome 11 [3, 4]. Molecular weights also vary and range from approximately 20 kDa for active MMP-7, which lacks a hemopexin-like domain, to 92 kDa for the gelatinase MMP-9. Following their release, MMPs are activated by removal of their pro domain or by factors that influence tertiary structure including oxidation and nitrosylation [5]. Reductions in soluble MMP activity can also be affected by interactions with endogenously expressed inhibitors known as tissue inhibitors of metalloproteinases (TIMPs)[6]. The MMP family also includes membrane-type MMPs (MT-MMPs), which possess a transmembrane domain as do the related “a disintegrin and metalloproteinase[s]” or ADAMs. The latter may be activated by intracellular signaling molecules including protein kinase C [7].
MMPs were named for their ability to process proteins of the extracellular matrix but are now appreciated to act on a variety of soluble molecules and cell surface receptors as well [8, 9]. These enzymes are expressed in the brain by varied cell types including neurons and glial cells [10, 11], and their expression, release and/or activity may be greatly enhanced in the setting of CNS infection or injury. MMPs typically have AP-1 and NF-κB transcription factor binding sites within their promoters, and their expression may also be regulated by hypoxia inducible transcription factor signaling [10]. MMPs have also been localized to preformed vesicular stores [12] from which their release may be facilitated by stimuli that increase intracellular calcium [13]. In addition, activation of released MMPs may be facilitated by injury-associated stimuli including serine proteases [14] and nitric oxide [5].
MMPs can overlap in terms of substrate specificity [15], though specificity for select substrates has been observed and the efficacy with which individual MMPs can act on specific substrates can vary. For example, one study noted relative selectivity of MMP-1 in terms of MMP dependent activation of the thrombin receptor protease activated receptor-1[16].
Studies of MMPs in the CNS have generally focused on their ability to target extracellular matrix proteins of the blood brain barrier [BBB]. Inflammatory cells may release MMPs to facilitate their transmigration through the blood brain barrier, and high levels of CNS derived MMPs likely diminish BBB integrity as well. Consistent with this, tight junction proteins are targeted by MMPs in the background of stroke [17], and CNS infiltration of inflammatory cells is reduced in MMP knockout animals [18]. MMPs have also been shown to target myelin proteins, and MMP inhibitors have thus been considered for the treatment of specific demyelinating disorders [19]. This review will, however, focus on another important target of MMP activity- cell adhesion molecules [CAMs].
CAMs are ideally positioned to be cleaved before MMP activity is squelched by endogenous soluble inhibitors. In addition, while basal shedding of CAMs occurs, CAM cleavage may be substantially increased in the setting of HIV infection and psychostimulant abuse. Moreover, as will be discussed, shed CAMs may interact with integrins to profoundly influence neuronal and glial function.
II. MMP LEVELS MAY BE SUBSTANTIALLY ELEVATED IN THE SETTING OF HIV INFECTION AND PSYCHOSTIMULANT USE
MMPs are elevated in association with HIV associated neurological disorders (HAND) [20–22]. CSF levels of MMP-2 and -9 in particular are increased. Moreover, HAND relevant stimuli including HIV proteins and TNF-α, can increase MMP release from CNS derived cells [20–23]. For example, injection of gp120 into rat caudate-putamen leads to increases in MMP-2 and -9 [24]. Stimulation of cultured cells with viral proteins is also linked to increases in MMP-2 and -9 [23, 25]. Moreover, HIV-1 Tat stimulates MMP-1 and -2 release from cultured neural cells [23] as well as MMP-9 from monocytes [26].
Similarly, methamphetamine (MA) has been associated with increase MMP levels in the CNS [27]. For example, chronic MA (2 mg/kg/day for five days) is associated with increased MMP-2 and -9 protein in the frontal cortex and nucleus accumbens of rats, and an acute high dose of MA (40 mg/kg) is followed by increased mRNA expression of MMP-9 in the CNS of mice [28]. We have also observed that acute MA increases MMP-9 levels in murine striatum and hippocampus [29].
MA is thought to increase extracellular monoamines [dopamine, serotonin and norepinephrine] through mechanisms that include a redistribution from synaptic vesicles to the cytoplasm and reverse flux through cell surface transporters [30, 31]. MA can also lead to an increase in extracellular glutamate levels [32]. MA associated increases in neurotransmitters may in turn lead to transmitter receptor dependent increased gene transcription. This mechanism is supported by published studies in which catecholamines have been shown to increase MMP expression [33–36].
Since recent studies also suggest that MMPs exist in perisynaptic vesicular stores [12], and that release of vesicular MMPs may be soluble NSF attachment protein receptor (SNARE) dependent [13], MA dependent changes in neurotransmitter levels might also stimulate SNARE dependent release of MMPs from vesicular stores. Consistent with this, a glutamate receptor agonist is associated with rapid MMP dependent substrate cleavage [37].
III. SPECIFIC CAMS, WITH KNOWN INTEGRIN BINDING MOTIFS, REPRESENT AN IMPORTANT CLASS OF MMP SUBSTRATES
CAMs represent an important class of MMP substrates, and CAM cleavage has been extensively investigated in the setting of cell migration and cancer biology. Though CAM cleavage has been less well studied in the CNS, the brain is rich in these molecules. Many are highly expressed in areas critical to brain function, including the neuronal synapse. Neuronal and glial CAMs include specific cadherin [Cdh] family members as well as CAMs with Ig-like domains. Among the latter are intercellular adhesion molecule- and -5 (ICAM-1 and ICAM-5), neural cell adhesion molecule (NCAM), and vascular cell adhesion molecule (VCAM). Shedding of these substrates can occur to a substantial extent, in that soluble shed forms of many CAMs can be detected in blood, brain, and CSF [38–41]. Shed forms of select CAMs including ICAM-5 have been described in control specimens, consistent with baseline shedding [29, 40, 41], and levels in blood or CSF are increased with CNS inflammation or other forms of neurological disease [38–41]. For a list of CAMs that are expressed in the CNS and targeted by MMPs, please see Table 1.
