Abstract
Canine bestrophinopathy (cBest) is an important translational model for BEST1-associated maculopathies in man that recapitulates the broad spectrum of clinical and molecular disease aspects observed in patients. Both human and canine bestrophinopathies are characterized by focal to multifocal separations of the retina from the RPE. The lesions can be macular or extramacular, and the specific pathomechanism leading to formation of these lesions remains unclear. We used the naturally occurring canine BEST1 model to examine factors that underlie formation of vitelliform lesions and addressed the susceptibility of the macula to its primary detachment in BEST1-linked maculopathies.
Keywords: BEST1, Bestrophinopathies, Canine model, Macula, Vitelliform lesion formation, RPE-photoreceptor interface, RPE microvilli, Cone photoreceptors, RPE apical domain, Insoluble interphotoreceptor matrix
38.1 Introduction
Bestrophinopathies are caused by mutations in the BEST1 gene expressed in the retinal pigment epithelium (RPE) (Petrukhin et al. 1998; Marmorstein et al. 2000). Among this group of disorders, Best vitelliform macular dystrophy (BVMD) and autosomal recessive bestrophinopathy (ARB) are the most common and best studied juvenile macular dystrophies, characterized by an excessive accumulation of lipofuscin within RPE, reduction in the electrooculogram (EOG) light peak, and formation of bilateral macular to multifocal subretinal lesions (Boon et al. 2009). Despite extensive research, the pathological mechanism of BEST1 still remains elusive, and factors leading to formation of macular lesions are largely unexplored. We used the spontaneous dog Best disease model, canine bestrophinopathy (cBest) (a.k.a. canine multifocal retinopathy, cmr) (Guziewicz et al. 2007; Zangerl et al. 2010), to study the architecture of the RPE-photoreceptor interface and define factors that promote development of vitelliform lesions.
38.2 Materials and Methods
38.2.1 Study Animals and Ethics Statement
cBest-affected and control dogs were genotyped and ophthalmoscopically examined, and ocular tissue was collected and processed as previously described (Guziewicz et al. 2007; Beltran et al. 2014). The studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH and in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (IACUC Protocol #s 801870, 803422). Adult dogs (age 1–3 years) after disease onset and young (aged 6wks) before development of clinical phenotype were selected for histological and immunohistochemical (IHC) analyses.
38.2.2 Histological and IHC Assessments
Retinal structure was examined in 10-μm-thick cryosections by H&E staining and IHC using a set of RPE- and photoreceptor-specific markers: 1/400 mouse anti-Best1 (ab2182), 1/400 mouse anti-EZR (ab4069), 1/500 rabbit anti-MCT1 (courtesy of N. Philp, Thomas Jefferson University), 1/500 rabbit anti-E2F1 (sc-193) or 1/10,000 rabbit anti-hCAR (courtesy of C. Craft, USC), and PNA (L-32458). Slides were evaluated with transmitted light microscopy, epifluorescence, and confocal laser scanning microscopy. All ex vivo analyses were carried out in comparison to the age-matched control eyes.
38.3 Results
38.3.1 Molecular Pathology of the RPE-Photoreceptor Interface in cBest
IHC evaluation of cBest retinae (age 1–3 years) revealed an abnormal RPE-photoreceptor interface with an apparent loss of cone-ensheathing RPE apical processes and compromised cone-associated insoluble interphotoreceptor matrix (IPM) (Fig. 38.1). Specific immunostaining against MCT1 (UniProt#P53985) was present in both the normal control and mutant retinae, but in the latter the labeling was limited to the RPE apical surface, but not along apical extensions (Fig. 38.1b). Further analysis with an alternative marker, E2F1 (UniProt#Q01094), confirmed these results in cBest (Fig. 38.1d) and exposed a bilayered extracellular compartmentalization of the cone-specific escheatment responsible for a normal apposition of RPE-cone OS (Fig. 38.1c, inset). This well-defined extracellular structure was lost in the affected retinae, revealing a dearth of the intrinsic RPE-associated apical microvillus layer accompanied by a fragmented external insoluble cone-specific matrix domain (Fig. 38.1d, inset). As a result cone OS stripped of their protective sheaths appeared undermined with no direct contact with the underlying RPE (Fig. 38.1d).
