Abstract
Background
The aim of this study was to compare the volume of blood loss and hemodynamic changes in patients undergoing lumbar discopathy, after continuous infusions of dexmedetomidine versus remifentanil during anesthesia with controlled low blood pressure.
Methods
In this randomized double-blind clinical trial, 60 patients aged 20 to 65 years were randomly assigned to control and intervention groups. The intervention group received a continuous infusion of dexmedetomidine at 0.3 - 0.7 µg/kg/hour plus propofol at 50 - 100 µg/kg/minute. The control group was given a continuous infusion of remifentanil at 0.1 to 1 µg/kg/minute plus the same dose of propofol as above. The primary outcome was the amount of patient's bleeding during surgery, and secondary outcomes were changes in the patient's systolic blood pressure, diastolic blood pressure, mean arterial pressure, and urinary output.
Results
Univariate and multivariate analyses of the main outcome in the control and intervention groups showed that there was no significant difference between the two drugs with regards to the volume of blood loss, mean arterial pressure, and systolic and diastolic blood pressure. Postoperative side effects were significantly lower in the intervention group (P = 0.002).
Conclusions
Administration of dexmedetomidine plus propofol in comparison with remifentanil plus propofol did not show any significant difference regarding blood loss and hemodynamic changes; however, it reduced some side effects after surgery and decreased analgesic requirement in the postoperative period. Taken together, the findings of this study do not support strong recommendations for dexmedetomidine infusion for all patients and the decision should be taken with caution on basis of the anesthesiologist’s expert opinion and the patient’s condition during surgery.
Keywords: Controlled hypotension, Dexmedetomidine, Remifentanil, Blood Loss, Hemodynamic Changes
1. Background
Controlled hypotension during surgery is an increasingly important method, in which arterial blood pressure is reduced predictably (1). During this process, systolic blood pressure (SBP) is reduced to 80 to 90 mmHg and mean arterial pressure (MAP) is reduced to 50 to 65 mmHg, meaning a 30% reduction in baseline MAP. The aim of this approach was to reduce blood loss and consequent blood transfusion requirements and also to improve the surgical field (2). This technique can provide a dry field for the surgeon and obviously lead to a better result in surgery, thus, in the recent years, there has been an increasing interest in its application. There are two main strategies for implementing controlled hypotension during anesthesia, increasing the depth of anesthesia and administering hypotensive drugs (1). A wide range of vasodilators, beta-blockers, and volatile anesthetics or combinations of two or more medications are used in various operation fields to induce hypotensive anesthesia (3). Drugs that can successfully be used alone include inhalation anesthetics, sodium nitroprusside, nitroglycerin, trimethaphan camsylate, alprostadil (prostaglandin E1), adenosine and remifentanil, and those that are best used adjunctively, include angiotensin-converting enzyme (ACE) inhibitors and clonidine (2).
Propofol, by dilating veins and hence facilitating the outflow of blood from the surgical field, can induce anesthesia and sedation. Dexmedetomidine is a selective α2-adrenergic receptor agonist, which can decrease mean arterial pressure (MAP) and heart rate by reducing norepinephrine release, and it is known to be an analgesic drug with sympatholytic, anesthetic-sparing, and hemodynamic-stabilizing properties. Dexmedetomidine may be an effective prophylactic agent that induces sedation and analgesia in patients with delayed extubation after craniotomy (4). Remifentanil is very fast acting with a rapid metabolism, which ensures rapid and predictable postoperative awakening and also maintains hemodynamic stability like other potent opioids. It can induce and maintain hypotension in an easy and safe way, even in pediatric patients; it can be a sufficient substitute for esmolol or nitroprusside in controlled hypotension, when it is combined with propofol or sevoflurane (3). Questions have been raised about the best choice for implementing controlled hypotensive anesthesia, and evidence shows that the ideal hypotensive agent must be easy to administer, have a short onset time, a rapid elimination without toxic metabolites, negligible effects on vital organs, and a predictable and dose-dependent effect (2). There are many different ways of combining anesthetic agents to obtain the best result, for example, induction with propofol and remifentanil and maintenance with remifentanil and inhalation agent with or without propofol infusion; or induction with propofol and fentanyl, maintenance with dexmedetomidine and inhalation agent with or without propofol; or induction with propofol and remifentanil, maintenance with remifentanil, dexmedetomidine and inhalation agent (1). In the recent years, there has been an increasing interest in using controlled hypotensive anesthesia in different surgery fields, and the results of studies vary. Debate continues about the best strategies for the management of blood loss during surgeries. Turgut Net al. concluded that propofol-remifentanil and propofol-dexmedetomidine were both suitable for elective supratentorial craniotomy and provided similar intraoperative hemodynamic responses and postoperative adverse events (5). These authors added that propofol-remifentanil allowed earlier cognitive recovery and consequently earlier demand for postoperative analgesics. In another study, dexmedetomidine was shown to be a safe and effective adjuvant for hypotensive anesthesia to decrease bleeding (6). Dexmedetomidine was also found to reduce bleeding, bleeding scores, and intraoperative fentanyl consumption during general anesthesia in septoplasty operations (7). Lee et al. observed that in the immediate postoperative period, remifentanil induced a faster recovery compared to dexmedetomidine (8).
