Abstract
In recent years, several therapeutic drugs have been rationally designed and synthesized based on the novel knowledge gained from investigating the actions of biologically active chemicals derived from foods, plants, and medicinal herbs. One of the major advantages of these naturalistic chemicals is their ability to interact with multiple targets in the body resulting in a combined beneficial effect. Ginseng is a perennial herb (Araliaceae family), a species within the genus Panax, and a highly valued and popular medicinal plant. Evidence for the medicinal and health benefits of Panax ginseng and its components in preventing neurodegeneration has increased significantly in the past decade. The beneficial effects of P. ginseng on neurodegenerative diseases have been attributed primarily to the antioxidative and immunomodulatory activities of its ginsenoside components. Mechanistic studies on the neuroprotective effects of ginsenosides revealed that they act not only as antioxidants but also as modulators of intracellular neuronal signaling and metabolism, cell survival/death genes, and mitochondrial function. The goal of the present paper is to provide a brief review of recent knowledge and developments concerning the beneficial effects as well as the mechanism of action of P. ginseng and its components in the treatment and prevention of neurodegenerative diseases.
Keywords: antioxidant, ginsenosides, neurodegenerative diseases, Panax ginseng
1. Introduction
1.1. Classification, structures, and chemical properties of ginseng components
Ginseng is a perennial herb (Araliaceae family), a species within the genus Panax, and a highly valued and popular medicinal plant [1]. The name “ginseng” originates from the Chinese words “Jen Sheng” and means “man herb” because of the human-like shape of the root or rhizome of the plant. The word Panax means “cure all” and describes the traditional belief that ginseng has properties that heal all bodily diseases. To date, 14 plants, including 12 species and two infraspecific taxa, have been classified under the genus Panax [2]. The three major commercial ginseng sorts are the Korean ginseng (Panax ginseng Meyer), the Chinese ginseng [Panax notoginseng (Burk.) F. H. Chen], and the American ginseng (Panax quinquefolius L.), and they have been used worldwide as herbal medicines for thousands of years [3].
Korean ginseng (P. ginseng Meyer) is a well-known medicinal herb cultivated in eastern Asian countries [4]. P. ginseng is native to China and Korea, but is now widely cultivated in other countries such as Japan, Russia, the United States, and Canada. Root of the Korean ginseng has traditionally been used to treat various diseases, particularly as an adaptogen since it is suggested to normalize body functions and increase physical strength [5], [6]. As fresh ginseng tends to be easily degraded at room temperature, it has traditionally been processed into white ginseng through air drying of the root or into red ginseng through root steaming followed by drying [7], [8], [9]. In Korea, red ginseng and various processed ginseng products are used popularly as functional foods or nutritional supplements. Based on recent studies, red ginseng has been reported to have biological benefits while inducing fewer side effects compared with fresh and white ginseng [7], [10], [11], [12], [13], [14]. In addition, Korean Red Ginseng is known to possess various biological activities including boosting the immune system, improving the blood circulation, enhancing memory, antifatigue effects, antioxidant effects, and positive effects on menopausal disorder [10], [11], [12], [13], [14].
Korean ginseng is known to have various therapeutic benefits mediated by its well-studied active components [15], [16], [17], [18], [19], [20], [21]. Indeed, Korean ginseng is reported to contain various functional constituents, including most notably ginseng saponins (also called ginsenosides), polyacetylenes, phenolic compounds, sesquiterpenes, alkaloids, polysaccharides, and oligopeptides [22].
1.2. Ginsenosides
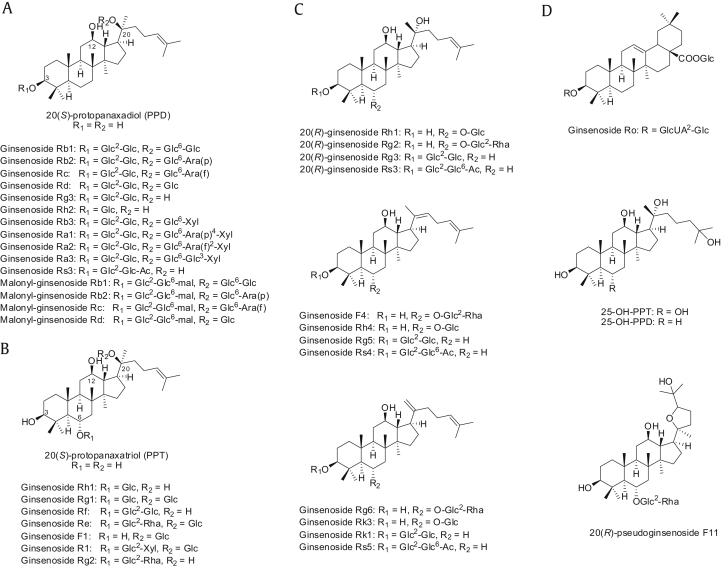
First attempts to isolate ginsenosides happened in the 1960s [23], [24] and most were identified from the Panax species. Ginsenosides are biosynthesized from 2,3-oxidosqualene, which leads to the formation of cycloartenol, dammarenediol-II, and β-amyrin by the action of three different enzymes. Dammarenediol-II and β-amyrin are eventually biotransformed into ginsenosides [2]. Based on their chemical structures, ginsenosides are typically divided into two groups: four-ring dammarane type and five-ring oleanane type. Dammarenediol-II is the precursor of the dammarane type, including ginsenosides Rb1, Rb2, Re, and Rg1, which account for a significant portion of ginsenosides found in ginseng species. Oleanane-type ginsenosides, on the contrary, are biosynthesized from β-amyrin. However, oleanane-type ginsenosides such as Ro are rare and often undetectable in P. ginseng. Dammarane-type ginsenosides are further classified into two groups: protopanaxadiols (PPDs) and protopanaxatriols (PPTs). Dammarenediol-II is hydroxylated into a PPD, 3β,12β,20-trihydroxydammar-24-ene. Consequently, a number of ginsenosides are biosynthesized by the O-glycosylation of PPDs, which involves the attachment of saccharides to carbon (C)-3 and/or C-20. PPD-type ginsenosides include Rb1, Rb2, Rc, Rd, Rg3, Rh2, and Rh3 (Fig. 1). Dammarenediol-II is further hydroxylated into a PPT, 3β,6α,12β,20-tetrahydroxydammar-24-ene. A variety of ginsenosides are biosynthesized by O-glycosylation of PPTs, which involves the linkage of saccharides to C-6 and/or C-20. Typically, the hydroxyl group at C-3 remains free in PPT-type ginsenosides. Typical PPT-type ginsenosides in P. ginseng are Re, Rf, Rg1, Rg2, and Rh1 (Fig. 1). While most naturally occurring ginsenosides are of the (S)-configuration at C-20, some artifactual ginsenosides exist in two epimeric forms at the carbon. The pseudoginsenoside F11 belongs to the PPT group although the carbon chain at C-20 is replaced by a tetrahydrofuran ring (Fig. 1). Several new ginsenosides such as 25-OH-PPD and 25-OH–PPT were recently isolated from ginseng berries [25]. Four malonyl derivatives of ginsenosides, Rb1, Rb2, Rc, and Rd, have also been reported [25]. The malonyl derivatives and ginsenoside Ro are also called “acidic” ginsenosides, while the others are called “neutral” ginsenosides [26].
Fig. 1.
