Abstract
Background
Studies have shown that curcumin from Curcuma longa has a wide range of medicinal and immunomodulatory properties. These activities have, however, been hindered by its low bioavailability. Meanwhile, incorporation of nanoparticles has been shown to increase bioavailability of certain drugs. This study was, therefore, conducted to comparatively evaluate the immunomodulatory activity of free and nanoparticulate curcumin in mice.
Methods
Healthy albino mice were sensitized with sheep red blood cells (SRBCs) and thereafter free and nanoparticulate curcumin were administered orally at doses of 5 mg/kg/day and 10 mg/kg/day for 10 days to the mice. The assessment of the immunomodulatory activity was carried out by determining the humoral and cell-mediated immune responses using hemagglutination and delayed-type hypersensitivity assays, respectively. Hematological components and some lymphoid organs of treated mice were further evaluated.
Results
The study showed that nanoparticulate curcumin stimulated higher early cell-mediated immune response at 5 mg/kg and 10 mg/kg when compared to control. While nanoparticulate curcumin significantly stimulated primary humoral immune response with 9.00 ± 1.00 antibody titre (p < 0.05), the free curcumin suppressed the immunity with 3.33 ± 0.67 antibody titre when compared to control. Similar result was observed with secondary humoral antibody titres. Production of white blood cells and weight of the lymphoid organs were also enhanced in the groups that received 10 mg/kg nanocurcumin.
Conclusion
This work showed that poly d,l-lactic-co-glycolic acid entrapped curcumin nanoparticle could increase bioavailability of curcumin for improved immunity.
Keywords: Bioavailability, Curcumin, Delivery system, Immune modulation
1. Introduction
The protection offered to the body system against specific diseases and infectious agents is achieved by the immune system.1 The ability of the immune system to elicit this function is closely associated with certain compounds called immune modulators, which exert significant biological and pharmacological effects on the immune cells. Immunomodulators become very useful in abating the pathological impacts of immune-alteration associated diseases such as asthma, cancer, parasitic diseases, arthritis and ulcerative colitis. Many synthetic immunomodulators have been described, but plant-derived immune modulators are still in a developing stage. The need for more of the latter becomes paramount owning to severe side effects the synthetic immunomodulators pose on the immune system. For example, cyclophosphamide, an immunosuppressant, causes myelosuppression, nephrotoxicity, neurotoxicity, induction of diabetes, hepatotoxicity and induction of hypertension.2 Medicinal plants are a rich source of substances that are known to induce paraimmunity, the non-specific immunomodulation of granulocytes, natural killer cells, macrophages and complement functions.3 Plants such as Punica granatum, Moringa oleifera, Phyllantus niruri, Cissus quandrangularis, Habenaria intermedia and Gymnema sylvestre have been explored for their immunostimulatory properties.4, 5, 6, 7
Turmeric (from Curcuma longa) is a mixture of curcumin [i.e., diferuloylmethane or 1,7-bis(4-hydroxy-3-methoxy-phenyl) hepta-1,6-diene-3,5-dione)], demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin.8 Curcumin has long been in use as a traditional remedy for diverse ailments, but its medicinal value was first documented in 1937 when used against biliary disease.9 Since then, its therapeutic potential has been widely explored. It has been reported to have anti-bacterial, anti-fungal, anti-viral, anti-oxidative, anti-inflammatory and anti-proliferative activities.10, 11, 12 The anti-cancer properties of curcumin against tumour cells have also been explored, and prevention of tumour initiation, promotion, metastasis and angiogenesis are the suggested mechanisms.13, 14 Curcumin has pleiotropic properties that modulate numerous targets including proteins (thioredoxin reductase, cyclooxygenase 2 (COX-2), protein kinase C (PKC), 5-lipoxygenase and tubulin), transcription factors, growth factors and their receptors, cytokines, enzymes and gene-regulating cell proliferation and apoptosis.15, 16, 17 This multi-targeted behaviour made it to be able to perform a wide spectrum of actions, while smart drugs or therapeutic drugs have only one target and are eliminated from the cells if they do not reach the right compartment.12 There have also been several clinical trials evaluating the possible anti-disease effect of curcumin in humans, as registered with the US National Institutes of Health, including studies on cancer, gastrointestinal diseases, cognitive disorders and psychiatric conditions.18
A large number of in vitro and animal studies have been conducted to evaluate the effect of curcumin on the immune system, and its adjunctive therapeutic potential for cerebral malaria management has been proposed.19 It has been found to act at various different levels of the arachidonic acid immunomodulatory cascade and through effects on various enzymes and cytokines.15, 20 Although curcumin has enjoyed a wide range of applications, its clinical development has not been fully harnessed due to its quick metabolism and hydrophobicity.21 Delivery systems such as liposome, biodegradable polymeric materials and chitosan are employed to increase the solubility and bioavailability of curcumin for improved biological actions.22, 23 The polymeric biodegradable nanomaterials have been especially favoured for the formulation of nanomedicines due to their grand bioavailability and controlled release.24 The aim of this study, therefore, was to improve the immunomodulatory properties of curcumin through encapsulation with poly d,l-lactic-co-glycolic acid nanomaterial.