Moreover, there have been no controlled and blinded studies comparing differences between remifentanil and dexmedetomidine, regarding hemodynamic changes of patients undergoing controlled hypotensive anesthesia in lumbar discopathy surgery, and there is still insufficient data for establishing the safety and efficacy of this approach.
2. Objectives
The objectives of this research were to compare volume of blood loss and hemodynamic changes between dexmedetomidine and remifentanil in controlled hypotensive anesthesia in lumbar discopathy surgery.
3. Methods
3.1. Trial design and Population
A double-blind randomized controlled trial was conducted on 60 male and female candidates for lumbar discopathy surgery, who were referred to Golestan hospital, Ahvaz, Iran, from Septamber to December 2017. This research was supported by the Pain Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, under proposal No.IR.AJUMS.REC.1396.311. All patients provided a written informed consent.
This study assessed the eligibility of 60 patients referred to the hospital, upon admission to the neurosurgery ward on the basis of the following criteria.
Inclusion criteria: age of 20 to 65 years old with American society of anesthesiologists (ASA) class I and II, scheduled for lumbar discopathy surgery at one or two levels.
Exclusion criteria: coagulative disorders, taking anticoagulant drugs, re-operative surgery, history of lumbar trauma, diabetes and uncontrolled hypertension.
3.2. Interventions
Patients were randomly assigned to the intervention group (dexmedetomidine infusion) or the control group (remifentanil infusion). In the intervention group, patients received a continuous infusion of dexmedetomidine (PFIZER, USA), 0.3 to 0.7 µg/kg/h, plus a continuous infusion of propofol (B.BRAUN, Germany), 50 to 100 µg/kg/min. In the control group, there was a continuous infusion of remifentanil (HAMELN, Germany), 0.1 to 1 µg/kg/h, plus a continuous infusion of propofol (B.BRAUN, Germany), 50 to 100 µg/kg/min.
Prior to surgery, patients were generally monitored by an anesthesiologist and equal medical regimens were used for induction of general anesthesia in both groups. Anesthesia was induced with 2 mg midazolam (Chemidarou, Iran), 2 to 3 µg/kg fentanyl (HAMELN, Germany), 4 to 5 mg/kg sodium thiopental (SANDOZ, Austria), and 0.5 mg/kg cisatracurium (HAMELN, Germany). The anesthesia procedure was the same for the two study groups.
Blood pressure readings, via an invasive arterial line (systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP)), heart rate (HR), arterial oxygen saturation (SpO2), and ECG were monitored continuously before and after starting the study drug and during surgery.
During surgery, 8 to 10 mL/kg/h of crystalloid intravenous fluid was prescribed for both groups, and 4 mL/kg/h after surgery. After ensuring hemodynamic stability, the patient was placed in the prone position and the intervention proceeded.
3.3. Randomization and Blinding
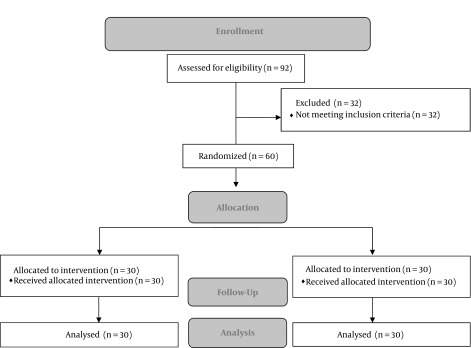
Participants were randomly allocated to two groups by using a computer-based randomization program (n = 30 in each group), which is depicted in Figure 1. This was a double-blind study, in which participants and outcome assessors were blinded yet not the investigators, due to the nature of the interventions.