Table 1.
Partial List of CNS CAMs Processed by Metalloproteinases
| Cell Adhesion Molecule | Cell Types for which Expression has been Reported | Metalloproteinase(s) that Mediate Shedding | Reference(s) |
|---|---|---|---|
| ICAM-1 | Astrocytes, oligodendrocytes, leukocytes, endothelial cells | ADAM-17 | [86–90] |
| ICAM-5 | Glutamatergic neurons of the telencephalon | MMP-2,-3,-7,-9 | [37, 75] |
| NCAM | Neurons, astrocytes, oligodendrocytes, microglia, endothelium | ADAM protease | [91–95] |
| L1-CAM | Neurons, oligodendrocytes, endothelial cells | ADAM-10, ADAM-17 | [96–99] |
| VCAM | Neurons, astrocytes, microglia, endothelial cells | ADAM-9 | [100–104] |
| N-Cadherin | Astrocytes, oligodendrocytes | ADAM-10, MMP-7 | [7, 105–108] |
| E-Cadherin | Neurons and endothelial cells | MMP-7, ADAM-10, ADAM-15 | [109–114] |
| VE-Cadherin | Neuronal stem cells, astrocytes and endothelial cells | MMP-7 | [115] |
| Nectin-1 | Neurons | ADAM-10 | [116–118] |
| Syndecan-1 | Neurons and astrocytes [expression upreulated with injury], endothelial cells | MT1-MMP, MMP-7, MMP-9 | [119–125] |
| SIRP-1α | Neurons, astrocytes, microglia, endothelial cells | Unknown metalloproteinase[s] | [126–130] |
CAM cleavage may have important consequences with respect to CNS function. Loss of adhesive contacts may impair cell or synaptic signaling or stability, with effects on cell survival. In addition, MMP dependent cleavage is often followed by intramembranous proteolysis mediated by gamma secretase, leading to the generation of specific CAM intracellular domains (ICDs). While these may be degraded by the proteosome system, some have the potential to alter cellular transcription. An example is provided by ICDs generated following cleavage of N-cadherin. Ectodomain shedding of N-cadherin is followed by intramembranous proteolysis which generates a C-terminal fragment known as CTF-2. This fragment binds the transcription factor CREB-binding protein (CBP) and promotes its proteosomal degradation so that CREB-mediated transcription is repressed [42].
A third consequence of CAM shedding relates to the generation of shed N-terminal domains that are bioactive. As mentioned, these shed domains are stable enough to be detectable in CSF. As we will discuss, many can also interact with integrins that play an important role in microglial activation and synaptic function.
Integrins are heterodimeric cell surface proteins containing two distinct subunits. These dimers represent an especially important receptor class for shed CAMs. For example, nectin [43], E-cadherin, VCAM (VLA4), ICAM-1 (LFA-1 and Mac-1), and ICAM-5 (LFA-1 and β1) [29, 44, 45] can all interact with integrins in cis or trans. Members of the immunoglobulin superfamily such as ICAMs and VCAMs in particular have been referred to as a main class of integrin binding ligands [45]. The binding of full length CAMs to integrins might promote stable cell-cell interactions, while the binding of shed CAM ectodomains to unengaged integrins might allow for rapid changes in integrin signaling. In studies focused on ICAM-5, both full length and shed forms were found to co-immunoprecipitate with β1 integrins. In addition, we have observed that soluble ICAM-5 stimulates integrin dependent phosphorylation of cofilin in neuronal cells, an event which typically allows for actin polymerization [29].
Integrin dependent effects mediated by soluble CAMs are not without precedent. For example, in one report focused on L1CAM shedding, soluble L1 ectodomain stimulated cell migration in an integrin dependent manner. The authors suggested that L1 shedding might influence cell migration in both an autocrine and paracrine manner [46].
IV. MMPS, CAM CLEAVAGE, AND MICROGLIAL ACTIVATION
Classical microglial activation, in which release of specific proinflammatory molecules is increased [47, 48], occurs in the setting of HAND with substance abuse and likely plays a role in associated neuropathology. In several studies, HIV infection has been associated with an increase in the products of classically activated microglia, and it has been suggested that microglial activation may be an important mediator of CNS injury in the setting of both simian immunodeficiency virus encephalitis (SIVE) and HAND [49–51]. Minocycline has been linked to reduced microglial activation and disease severity with SIV infection, and SIV disease progresses more rapidly in animals showing an increase in markers of microglial activation. Increased activation of microglial cells has also been linked to the severity of HAND. Markers of macrophage/microglial cell activation are increased in association with disease severity, and apoptotic neurons are closely associated with markers of microglial activation [52]. Microglial activation also occurs with MA. Dopamine release and dopamine quinones in particular may be contributory [53]. Increased levels of reactive oxygen and nitrogen species may also play a role in that MA toxicity is reduced in copper/zinc superoxide dismutase transgenic mice [54].
