Abstract
Cardiovascular diseases are the leading cause of morbidity and mortality worldwide. Regenerative therapy has been applied to restore lost cardiac muscle and cardiac performance. Induced pluripotent stem cells (iPSCs) can provide an unlimited source of cardiomyocytes and therefore play a key role in cardiac regeneration. Despite initial encouraging results from pre-clinical studies, progress toward clinical applications has been hampered by issues such as tumorigenesis, arrhythmogenesis, immune rejection, scalability, low graft-cell survival, and poor engraftment. Here, we review recent developments in iPSC research on regenerating injured heart tissue, including novel advances in cell therapy and potential strategies to overcome current obstacles in the field.
Keywords: pluripotent stem cells, cardiomyocytes, regenerative medicine, tissue engineering, tumorigenicity, immune rejection, arrhythmogenicity, engraftment
The focus of this review is to present recent developments in iPSC research on cardiac regeneration and discuss novel developments in iPSC-cardiomyocyte (CM) cell therapy application and potential approaches to overcome current hurdles to reach clinical practice.
Main Text
The prevalence of cardiovascular diseases (CVDs) globally has reached alarming levels.1, 2, 3 In the United States, the economic burden of CVD is expected to reach over $1 trillion dollars annually by 2035.4 Despite significant progress in prevention, diagnosis, and treatment of CVD and heart failure (HF), in severe cases, there is no alternative for patients other than heart transplantation.5, 6 The limited regenerative capacity of the adult heart is insufficient for repairing the massive loss of cardiac tissue and particularly of cardiomyocytes (CMs), leading to maladaptive cardiac remodeling and the subsequent development of HF.7, 8 Therefore, the adult heart is a prime target for the development of cardiac regenerative strategies, with the ultimate goal of replacing the lost myocardial tissue with functionally active and electrically coupled CMs.
Overview of Cardiac Regeneration
Numerous strategies have been developed to use diverse cell types to serve as potential sources of newly formed CMs. These cell types include skeletal myoblasts, bone-marrow-derived mononuclear cells (BMMNCs), mesenchymal stem cells (MSCs), and adult cardiac stem cells.9 The abilities of these cells to repair myocardial tissue have been tested mainly in pre-clinical models. Some of these studies have led to clinical trials, but while safe, so far these approaches have only been marginally efficacious.10, 11 Therefore, additional strategies are needed to address the unmet medical need to treat heart failure. Potential novel methods include induction of endogenous cardiac progenitors,12 direct reprogramming of resident cardiac fibroblasts to cardiomyocytes,13 as well as generation of cardiomyocytes from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).14 Direct reprogramming of cardiac fibroblasts represents an attractive avenue toward cardiac regeneration because it would create non-immunogenic, autologously derived CMs adjacent to the injury sites. Further work is required to improve the efficiency of reprogramming, cardiac tropism, and the maturation of CMs.15 Similarly, further research is needed to establish paracrine factors that can activate proliferation and differentiation programs of endogenous cardiac progenitors toward CMSs.
Pluripotent Stem Cells as Candidates for Cell Therapy Trials
Since the discovery of iPSCs in 2006, there has been a great interest in their potential therapeutic application in numerous diseases, including various heart diseases.16 In 2014, the world’s first transplantation of autologous iPSC-derived cells was carried out on a patient with age-related macular degeneration.17 During the first year after transplantation, there was no sign of teratoma formation or immune rejection of the transplanted iPSC-derived retinal pigment epithelium (RPE) cells in the absence of immunosuppressants, but the visual acuity of the patient neither improved nor declined without additional treatment. More recently, the same group reported the first clinical case of allogeneic transplantation of iPSC-RPE using histocompatibility leukocyte antigen (HLA)-matched allogeneic iPSCs.18 Although clinical trials for heart repair using iPSCs have yet to begin, these studies encourage parallel efforts toward novel iPSC-based cardiac regeneration therapies.