Figure 1. Flow diagram of the study.
3.4. Outcomes
The primary outcome of this study was blood loss volume (in milliliters). Total blood loss was estimated during the entire procedure, and after serial blood gas analysis, patients received blood transfusion if hemoglobin concentration was less than 8 g/dL.
As secondary outcomes, the researchers measured changes in the systolic blood pressure, diastolic blood pressure, and mean arterial pressure at 0, 10, 30, and 60 minutes after infusion and also after extubation; in both the intervention and control group. Other secondary outcomes were changes in heart rate (at 0, 10, 30, and 60 minutes after infusion and also after extubation), urinary output (post-surgery, in milliliters), interval between extubation to full orientation (in minutes), and the need for an analgesic agent in the post-operative period.
Patients were followed until the end of the recovery time.
3.5. Sample Size
The sample size was determined according to a similar previous study (8). The sample size, considering attrition, was calculated as 30 in each group (power: 80% and α: 5%).
3.6. Ethical Considerations
Informed written consent was obtained from all participants, and the ethical committee of Ahvaz Jundishapur University of Medical Sciences approved this study in 2017 (Ref. No.IR.AJUMS.REC.1396.311). The study protocol conformed to the ethical guidelines of the 2008 declaration of Helsinki and it has been registered at the Iranian registry of clinical trials (IRCT ID: IRCT2017083136009N1).
Statistical methods: All statistical analyses were performed using SPSS version 22. All main outcomes are presented as mean (± SD) and frequency (%) for continuous and categorical variables, respectively. The statistical significance level was set at a two-tailed type I error of 0.05, and deviation from abnormal distribution was determined by the Kolmogorov-Smirnov test.
Univariate analysis was done by the Student t-test and Mann-Whitney, chi-square, and repeated measures tests. Mixed model, linear regression, and logistic regression were used to evaluate the data in multivariate analysis.
4. Results
No statistically significant differences were found between the two groups in the patient's demographic and clinical characteristics (Table 1). There was no significant difference between the two groups at induction and maintenance of anesthesia regarding hemodynamic parameters, yet heart rate, MAP, SBP, and DBP gradually decreased from the first to 60th minute and then increased after extubation, and there were significantly in each group (P < 0.001) (Table 2).
Table 1. Baseline Characteristics of Participantsa.
| Patients Characteristics | Intervention Group (Dexmedetomidine Infusion) (n = 30) | Control Group (Remifentanil Infusion) (n = 30) | P Value |
|---|---|---|---|
| Age, y | 36.57 ± 8.04 | 37.97 ± 9.58 | 0.542 |
| Gender (male/female) | 18/12 | 16/14 | |
| Weight, kg | 73.93 ± 7.23 | 74.10 ± 6.95 | 0.927 |
| ASA I/II | 21/9 | 22/8 | 0.58 |
| Duration of surgery, min | 46.3 ± 4.72 | 50.19 ± 4.91 | 0.83 |
Abbreviations: ASA, American society of anesthesiologist’s status; SD, standard deviation.
aValues are expressed as mean ± SD or No. (%), where applicable value calculated using independent sample t-test or Chi-square test.
Table 2. Comparison of Hemodynamic Variables During Surgery, at Different Time Points, Between the Two Groupsa.