2. Methods
2.1. Chemicals/drugs
Curcumin (from C. longa Linn), polyvinyl alcohol (PVA) (MW = 30–70 kDa) and d-mannitol were purchased from Sigma–Aldrich Ltd (St. Louis, MO, USA). Poly d,l-lactic-co-glycolic acid (PLGA) (intrinsic viscosity η = 0.41 dL/g, copolymer ratio 50:50, 45 kDa) was purchased from Purac Biochem, Holland. Dichloromethane and acetone were procured from Merck Serono Ltd (Middlesex, United Kingdom). Water purified by Milli-Qplus system from Millipore (MQ water) (Missouri, USA) was used.
2.2. Preparation of nanoparticulate curcumin
Curcumin-loaded PLGA nanoparticle (size: 291.2 ± 82.1 nm) was formulated by the method described by Busari et al.25 Briefly, 5 mg of curcumin was added to the organic phase containing 50 mg of polymer dissolved in 3.5 mL of dichloromethane and 0.5 mL of acetone to constitute 1:10 (drug-to-polymer) ratio. The emulsion was continuously stirred at 300 rpm for 6 hours to evaporate the solvent, leaving behind the colloidal suspension of the drug-encapsulated nanoparticle in aqueous phase. The formulation was centrifuged at 16,000 rpm for 15 minutes and then washed three times. Dry powder was obtained by lyophilization of frozen sample in the presence of 5% mannitol as cryoprotectant.
2.3. Experimental animals
Swiss Albino male mice weighing between 20 g and 25 g were procured from colonies maintained at the Department of Pharmacology, University of Ibadan, Nigeria. All animal experiments complied with the International Guiding Principles for Biomedical Research involving Animals (CIOMS and ICLAS, 2012). The protocol was approved by the Animal Care Use and Research Ethics Committee (ACUREC) of the University of Ibadan, Nigeria. The animals were housed in clean cages and fed with standard pellet diet, with water ad libitum. The cages were maintained under standard environmental conditions in the Animal House at the Department of Zoology, University of Ibadan. Animals were weighed before and after the experiments.
2.4. Preparation of antigen (sheep red blood cells, SRBCs)
Fresh sheep blood (50 mL) was collected in Alsever's solution (1:1) from sheep killed at the Abattoir, Bodija market, Ibadan. The fresh blood was centrifuged at 3000 rpm for 10 minutes. The SRBCs were obtained by washing the blood three times in large volume of pyrogen-free 0.9% normal saline. The SRBCs were then suspended in 10% saline solution and stored at 4°C until use. The SRBCs were used throughout the experiments for uniformity and adjusted to 5% for sensitization and challenge of animals.
2.5. Drug administration
All mice were randomly divided into 10 groups with 5 animals in each; 5 groups per hemagglutination and delayed-type hypersensitivity assays. Nanoparticulate (5 mg/kg and 10 mg/kg), free curcumin (5 mg/kg and 10 mg/kg) and Tween 80 (0.2 mL) were orally administered to the different groups of animals (Table 1, Table 2). The 5 mg/kg and 10 mg/kg free and nanotized curcumin corresponded with the doses used against Plasmodium berghei in a murine model in a previous study.25
Table 1.
The experimental design of the hemagglutination assay
| Days | Groups | Treatment/activities |
|---|---|---|
| Day 0 | Groups I–V | Sensitization of animals intraperitoneally with 0.2 mL of 5% SRBCs |
| Day 1–Day 5 | Drug administration: | |
| Group I | Tween-80 (control) | |
| Group II | Nanoparticulate curcumin at 5 mg/kg | |
| Group III | Nanoparticulate curcumin at 10 mg/kg | |
| Group IV | Free curcumin at 5 mg/kg | |
| Group V | Free curcumin at 10 mg/kg | |
| Day 6 | Groups I–V | Collection of blood via ocular puncture (primary antibody titre). |
| Day 7 | Groups I–V | Challenge of animals with 0.1 mL of 5% SRBC intraperitoneally. |
| Day 8– Day 12 | Groups I–V | Treatment (same as that on days 1–5 with each group and its corresponding treatment). |
| Day 13 | Groups I–V | Collection of blood via ocular puncture (secondary antibody titre) |
Table 2.