Properties of ESC- and iPSC-Derived Cardiomyocytes
Human PSCs, which include both ESCs and iPSCs, are alternative sources of CMs and effective candidates for the remuscularization of the diseased human heart. Recent progress in the differentiation protocols of ESCs and iPSCs has led to the identification of serum-free, chemically defined media supplements that give rise to populations of human CMs with high yield and purity.19 These protocols produce CMs that possess critical functional and structural properties, such as expression of contractile apparatus components, calcium handling machinery, electrical connectivity, and excitability. However, ESC- and iPSC-derived CMs (iPSC-CMs) also exhibit immature cell characteristics such as small size, lack of T-tubules, immature myofibril ultrastructure alignment, inefficient Ca2+ handling, and fetal metabolic phenotype. Moreover, they represent mixed populations of ventricular, atrial, and nodal CMs.20
Applications of iPSC-Derived CMs in Disease Modeling and Drug Discovery
The generation of patient-derived iPSCs and iPSC-CMs has provided an extraordinary opportunity to create relevant in vitro models for human diseases and test therapeutic compounds in a personalized manner. Various forms of inherited cardiac disease, including predominantly arrhythmogenic syndromes and ion-channel disorders (channelopathies), such as arrhythmogenic right ventricular dysplasia (ARVD),21, 22 familial long-QT syndrome (LQT1,23, 24, 25 LQT2,26, 27, 28 LQT3,29 LQT8,30 LQT14,31 and LQT5 32), and catecholaminergic polymorphic ventricular tachycardia (CPVT),33, 34, 35 have been successfully modeled by iPSC-CMs. Moreover, iPSC-CMs have been successfully used to study the molecular characteristics of cardiac diseases related to sarcomeric and cytoskeletal proteins, such as hypertrophic cardiomyopathy (HCM)36 and dilated cardiomyopathy (DCM).37, 38 In addition, patient-derived iPSC-CMs have been used to model cardiometabolic disorders, such as diabetic cardiomyopathy,39 Dannon disease,40 Barth syndrome,41 aldehyde dehydrogenase 2 (ALDH-2) deficiency,42 and Pompe disease.43 Furthermore, iPSC-CMs have been used to study differential sensitivity of breast cancer patients to doxorubicin-induced cardiomyopathy.44
In addition to disease modeling, iPSC-CMs are valuable tools for assessing cardiotoxicity of drugs as well as identifying novel therapeutic compounds. For example, Sharma et al.45 developed a cardiotoxicity safety index for anticancer tyrosine kinase inhibitors in control versus patient-derived iPSC-CMs. Additionally, recent years have seen tremendous efforts devoted to the integration of iPSC-CMs into high-throughput platforms and development of assays to monitor cell phenotyping,46 contractile properties,47 electrophysiological parameters,48, 49 and Ca+2 transients.50, 51, 52, 53 Despite considerable success in reproducing clinical phenotypes of cardiovascular diseases using patient-derived iPSC-CMs, there has been relatively little progress in identifying novel therapeutic compounds for these diseases. To fill this gap, increased efforts should be focused on the development of more mature iPSC-CMs and their integration in high-throughput platforms that will facilitate screening of novel compounds.
Cell Therapy Applications of iPSC-Derived CMs
Although the differences in regenerative potential between iPSCs and ESCs are still unclear,54 studies examining the therapeutic effect of ESC-derived cells have provided valuable information to develop iPSC-derived cells as therapeutic tools in the clinic. In this review, we discuss the latest advances and challenges in the clinical applications of human pluripotent stem cell-derived CMs (PSC-CMs) for cardiac repair and regeneration, focusing on recent studies that examine the therapeutic effect of human PSC-CM transplantation in the treatment of heart failure (Figure 1). This review is not intended to give an exhaustive description of the historical development in this area, for which excellent review articles are available.16, 55
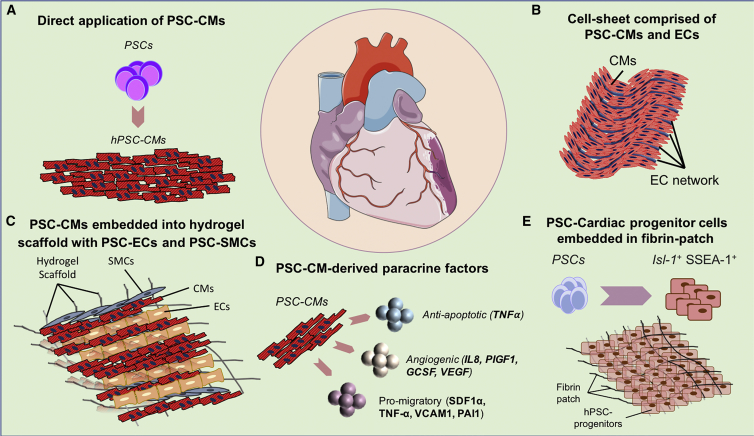
Figure 1.
Cardiac Cell Therapy Using PSC-CMs
Illustration of recent cell therapy approaches to recover lost cardiac muscle following severe myocardial injury. (A) As alternatives to direct injection of PSC-CMs in the injured heart, (B) tissue engineering approaches have been employed to increase the survival and functional engraftment of cells following delivery. (C) The integration of endothelial cells forming vascular networks into PSC-CM cell sheets facilitates delivery of oxygen and nutrients to the graft, greatly augmenting its engraftment and function as a novel contractile unit. Providing a structural framework with hydrogel and other cell types complementary to CMs such as ECs and smooth muscle cells may boost functional integration into the host myocardium. (D) The secretion of growth factors and cytokines represents another way that administered PSC-CMs might benefit cardiac performance following injury. (E) Finally, the use of scaffolds formulated from fibrin patches containing PSC-derived cardiac progenitor cells (CPCs) is another therapeutic application. PSCs, pluripotent stem cells; PSC-CMs, PSC-derived cardiomyocytes; ECs, endothelial cells; SMCs, smooth muscle cells; Isl-1, Islet-1; SSEA-1, stage-specific embryonic antigen-1.