Varied neuronal subpopulations may be vulnerable to neurotoxicity occurring as a result of microglial activation. These include glutamatergic neurons of the cortex and dopaminergic neurons of the substantia nigra. In animal models of Parkinson’s disease, microglial activation has been well linked to dopaminergic cell loss [55, 56]. Bacterial lipopolysaccharide, the prototypical inducer of strong microglial activation, also stimulates dopaminergic cell loss [57].
Studies suggest that MMP activity plays an important role in microglial activation. This claim is supported by studies in varied disease models. In a model of Parkinson’s disease, microglial activation, superoxide production and dopaminergic neuronal cell death were largely attenuated in MMP-3 null mice [55]. It has also been shown that inhibition of MMP-3 and -9 decreases LPS associated microglial activation [58]. Additional evidence for the importance of MMPs to microglial activation comes from studies with minocycline. In studies related to varied diseases and disease models, including Parkinson’s disease and multiple sclerosis, minocycline has shown neuroprotective effects [59]. Minocycline and other tetracycline derivatives can reduce MMP activity by at least two mechanisms including inhibition of microglial activation [49], which would reduce expression of microglial-derived MMPs, and direct inhibition of preformed MMPs via chelation of the active site zinc atom [60].
While the mechanisms by which MMPs activate microglia have not been well characterized, their ability to generate soluble integrin binding ligands may be particularly important. Microglia abundantly express varied integrins including the β2 integrins αLβ2 (LFA-1) and αMβ2 (Mac-1). Microglia also express β1 integrins α4β1 (VLA-4), α5β1, and α6β1 as well as the αv integrin αvβ6. Expression of α1, α2, αx, β4, and β7 has not been detected [61]. In terms of integrins that play a role in microglial activation, β2 containing integrins may be particularly important. A recent study showed that Mac-1 [αMβ2] was critical to the microglial activation of in a Parkinson’s Disease model. Activation of Mac-1 was associated with neurotoxin release and inhibition of Mac-1 signaling prevented neurodegeneration [62]. In a related study, Mac-1 was essential to β-amyloid induced microglial activation, production of superoxide, and neurotoxicity [63]. LFA-1 [αLβ2] also plays an important role in microglial activation. Microglial cells are the principal resident cells of the CNS to express LFA-1 [64]. Engagement of LFA-1 has been linked to microglial spreading and to transcription of AP-1 responsive genes [64], which may lead to an activated phenotype. Similar to β2 containing integrins, those containing β1 might also be important to microglial activation. β1 ligands can stimulate microglial cell activation and expression of pro-MMP-9 [65].
Shed CAMs interact with β integrins expressed on microglia. For example, VCAM has been shown to engage α4β1/VLA4, ICAM-1 has been shown to interact with LFA-1 and Mac-1, and ICAM-5 to interact with both both LFA-1 and β1 [29, 44, 45]. As stated earlier, in recently published work, we have observed that soluble ICAM-5 stimulates integrin dependent phosphorylation of cofilin, an event which allows for actin polymerization. Actin polymerization occurs with microglial activation and, as will be discussed, is also thought to occur with changes in the structure of postsynaptic structures known as dendritic spines.
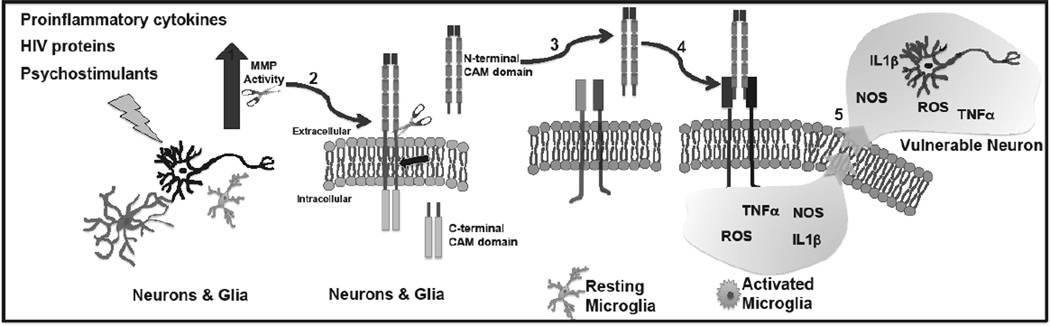
The expression of microglial integrins may be upregulated by inflammatory stimuli known to be elevated with HAND. Thus, an increase in MMP generated integrin binding ligands might assume greater significance in the setting of HAND. TNF-α can enhance surface expression of α4 and LFA-1, while both IL-1 and TNF-α can increase expression of Mac-1 [61]. Moreover, integrin affinity for ligands is regulated by “inside out signaling” in which select stimuli influence the intracellular milieu to increase an integrin’s ability to engage its ligand [45]. Like expression, integrin affinity may also be increased in the setting of inflammation [66]. For a schematic depicting CAM cleavage and subsequent effects on microglia, please see Fig. (1).
Fig. (1). MMPs generate integrin binding ligands to activate microglia.
Schematic diagram showing: 1. Increased MMP activity in the setting of HAND with substance abuse; 2. MMP- dependent shedding of CAM ectodomains. Note that following CAM shedding, remaining C-terminal fragments may be further processed by intramembranous proteolysis and degradation; 3 and 4. CAM ectodomain engagement of microglial integrins to stimulate classical activation with 5. increased release of potentially neurotoxic molecules.
V. MMPS, CAM CLEAVAGE, AND SYNAPTIC FUNCTION
While studies of MMPs in the CNS have generally focused on CNS inflammation and injury, recent evidence suggests that MMPs play a critical role in normal CNS physiology [67].