Structure of selected ginsenosides. (A) PPDs. (B) PPTs. (C) Derivatives of PPDs and PPTs. (D) Other ginsenosides. Ac, acetyl; Ara(f), α-L-arabinose(furanose); Ara(p), α-L-arabinose(pyranose); Glc, β-D-glucose; GlcUA, β-D-glucuronic acid; mal, malonyl; PPD, protopanaxadiol; PPT, protopanaxatriols; Rha, α-L-rhamnose; Xyl, β-D-xylose.
Heat treatment induces deglycosylation of ginsenosides. As a result, red ginseng has relatively high concentrations of the less polar ginsenosides transformed from fresh ginseng ginsenosides. Red ginseng contains ginsenosides Rg2, Rg6, F4, 20(E)-F4, Rh1, Rh4, Rk3, Rg3, Rg5, Rz1, Rk1, Rg9, and Rg10, which are converted from the major ginsenosides Rb1, Rb2, Rc, Rd, Rg1, and Re [7]. Generally, ginsenoside deglycosylation during the process of red ginseng production results in these conversions: [Rg1 → Rh1 → (Rh4, Rk3)], [Re → Rg2 → (F4, Rg6)], [Rf → (Rg9, 20Z-Rg9, Rg10)], and [(Rb1, Rc, Rb2, Rd) → Rg3 → (Rg5, Rk1, Rz1)] [7]. These results are consistent with the experimental evidence that the levels of the less polar ginsenosides such as Rg2, Rh1, and Rg3 progressively increase, whereas the levels of the natural ginsenosides such as Rg1, Re, Rb1, Rc, and Rd progressively decrease during the heat-processed red ginseng production [27].
1.3. Polyacetylenes
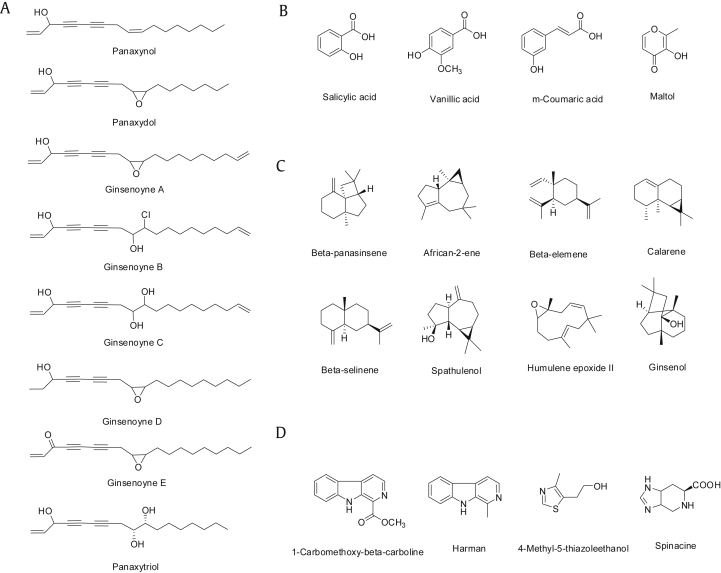
Polyacetylenes are representative nonsaponin components of ginseng. The first polyacetylene identified and extracted from P. ginseng was panaxynol [28]. Since then, many polyacetylenic substances, including panaxydol and ginsenoynes A–E, have been identified and extracted from P. ginseng (Fig. 2) [22]. Panaxytriol is a hydrated compound with an epoxy ring derived from panaxydol by heat and acid treatment (Fig. 2). These P. ginseng polyacetylenes are believed to possess anticancer properties. However, their in vivo efficacy has not been determined due to their chemical instability.
Fig. 2.
Structure of selected nonsaponin constituents. (A) Polyacetylenes. (B) Phenolic compounds. (C) Sesquiterpenes. (D) Alkaloids.
1.4. Phenolic compounds
Phenolic compounds generally possess antioxidative and anticancer biological properties. However, phenolic compounds found in ginseng are relatively less investigated. More than 10 phenolic compounds have previously been reported in fresh and/or processed ginseng (Fig. 2). These include salicylic acid, vanillic acid, ascorbic acid, p-coumaric acid, ferulic acid, caffeic acid, gentisic acid, p-hydroxybenzoic acid, maltol, cinnamic acid, protocatechuic acid, syringic acid, and quercetin [29]. A recent study revealed that chlorogenic acid, gentisic acid, p- and m-coumaric acid, and rutin are the major phenolic compounds in 3–6-yr-old ginseng fruits, leaves, and roots [30]. Korean ginseng, which is suggested to provide more health benefits than other ginseng species, usually contains more phenolic compounds than Chinese ginseng [31].
1.5. Sesquiterpenes
A number of sesquiterpene hydrocarbons as well as oxygenated sesquiterpenes have been identified as volatile constituents of P. ginseng. More than 15 sesquiterpenes have been identified as volatile constituents of P. ginseng. These include sesquiterpene hydrocarbons such as β-panasinsene, african-2-ene, β-elemene, calarene, (E)-β-farnesene, α-humulene, α-neoclovene, 2-epi-(E)-β-caryophyllene, β-neoclovene, β-selinene, and bicyclogermacrene, and oxygenated sesquiterpenes such as spathulenol, humulene epoxide II, ginsenol, hexadecanoic acid, and falcarinol (Fig. 2) [32], [33].
1.6. Alkaloids
Alkaloids are another nonsaponin component of Korean ginseng and include 1-carbomethoxy-β-carboline, N9-formylharman, harman, norharman, perlolyrine, 4-methyl-5-thiazoleethanol, and spinacine (Fig. 2) [22]. Recently, a new indole alkaloid, ginsenine, with a seven-membered lactam unit, was isolated from P. ginseng berries [34]. These alkaloids are minor components of P. ginseng and their biological activities are also limited.
1.7. Polysaccharides
Korean ginseng contains various polysaccharides. The hypoglycemic glycans, panasans A–E, and panaxans I–L, M–P, and Q–U have been isolated from the roots of P. ginseng [3], [35], [36], [37]. It has been recognized that the composition of the polysaccharides varies depending on strains and/or places of production [35], [37]; however, acid hydrolysis, reduction, acetylation followed by gas–liquid chromatography of these glycans showed that they consist of diverse combinations of neutral sugars including rhamnose, mannose, galactose, arabinose, galactose, and glucose. In addition, the immunomodulating glycans ginsenan PA and ginsenan PB were identified in P. ginseng root [38]. These immunomodulating glycans are composed of L-arabinose, D-galactose, L-rhamnose, D-galacturonic acid, and D-glucuronic acid, but their exact structure is still unknown. Other immunomodulating glycans, such as acidic polysaccharide ginsenan S-IA and ginsenan S-II A, have also been identified. By contrast, ginseng polysaccharides are mainly composed of neutral polysaccharides (starch-like glucans) and acidic substances (ginseng pectin) [39]. Ginseng pectins have been reported to show a wider range of pharmacological activities compared with neutral polysaccharides [40], [41], and they are known to be composed of galacturonic acid, galactose, glucose, arabinose, rhamnose, glucuronic acid, and mannose [41]; however, their exact structure is also unknown.