| Variable | Beginning | 10th min | 30th min | 60th min | Extubation | P Value |
|---|---|---|---|---|---|---|
| Heart rate, beat/min | ||||||
| Intervention group | 79.47 ± 11.34 | 74.33 ± 9.56 | 68.33 ± 10.02 | 63.43 ± 10.82 | 92.93 ± 12.77 | < 0.001 |
| Control group | 82.33 ± 12.15 | 77.5 ± 12.09 | 69.60 ± 9.94 | 65.23 ± 8.17 | 94.73 ± 13.78 | < 0.001 |
| P Value | 0.35 | 0.26 | 0.62 | 0.47 | 0.60 | |
| Systolic blood pressure, mmHg | ||||||
| Intervention group | 114.6 ± 12.32 | 110.3 ± 12.06 | 103.5 ± 12.04 | 99.2 ± 12.44 | 132.63 ± 15.44 | < 0.001 |
| Control group | 119.1 ± 13.78 | 112.47 ± 12.81 | 103.53 ± 10.1 | 99.78 ± 11.01 | 135.37 ± 13.55 | < 0.001 |
| P Value | 0.19 | 0.50 | 0.99 | 0.83 | 0.47 | |
| Diastolic blood pressure, mmHg | ||||||
| Intervention group | 74.60 ± 9.86 | 73.83 ± 10.16 | 70.60 ± 10.08 | 68.70 ± 10.71 | 85.03 ± 10.13 | < 0.001 |
| Control group | 76.87 ± 9.97 | 74.93 ± 9.62 | 68.77 ± 8.03 | 68.93 ± 7.64 | 86.83 ± 8.38 | < 0.001 |
| P Value | 0.38 | 0.67 | 0.44 | 0.92 | 0.46 | |
| Mean arterial blood pressure, mmHg | ||||||
| Intervention group | 87.93 ± 9.36 | 85.99 ± 9.61 | 81.57 ± 9.72 | 78.87 ± 10.64 | 100.9 ± 10.52 | < 0.001 |
| Control group | 90.94 ± 10.54 | 87.44 ± 10.04 | 80.36 ± 8.13 | 79.24 ± 8.02 | 103.01 ± 8.85 | < 0.001 |
| P Value | 0.25 | 0.57 | 0.60 | 0.88 | 0.40 |
aValues are expressed as mean ± SD.
There was no significant interaction between time points and groups regarding heart rate. Multivariate analysis of heart rate showed that by adjusting ASA class, age, weight, and baseline heart rate, there was no significant difference between the two groups (P = 0.59).
The mean and standard deviation of blood loss, urinary output, interval between discontinuation of maintenance to full orientation, frequency of need for analgesic drugs, and percentage of side effects are presented in Table 3. Blood loss and urinary output did not show any difference between the two groups, yet there were significant differences between the two groups in the interval between discontinuation of maintenance and full orientation (P < 0.001), analgesic requirement (P = 0.002) and postoperative side effects (P = 0.002).
Table 3. The Comparison of Blood Loss, Urinary Output, Time Between Discontinue of Anesthesia to Full Orientation, and Analgesic Requirement Between the Two Groupsa.
| Outcomes | Dexmedetomidine Group (n = 30) | Control Group (n = 30) | P Value |
|---|---|---|---|
| Blood loss, mL | 268.33 ± 132.67 | 255.67 ± 84.96 | 0.795 |
| Urinary output, mL | 255.17 ± 200.03 | 247.67 ± 170.65 | 0.706 |
| Discontinue of anesthesia to full orientation, min | 32.67 ± 5.06 | 24.83 ± 4.63 | < 0.001 |
| Post-Operative Analgesic requirements | 0.002 | ||
| Yes | 11 (36.7) | 23 (76.7) | |
| No | 19 (63.3) | 7 (23.3) |
aValues are expressed as mean ± SD.
Table 4 shows the type and frequency of each side effect observed in both the intervention group and the control group. Shivering was more common in the control group than the intervention group, which was significant (P = 0.004); and nausea and vomiting was more common in the control group than the intervention group yet the difference was non-significant (P = 0.209). Bradycardia and need for administration of atropine was more frequent in the intervention group; this was not significant (P = 0.299).
Table 4. The Comparison of Particular Side Effects Between the Intervention and Control Groupa.
| Side Effects | Dexmedetomidine Group (n = 30) | Control Group (n = 30) | P Value |
|---|---|---|---|
| Shivering | 0.004 | ||
| Yes | 7 (23.3) | 18 (60) | |
| No | 23 (76.7) | 12 (40) | |
| Nausea and Vomiting | 0.209 | ||
| Yes | 4 (13.3) | 9 (30) | |
| No | 26 (86.7) | 21 (70) | |
| Bradycardia (Atropine administered) | 0.299 | ||
| Yes | 7 (23.3) | 3 (10) | |
| No | 23 (76.7) | 27 (90) |
aValues are expressed as No. (%).
In all cases of bradycardia, atropine (0.5 mg. IV. bolus) was used only once and there was no need for a repeat.
5. Discussion
The present study was a double-blind randomized clinical trial that was designed to determine the differences between dexmedetomidine and remifentanil infusion with regards to blood loss volume and hemodynamic changes in lumbar discopathy surgery. It was hypothesized that all candidates for this surgery would benefit from dexmedetomidine infusion in a controlled hypotensive anesthesia.