Neuronal activity has been linked to increased MMP release [37, 68–70] and in recent experiments, we have detected MMP dependent shedding of a neuronal substrate within 5 minutes of N-methyl-D-aspartic acid (NMDA) treatment [70]. Recent studies suggest that MMPs exist in perisynaptic vesicular stores [12] and that vesicular MMPs from fibroblasts may be soluble NSF attachment protein receptor [SNARE] dependent [13]. As suggested above, if neuronal release is also SNARE dependent, it may occur with select stimuli such as neurotransmitters that increase intracellular calcium.
While a relatively new area of investigation, MMP activity has been shown to influence dendritic spine morphology as well as learning and memory [71–75]. An increase in the size of spines, allowing for increased post synaptic neurotransmitter receptor abundance, occurs in many studies of long-term potentiation (LTP). Though effects may be influenced by MMP dose/duration, and the developmental stage of neurons, these enzymes have the potential to increase spine size and hippocampal dependent memory. For example, MMP inhibition has been shown to reduce multiple forms of hippocampal CA1 plasticity [76], and MMP-9 activity has been implicated in the maintenance of late LTP [73]. In addition, MMP antisense constructs have been shown to prevent acquisition in the Morris water maze test [74], and at least one MMP can increase dendritic spine size in a manner that is temporally coordinated with LTP [72].
MMPs may also play a role in the maladaptive sort of learning that underlies addiction. That MA associated changes in MMPs contribute to addiction is supported by behavioral studies. For example, MA-induced behavioral sensitization and conditioned place preference, a measure of the rewarding effect of a drug, is reduced in mice lacking MMP-2 or MMP-9 [77]. Of interest, MMPs also play a critical role in cocaine associated conditioned place preference [78–80].
Though the mechanisms by which MMPs affect synaptic structure and function are not well understood, it is tempting to speculate that CAM cleavage plays a role. For example, ICAM-5 is highly expressed on thin dendritic spines and MMP dependent ICAM-5 shedding has been linked to spine maturation. Shedding of specific CAMs may allow for spine expansion. A non-mutually exclusive possibility that we have investigated is the possibility that the shed ectodomain can bind to unengaged postsynaptic integrins to stimulate dendritic actin polymerization and spine expansion. Integrin signaling is critical during developmental changes in spine morphology [81], and multiple forms of learning associated plasticity, including that mediated by MMPs, are thought to be integrin dependent [73, 76].
While relatively low levels of MMPs and/or MMPs released in a physiologically localized manner may enhance learning and memory, it should be noted that in HAND with superimposed substance abuse, levels of these enzymes may be pathologically elevated. This could stimulate excessive cleavage of CAMs that otherwise maintain synaptic structure and neuronal survival. It could also lead to a situation in which processes governed by physiologically appropriate MMP release go awry.
Several publications support the possibility that high levels of MMPs may be toxic. While this can occur by indirect mechanisms, in which BBB integrity is first disrupted, relatively direct mechanisms occur as well. For example, exogenous MMP-1 has been linked to neuronal death in dissociated and organotypic neuronal cultures. MMP-9 has also been shown to be directly neurotoxic. Moreover, high levels of exogenous MMP-7, which has a broad substrate range and cleaves varied synaptic CAMs, can stimulate synaptic injury as detected by changes including a reduction in the area of the post synaptic density [82]. Consistent with this, synaptic injury in the setting of brain trauma has been observed with increased MMP levels, and trauma associated reduction in synaptophysin immunoreactivity were diminished by MMP inhibition [11]. Synaptic injury occurs in HIV with substance abuse [83, 84], and it is tempting to speculate that MMPs, and the cleavage of specific CAMs in particular, play a role. This could occur both through the activation of microglia and possibly, by excessive proteolysis of neuronal and synaptic CAMs. Cell death has indeed been observed as a result of a disruption in adhesion, a process known as anoikis [85].
VI. CONCLUSIONS
MMP levels may be elevated with HIV infection and MA abuse. CAMs represent an important class of MMP substrates, which are ideally positioned to be processed by cell surface and secreted MMPs. Since the integrity of CAMs is critical to synaptic stability, excess cleavage of synaptic adhesion molecules may play a role in HIV/MA associated synaptic and neuronal injury. In addition, shed CAM fragments may interact with microglial integrins to stimulate classical activation of this cell type. The products of classically activated microglia can in turn compound neuronal injury. Future studies are warranted to determine whether MMP inhibitors or specific microglial integrin antagonists might be beneficial for the treatment of CNS inflammation occurring with HIV and MA.