2. Basic and clinical evidence on the beneficial effects of P. ginseng on neurodegenerative diseases
2.1. Alzheimer's disease
Alzheimer’s disease is a neurodegenerative disorder that affects the central nervous system and results in a loss of memory and basic motor functions. It is one of the most common causes of mental deterioration in elderly people and accounts for around 50–60% of the overall cases of dementia [42], [43]. The major brain areas affected in Alzheimer’s disease include the cerebral cortex, locus coeruleus, nucleus basalis of Meynert, and hippocampus [44]. The pathological characteristics include extracellular deposits of amyloid β (derived from amyloid precursor protein) in senile plaques, intracellular formation of neurofibrillary tangles (containing an abnormally phosphorylated form of tau, a microtubule-associated protein), and loss of neuronal synapses and pyramidal neurons [42], [45].
Since the exact mechanism underlying Alzheimer's disease is not fully understood, current therapies are largely based on a number of theories/hypotheses regarding the pathogenesis of the disease. The cholinergic hypothesis of Alzheimer's disease is based on the reported presynaptic deficits observed in Alzheimer's disease-affected brains and on the role of the cholinergic system in animal and human behavior [42], [46]. The most common treatment strategy in Alzheimer's disease involves acetylcholine (ACh), an important neurotransmitter in cognition and memory processes that is known to be decreased in Alzheimer's disease. Treatment options include the use of ACh precursors, ACh-releasing agents, and acetylcholinesterase (AChE) inhibitors [47], [48]. Other therapeutic interventions, although with fewer proven beneficial effects, also involve antioxidative agents that scavenge free radicals and anti-inflammatory agents that treat the amyloid β cascade [48].
Basic and clinical evidence of the beneficial effects of P. ginseng on Alzheimer's disease is summarized in Table 1. In male Sprague-Dawley rats, P. ginseng extracts have been reported to exert neuroprotective effects by ameliorating the advanced glycation end product-induced memory impairment and mitigating the Alzheimer-like pathophysiological changes through downregulation of the RAGE/NF-κB pathway [51]. Moreover, ginsenosides attenuated d-galactose- and aluminium chloride (AlCl3)- induced spatial memory impairment and Alzheimer-like pathophysiological changes in male Wistar rats through restoration of amyloid β formation, tau phosphorylation, and function of various neurotransmitters including glutamate (Glu), aspartate (Asp), gamma-aminobutyric acid (GABA), acetylcholine (ACh), dopamine (DA), glycine (Gly), and 5-hydroxytryptamine (5-HT) [52]. Ginsenosides are reported to improve memory loss in C57BL/6J mice with severe hippocampal damage and in aged SAMP8 mice (senescence-accelerated mouse) by upregulating plasticity-related proteins such as postsynaptic density protein-95 (PSD-95), gamma isotype of protein kinase C (PKCγ), and brain-derived neurotrophic factor (BDNF) [54].
Table 1.
Effects of P. ginseng and its active ingredient on Alzheimer's disease
| Active ingredient | Target molecules | Cell lines or animal strain (toxicants) | Effective doses (treatment time) | References |
|---|---|---|---|---|
| Rg1 | TNF-α, IFN-β, iNOS, TLR3, TLR4, NF-κB, and TRAF-6 | NG108-15 cells (amyloid β peptide 25–35) | 8 μg/mL, 16 μg/mL, and 32 μg/mL (24 h) | [49] |
| Rb1 | CAP1, CAPZB, TOMM40, DSTN, PARP-1, and Bax | SH-SY5Y cells (amyloid β) | 100μM (24 h) | [50] |
| P. ginseng extract | RAGE and NF-κB | Male Sprague-Dawley rats (advanced glycation end product) | 0.25 g/kg/d, 0.5 g/kg/d, and 1 g/kg/d (30 d) | [51] |
| Ginseng total saponin | Aβ, tau, Glu, Asp, GABA, Ach, DA, Gly, and 5-HT | Male Wistar rats (d-galactose with AlCl3) | 2 g/kg/d (30 d) | [52] |
| Ginseng total saponin | PSD-95, PKCγ, and BDNF | Female C57BL/6J mice (aged mice: 12 mo old) | 0.056% and 0.112% (w/v) (8 mo) | [53] |
| Ginseng total saponin | PSD-95, p-NMDAR1, p-CaMKII, p-PKA Cβ, PKCγ, p-CREB, and BDNF | Male SAMP8 and SAMR1 mice (aged mice: 4 mo old) | 100 mg/kg/d and 200 mg/kg/d (7 mo) | [54] |
| Rh1 | BDNF | Male ICR mice (aged mice: 6 mo old) | 10 mg/kg/d (3 mo) | [55] |
| Rg5 | TNF-α, IL-1β, IGF-1, BDNF, COX-2, iNOS, and Aβ | Wistar rats (streptozotocin) | 10 mg/kg/d and 20 mg/kg/d (28 d) | [56] |
| Rg5 and Rh3 | BDNF and CREB | Male ICR mice (scopolamine) | 10 mg/kg (1 h) | [57] |
| Rg1 | GSK3β and tau | Male Sprague-Dawley rats (okadaic acid) | 20 mg/kg/d (25 d) | [58] |
Ginsenoside Rb1 protected against amyloid β-induced neurotoxicity in SH-SY5Y cells by regulating the adenylate cyclase-associated protein 1 (CAP1), capping protein (actin filament) muscle Z-line beta (CAPZB), translocase of outer mitochondrial membrane 40 homolog (TOMM40), and destrin (DSTN) proteins related to actin cytoskeleton organization and by decreasing the levels of apoptotic proteins such as poly (ADP-ribose) polymerase 1 (PARP-1) and Bax [50]. The expression of BDNF, a key modulator of neuronal survival, activity, and synaptic transmission, and a key player in hippocampal-dependent learning and memory, was increased in male ICR mice treated with ginsenoside Rh1, resulting in enhanced survival of dentate gyrus cells. Ginsenoside Rh1 was also reported to protect newborn neurons from death during the neuronal differentiation process [53], [55].
Ginsenoside Rg5 improved cognition and amyloid β deposition in a Wistar rat model by increasing insulin-like growth factor 1 (IGF-1) and BDNF levels and decreasing tumor necrosis factor-alpha (TNF-α) and interleukin 1 beta (IL-1β) as well as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) levels [56]. Ginsenoside Rg5 and Rh3 reversed scopolamine-induced memory deficits in male ICR mice by inhibiting AChE activity and increasing BDNF expression and cAMP response element binding protein (CREB) activation [57]. Ginsenoside Rg1 reduced the mRNA and protein expressions of Toll-like receptor (TLR3), TLR4, nuclear factor kappa B (NF-κB), and TNF receptor associated factor-6 (TRAF-6) and downregulated the levels of TNF-α and interferon beta-1 (IFN-β) in an NG108-15 neuroglial cell line stimulated by amyloid β peptide 25–35 [49]. Moreover, ginsenoside Rg1 attenuated okadaic acid-induced memory impairment in male Sprague-Dawley rats through the glycogen synthase kinase 3 beta (GSK3β)/tau signaling pathway and the prevention of amyloid β formation [58].