Fig. 38.1.
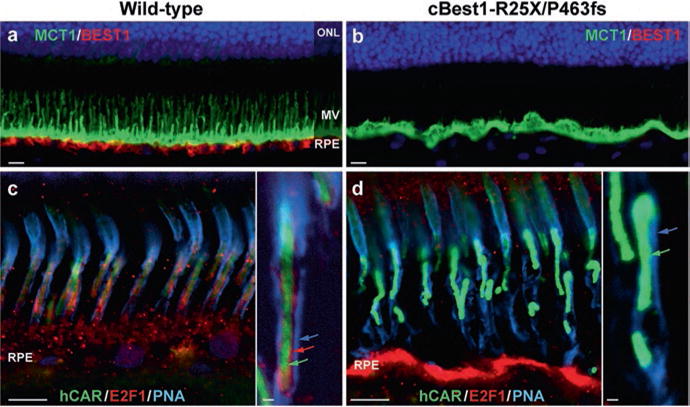
Abnormal RPE-photoreceptor interface in canine bestrophinopathies (a, b) Representative images of anti-MCT1 (monocarboxylate transporter 1) and anti-Best1 double- labeling in 2-yr-old cBest1-R25X/P463fs-affected retina (b) vs age-matched wild-type control (a). Note the absence of Best1 basolateral labeling and lack of MCT1-positive apical RPE microvillous (MV) extensions in the mutant retina (b). (c, d) Direct comparison of multilabeled (hCAR human cone arrestin, E2F1 transcription factor 1, PNA peanut agglutinin lectin) confocal photomicrographs showing the absence of E2F1-positive cone-associated RPE microvilli (d) when compared to the wild-type control (c, inset, red arrow) and compromised insoluble interphotoreceptor matrix domain (insets, blue arrow) in the mutant retina (d). Cone outer segments (insets, green arrows) appeared disordered (d) in comparison to the control tissue (c). Scale bars: 10 μm (a–d) and 1 μm (insets)
38.3.2 Underdeveloped RPE Apical Domain Promotes Retinal Detachment in cBest
Comparable studies on the RPE-photoreceptor interface were performed in 6-week- old retinae, at the time when the canine retina completes its development (Acland and Aguirre 1987) (Fig. 38.2). Immunolabeling with MCT1 and Ezrin (UniProt#P15311) exposed underdeveloped RPE apical domain in the young cBest retina, with only a few longer RPE microvilli and a highly disorganized distribution of Ezrin (Fig. 38.2a–d). In comparison to the age-matched controls, the 6-wk-old cBest retina showed diminished expression of Ezrin at the RPE apical surface, accompanied by an increase of Ezrin-positive signals associated with microvilli of Müller cells (Fig. 38.2d). Although the young cBest retinae appeared histologically intact, cone photoreceptor outer segments (hCAR) appeared compromised, further suggesting perturbations in the homeostatic mechanism of subretinal space in bestrophinopathies.
Fig. 38.2.