ACKNOWLEDGEMENTS
We are thankful for support from NIH (R21NS074916; STL and R01ES014470; KMZ), and from the von Matsch Professorship in Neurological Disease (KC).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Handsley MM, Cross J, Gavrilovic J, Edwards DR. Gottschall PE, Conant K. Matrix Metalloproteinases in the Central Nervous System. Vol.1. London: Imperial College Press; 2005. The Matrix Metalloproteinases and Their Inhibitors; pp. 3–16. [Google Scholar]
- 2.Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nature reviews. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 3.Knox JD, Boreham DR, Walker JA, et al. Mapping of the metalloproteinase gene matrilysin (MMP7) to human chromosome 11q21-->q22. Cytogenet Cell Genet. 1996;72:179–182. doi: 10.1159/000134181. [DOI] [PubMed] [Google Scholar]
- 4.Pendas AM, Balbin M, Llano E, et al. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13) Genomics. 1997;40:222–233. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- 5.Gu Z, Kaul M, Yan B, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 6.Clutterbuck AL, Asplin KE, Harris P, et al. Targeting matrix metalloproteinases in inflammatory conditions. Curr Drug Targets. 2009;10:1245–1254. doi: 10.2174/138945009789753264. [DOI] [PubMed] [Google Scholar]
- 7.Kohutek ZA, diPierro CG, Redpath GT, Hussaini IM. ADAM-10-mediated N-cadherin cleavage is protein kinase C-alpha dependent and promotes glioblastoma cell migration. J Neurosci. 2009;29:4605–4615. doi: 10.1523/JNEUROSCI.5126-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 9.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 10.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding JY, Kreipke CW, Schafer P, et al. Synapse loss regulated by matrix metalloproteinases in traumatic brain injury is associated with hypoxia inducible factor-1alpha expression. Brain Res. 2009;1268:125–134. doi: 10.1016/j.brainres.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Sbai O, Ferhat L, Bernard A et al. Vesicular trafficking and secretion of matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol Cell Neurosci. 2008;39:549–568. doi: 10.1016/j.mcn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Kean MJ, Williams KC, Skalski M, et al. VAMP3, syntaxin-13 and SNAP23 are involved in secretion of matrix metalloproteinases, degradation of the extracellular matrix and cell invasion. J Cell Sci. 2009;122:4089–4098. doi: 10.1242/jcs.052761. [DOI] [PubMed] [Google Scholar]
- 14.Salles FJ, Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J Neurosci. 2002;22:2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottschall PESJ, Zimmerman DR. Substrates for Metalloendopeptidases in the Central Nervous System. In: Gottschall PE, Conant K, editors. Matrix Metalloproteinases in the Central Nervous System. Vol. 1. London: Imperial College Press; 2005. pp. 87–118. [Google Scholar]
- 16.Boire A, Covic L, Agarwal A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Estrada EY, Thompson JF, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 18.Buhler LA, Samara R, Guzman E, et al. Matrix metalloproteinase-7 facilitates immune access to the CNS in experimental autoimmune encephalomyelitis. BMC neuroscience. 2009;10:17. doi: 10.1186/1471-2202-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brundula V, Rewcastle NB, Metz LM, et al. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- 20.Ghorpade A, Persidskaia R, Suryadevara R, et al. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 2001;75:6572–6583. doi: 10.1128/JVI.75.14.6572-6583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conant K, McArthur JC, Griffin DE, et al. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Sporer B, Paul R, Koedel U, et al. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:854–857. doi: 10.1086/515342. [DOI] [PubMed] [Google Scholar]
- 23.Conant K, St Hillaire C, Anderson C, et al. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol. 2004;10:21–28. doi: 10.1080/13550280490261699. [DOI] [PubMed] [Google Scholar]
- 24.Louboutin JP, Reyes BA, Agrawal L, et al. HIV-1 gp120 upregulates matrix metalloproteinases and their inhibitors in a rat model of HIV encephalopathy. Eur J Neurosci. 2011;34:2015–2023. doi: 10.1111/j.1460-9568.2011.07908.x. [DOI] [PubMed] [Google Scholar]
- 25.Misse D, Esteve PO, Renneboog B, et al. HIV-1 glycoprotein 120 induces the MMP-9 cytopathogenic factor production that is abolished by inhibition of the p38 mitogen-activated protein kinase signaling pathway. Blood. 2001;98:541–547. doi: 10.1182/blood.v98.3.541. [DOI] [PubMed] [Google Scholar]
- 26.Lafrenie RM, Wahl LM, Epstein JS, et al. HIV-1-Tat modulates the function of monocytes and alters their interactions with microvessel endothelial cells. A mechanism of HIV pathogenesis. J Immunol. 1996;156:1638–1645. [PubMed] [Google Scholar]
- 27.Mizoguchi H, Yamada K, Nabeshima T. Neuropsychotoxicity of abused drugs: involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J Pharmacol Sci. 2008;106:9–14. doi: 10.1254/jphs.fm0070139. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Brown S, Shaikh J, et al. Relationship between methamphetamine exposure and matrix metalloproteinase 9 expression. Neuroreport. 2008;19:1407–1409. doi: 10.1097/WNR.0b013e32830dd606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conant K, Lonskaya I, Szklarczyk A et al. Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J Neurochem. 2011;118:521–532. doi: 10.1111/j.1471-4159.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cozzi NV, Sievert MK, Shulgin AT, et al. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- 31.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci. 2007;27:6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 34.Speidl WS, Toller WG, Kaun C, et al. Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. Faseb J. 2004;18:603–605. doi: 10.1096/fj.03-0454fje. [DOI] [PubMed] [Google Scholar]