The potential efficacy of a heat-processed form of ginseng on cognitive function and behavioral symptoms has recently been reported in a clinical study in patients with moderately severe Alzheimer's disease. Indeed, ginseng-treated patients showed a significant improvement on the Mini-Mental State Examination (MMSE) and Alzheimer's Disease Assessment Scale (ADAS). Moreover, patients treated with higher ginseng doses (4.5 g/d) showed further improvements in their ADAS cognitive, ADAS noncognitive, and MMSE scores as early as 12 wk following treatment. This improvement was sustained over a follow-up period of 24 wk [59]. However, the effects of ginseng on Alzheimer’s disease remain inconclusive as reported in a recent meta-analysis study including seven main databases for randomized clinical trials [60]. The main limitations of the available studies are small sample size, poor methodological qualities, and the absence of placebo controls [60].
2.2. Parkinson's disease
Parkinson's disease is a neurodegenerative disorder commonly affecting the elderly. It is characterized by degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) as well as other regions of the central nervous system [61], [62], [63], [64], [65]. Dopaminergic degeneration is typically associated with the presence of protein deposits, called Lewy bodies, in the neuronal cytoplasm and thread-like proteinaceous inclusions, called Lewy neurites, within neuronal neurites [66], [67], [68]. The main clinical symptoms include resting tremor, bradykinesia, rigidity, and postural instability, which result in impaired movement and other neurological dysfunctions. The current understanding of the pathophysiology of Parkinson's disease largely stemmed from elegant neurochemical investigations in the 1950–1960s that demonstrated over 80% reduction in striatal dopamine along with the loss of SNpc dopaminergic neurons in most Parkinson's disease patients.
While the pathogenic mechanism of human Parkinson's disease is still not fully understood, oxidative stress and cytotoxicity are thought to play an important role in the degeneration of dopaminergic neurons [63], [64], [69], [70], [71], [72], [73], [74]. Mechanistically, it is known that dopamine neurotransmitter is chemically labile and its oxidation products, such as dopamine quinones and semiquinones, are highly cytotoxic to neurons in general and dopaminergic neurons in particular [63], [64], [69]. Elevated formation of these neurotoxic intermediates contributes to neuronal damage and degeneration. This mechanistic hypothesis is supported by many in vitro as well as in vivo studies.
Basic and clinical evidence of the beneficial effects of P. ginseng on Parkinson's disease is summarized in Table 2. Extracts of P. ginseng had neuroprotective effects on 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis in SH-SY5Y cells through decreasing the levels of apoptotic proteins such as Bax, Bcl-2, cytochrome c, and cleaved caspase-3 [75]. In both in vivo (C57BL/6J mice) and in vitro (PC12 cells) models of Parkinson's disease, ginsenoside Rg1 exerted neuroprotective effects through the Wnt/β-catenin signaling pathway including Wnt-1, β-catenin, GSK-3β, and p-GSK-3β. Neuroprotective effects of ginsenoside Rg1 on MPP+-induced apoptosis in PC12 cells were also mediated through the decrease in apoptotic proteins levels including Bcl-xL and cleaved caspase-3 [76]. Ginsenoside Rd was also shown to exert neuroprotective effects on MPP+-induced apoptosis in SH-SY5Y cells by decreasing the levels of apoptotic proteins including p-Akt, Bax, and Bcl-2 [77].
Table 2.
Effects of P. ginseng and its active ingredient on Parkinson's disease
| Active ingredient | Target molecules | Cell lines or animal strain (toxicants) | Effective doses (treatment time) | References |
|---|---|---|---|---|
| P. ginseng extract | Bax, Bcl-2, cytochrome c, and cleaved caspase-3 | SH-SY5Y cells (MPP+) | 0.2 mg/mL (60 h) | [75] |
| Rg1 | Wnt-1, β-catenin, GSK-3β and p-GSK-3β, cleaved caspase-3, and Bcl-xL | PC12 cells (MPP+) | 20μM (24 h) | [76] |
| Rd | Bax, Bcl-2, and p-Akt | SH-SY5Y cells (MPP+) | 1μM and 10μM (72 h) | [77] |
| Rg1 | Wnt-1, β-catenin, GSK-3β, and p-GSK-3β | Male C57BL/6J mice (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) | 5 mg/kg/d, 10 mg/kg/d, and 20 mg/kg/d (15 d) | [76] |
MPP+, 1-methyl-4-phenylpyridinium ion
2.3. Brain ischemia and stroke
Stroke is the third leading cause of death in the industrialized world and the leading cause of disability [78]. There are two mechanistically distinct modes of cerebral ischemia, namely, global and focal ischemia. Global ischemia commonly develops after transient cardiac arrest. The typical histological picture following global ischemic insults is described by delayed neuronal death sparing glial cells. Under normothermic conditions, 10 min of global brain ischemia is lethal in humans. Focal ischemia occurs after transient or permanent flow reduction in the territory of a cerebral artery resulting from embolic or thrombotic vessel occlusion. The typical histological picture following focal ischemia is a pan-necrosis that includes all brain cell types [78].
Basic and clinical evidence of the beneficial effects of P. ginseng on stroke is summarized in Table 3. In male Sprague–Dawley rats, ginsenoside Rd has been reported to protect against ischemic cerebral damage by promoting clearance of extracellular glutamate through the upregulation of glutamate transporter 1 (GLT-1) expression via phosphatidylinositol 3-kinase (PI3K)/Akt and extracellular signal–regulated kinase (ERK)1/2 pathways [80]. Moreover, administration of ginsenoside Rd increased the expression of nonselective cation channels including transient receptor potential cation channel subfamily M member 7 (TRPM7), acid-sensing ion channel (ASIC) 1a, and ASIC2a [81], and decreased the levels of apoptotic proteins such as cytochrome c (CytoC), apoptosis inducing factor (AIF), and caspase-3 [82]. Postischemic synthesis of two damaging enzymes, COX-2 and iNOS, were also significantly decreased by ginsenoside Rd [83]. Ginsenoside Rb1 was also reported to promote extracellular glutamate clearance by upregulating GLT-1 expression via PI3K/Akt and ERK1/2 pathways in male Sprague-Dawley rats [79].
Table 3.
Effects of P. ginseng and its active ingredient on brain ischemia and stroke
| Active ingredient | Target molecules | Cell lines or animal strain (toxicants) | Effective doses (treatment time) | References |
|---|---|---|---|---|
| Rb1 | ERK1/2 | C57BL/6J mice (aged mice: 12 mo old) | 5 mg/kg/every 3 d (1 yr) | [79] |
| Rd | GLT-1, PI3K/Akt, and ERK1/2 | Male Sprague-Dawley rats (MCAO) | 30 mg/kg (1 h) | [80] |
| Rd | TRPM-1, TRPM-2, TRPM-3, TRPM-4, TRPM-5, TRPM-6, TRPM-7, ASIC1a, ASIC2a, NR1, NR2A, and NR2B | Male Sprague-Dawley rats (MCAO) | 10 mg/kg (15 min) | [81] |
| Rd | CytoC, AIF, and Caspase-3 | Male Sprague-Dawley rats (MCAO) | 50 mg/kg (30 min) | [82] |
| Rd | COX-2 and iNOS | Male Sprague-Dawley rats (MCAO) | 50 mg/kg (30 min) | [83] |
MCAO, middle cerebral artery occlusion
Although no prospective clinical trials are available for P. ginseng, a multicenter, double-blinded, and randomized controlled clinical trial of 140 Chinese patients demonstrated that a low dose of aspirin (50 mg/d) combined with notoginseng capsules (200 mg, three times/d) significantly ameliorated neurological deficits and improved daily life activities compared with treatment with aspirin alone [84].