The experimental design of the delayed-type hypersensitivity assay
| Days | Groups | Treatment |
|---|---|---|
| Day 0 | Groups I, II, III, IV and V | Sensitization of each animal with 0.2 mL of 5% SRBC intraperitoneally. |
| Day 1–Day 5 | Group I | Tween 80 (control) |
| Group II | Nanoparticulate curcumin at 5 mg/kg | |
| Group III | Nanoparticulate curcumin at 10 mg/kg | |
| Group IV | Free curcumin at 5 mg/kg | |
| Group V | Free curcumin at 10 mg/kg | |
| Day 6 | Groups I–V | Measurement of paw thickness |
| Groups I–V | Challenge with 0.04 mL of 5% SRBC on left footpad and 0.04 mL of normal saline on right footpad (control) | |
| Groups I–V | Measurement of paw thickness after 4 h | |
| Day 7–Day 8 | Groups I–V | Measurement of paw thickness at 24 and 48 h. |
| Day 8–Day 12 | Groups I–V | Treatment (same as that of days 1–5) |
| Day 13 | Groups I–V | Collection of whole blood via ocular puncture into EDTA bottles for extraction of plasma sample for analyses of haematological parameters. Sacrificing of animals by cervical dislocation and extraction of liver and spleen. |
2.6. Experimental design of the hemagglutination assay
The method described by Ahirwal et al7 was used to determine hemagglutinating antibody (HA) titre. On the first day (day 0) of the experiment, mice were sensitized by injecting 0.2 mL of the 5% SRBCs intraperitoneally. Drugs were administered to animals for five days from days 1 to 5. The experimental design was as summarized in Table 1.
2.7. Blood sample collection and processing
Blood samples were collected from all mice by retro-orbital bleeding into clean Eppendorf tubes on the 6th and 13th days for the analysis of primary and secondary antibody titres, respectively. Blood samples were kept at room temperature for 30 minutes to clot. Anti-sera were obtained by centrifuging the clots at 3000 rpm for 20 minutes.
2.8. Hemagglutination assay
The anti-sera obtained from the blood were titrated against the erythrocyte antigen (1% SRBCs) in a 96-well microtitre plate according to Mazumder et al26 Briefly, twofold serial dilution of anti-serum (50 μL) was made in 25 μL of 0.9% pyrogen-free normal saline. Thereafter, 25 μL of 1% SRBCs was added to each well, and microtitre plates were mixed gently by handshaking and incubated at room temperature (25°C) for two hours. The plates were then macroscopically observed under white tile for haemaglutination. The plates were left overnight for further observation. The value of antibody titre was assigned to the highest serum dilution showing at least 50% of visible haemaglutination and was expressed as the log 2 of the reciprocal of the dilution factors. Antibody titres obtained on the 6th and 13th days were considered as primary and secondary humoral immune responses, respectively.
2.9. Delayed-type hypersensitivity (DTH) and measurement of haematological parameters and organ coefficient
The DTH response was determined using the method of Doherty27 and Verma et al28 The day of sensitization was designated as day 0, after which drugs were orally administered for five days. At intervals of 24 and 48 hours after the challenge, the paw thickness of the mice was measured by measuring the increase in dorso-ventral thickness using digital Vernier caliper. Thereafter, animals were further treated with drugs for five days (Table 2), and blood was collected from each mouse for determination of haematological parameters using standard methods. The blood parameters analyzed included total and differential white blood cell counts (WBC), packed cell volume (PCV), red blood cell counts (RBC), platelets as well as haemoglobin contents (HB). After blood collection, animals were sacrificed; the liver and spleen of each animal were extracted and weighed using a sensitive weighing balance, and organ coefficient (relative organ weight) was calculated as follows:
2.10. Statistical analysis
The data obtained from animal experiments were expressed as mean ± standard error of mean (mean ± SEM). Results obtained were statistically analyzed by using one-way ANOVA followed by Dunnett multiple comparison tests. p < 0.05 were considered statistically significant.