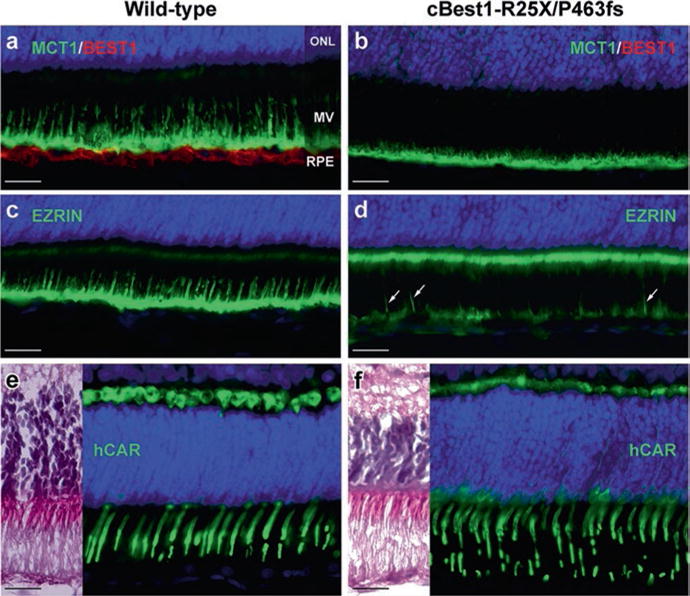
Underdeveloped RPE apical domain promotes formation of subretinal lesions in bestrophinopathies. Immunohistochemical evaluation of 6-week-old wild-type (a, c, e) and cBest1-R25X/P463fs-affected (b, d, f) retinae revealed underdevelopment of RPE apical domain of cBest-mutant retina, demonstrated by anti-MCT1 (a, b), and anti-Ezrin (c, d) labeling. Note the absence of Best1 basolateral labeling (b), reduced Ezrin immunostaining at the RPE apical surface with only a few Ezrin-positive RPE processes (d, arrows), and a increase of Ezrin labeling associated with microvilli of Müller cells (d). Cone outer segments (green) appear compromised (f, hCAR) when compared to the wild-type retina (e). Scale bar: 20 μm applies to all panels
38.4 Discussion
We have previously shown that canine bestrophinopathy (cBest or cmr) serves as an important translational model for BEST1-associated maculopathies in man, as it recapitulates all fundamental aspects of human disease, including the salient predilection of subretinal lesions to the canine macular region (Guziewicz et al. 2007; Beltran et al. 2014; Singh et al. 2015). In this study, cBest model proved instrumental for defining the early structural alterations at the RPE-photoreceptor interface, which may explain the susceptibility of the macula to its primary detachment in BEST1-associated maculopathies.
Apical microvilli, an integral structure of RPE cells, are essential for mediating basic RPE functions, including daily phagocytosis of OS and transport of nutrients into and removal of byproducts from the photoreceptor cells (Bonilha et al. 2004, 2006). It is important to note that these RPE projections greatly increase the apical surface area and, consequently, the transport of signaling molecules it contains, thereby enhancing the epithelial functional capacity (Bonilha et al. 2004). Thus, it is very likely that most of the major hallmarks of bestrophinopathies, e.g., disrupted ion transport, impaired phagocytosis, abnormal accumulation of autofluorescent debris in the subretinal space, and finally RPE-neuroretinal detachment and formation of serous lesions (Boon et al. 2009; Singh et al. 2015), arise directly from the dearth of properly formed microvilli and impaired interaction/adhesiveness with OS. The report by Bonilha et al. provided strong evidence that Ezrin is a major determinant in the maturation of surface differentiations of RPE, promoting morphogenesis of apical microvilli (Bonilha et al. 1999). Our findings in the canine BEST1 model support these results and strongly suggest that underdevelopment of cone-associated apical projections constitutes a key factor that underlies formation of macular lesions in bestrophinopathies. The fact that these prominent structural alterations associated with cone ensheathment are observed in cBest dogs as early as 6 weeks of age would also explain the early onset of ARB and BVMD along with the predilection of the cone-packed macular region to its primary detachment in affected dogs and patients.