2.4. Huntington's disease
Huntington's disease is a neurodegenerative disorder caused by a CAG trinucleotide repeat expansion in gene encoding for the huntingtin protein [85], [86]. Clinical symptoms of Huntington's disease comprise adult-onset personality changes, generalized motor dysfunctions, and cognitive decline. The peak age of adult-onset Huntington's disease is between 35 yr and 50 yr [85], [87]. Commonly reported symptoms include progressive weight loss, alterations in sexual behavior, and disturbances in the wake–sleep cycle, which occur very early during the course of the disease possibly due to hypothalamic dysfunction [88]. At later disease stages, characteristic symptoms include motor impairments, progressive dementia, and gradual impairment of mental processes involved in comprehension, reasoning, judgment, and memory [89], [90]. Most affected patients eventually succumb to the disease due to aspiration pneumonia caused by swallowing difficulties [89].
Basic evidence of the beneficial effects of P. ginseng on Parkinson's disease is summarized in Table 4. In a cellular model of Huntington's disease with primary medium spiny striatal neuronal cultures, ginsenosides Rb1, Rc, and Rg5 exerted protective effects on glutamate-induced apoptosis and were suggested as a potential treatment choice [92]. In a Sprague-Dawley rat model of Huntington's disease, PPTs were reported to have neuroprotective effects on 3-nitropropionic acid-induced oxidative stress in males. Oral administration of PPTs resulted in marked improvements in body weight and locomotor activity. Beneficial effects of PPTs were mediated by increasing the nuclear factor erythroid 2-related factor 2 (Nrf2) entry into the nucleus while enhancing the expression of heme oxygenase-1 (HO-1) and nicotinamide adenine dinucleotide phospate (NAD(P)H) quinone oxidase 1 in the striatum [91].
Table 4.
Effects of P. ginseng and its active ingredient on Huntington's disease
| Active ingredient | Target molecules | Cell lines or animal strain (toxicants) | Effective doses (treatment time) | Reference |
|---|---|---|---|---|
| Protopanaxtriol | Nrf2, HO-1, NQO1, and PCNA | Male Sprague-Dawley rats (3-nitropropionic acid) | 20 mg/kg (30 min) | [91] |
3. Concluding remarks and future perspectives
Oxidative stress and dysregulation of the inflammatory network are being recognized as important components in the pathogenesis of neurodegenerative diseases [93], [94], [95]. Oxidative stress has been linked to neuronal cell death associated with certain neurodegenerative conditions [96], [97]. Owing to its high metabolic rate and relatively reduced capacity for cellular regeneration compared with other organs, the brain is believed to be particularly susceptible to the damaging effects of reactive oxygen species (ROS). An acute oxidative insult to brain tissue can amplify ROS generation, increase the accumulation of oxidized biomolecules, and promote oxidative stress [98]. Accumulation of ROS in the brain stimulates the oxidation of lipids [99], protein [100], and DNA [101], which are characteristic changes of many neuronal pathologies.
In the case of Parkinson's and Alzheimer's diseases, various indices of ROS damage have been reported within specific brain regions that undergo selective neurodegeneration [102], [103], [104]. Many researchers in the neurodegenerative field are seeking ways to modulate or emulate the protective effects of key enzymatic components that regulate oxidative stress, with the aim of developing rational drugs or genetic therapies [105], [106].
A growing number of studies have demonstrated the efficacy of ginseng components extracted from ginseng fruits, roots, and leaves in reducing or blocking neuronal death in various experimental neurodegeneration models [57], [60]. Ginseng components, particularly ginsenosides, are capable of protecting neurons both in vitro and in vivo by modulating biological processes including oxidative stress, excitotoxicity, apoptotic neuronal death, and the kinase and ubiquitin–proteasome signaling pathways [20], [107]. Indeed, ginsenosides are receiving increasing interest from consumers as well as researchers because of their unique ability to prevent neurodegeneration [108], [109]. Extensive research over the last 10 yr has indicated that components derived from P. ginseng target ROS and, therefore, may prevent neurodegenerative diseases [20], [110].
Evidence for the medicinal and health benefits of P. ginseng and its components in preventing neurodegenerative diseases is increasing [20], [57], [111], [112], [113]. The current clinical results did not report any serious adverse effects of ginseng [60], but it may alter blood hemostasis and anticoagulation with warfarin [108]. The beneficial effects of ginseng have been attributed to the presence of ginsenosides that are powerful antioxidants and free iron scavengers. Mechanistic studies on the neuroprotective effects of ginsenosides revealed that they act not only as antioxidant metal chelators, but also as modulators of intracellular neuronal signaling and metabolism, cell survival/death genes, and mitochondrial function. It has been shown that ginsenosides modulate caspase-dependent and caspase-independent programmed cell death. Indeed, several ginsenosides significantly inhibit the activation of caspase-3, a key apoptotic player, and are able to modulate mitogen-activated protein kinases known to play an important role in neuronal apoptosis. However, findings from clinical studies on ginseng for neurodegenerative diseases showed that the effects of ginseng were still inconclusive. The main limitations of the available studies were small sample size, poor methodological qualities, and absence of placebo controls. Larger, well-designed clinical studies are a prerequisite to successfully elucidate the effect of ginseng on neurodegenerative diseases.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016R1C1B1012787).
Contributor Information
Kiwon Jung, Email: pharmj@cha.ac.kr.
Ki Sung Kang, Email: kkang@gachon.ac.kr.