3. Results
3.1. Humoral antibody (HA) titre response to SRBCs
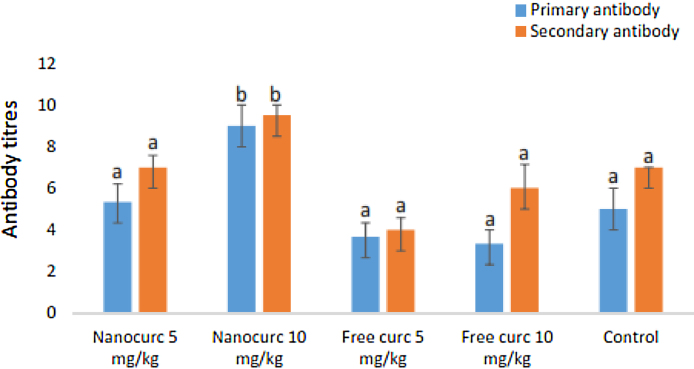
The primary and secondary antibody titres showing humoral immune response are shown in Fig. 1. The primary (9.00 ± 1.00) and the secondary (9.50 ± 0.50) antibody titres in the nanoparticulate curcumin group at 10 mg/kg were significantly higher than the values recorded in the free curcumin groups and other groups. The antibody titres in the secondary humoral immune response group were generally higher than the values recorded for the primary immune response (Fig. 1).
Fig. 1.
Primary and secondary antibody titres across all the groups.
Nanocurc, nanocurcumin; Free-curc, free curcumin. Similar superscripts denote no significant difference (p > 0.05) while different superscripts denote significant difference (p < 0.05).
3.2. Effect of free curcumin and nanocurcumin on mean footpad oedema in DTH model
Effect of free and nano-sized curcumin on cell-mediated immune responses by DTH-induced footpad oedema is shown in Table 3. The mice treated in the experimental groups showed increase response in footpad oedema when compared to the negative control. Percentage increase in footpad oedema in mice treated with nanocurcumin 5 mg/kg after 4 hours and 24 hours (48.0, 26.0%) was lower than in the corresponding free curcumin group (65.0, 34.0%). Nanocurcumin at 10 mg/kg (62.0, 36.0%), however, showed higher percentage increase in footpad oedema compared to treatment with 10 mg/kg free curcumin (39.0, 31.0%). After 24 hours, a sharp decline was seen in the occurrence of footpad oedema across all the groups.
Table 3.
Delayed-type hypersensitivity responses of the animals and the corresponding percentage increase in paw volume to the treatments at 4 h, 24 h and 48 h
| Treatment | Dose (mg/kg) | Initial footpad thickness (mm) | After 4 h |
After 24 h |
After 48 h |
|||
|---|---|---|---|---|---|---|---|---|
| Footpad thickness (mm) | Percentage increase (%) | Footpad thickness (mm) | Percentage increase (%) | Footpad thickness (mm) | Percentage increase (%) | |||
| Nanocurc | 5 | 2.21 ± 0.04a | 3.26 ± 0.07a | 48.0 | 2.78 ± 0.10a | 26.0 | 2.60 ± 0.03a | 18.0 |
| Nanocurc | 10 | 2.20 ± 0.04a | 3.56 ± 0.05a | 62.0 | 2.99 ± 0.10a | 36.0 | 2.58 ± 0.09a | 17.0 |
| Free-curc | 5 | 2.18 ± 0.05a | 3.58 ± 0.22a | 65.0 | 2.90 ± 0.09a | 34.0 | 2.61 ± 0.01a | 20.0 |
| Free-curc | 10 | 2.26 ± 0.04a | 3.13 ± 0.06a | 39.0 | 2.96 ± 0.07a | 31.0 | 2.70 ± 0.11a | 19.0 |
| Control | – | 2.23 ± 0.04a | 3.01 ± 0.07a | 35.0 | 2.69 ± 0.06a | 20.0 | 2.66 ± 0.01a | 19.0 |
Nanocurc, nanocurcumin; Free-curc, free curcumin. Similar superscripts denote no significant difference (p > 0.05) while different superscripts denote significant difference (p < 0.05).
3.3. Effects of treatment on haematological parameters
Generally, there were no significant differences in mean values of haematological parameters in different treatment groups (p > 0.05). However, higher values were recorded in PCV, HB and RBC in the negative control (Table 4). Platelets (123,000.0) and WBC (6770.0) counts were higher in groups that were administered 10 mg/kg free curcumin (p > 0.05). The highest value of neutrophil (31.8 ± 2.36) count was recorded in the 5 mg/kg free curcumin group (Table 4). Lymphocyte (66.0 ± 1.78) and eosinophil (2.8 ± 0.85) counts were higher in the 10 mg/kg nanocurcumin group than in the free drug group.
Table 4.