The main question in this research was whether dexmedetomidine reduces blood loss during controlled hypotensive anesthesia.The results of this study indicated that blood loss did not differ between the two study groups (P = 0.795). Durmus et al. evaluated the effect of dexmedetomidine on bleeding during tympanoplasty or septorhinoplasty and they showed a reduction in blood loss in the group receiving this drug in comparison to placebo (P < 0.05) (9). Mizrak et al. evaluated the effect of dexmedetomidine on intraoperative blood loss inpediatric adenotonsillectomy, and the results demonstrated that surprisingly, the total amount of blood loss with dexmedetomidine was significantly higher than the placebo group (P < 0.05) (10). In a study by Ibraheim et al. on the effects of dexmedetomidine compared to esmolol on blood loss and hemodynamic changes in scoliosis surgery, they observed a significant reduction in blood loss in patients, who received dexmedetomidine (465 mL versus 667 mL, P < 0.05) (11). The results of previous studies on blood loss are controversial and this may be due to the different types of surgeries.
In this study, there was no significant difference in MAP between these two drugs. However, the findings of the current study did not support the findings of an earlier work by Durum et al. In a study by Koroglu et al. the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol were compared in children undergoing magnetic resonance imaging procedures. Mean Arterial Pressure was significantly lower in the propofol group (12).
The results of this study was in contrast with the findings of some previous works in this field. In the present study, there was no significant correlation between times and groups regarding DBP, SBP, and Heart Rate. In a comparative study of hemodynamic effects of four different intravenous doses of dexmedetomidine (0.25, 0.5, 1.0 and 2.0 µg/kg), heart rate of patients decreased by 17% at 150 minutes. The comparison showed no benefit for higher doses and all doses were well-tolerated. In another study by Aho et al. (13), dexmedetomidine (0.3 or 0.6 µg/kg) was compared with fentanyl 2.0 µg/kg or saline as a single intravenous bolus. The results showed that post-intubation increase in heart rate was significantly less in the group that received the higher dose of dexmedetomidine than all other groups (14). There are several possible explanations for this result. In a recent study that was conducted to compare the effect of dexmedetomidine and remifentanil on intraoperative field conditions and recovery during endoscopic sinus surgery, Lee et al. did not detect any significant differences between the two groups with respect to surgical field conditions, blood loss, hemodynamic parameters, and time to extubation (8). The results of this study are in line with the current.
It has been reported that dexmedetomidine has anesthetic and analgesic effects in addition to its sedative effects (5). The current study showed that analgesic requirement in the postoperative period was significantly reduced with dexmedetomidine-propofol infusion compared to remifentanil-propofol infusion (P = 0.002)
As for anesthesia emergence characteristics, a study by Namigar et al. showed an advantage for remifentanil with respect to PACU discharge time, where dexmedetomidine patients required a longer time compared to remifentanil patients to achieve their first normal neurological score (33 versus 31 minutes) (5). The current observation was directed at the interval time between discontinuation of maintenance of anesthetic drugs to full orientation status. This interval was longer with dexmedetomidine infusion (32.6 minutes) than remifentanil infusion (24.8 minutes) (P < 0.001). In Safinaz Karabayirli et al.’s study, it was concluded that in comparison with remifentanil, dexmedetomidine during functional endoscopic sinus surgery (FESS) for controlled hypotension, is of limited value as it has no additional benefits in terms of control of hypotension and amount of bleeding in the surgical field and it is associated with higher recovery time and first-hour postoperative sedation scores (15, 16). The results of these studies are in line with that of the current study.
5.1. Limitations
The researchers were not able to assign all surgical procedures to a single surgeon, because Golestan Hospital is an educational hospital with many surgeons working on different days.
It was better to compare the anesthetic depth between the two groups. The Bi-Spectral Index device is used for this purpose. Due to some technical difficulties, it was not possible to use this hospital device.
5.2. Conclusions
The findings of this study have a number of important implications for future practice. Administration of dexmedetomidine plus propofol compared to remifentanil plus propofol did not show any significant difference regarding hemodynamic changes and blood loss; however, it reduced some side effects after surgery and decreased analgesic requirement in the postoperative period. Taken together, the findings of this study do not support strong recommendations for dexmedetomidine infusion for all patients, and the decision should be taken with caution on the basis of the anesthesiologist’s expert opinion and patient’s condition during surgery.
Acknowledgments
This paper was part of a thesis project (project code: PAIN-9605). Financial support for this study was provided by Ahvaz Jundishapur University of Medical Sciences. The authors sincerely thank the patients, who cooperated in this project and supported the research team.