References
- 1.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 2.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D.H. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J.D., Rhee D.K., Lee Y.H. Biological activities and chemistry of saponins from Panax ginseng CA Meyer. Phytochem Rev. 2005;4:159–175. [Google Scholar]
- 5.Liu C.X., Xiao P.G. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Li S., Fan Y., Chen Y., Liu D., Cheng H., Gao X., Zhou Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. J Ethnopharmacol. 2010;130:421–423. doi: 10.1016/j.jep.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M.S., Han I.H., Lee D., An J.M., Kim S.N., Shin M.S., Yamabe N., Hwang G.S., Yoo H.H., Choi S.J. Beneficial effects of fermented black ginseng and its ginsenoside 20 (S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J Ginseng Res. 2016;40:135–140. doi: 10.1016/j.jgr.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J.Y., Choi P., Kim H.K., Kang K.S., Ham J. Increase in apoptotic effect of Panax ginseng by microwave processing in human prostate cancer cells: in vitro and in vivo studies. J Ginseng Res. 2016;40:62–67. doi: 10.1016/j.jgr.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babiker L.B., Gadkariem E.A., Alashban R.M., Aljohar H.I. Investigation of stability of Korean ginseng in herbal drug product. Am J Appl Sci. 2014;11:160–170. [Google Scholar]
- 11.Zhang D., Yasuda T., Yu Y., Zheng P., Kawabata T., Ma Y., Okada S. Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996;20:145–150. doi: 10.1016/0891-5849(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 12.Yun T.K., Choi S.Y., Yun H.Y. Epidemiological study on cancer prevention by ginseng: are all kinds of cancers preventable by ginseng? J Korean Med Sci. 2001;16:S19–S27. doi: 10.3346/jkms.2001.16.S.S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo S.S., Won T.J., Lee D.I. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire, and their potential roles in neuroprotection in vitro. Planta Med. 2005;71:476–481. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- 14.Jung C.H., Seog H.M., Choi I.W., Choi H.D., Cho H.Y. Effects of wild ginseng (Panax ginseng CA Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol. 2005;98:245–250. doi: 10.1016/j.jep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Fang Y., Shen N., Chen X. Beneficial changes in prostacyclin and thromboxane A2 induced by ginsenosides in myocardial infarction and reperfusion injury in dogs. Zhongguo Yao Li Xue Bao. 1986;7:226–230. [PubMed] [Google Scholar]
- 16.Sakata T., Etou H., Fujimoto K., Ockuma K., Hayashi T., Arichi S. Korea–Japan Panax ginseng Symposium. 1987. Central effects of ginsenosides on the feeding behavior and response to stress in rats; pp. 20–28. [Google Scholar]
- 17.Fujimoto K., Sakata T., Ishimaru T., Etou H., Ookuma K., Kurokawa M., Machidori H. Attenuation of anorexia induced by heat or surgery during sustained administration of ginsenoside Rg1 into rat third ventricle. Psychopharmacology. 1989;99:257–260. doi: 10.1007/BF00442819. [DOI] [PubMed] [Google Scholar]
- 18.Xie J.T., McHendale S., Yuan C.S. Ginseng and diabetes. Am J Chin Med. 2005;33:397–404. doi: 10.1142/S0192415X05003004. [DOI] [PubMed] [Google Scholar]
- 19.Yang G., Park D., Lee J., Song B.S., Jeon T.H., Kang S.J., Jeon J.H., Shin S., Jeong H.S., Lee H.J. Suppressive effects of red ginseng preparations on SW480 colon cancer xenografts in mice. Food Sci Biotechnol. 2011;20:1649–1653. [Google Scholar]
- 20.Cho I.H. Effects of Panax ginseng in neurodegenerative diseases. J Ginseng Res. 2012;36:342. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang S.W., Min H.Y. Ginseng, the 'immunity boost': the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J. Recent studies on the chemical constituents of Korean ginseng (Panax ginseng CA Meyer) Korean J Ginseng Sci. 1996;20:389–415. [Google Scholar]
- 23.Elyakov G., Strigina L., Uvarova N., Vaskovsky V., Dzizenko A., Kochetkov N. Glycosides from ginseng roots. Tetrahedron Lett. 1964;5:3591–3597. [Google Scholar]
- 24.Shibata S., Fujita M., Itokawa H., Tanaka O., Ishii T. Studies on the constituents of Japanese and Chinese crude drugs. XI. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull. 1963;11:759–761. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 25.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 27.Lee S.M. Thermal conversion pathways of ginsenoside in red ginseng processing. Nat Prod Sci. 2014;20:119–125. [Google Scholar]
- 28.Takahashi M., Yoshikura M. Studies on the components of Panax ginseng CA Meyer. V. On the structure of a new acetylene derivative “panaxynol” (3). Synthesis of 1,9-(cis)-heptadecadiene-4,6-diyn-3-ol. Yakugaku Zasshi. 1966;86:1053–1056. doi: 10.1248/yakushi1947.86.11_1053. [DOI] [PubMed] [Google Scholar]
- 29.Kong Y.H., Lee Y.C., Choi S.Y. Neuroprotective and anti-inflammatory effects of phenolic compounds in Panax ginseng CA Meyer. J Ginseng Res. 2009;33:111–114. [Google Scholar]
- 30.Chung I.M., Lim J.J., Ahn M.S., Jeong H.N., An T.J., Kim S.H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J Ginseng Res. 2016;40:68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H., Yoo B., Byun S. Differences in phenolic acids between Korean ginsengs and mountain ginsengs. Korean J Biotechnol Bioeng. 2000;15:323–328. [Google Scholar]
- 32.Iwabuchi H., Yoshikura M., Kamisako W. Studies on the sesquiterpenoids of Panax ginseng C. A. Meyer. II. Isolation and structure determination of ginsenol, a novel sesquiterpene alcohol. Chem Pharm Bull (Tokyo) 1988;36:2447–2451. doi: 10.1248/cpb.36.2447. [DOI] [PubMed] [Google Scholar]
- 33.Richter R., Basar S., Koch A., König W.A. Three sesquiterpene hydrocarbons from the roots of Panax ginseng CA Meyer (Araliaceae) Phytochemistry. 2005;66:2708–2713. doi: 10.1016/j.phytochem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Wang J.Y., Li X.G., Yang X.W. Ginsenine, a new alkaloid from the berry of Panax ginseng CA Meyer. J Asian Nat Prod Res. 2006;8:605–608. doi: 10.1080/10286020500208444. [DOI] [PubMed] [Google Scholar]
- 35.Konno C., Murakami M., Oshima Y., Hikino H. Isolation and hypoglycemic activity of panaxans Q, R, S, T and U, glycans of Panax ginseng roots. J Ethnopharmacol. 1985;14:69–74. doi: 10.1016/0378-8741(85)90030-3. [DOI] [PubMed] [Google Scholar]
- 36.Konno C., Sugiyama K., Kano M., Takahashi M., Hikino H. Isolation and hypoglycaemic activity of panaxans A, B, C, D and E, glycans of Panax ginseng roots. Planta Med. 1984;50:434–436. doi: 10.1055/s-2007-969757. [DOI] [PubMed] [Google Scholar]
- 37.Oshima Y., Konno C., Hikino H. Isolation and hypoglycemic activity of panaxans I, J, K and L, glycans of Panax ginseng roots. J Ethnopharmacol. 1985;14:255–259. doi: 10.1016/0378-8741(85)90091-1. [DOI] [PubMed] [Google Scholar]
- 38.Tomoda M., Takeda K., Shimizu N., Gonda R., Ohara N., Takada K., Hirabayashi K. Characterization of two acidic polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull. 1993;16:22–25. doi: 10.1248/bpb.16.22. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X., Yu L., Bi H.T., Li X.H., Ni W.H., Han H., Li N., Wang B.Q., Zhou Y.F., Tai G.H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. Carbohyd Polym. 2009;77:544–552. [Google Scholar]
- 40.Fan Y.Y., Sun C.X., Gao X.G., Wang F., Li X.Z., Kassim R.M., Tai G.H., Zhou Y.F. Neuroprotective effects of ginseng pectin through the activation of ERK/MAPK and Akt survival signaling pathways. Mol Med Rep. 2012;5:1185–1190. doi: 10.3892/mmr.2012.811. [DOI] [PubMed] [Google Scholar]