- 35.Takemoto Y, Yoshiyama M, Takeuchi K, et al. Increased JNK, AP-1 and NF-kappa B DNA binding activities in isoproterenol-induced cardiac remodeling. J Mol Cell Cardiol. 1999;31:2017–2030. doi: 10.1006/jmcc.1999.1033. [DOI] [PubMed] [Google Scholar]
- 36.Guillaumond F, Becquet D, Bosler O, Francois-Bellan AM. Adrenergic inducibility of AP-1 binding in the rat pineal gland depends on prior photoperiod. J Neurochem. 2002;83:157–166. doi: 10.1046/j.1471-4159.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 37.Conant K, Wang Y, Szklarczyk A, et al. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166:508–521. doi: 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vawter MP, Usen N, Thatcher L, et al. Characterization of human cleaved N-CAM and association with schizophrenia. Exp Neurol. 2001;172:29–46. doi: 10.1006/exnr.2001.7790. [DOI] [PubMed] [Google Scholar]
- 39.Guo H, Tong N, Turner T, et al. Release of the neuronal glycoprotein ICAM-5 in serum after hypoxic-ischemic injury. Ann Neurol. 2000;48:590–602. [PubMed] [Google Scholar]
- 40.Eugenin EA, Gamss R, Buckner C, et al. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J Leukoc Biol. 2006;79:444–452. doi: 10.1189/jlb.0405215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsberg PJ, Launes J, Tian L, et al. Release of soluble ICAM-5, a neuronal adhesion molecule, in acute encephalitis. Neurology. 2002;58:446–451. doi: 10.1212/wnl.58.3.446. [DOI] [PubMed] [Google Scholar]
- 42.Marambaud P, Wen PH, Dutt A, et al. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto Y, Ogita H, Hirota T, et al. Interaction of integrin alpha(v)beta3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J Biol Chem. 2006;281:19631–19644. doi: 10.1074/jbc.M600301200. [DOI] [PubMed] [Google Scholar]
- 44.Gahmberg CG, Tian L, Ning L, Nyman-Huttunen H. ICAM-5--a novel two-facetted adhesion molecule in the mammalian brain. Immunol Lett. 2008;117:131–135. doi: 10.1016/j.imlet.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Marciano DK, Denda S, Reichardt LF. Methods for identifying novel integrin ligands. Methods in enzymology. 2007;426:223–237. doi: 10.1016/S0076-6879(07)26011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mechtersheimer S, Gutwein P, Agmon-Levin N, et al. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001;155:661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 48.Jayadev S, Nesser NK, Hopkins S, et al. Transcription factor p53 influences microglial activation phenotype. Glia. 2011;59:1402–1413. doi: 10.1002/glia.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell JH, Burdo TH, Autissier P, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV NeuroAIDS. PLoS One. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zink MC, Uhrlaub J, DeWitt J, et al. Neuroprotective and antihuman immunodeficiency virus activity of minocycline. Jama. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- 51.Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- 52.Gray F, Adle-Biassette H, Brion F, et al. Neuronal apoptosis in human immunodeficiency virus infection. J Neurovirol. 2000;6(Suppl 1):S38–S43. [PubMed] [Google Scholar]
- 53.Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- 54.Cadet JL, Sheng P, Ali S, et al. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;62:380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- 55.Kim YS, Choi DH, Block ML, et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. Faseb J. 2007;21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 56.Hamill CE, Caudle WM, Richardson JR, et al. Exacerbation of dopaminergic terminal damage in a mouse model of Parkinson's disease by the G-protein-coupled receptor protease-activated receptor 1. Mol Pharmacol. 2007;72:653–664. doi: 10.1124/mol.107.038158. [DOI] [PubMed] [Google Scholar]
- 57.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 58.Woo MS, Park JS, Choi IY, et al. Inhibition of MMP-3 or-9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106:770–780. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- 59.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 60.Duivenvoorden WC, Hirte HW, Singh G. Use of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cells. Invasion & metastasis. 1997;17:312–322. [PubMed] [Google Scholar]
- 61.Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–3858. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- 62.Gao HM, Zhou H, Zhang F, et al. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang D, Hu X, Qian L, et al. Microglial MAC1 receptor and PI3K are essential in mediating beta-amyloid peptide-induced microglial activation and subsequent neurotoxicity. J Neuroinflammation. 2011;8:3. doi: 10.1186/1742-2094-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuno T, Yoshihara Y, Kagamiyama H, et al. Neuronal adhesion molecule telencephalin induces rapid cell spreading of microglia. Brain Res. 1999;849:58–66. doi: 10.1016/s0006-8993(99)01984-8. [DOI] [PubMed] [Google Scholar]
- 65.Milner R, Crocker SJ, Hung S, et al. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins alpha5beta1 and alphavbeta5. J Immunol. 2007;178:8158–8167. doi: 10.4049/jimmunol.178.12.8158. [DOI] [PubMed] [Google Scholar]