Mean hematological parameters for the DTH mice across all the groups
| Group | Dose (mg/kg) | PCV | HB | RBC | WBC | Platelet | LYM | NEUT | MONO | EOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Nanocurc | 5 | 37.5 ± 0.50a | 12.4 ± 0.13a | 6.3 ± 0.04a | 4037.5 ± 1453.4a | 73500.0 ± 25632.0a | 68.3 ± 3.71a | 28.0 ± 3.16a | 1.8 ± 0.48a | 2.0 ± 0.41a |
| Nanocurc | 10 | 37.0 ± 2.35a | 12.1 ± 0.76a | 6.2 ± 0.37a | 4937.5 ± 1205.1a | 79000.0 ± 15874.5a | 66.0 ± 1.78a | 29.3 ± 1.93a | 2.0 ± 0.41a | 2.8 ± 0.85a |
| Free-curc | 5 | 37.8 ± 1.20a | 12.1 ± 0.24a | 6.0 ± 0.32a | 4433.3 ± 1749.4a | 84500.0 ± 33744.1a | 64.0 ± 2.80a | 31.8 ± 2.36a | 2.0 ± 0.58a | 2.3 ± 0.25a |
| Free-curc | 10 | 38.0 ± 1.47a | 12.7 ± 0.47a | 6.2 ± 0.33a | 6770.0 ± 3634.6a | 123000.0 ± 72263.4a | 64.5 ± 1.04a | 31.3 ± 1.31a | 1.8 ± 0.48a | 2.5 ± 0.29a |
| Control | – | 40.8 ± 1.03a | 13.5 ± 0.45a | 6.8 ± 0.22a | 5000.0 ± 2454.9a | 90250.00 ± 32907.7a | 65.8 ± 4.55a | 30.8 ± 4.82a | 1.8 ± 0.25a | 1.8 ± 0.48a |
Nanocurc, nanocurcumin; Free-curc, free curcumin, PCV, packed cell volume; HB, haemoglobin; RBC, red blood cells; WB, white blood cells; EOS, eosinophils; MONO, monocyte; LYM, lymphocyte; NEUT, neutrophil. Similar superscripts denote no significant difference (p > 0.05) while different superscripts denote significant difference (p < 0.05).
3.4. Morphometric measures of mice in different treatments
Table 5 shows the morphometric measures of mice treated with curcumin nanoparticulate and free curcumin. The liver (1.31 ± 0.08 g) and spleen (0.15 ± 0.01 g) weight of mice exposed to 10 mg/kg nanocurcumin was significantly higher than that of the negative control.
Table 5.
Morphometric measures of mice treated with free, nanoparticulate curcumin and the control groups
| Treatment | Dose (mg/kg) | Weight of organs (g) |
Organ coefficient (%) |
|||
|---|---|---|---|---|---|---|
| Body weight (g) | Liver | Spleen | Liver | Spleen | ||
| Nanocurc | 5 | 24.90 ± 0.20a | 1.40 ± 0.05b | 0.12 ± 0.01a | 5.62a | 0.48a |
| Nanocurc | 10 | 26.65 ± 1.21a | 1.31 ± 0.08b | 0.15 ± 0.01b | 4.92a | 0.56b |
| Free-curc | 5 | 26.53 ± 0.77a | 1.22 ± 0.02a | 0.12 ± 0.01a | 4.60a | 0.45a |
| Free-curc | 10 | 25.18 ± 0.47a | 1.14 ± 0.06a | 0.11 ± 0.00a | 4.53a | 0.44a |
| Control | – | 24.25 ± 1.00a | 0.97 ± 0.07a | 0.10 ± 0.00a | 4.00a | 0.41a |
Nanocurc, nanocurcumin; Free-curc, free curcumin. Similar superscripts denote no significant difference (p > 0.05) while different superscripts denote significant difference (p < 0.05).