- 41.Fan Y.Y., Cheng H.R., Liu D., Zhang X., Wang B., Sun L., Tai G.H., Zhou Y.F. The inhibitory effect of ginseng pectin on L-929 cell migration. Arch Pharm Res. 2010;33:681–689. doi: 10.1007/s12272-010-0506-9. [DOI] [PubMed] [Google Scholar]
- 42.Francis P.T., Palmer A.M., Snape M., Wilcock G.K. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong L.M., Fong H., Huang Y. Stem cell therapy for Alzheimer’s disease and related disorders: current status and future perspectives. Exp Mol Med. 2015;47:e151. doi: 10.1038/emm.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musiek E.S., Xiong D.D., Holtzman D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciechanover A., Kwon Y.T. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajda M., Guzior N., Ignasik M., Malawska B. Multi-target-directed ligands in Alzheimer's disease treatment. Curr Med Chem. 2011;18:4949–4975. doi: 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- 48.Mancuso C., Bates T.E., Butterfield D.A., Calafato S., Cornelius C., Lorenzo A.D., Dinkova Kostova A.T., Calabrese V. Natural antioxidants in Alzheimer's disease. Expert Opin Investig Drugs. 2007;16:1921–1931. doi: 10.1517/13543784.16.12.1921. [DOI] [PubMed] [Google Scholar]
- 49.Zhao B.S., Liu Y., Gao X.Y., Zhai H.Q., Guo J.Y., Wang X.Y. Effects of ginsenoside Rg1 on the expression of toll-like receptor 3, 4 and their signalling transduction factors in the NG108-15 murine neuroglial cell line. Molecules. 2014;19:16925–16936. doi: 10.3390/molecules191016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang J.Y., Shim J.S., Song M.Y., Yim S.V., Lee S.E., Park K.S. Proteomic analysis reveals that the protective effects of ginsenoside Rb1 are associated with the actin cytoskeleton in β-amyloid-treated neuronal cells. J Ginseng Res. 2016;40:278–284. doi: 10.1016/j.jgr.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan X., Gu J., Zhao B., Wang S., Yuan J., Wang C., Chen J., Liu J., Feng L., Jia X. Ginseng improves cognitive deficit via the RAGE/NF-κB pathway in advanced glycation end product-induced rats. J Ginseng Res. 2015;39:116–124. doi: 10.1016/j.jgr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Pi Z., Song F., Liu Z. Ginsenosides attenuate d-galactose-and AlCl 3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer's disease. J Ethnopharmacol. 2016;194:188–195. doi: 10.1016/j.jep.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhao H., Li Q., Li Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. Neuroscience. 2011;183:189–202. doi: 10.1016/j.neuroscience.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 54.Zhao H., Li Q., Zhang Z., Pei X., Wang J., Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 55.Hou J., Xue J., Lee M., Yu J., Sung C. Long-term administration of ginsenoside Rh1 enhances learning and memory by promoting cell survival in the mouse hippocampus. Int J Mol Med. 2014;33:234–240. doi: 10.3892/ijmm.2013.1552. [DOI] [PubMed] [Google Scholar]
- 56.Chu S., Gu J., Feng L., Liu J., Zhang M., Jia X., Liu M., Yao D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int Immunopharmacol. 2014;19:317–326. doi: 10.1016/j.intimp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Kim E.J., Jung I.H., Van Le T.K., Jeong J.J., Kim N.J., Kim D.H. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol. 2013;146:294–299. doi: 10.1016/j.jep.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 58.Song X.Y., Hu J.F., Chu S.F., Zhang Z., Xu S., Yuan Y.H., Han N., Liu Y., Niu F., He X. Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3β/tau signaling pathway and the Aβ formation prevention in rats. Eur J Pharmacol. 2013;710:29–38. doi: 10.1016/j.ejphar.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 59.Heo J.H., Lee S.T., Chu K., Oh M.J., Park H.J., Shim J.Y., Kim M. Heat-processed ginseng enhances the cognitive function in patients with moderately severe Alzheimer's disease. Nutr Neurosci. 2012;15:278–282. doi: 10.1179/1476830512Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y., Yang G., Gong J., Lu F., Diao Q., Sun J., Zhang K., Tian J., Liu J. Ginseng for Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Curr Top Med Chem. 2016;16:529–536. doi: 10.2174/1568026615666150813143753. [DOI] [PubMed] [Google Scholar]
- 61.Hornykiewicz O. Dopamine in the basal ganglia: its role and therapeutic implications (including the clinical use of L-DOPA) Br Med Bull. 1973;29:172–178. doi: 10.1093/oxfordjournals.bmb.a070990. [DOI] [PubMed] [Google Scholar]
- 62.Hornykiewicz O. How L-DOPA was discovered as a drug for Parkinson's disease 40 years ago. Wien Klin Wochenschr. 2001;113:855–862. [PubMed] [Google Scholar]
- 63.Mizuno Y., Mori H., Kondo T. Parkinson's disease: from etiology to treatment. Int Med. 1995;34:1045–1054. doi: 10.2169/internalmedicine.34.1045. [DOI] [PubMed] [Google Scholar]
- 64.Sherer T., Betarbet R., Greenamyre J. Pathogenesis of Parkinson's disease. Curr Opin Investig Drugs. 2001;2:657–662. [PubMed] [Google Scholar]
- 65.Bae J.R., Lee B.D. Function and dysfunction of leucine-rich repeat kinase 2 (LRRK2): Parkinson’s disease and beyond. BMB Rep. 2015;48:243–248. doi: 10.5483/BMBRep.2015.48.5.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollanen M.S., Dickson D.W., Bergeron C. Pathology and biology of the Lewy body. J Neuropathol Exp Neurol. 1993;52:183–191. doi: 10.1097/00005072-199305000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Kuzuhara S., Mori H., Izumiyama N., Yoshimura M., Ihara Y. Lewy bodies are ubiquitinated. Acta Neuropathol. 1988;75:345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- 68.Chung Y.C., Shin W.H., Baek J.Y., Cho E.J., Baik H.H., Kim S.R., Won S.Y., Jin B.K. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson's disease. Exp Mol Med. 2016;48:e205. doi: 10.1038/emm.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu B.T. CNS dopamine oxidation and catechol-O-methyltransferase: importance in the etiology, pharmacotherapy, and dietary prevention of Parkinson's disease. Int J Mol Med. 2004;13:343–354. [PubMed] [Google Scholar]
- 70.Fahn S., Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 71.Fahn S., Bressman S.B. Should levodopa therapy for parkinsonism be started early or late? Evidence against early treatment. Can J Neurol Sci. 1984;11:200–205. doi: 10.1017/s0317167100046412. [DOI] [PubMed] [Google Scholar]
- 72.Alam Z.I., Daniel S.E., Lees A.J., Marsden D.C., Jenner P., Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 73.Diamond S.G., Marchkham C.H., Hoehn M.M., McDowell F.H., Muenter M.D. Multi-center study of Parkinson mortality with early versus later dopa treatment. Ann Neurol. 1987;22:8–12. doi: 10.1002/ana.410220105. [DOI] [PubMed] [Google Scholar]
- 74.Parodi J., Ormeño D., Ochoa-de la Paz L.D. Amyloid pore-channel hypothesis: effect of ethanol on aggregation state using frog oocytes for an Alzheimer’s disease study. BMB Rep. 2015;48:13–18. doi: 10.5483/BMBRep.2015.48.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu S., Han R., Mak S., Han Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng CA Meyer) in SH-SY5Y cells. J Ethnopharmacol. 2011;135:34–42. doi: 10.1016/j.jep.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Zhou T., Zu G., Zhang X., Wang X., Li S., Gong X., Liang Z., Zhao J. Neuroprotective effects of ginsenoside Rg1 through the Wnt/β-catenin signaling pathway in both in vivo and in vitro models of Parkinson's disease. Neuropharmacology. 2016;101:480–489. doi: 10.1016/j.neuropharm.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y., Zhang R.Y., Zhao J., Dong Z., Feng D.Y., Wu R., Shi M., Zhao G. Ginsenoside Rd protects SH-SY5Y cells against 1-methyl-4-phenylpyridinium induced injury. Int J Mol Sci. 2015;16:14395–14408. doi: 10.3390/ijms160714395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodruff T.M., Thundyil J., Tang S.C., Sobey C.G., Taylor S.M., Arumugam T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6:11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong X., Zheng L., Lu S., Yang Y. Neuroprotective effects of pretreatment of ginsenoside Rb1 on severe cerebral ischemia-induced injuries in aged mice: involvement of anti-oxidant signaling. Geriatr Gerontol Int. 2017;17:338–345. doi: 10.1111/ggi.12699. [DOI] [PubMed] [Google Scholar]