Footnotes
Financial Disclosure:The corresponding author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and so registered) have been explained. The reporting of this work is compliant with CONSORT guidelines.
Conflict of Interests:All authors affirm that there were no conflicts of interest.
Funding/Support:Pain research center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Contributor Information
Mansoor Soltanzadeh, Email: sultanman84@yahoo.com.
Amir Salari, Email: salari@ajums.ac.ir.
References
- 1.Upadhyay SP, Samant U, Tellicherry SS, Mallik S, Saikia PP, Mallick PN. Controlled hypotension in modern anaesthesia: A review and update. Int J Bio Pharm Res. 2015;6:532–42. [Google Scholar]
- 2.Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007;67(7):1053–76. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 3.Shah H, Kulkarni A. A comparative study between Dexmedetomidine infusion and Propofol infusion for maintenance in patients undergoing Functional Endoscopic Sinus Surgery under General Anaesthesia. IOSR-JDMS. 2016;15(3):82–6. [Google Scholar]
- 4.Zhao LH, Shi ZH, Chen GQ, Yin NN, Chen H, Yuan Y, et al. Use of Dexmedetomidine for Prophylactic Analgesia and Sedation in Patients With Delayed Extubation After Craniotomy: A Randomized Controlled Trial. J Neurosurg Anesthesiol. 2017;29(2):132–9. doi: 10.1097/ANA.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turgut N, Turkmen A, Ali A, Altan A. Remifentanil-propofol vs dexmedetomidine-propofol--anesthesia for supratentorial craniotomy. Middle East J Anaesthesiol. 2009;20(1):63–70. [PubMed] [Google Scholar]
- 6.Gupta P, Choudhary R, Ojha T, Jethava D. Dexmedetomidine as an adjuvant for Hypotensive Anaesthesia during Functional Endoscopic Sinus Surgery (FESS). IOSR J Dent Med Sci. 2016;15(08):143–6. doi: 10.9790/0853-150802143146. [DOI] [Google Scholar]
- 7.Ayoglu H, Yapakci O, Ugur MB, Uzun L, Altunkaya H, Ozer Y, et al. Effectiveness of dexmedetomidine in reducing bleeding during septoplasty and tympanoplasty operations. J Clin Anesth. 2008;20(6):437–41. doi: 10.1016/j.jclinane.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Kim Y, Park C, Jeon Y, Kim D, Joo J, et al. Comparison between dexmedetomidine and remifentanil for controlled hypotension and recovery in endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2013;122(7):421–6. doi: 10.1177/000348941312200702. [DOI] [PubMed] [Google Scholar]
- 9.Durmus M, But AK, Dogan Z, Yucel A, Miman MC, Ersoy MO. Effect of dexmedetomidine on bleeding during tympanoplasty or septorhinoplasty. Eur J Anaesthesiol. 2007;24(5):447–53. doi: 10.1017/S0265021506002122. [DOI] [PubMed] [Google Scholar]
- 10.Mizrak A, Karatas E, Saruhan R, Kara F, Oner U, Saricicek V, et al. Does dexmedetomidine affect intraoperative blood loss and clotting tests in pediatric adenotonsillectomy patients? J Surg Res. 2013;179(1):94–8. doi: 10.1016/j.jss.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Ibraheim OA, Abdulmonem A, Baaj J, Zahrani TA, Arlet V. Esmolol versus dexmedetomidine in scoliosis surgery: study on intraoperative blood loss and hemodynamic changes. Middle East J Anaesthesiol. 2013;22(1):27–33. [PubMed] [Google Scholar]
- 12.Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103(1):63–7. doi: 10.1213/01.ANE.0000219592.82598.AA. table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74(6):997–1002. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77(6):1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Karabayirli S, Ugur KS, Demircioglu RI, Muslu B, Usta B, Sert H, et al. Surgical conditions during FESS; comparison of dexmedetomidine and remifentanil. Eur Arch Otorhinolaryngol. 2017;274(1):239–45. doi: 10.1007/s00405-016-4220-1. [DOI] [PubMed] [Google Scholar]
- 16.Taghipour Anvari Z, Afshar-Fereydouniyan N, Imani F, Sakhaei M, Alijani B, Mohseni M. Effect of Clonidine Premedication on Blood Loss in Spine Surgery. Anesth Pain Med. 2012;1(4):252–6. doi: 10.5812/aapm.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]