4. Discussion
Curcumin has been widely exploited for the treatment of various ailments. In a bid to increase the spectrum of activities and potency of curcumin, we have recently incorporated the compound in polymeric nanomaterial for possible treatment of malaria.25 Besides the immunomodulatory potentials of curcumin, the adjuvanticity of poly (lactic-co-glycolic acid),29 the polymeric material used for its encapsulation, could potentiate its medicinal activities. The size of the curcumin nanoparticle used in this study is suitable for easy and efficient transportation of drug to target tissues through the epithelia and other biological barriers.30
The significantly higher antibody production in 10 mg/kg nanocurcumin group compared with the control group observed in our study suggests immunostimulatory potential of the nanosynthesized drug. This immune-enhancing activity compared with the supposed immunosuppressive potential of free curcumin could have been due to PLGA adjuvanticity properties as earlier mentioned. The increase in secondary humoral antibody levels confirmed the true ability of the drug used in this study to stimulate the immune system and further suggests that many B-lymphocytes and other antibody secreting cells have become memory cells to the antigen (SRBCs). In addition to increase in B-lymphocytes activation by nanocurcumin, there could also be possible enhancement in the level of IgM and IgG.31 Other immunological processes that could have been influenced by nanocurcumin are release of mediators of hypersensitivity reactions and tissue responses to these mediators in the target organs.32 Unlike nanocurcumin, the low antibody response in free curcumin compared with the negative control suggests immunosuppressive property of the free drug. Curcumin has been known to suppress multiple immunological mechanisms that potentiate certain diseases or disorders such as arthritis, multiple sclerosis, Alzheimer disease, inflammatory bowel disease, psoriasis, wound healing and inflammatory type II diabetes.33, 34, 35, 36, 37
While this study suggested the immunosuppressiveness of free curcumin on B-lymphocytes evident in lower antibody production, the delayed-type hypersensitivity (DTH) was enhanced. This indicates the ability of curcumin to boost cell-mediated immune responses, an observation which has been reported in many plant products.5, 32 It was found that both nanoparticulate and free curcumin potentiated DTH reactions induced by SRBCs. The DTH reactions, which usually occur between 24 and 72 hours, are stimulated by memory T cells, macrophages as well as CD4 and CD8 T cells.38, 39 The role of the cytokine interferon gamma (IFNγ) in induction of DTH reactions has also been emphasized.5 Recruitment of T cells into tissues followed by activation by antigen-presenting cells (APCs) leading to production of cytokine that mediates local inflammation is fundamental to DTH reactions.40 This response has been particularly important for wading off intracellular pathogens, in transplant rejection and tumour immunity.38 The study showed that free curcumin and nanocurcumin modulated cell-mediated adaptive immune response in mice through decrease in DTH reactions after 24 hours. The higher increase in footpad oedema in mice fed on to treated with a low-dose free curcumin compared to the corresponding nanocurcumin in our results could be attributed to fast metabolism and moderate localization or aggregation of the drug on immune cells leading to corresponding boost in cell-mediated immune response. The decrease in cellular immune response observed in the mice fed on to treated with a higher dose of free curcumin compared with the low-dose of curcumin could be due to drug burden resulting in suppression of cellular immune response. The controlled release of curcumin from the higher dosage of the nanotized form, however, was able to prevent immune burden and thus potentiate the cell-mediated immune response.
The white blood cells are an important component of the blood, which to a large extent affect the immune response. The change in white blood cell and differential counts is an important marker of immune response. While there were no significant differences in haematological parameters across different treatment groups, an increase in counts of lymphocytes, monocytes and eosinophils in the mice treated with nanocurcumin compared with the free curcumin further confirms its greater immunomodulatory potential. Neutrophils are phagocytes that are non-specific in action and are involved in innate surveillance. In microbial or parasitic infections, the neutrophils play a crucial role as killer cells in antigenic challenges.41 The cells migrate towards a challenge and kill pathogens intracellularly through formation of oxygen radicals.42 The increase in eosinophils in the 10 mg/kg nanocurcumin-exposed group suggests the suitability of the nanoformulation in combating multicellular parasites and other infections in vertebrates. The anti-filarial efficacy of nanocurcumin against Brugia malayi has been reported.43 Eosinophils accumulate and degranulate around tissue-invading helminth larvae secreting proteins, which are thought to be responsible for the death of the worm.44 White blood cell count (WBC) and platelet count increase in the higher-dose free curcumin is an implication of systemic inflammatory reaction within the body45, an observation which could be responsible for the decrease in the cellular immune response as earlier discussed. This inflammatory process could be due to high-drug burden associated with free curcumin at high dose. The decrease observed in the count of WBC and platelets in mice fed on to treated with high-dose nanotized curcumin suggested that it possessed some anti-inflammatory effects. The larger size of spleen in mice fed on to treated with nanocurcumin (10 mg/kg) is supported by the higher antibody production and white blood cell counts, all of which contributed to greater immune response observed in the group.
This study showed that nanocurcumin induced higher humoral immune response compared with the free curcumin. The cellular-mediated immune response was similarly potentiated by low-dose free curcumin and high-dose nanocurcumin. The bioavailability and controlled release of the higher-dose nanocurcumin could be responsible for such increase in humoral and cellular immune responses. Although the cellular-mediated immunomodulatory effect of curcumin nanoparticles on CD3, CD4, CD8 and CD24 in splenocytes could provide more information on its involvement in cellular immunity, this was not evaluated. Its ability to stimulate production of leukocytes especially eosinophils could make it an important future anti-helminthic drug. It is believed that this may augment the anti-helminthic properties of free curcumin reported in previous studies46, 47 and could replace synthetic drugs that have been proven to constitute harmful side effects on the immune system.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The nanoparticle used for this study was formulated at the Product Cell Development Laboratory II, National Institute of Immunology, New Delhi, India.