- 66.Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- 67.Milward EA, Fitzsimmons C, Szklarczyk A, Conant K. The matrix metalloproteinases and CNS plasticity: an overview. J Neuroimmunol. 2007;187:9–19. doi: 10.1016/j.jneuroim.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Michaluk P, Kolodziej L, Mioduszewska B, et al. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- 69.Pauly T, Ratliff M, Pietrowski E, et al. Activity-dependent shedding of the NMDA receptor glycine binding site by matrix metalloproteinase 3: a PUTATIVE mechanism of postsynaptic plasticity. PLoS One. 2008;3:e2681. doi: 10.1371/journal.pone.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagy V, Bozdagi O, Matynia A, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang XB, Bozdagi O, Nikitczuk JS, et al. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagy V, Bozdagi O, Matynia A, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meighan SE, Meighan PC, Choudhury P, et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- 75.Tian L, Stefanidakis M, Ning L, et al. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meighan PC, Meighan SE, Davis CJ, et al. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- 77.Mizoguchi H, Yamada K, Mouri A, et al. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007;102:1548–1560. doi: 10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- 78.Brown TE, Forquer MR, Cocking DL, et al. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learning & memory (Cold Spring Harbor, N.Y. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown TE, Forquer MR, Harding JW, et al. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- 80.Maiya R, Zhou Y, Norris EH, et al. Tissue plasminogen activator modulates the cellular and behavioral response to cocaine. Proc Natl Acad Sci USA. 2009;106:1983–1988. doi: 10.1073/pnas.0812491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sfakianos MK, Eisman A, Gourley SL, et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J Neurosci. 2007;27:10982–10992. doi: 10.1523/JNEUROSCI.0793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szklarczyk A, Conant K, Owens DF, et al. Matrix metalloproteinase-7 modulates synaptic vesicle recycling and induces atrophy of neuronal synapses. Neuroscience. 2007;149:87–98. doi: 10.1016/j.neuroscience.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 83.Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;2:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 84.Langford D, Adame A, Grigorian A, et al. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- 85.Valentijn AJ, Zouq N, Gilmore AP. Anoikis. Biochem Soc Trans. 2004;32:421–425. doi: 10.1042/BST0320421. [DOI] [PubMed] [Google Scholar]
- 86.Vitolo D, Paradiso P, Uccini S, et al. Expression of adhesion molecules and extracellular matrix proteins in glioblastomas: relation to angiogenesis and spread. Histopathology. 1996;28:521–528. doi: 10.1046/j.1365-2559.1996.d01-471.x. [DOI] [PubMed] [Google Scholar]
- 87.Tsakadze NL, Sithu SD, Sen U, et al. Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2006;281:3157–3164. doi: 10.1074/jbc.M510797200. [DOI] [PubMed] [Google Scholar]
- 88.Lyons PD, Benveniste EN. Cleavage of membrane-associated ICAM-1 from astrocytes: involvement of a metalloprotease. Glia. 1998;22:103–112. doi: 10.1002/(sici)1098-1136(199802)22:2<103::aid-glia1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 89.Clark WM, Lauten JD, Lessov N, et al. Time course of ICAM-1 expression and leukocyte subset infiltration in rat forebrain ischemia. Molecular and chemical neuropathology / sponsored by the International Society for Neurochemistry and the World Federation of Neurology and research groups on neurochemistry and cerebrospinal fluid. 1995;26:213–230. doi: 10.1007/BF02815139. [DOI] [PubMed] [Google Scholar]
- 90.Satoh J, Kastrukoff LF, Kim SU. Cytokine-induced expression of intercellular adhesion molecule-1 (ICAM-1) in cultured human oligodendrocytes and astrocytes. J Neuropathol Exp Neurol. 1991;50:215–226. doi: 10.1097/00005072-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Rutishauser U, Acheson A, Hall AK, et al. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988;240:53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- 92.Bhat S, Silberberg DH. Developmental expression of neural cell adhesion molecules of oligodendrocytes in vivo and in culture. J Neurochem. 1988;50:1830–1838. doi: 10.1111/j.1471-4159.1988.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Neumann H. Alleviation of neurotoxicity by microglial human Siglec-11. J Neurosci. 30:3482–3488. doi: 10.1523/JNEUROSCI.3940-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinkle CL, Diestel S, Lieberman J, Maness PF. Metalloprotease-induced ectodomain shedding of neural cell adhesion molecule (NCAM) J Neurobiol. 2006;66:1378–1395. doi: 10.1002/neu.20257. [DOI] [PubMed] [Google Scholar]
- 95.Niquet J, Jorquera I, Ben-Ari Y, Represa A. NCAM immunoreactivity on mossy fibers and reactive astrocytes in the hippocampus of epileptic rats. Brain Res. 1993;626:106–116. doi: 10.1016/0006-8993(93)90569-9. [DOI] [PubMed] [Google Scholar]
- 96.Gutwein P, Stoeck A, Riedle S, et al. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res. 2005;11:2492–2501. doi: 10.1158/1078-0432.CCR-04-1688. [DOI] [PubMed] [Google Scholar]
- 97.Maretzky T, Schulte M, Ludwig A, et al. L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol. 2005;25:9040–9053. doi: 10.1128/MCB.25.20.9040-9053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nolte C, Moos M, Schachner M. Immunolocalization of the neural cell adhesion molecule L1 in epithelia of rodents. Cell Tissue Res. 1999;298:261–273. doi: 10.1007/s004419900063. [DOI] [PubMed] [Google Scholar]
- 99.Itoh K, Sakurai Y, Asou H, Umeda M. Differential expression of alternatively spliced neural cell adhesion molecule L1 isoforms during oligodendrocyte maturation. J Neurosci Res. 2000;60:579–586. doi: 10.1002/(SICI)1097-4547(20000601)60:5<579::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 100.Guaiquil V, Swendeman S, Yoshida T, et al. ADAM9 is involved in pathological retinal neovascularization. Mol Cell Biol. 2009;29:2694–2703. doi: 10.1128/MCB.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wassmer SC, Moxon CA, Taylor T, et al. Vascular endothelial cells cultured from patients with cerebral or uncomplicated malaria exhibit differential reactivity to TNF. Cellular microbiology. 2011;13:198–209. doi: 10.1111/j.1462-5822.2010.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peterson JW, Bo L, Mork S, et al. VCAM-1-positive microglia target oligodendrocytes at the border of multiple sclerosis lesions. J Neuropathol Exp Neurol. 2002;61:539–546. doi: 10.1093/jnen/61.6.539. [DOI] [PubMed] [Google Scholar]