As evinced by numerous studies, IPM is critical for maintaining the homeostatic milieu of the subretinal space, serving a range of biochemical and physical functions; these include the regulation of oxygen, nutrients, and retinoid transport, preservation of retinal adhesion by providing electrostatic support for photoreceptors, and participating in cytoskeletal organization in the surrounding cells (Ishikawa et al. 2015). Thus, together with the impaired microvillar ensheathment, the compromised IPM accounts for the two major pathological culprits responsible for weakening of the RPE-neuroretina interactions in bestrophinopathies. We posit that these salient interface defects may also be responsible for the difference in photoreceptor equivalent thickness found in BVMD patients (Kay et al. 2012) and the abnormal EOG and Arden ratio in bestrophinopathy patients. As such, this study provides new critical insights into the molecular basis of bestrophinopathies that would assist in the development of outcome measures for assessing therapeutic strategies in ARB and BVMD patients.
Acknowledgments
This study was supported by FFB-TRAP and FFB-Facility grants, MVRF, NEI/NIH EY06855, EY10420, and Hope for Vision.
Contributor Information
Karina E. Guziewicz, Department of Clinical Studies-Philadelphia, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA; University of Pennsylvania, Ryan Veterinary Hospital, Philadelphia, PA, USA
Emily McTish, Department of Clinical Studies-Philadelphia, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Valerie L. Dufour, Department of Clinical Studies-Philadelphia, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA
Kathryn Zorych, Department of Clinical Studies-Philadelphia, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Anuradha Dhingra, Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Kathleen Boesze-Battaglia, Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Gustavo D. Aguirre, Department of Clinical Studies-Philadelphia, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA
References
- Acland GM, Aguirre GD. Retinal degenerations in the dog: IV. Early retinal degeneration (erd) in Norwegian elkhounds. Exp Eye Res. 1987;44:491–521. doi: 10.1016/s0014-4835(87)80160-4. [DOI] [PubMed] [Google Scholar]
- Beltran WA, Cideciyan AV, Guziewicz KE, et al. Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PLoS One. 2014;3:e90390. doi: 10.1371/journal.pone.0090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL, Finnemann SC, Rodriguez-Boulan E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;147:1533–1548. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL, Bhattacharya SK, West KA, et al. Support for a proposed retinoid-processing protein complex in apical retinal pigment epithelium. Exp Eye Res. 2004;79:419–422. doi: 10.1016/j.exer.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bonilha VL, Rayborn ME, Bhattacharya SK, et al. The retinal pigment epithelium apical microvilli and retinal function. Adv Exp Med Biol. 2006;572:519–524. doi: 10.1007/0-387-32442-9_72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon CJ, Klevering BJ, Leroy BP, et al. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009;28:187–205. doi: 10.1016/j.preteyeres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Guziewicz KE, Zangerl B, Lindauer SJ, et al. Bestrophin gene mutations cause canine multifocal retinopathy: a novel animal model for best disease. Invest Ophthalmol Vis Sci. 2007;48:1959–1967. doi: 10.1167/iovs.06-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Sawada Y, Yoshitomi T. Structure and function of the interphotoreceptor matrix surrounding retinal photoreceptor cells. Exp Eye Res. 2015;133:3–18. doi: 10.1016/j.exer.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Kay CN, Abramoff MD, Mullins RF, et al. Three-dimensional distribution of the vitelliform lesion, photoreceptors and retinal pigment epithelium in the macula of patients with best vitelliform macular dystrophy. Arch Ophthalmol. 2012;130:357–364. doi: 10.1001/archophthalmol.2011.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Rayborn M, et al. Bestrophin the product of the best vitelliform macular dystrophy gene (VMD2) localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000;97:12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrukhin K, Koisti MJ, Bakall B, et al. Identification of the gene responsible for best macular dystrophy. Nat Genet. 1998;19:241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- Singh R, Kuai D, Guziewicz KE, et al. Pharmacological modulation of photoreceptor outer segment degradation in a human iPS cell model of inherited macular degeneration. Mol Ther. 2015;23:1700–1711. doi: 10.1038/mt.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangerl B, Wickström K, Slavik J, et al. Assessment of canine BEST1 variations identifies new mutations and establishes an independent bestrophinopathy model (cmr3) Mol Vis. 2010;16:2791–2804. [PMC free article] [PubMed] [Google Scholar]