References
- 1.Agarwal S.S., Singh V.K. Immunomodulators: a review of studies on Indian medicinal plants and synthetic peptides. Part 1: Medicinal plants. PINSA. 1999;B65:179–204. [Google Scholar]
- 2.Waldmann H. The new immunosuppression. Curr Opin Chem Biol. 2003;7:476–480. doi: 10.1016/s1367-5931(03)00076-0. [DOI] [PubMed] [Google Scholar]
- 3.Sainis K.B., Sumariwalla P.F., Goel A., Chintalwar G.J., Sipahimalani A.T., Banerji A. Immunomodulatory properties of stem extracts of Tinospora cordifolia: cell targets and active principles. In: Upadhyay S.N., editor. Immunomodulation. Narosa Publishing House; New Delhi, India: 1997. p. 95. [Google Scholar]
- 4.Sibi P.I., Varghese P. Evaluation of in vivo immunomodulatory activity of Punica granatum Linn. Int J Res Ayurveda Pharm. 2014;5:175–178. [Google Scholar]
- 5.Eze C.O., Nworu C.S., Esimone C.O., Okore V.C. Immunomodulatory activities of methanol extract of the whole aerial part of Phyllantus niruri L. J Pharmacog Phytother. 2014;6:41–46. [Google Scholar]
- 6.Yadav P., Ganeshpurkar A., Sonkar N., Bansal D., Dubey N. Experimental studies on immunomodulatory potential of Cissus quadrangularis Linn. Niger J Exp Clin Biosci. 2014;2:49–53. [Google Scholar]
- 7.Ahirwal L., Singh S., Dubey M.K., Bharti V., Mehta M., Shukla S. In vivo immunomodulatory effects of the methanolic leaf extract of Gymnema sylvestre in Swiss albino mice. Arch Biol Sci Belgrade. 2015;67:561–570. [Google Scholar]
- 8.Singh S., Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava R.M., Singh S., Dubey S.K., Misra K., Khar A. Immunomodulatory and therapeutic activity of curcumin. Inter Immunopharmacol. 2011;11:331–341. doi: 10.1016/j.intimp.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Gullett N.P., Ruhul Amin A.R., Bayraktar S. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Visioli F., De La, Lastra C.A. Polyphenols and human health: a prospectus. Crit Rev Food Sci Nutr. 2011;51:524–546. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- 12.Hatcher H. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-Sela G., Epelbaum R., Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17:190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- 14.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 15.Basnet P., Skalko-Basnet N. Curcumn: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2010;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teiten M.H., Eifes S., Dicato M., Diederich M. Curcumin – the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins (Basel) 2010;2:128–162. doi: 10.3390/toxins2010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilken R. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical Trial (CTG) US National Institutes of Health, Clinical Trial Registry; June 2015. ClinicalTrials.gov: current clinical trials on curcumin. [Google Scholar]
- 19.Mimche P.N., Taramelli D., Vivas L. The plant based immunomodulatory curcumin as a potential candidate for the development of an adjunctive therapy for cerebral malaria. Mal J. 2011;10:S10. doi: 10.1186/1475-2875-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel A., Boland C.R., Chauhan D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 21.Tonnesen H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Die Pharm. 2002;57:820–824. [PubMed] [Google Scholar]
- 22.Bisht S., Feldmann G., Soni S., Ravi R., Karikar C., Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol. 2007;5:1–18. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan J., Zhang Y., Han S., Chen Y., Li B., Liao M. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly (butyl cyanoacrylate) nanoparticles. Int J Pharm. 2010;400:211–220. doi: 10.1016/j.ijpharm.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen H.T., Tran T.H., Kim J.O., Yong C.S., Nguyen C.N. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly d,l-lactide-co-glycolide (PLGA) nanoparticles. Arch Pharm Res. 2015;38:716–724. doi: 10.1007/s12272-014-0424-3. [DOI] [PubMed] [Google Scholar]
- 25.Busari Z.A., Dauda K.A., Morenikeji O.A., Afolayan F., Oyeyemi O.T., Meena J. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front Pharmacol. 2017:622. doi: 10.3389/fphar.2017.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazumder A.C., Khatun S., Nooruzzaman M., Chowdhury E.H., Das P.M., Islam M.R. Isolation and identification of Newcastle disease viruses from field outbreaks in chickens and pigeons. Bangladesh Vet. 2012:41–48. [Google Scholar]