- 103.Birdsall HH, Lane C, Ramser MN, Anderson DC. Induction of VCAM-1 and ICAM-1 on human neural cells and mechanisms of mononuclear leukocyte adherence. J Immunol. 1992;148:2717–2723. [PubMed] [Google Scholar]
- 104.Kaluza J, Krupinski J, Kumar P, et al. VCAM-1 expression on reactive and tumour astrocytes. Folia Histochem Cytobiol. 1994;32(1):17–20. [PubMed] [Google Scholar]
- 105.Reiss K, Maretzky T, Ludwig A, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. Embo J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Payne HR, Hemperly JJ, Lemmon V. N-cadherin expression and function in cultured oligodendrocytes. Brain Res Dev Brain Res. 1996;97:9–15. doi: 10.1016/s0165-3806(96)00124-1. [DOI] [PubMed] [Google Scholar]
- 107.Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J Cell Biol. 1988;107:1177–1187. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams H, Johnson JL, Jackson CL, et al. MMP-7 mediates cleavage of N-cadherin and promotes smooth muscle cell apoptosis. Cardiovascular research. 2010;87:137–146. doi: 10.1093/cvr/cvq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maretzky T, Reiss K, Ludwig A, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee KH, Choi EY, Hyun MS, et al. Association of extracellular cleavage of E-cadherin mediated by MMP-7 with HGF-induced in vitro invasion in human stomach cancer cells. European surgical research. Eur Surg Res. 2007;39(4):208–215. doi: 10.1159/000101452. [DOI] [PubMed] [Google Scholar]
- 111.Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem. 2008;283:18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maretzky T, Scholz F, Koten B, et al. ADAM10-mediated E-cadherin release is regulated by proinflammatory cytokines and modulates keratinocyte cohesion in eczematous dermatitis. J Invest Dermatol. 2008;128:1737–1746. doi: 10.1038/sj.jid.5701242. [DOI] [PubMed] [Google Scholar]
- 113.Mizuhara E, Minaki Y, Nakatani T, et al. Purkinje cells originate from cerebellar ventricular zone progenitors positive for Neph3 and E-cadherin. Developmental biology. 2010;338:202–214. doi: 10.1016/j.ydbio.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 114.Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- 115.Ichikawa Y, Ishikawa T, Momiyama N, et al. Matrilysin (MMP-7) degrades VE-cadherin and accelerates accumulation of beta-catenin in the nucleus of human umbilical vein endothelial cells. Oncology reports. 2006;15:311–315. [PubMed] [Google Scholar]
- 116.Kim J, Lilliehook C, Dudak A. Activity-dependent alpha-cleavage of nectin-1 is mediated by a disintegrin and metalloprotease 10 (ADAM10) J Biol Chem. 2010;285:22919–22926. doi: 10.1074/jbc.M110.126649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim DY, Ingano LA, Kovacs DM. Nectin-1alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem. 2002;277:49976–49981. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 118.Mizoguchi A, Nakanishi H, Kimura K, et al. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol. 2002;156:555–565. doi: 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Su G, Blaine SA, Qiao D, Friedl A. Membrane type 1 matrix metalloproteinase-mediated stromal syndecan-1 shedding stimulates breast carcinoma cell proliferation. Cancer Res. 2008;68:9558–9565. doi: 10.1158/0008-5472.CAN-08-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hayashida K, Stahl PD, Park PW. Syndecan-1 ectodomain shedding is regulated by the small GTPase Rab5. J Biol Chem. 2008;283:35435–35444. doi: 10.1074/jbc.M804172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 122.Chen P, Abacherli LE, Nadler ST, et al. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting alpha(2)beta(1) integrin activation. PLoS One. 2009;4:e6565. doi: 10.1371/journal.pone.0006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brule S, Charnaux N, Sutton A, et al. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16:488–501. doi: 10.1093/glycob/cwj098. [DOI] [PubMed] [Google Scholar]
- 124.Murakami K, Namikawa K, Shimizu T, et al. Nerve injury induces the expression of EXT2, a glycosyltransferase required for heparan sulfate synthesis. Neuroscience. 2006;141:1961–1969. doi: 10.1016/j.neuroscience.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 125.Properzi F, Lin R, Kwok J, et al. Heparan sulphate proteoglycans in glia and in the normal and injured CNS: expression of sulphotransferases and changes in sulphation. Eur J Neurosci. 2008;27:593–604. doi: 10.1111/j.1460-9568.2008.06042.x. [DOI] [PubMed] [Google Scholar]
- 126.Ohnishi H, Kobayashi H, Okazawa H, et al. Ectodomain shedding of SHPS-1 and its role in regulation of cell migration. J Biol Chem. 2004;279:27878–27887. doi: 10.1074/jbc.M313085200. [DOI] [PubMed] [Google Scholar]
- 127.Comu S, Weng W, Olinsky S, et al. The murine P84 neural adhesion molecule is SHPS-1, a member of the phosphatase-binding protein family. J Neurosci. 1997;17:8702–8710. doi: 10.1523/JNEUROSCI.17-22-08702.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kusakari S, Ohnishi H, Jin FJ, et al. Trans-endocytosis of CD47 and SHPS-1 and its role in regulation of the CD47-SHPS-1 system. J Cell Sci. 2008;121:1213–1223. doi: 10.1242/jcs.025015. [DOI] [PubMed] [Google Scholar]
- 129.Tomizawa T, Kaneko Y, Kaneko Y, et al. Resistance to experimental autoimmune encephalomyelitis and impaired T cell priming by dendritic cells in Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 mutant mice. J Immunol. 2007;179:869–877. doi: 10.4049/jimmunol.179.2.869. [DOI] [PubMed] [Google Scholar]
- 130.Johansen ML, Brown EJ. Dual regulation of SIRPalpha phosphorylation by integrins and CD47. J Biol Chem. 2007;282:24219–24230. doi: 10.1074/jbc.M701565200. [DOI] [PubMed] [Google Scholar]