- 80.Zhang X., Shi M., Bjoras M., Wang W., Zhang G., Han J., Liu Z., Zhang Y., Wang B., Chen J. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front Pharmacol. 2013;4:152. doi: 10.3389/fphar.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Zhou L., Zhang X., Bai J., Shi M., Zhao G. Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci. 2012;33:1125–1131. doi: 10.1007/s10072-011-0916-6. [DOI] [PubMed] [Google Scholar]
- 82.Ye R., Zhang X., Kong X., Han J., Yang Q., Zhang Y., Chen Y., Li P., Liu J., Shi M. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 83.Ye R., Yang Q., Kong X., Han J., Zhang X., Zhang Y., Li P., Liu J., Shi M., Xiong L. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 84.He L., Chen X., Zhou M., Zhang D., Yang J., Yang M., Zhou D. Radix/rhizoma notoginseng extract (sanchitongtshu) for ischemic stroke: a randomized controlled study. Phytomedicine. 2011;18:437–442. doi: 10.1016/j.phymed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 85.Rub U., Vonsattel J.P.G., Heinsen H., Korf H.W. The neuropathology of Huntington's disease: classical findings, recent developments and correlation to functional neuroanatomy conclusions and outlook. Adv Anat Embryol Cell Biol. 2015;217:1–46. [PubMed] [Google Scholar]
- 86.Saudou F., Humbert S. The biology of huntingtin. Neuron. 2016;89:910–926. doi: 10.1016/j.neuron.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 87.Zuccato C., Valenza M., Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 88.Politis M., Pavese N., Tai Y.F., Tabrizi S.J., Barker R.A., Piccini P. Hypothalamic involvement in Huntington's disease: an in vivo PET study. Brain. 2008;131:2860–2869. doi: 10.1093/brain/awn244. [DOI] [PubMed] [Google Scholar]
- 89.Bates G., Jones L. Oxford University Press; Oxford: 2002. Huntington's disease. CIT0001. [Google Scholar]
- 90.Rosenblatt A. Neuropsychiatry of Huntington's disease. Dialogues Clin Neurosci. 2007;9:191–197. doi: 10.31887/DCNS.2007.9.2/arosenblatt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Y., Chu S.F., Li J.P., Zhang Z., Yan J.Q., Wen Z.L., Xia C.Y., Mou Z., Wang Z.Z., He W.B. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease. Acta Pharmacol Sin. 2015;36:311–322. doi: 10.1038/aps.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu J., Jeong H.K., Bulin S.E., Kwon S.W., Park J.H., Bezprozvanny I. Ginsenosides protect striatal neurons in a cellular model of Huntington's disease. J Neurosci Res. 2009;87:1904–1912. doi: 10.1002/jnr.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amor S., Puentes F., Baker D., Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kannappan R., Gupta S.C., Kim J.H., Reuter S., Aggarwal B.B. Neuroprotection by spice-derived nutraceuticals: you are what you eat! Mol Neurobiol. 2011;44:142–159. doi: 10.1007/s12035-011-8168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchetti B., Abbracchio M.P. To be or not to be (inflamed)—is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 96.Andersen J.K. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10:18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 97.Son J.H., Shim J.H., Kim K.H., Ha J.Y., Han J.Y. Neuronal autophagy and neurodegenerative diseases. Exp Mol Med. 2012;44:89–98. doi: 10.3858/emm.2012.44.2.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrante R.J., Shinobu L.A., Schulz J.B., Matthews R.T., Thomas C.E., Kowall N.W., Gurney M.E., Beal M.F. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann Neurol. 1997;42:326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- 99.Adibhatla R.M., Hatcher J.F., Dempsey R.J. Phospholipase A2, hydroxyl radicals, and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal. 2003;5:647–654. doi: 10.1089/152308603770310329. [DOI] [PubMed] [Google Scholar]
- 100.Hall N., Carney J., Cheng M., Butterfield D. Ischemia/reperfusion-induced changes in membrane proteins and lipids of gerbil cortical synaptosomes. Neuroscience. 1995;64:81–89. doi: 10.1016/0306-4522(94)00385-i. [DOI] [PubMed] [Google Scholar]
- 101.Won M.H., Kang T.C., Jeon G.S., Lee J.C., Kim D.Y., Choi E.M., Lee K.H., Do Choi C., Chung M.H., Cho S.S. Immunohistochemical detection of oxidative DNA damage induced by ischemia–reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999;836:70–78. doi: 10.1016/s0006-8993(99)01611-x. [DOI] [PubMed] [Google Scholar]
- 102.Dexter D., Carter C., Wells F., Javoy-Agid F., Agid Y., Lees A., Jenner P., Marsden C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 103.Hensley K., Maidt M.L., Yu Z., Sang H., Markesbery W.R., Floyd R.A. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J Neurosci. 1998;18:8126–8132. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Butterfield D.A., Castegna A., Lauderback C.M., Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 105.Yang H.Y., Lee T.H. Antioxidant enzymes as redox-based biomarkers: a brief review. BMB Rep. 2015;48:200–208. doi: 10.5483/BMBRep.2015.48.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye M., Chung H.S., Lee C., Song J.H., Shim I., Kim Y.S., Bae H. Bee venom phospholipase A2 ameliorates motor dysfunction and modulates microglia activation in Parkinson's disease alpha-synuclein transgenic mice. Exp Mol Med. 2016;48:e244. doi: 10.1038/emm.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Radad K., Moldzio R., Rausch W.D. Ginsenosides and their CNS targets. CNS Neurosci Ther. 2011;17:761–768. doi: 10.1111/j.1755-5949.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen C.T., Luong T.T., Kim G.L., Pyo S., Rhee D.K. Korean Red Ginseng inhibits apoptosis in neuroblastoma cells via estrogen receptor β-mediated phosphatidylinositol-3 kinase/Akt signaling. J Ginseng Res. 2015;39:69–75. doi: 10.1016/j.jgr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim S., Kim M.S., Park K., Kim H.J., Jung S.W., Nah S.Y., Han J.S., Chung C. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40:55–61. doi: 10.1016/j.jgr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kang K.S., Ham J., Kim Y.J., Park J.H., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.González-Burgos E., Fernandez-Moriano C., Gómez-Serranillos M.P. Potential neuroprotective activity of ginseng in Parkinson's disease: a review. J Neuroimmune Pharmacol. 2015;10:14–29. doi: 10.1007/s11481-014-9569-6. [DOI] [PubMed] [Google Scholar]
- 112.Ong W.Y., Farooqui T., Koh H.L., Farooqui A.A., Ling E.A. Protective effects of ginseng on neurological disorders. Front Aging Neurosci. 2015;7:129. doi: 10.3389/fnagi.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li N., Liu Y., Li W., Zhou L., Li Q., Wang X., He P. A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer's disease. J Ginseng Res. 2016;40:9–17. doi: 10.1016/j.jgr.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]