- 27.Doherty N.S. Selective effects of immunosuppressive agents against the delayed hypersensitivity response and humoral response to sheep red blood cells in mice. Agents Action. 1981;11:237–242. doi: 10.1007/BF01967620. [DOI] [PubMed] [Google Scholar]
- 28.Verma A., Sahu M.S., Sahu R.A. Immunomodulatory activity of alcoholic extract of Habenaria intermedia in mice. Inter J Pharm Pharm Sci. 2013;5:406–409. [Google Scholar]
- 29.Silva A.L., Soema P.C., Slütter D., Ossendorp F., Jiskoot W. PLGA particulate delivery systems for subunit vaccines: linking particle properties to immunogenicity. Hum Vacc Immunotherap. 2016;12:1056–1069. doi: 10.1080/21645515.2015.1117714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manolova V., Flace A., Bauer M., Schwarz K., Saudan P., Bachmann M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 31.Arshami J., Pilevar M., Azghadi M.A., Raji A.R. Hypolipidemic and antioxidative effects of curcumin on blood parameters, humoral immunity, and jejunum histology in Hy-line hens. Avicenna J Phytomed. 2012;3:178–185. [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain A., Shadma W., Maksood A., Ansari S.H. Protective effects of Picrorhiza kurroa on cyclophosphamide-induced immunosuppression in mice. Pharmacognosy Res. 2013;5:30–35. doi: 10.4103/0974-8490.105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisberg S.P., Leibel R., Tortoriello D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie L., Li X.K., Funeshima-Fuji N., Kimura H., Matsumoto Y., Isaka Y. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int Immunopharmacol. 2009;9:575–581. doi: 10.1016/j.intimp.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 35.Rajakrishnan V., Shiney S.J., Sudhakaran P.R., Menon V.P. Effect of curcumin on ethanol-induced stress on mononuclear cells. Phytother Res. 2002;16:171–173. doi: 10.1002/ptr.741. [DOI] [PubMed] [Google Scholar]
- 36.Lai J.J., Lai K.P., Chuang K.H., Chang P., Yu I.C., Lin W.J. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giri R.K., Rajagopal V., Kalra V.K. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. J Neurochem. 2004;91:1199–1210. doi: 10.1111/j.1471-4159.2004.02800.x. [DOI] [PubMed] [Google Scholar]
- 38.Allen I.C. Delayed-type hypersensitivity models in mice. Methods Mol Biol. 2013;1031:101–107. doi: 10.1007/978-1-62703-481-4_13. [DOI] [PubMed] [Google Scholar]
- 39.Sachdeva H., Sehgal R., Kaur S. Asparagus racemosus ameliorates cisplatin induced toxicities and augments its antileishmanial activity by immunomodulation in vivo. Parasitol Int. 2014;63:21–30. doi: 10.1016/j.parint.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Kalish R.S., Askenase P.W. Molecular mechanisms of CD8+T cell-mediated delayed hypersensitivity: implications for allergies, asthma and automimmunity. J Allergy Clin Immunol. 1999;103:192–199. doi: 10.1016/s0091-6749(99)70489-6. [DOI] [PubMed] [Google Scholar]
- 41.Basaran A.A., Ceriioghu I., Undeger U., Basaran N. Immunmodulatory activities of some Turkish medicinal plants. Phytother Res. 1997;11:609–611. [Google Scholar]
- 42.Badway J.A., Karnovski M.L. Active oxygen species and functions of phagocytic leukocytes. Ann Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- 43.Ali M., Afzal M., Abdul Nasim S., Ahmad I. Nanocurcumin: a novel antifilarial agent with DNA topoisomerase II inhibitory activity. J Drug Targ. 2014;22:395–407. doi: 10.3109/1061186X.2013.869823. [DOI] [PubMed] [Google Scholar]
- 44.Lange A.M., Yutanawiboonchai W., Scott P., Abraham D. IL-4- and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994;153:205–211. [PubMed] [Google Scholar]
- 45.Kumar V., Abbas A.K., Fausto N. 7th ed. Saunders; Philadelphia: 2004. Robbins & Cotran pathologic basis of disease. [Google Scholar]
- 46.Yallapu M.M., Gupta B.K., Jaggi M., Chauhan S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interf Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Luz P.P., Magalhães L.G., Pereira A.C., Cunha W.R., Rodrigues V., Andrade e Silva M.L. Curcumin-loaded into PLGA nanoparticles: preparation and in vitro schistosomicidal activity. Parasitol Res. 2012;351:593–598. doi: 10.1007/s00436-011-2527-9. [DOI] [PubMed] [Google Scholar]