Preclinical Large Animal Studies
To date, many studies have already demonstrated the feasibility and efficacy of cardiac stem cell therapy in small animal models. Recent studies therefore have shifted toward large animal models prior to translation into human trials.56 In the first clinical-scale transplantation of human PSC-CMs by Chong et al.,57 one billion human ESC-derived CMs (ESC-CMs) were intra-myocardially injected into the heart of adult pigtail macaques (Macaca nemestrina) 2 weeks after acute myocardial ischemia reperfusion (I/R) injury under immunosuppressive treatment. These investigators found that a substantial number of grafted cells survived in non-human primate (NHP) hearts with blood supply from host vessels over a 3-month period after injection. In addition, grafted CMs were capable of electrical coupling with the host myocardium. Teratoma formation or cell engraftment in the off-target organs was not detected. However, the authors could not conclude whether transplantation of human ESC-CMs ameliorated cardiac dysfunction or subsequent heart failure after I/R injury, mainly due to the small number of animals used in the study. An additional concern regarding future clinical applications is the determination of the optimal number of iPSC-CMs to be injected in the damaged human myocardium. Extrapolating these numbers to the human heart, which is roughly ten times larger than a macaque’s heart and contains 2–3 billion CMs, would lead to an unrealistic number of required iPSC-CMs to be injected (approximately 10 billion).58 Further studies are required to determine the optimal feasible number of iPSC-CMs that needs to be injected into the human myocardium.
More recently, Zhu et al.59 examined the effectiveness and safety of transplantation of human PSC-derived cardiovascular progenitor cells (PSC-CVPCs) in an NHP model. In this study, PSC-CVPCs were differentiated from human ESCs using BMP-4, Activin A, and CHIR99021 (GSK-3 inhibitor) for 3 days. Following the injection of 10 million PSC-CVPCs into a cynomolgus monkey heart 30 min after myocardial infarction (MI) induced by permanent ligation of the left anterior descending (LAD) artery, they observed recovery of cardiac function (left ventricular ejection fraction [LVEF]; from 37.5% to 43.5% in the CVPC group), but no transplanted cells were found after treatment with multiple immunosuppressive agents at 20 weeks after transplantation. Although the exact reasons for the discrepancy in graft survival between the Chong and Zhu studies are not clear, the experiments differed in various aspects, such as the disease model (I/R versus MI), timing of cell delivery (2 weeks versus 30 min after injury), number of transplanted cells (1 billion ESC-CMs versus 10 million PSC-CVPCs), and data interpretation, as the authors noted.59
Tissue Engineering Approach
Despite efforts to obtain better cell engraftment after direct myocardial injection, high injection pressures and harsh tissue environment compromise cell survival, especially in areas of scar tissue, which are less expandable and provide fewer nutrients compared to non-injured healthy muscle tissue. To circumvent these problems, tissue engineering approaches have been applied to construct engineered heart tissue (EHT) for implantation onto the surface of the infarcted area. EHT is expected to enhance the efficacy of cell therapy by reducing heart wall stress and prolonging paracrine secretion. However, the optimal combination of cell types and scaffold materials employed in EHT has yet to be established for clinical application. Two main types of EHT, hydrogel-based EHT and cell sheets, are currently being investigated for cardiac regeneration.
Hydrogel-Based EHT
In hydrogel-based EHT, various extracellular matrix (ECM) proteins, such as collagen I, Matrigel, and fibrin, are commonly used as scaffold material. Riegler et al.60 reported the transplantation of a collagen-based EHT containing 2.5 million human ESC-CMs into nude athymic rats 1 month following I/R injury. A high survival rate of CMs in the implanted graft was seen at 1 month. However, compared to the patch containing nonviable ESC-CMs (following lethal irradiation), transplantation with a patch containing viable ESC-CMs showed no significant differences in LVEF. This finding is consistent with the anticipated lack of electric integration of human xenografts in rat hearts, but also highlights the possibility that cell-independent effects (e.g., activation of immune cells and mechanical stabilization) could also elicit therapeutic effects. In another study, Weinberger et al.61 implanted a fibrin-based EHT containing 5 million human iPSC-CMs and 2 million human endothelial cells (ECs) into guinea pig hearts 1 week after cryoinjury under immunosuppressive treatment. Interestingly, the EHT improved LV contraction, with robust survival of iPSC-CMs 1 month after transplantation compared to the cell-free patch. They also observed electrical coupling between the EHT and the host myocardium, albeit only in a subset of engrafted cells. More recently, a fibrin-based EHT of clinically relevant size (4 cm × 2 cm × 1.25 mm) was generated by Gao et al.62 using human iPSC-CMs, iPSC-ECs, and iPSC-derived smooth muscle cells (iPSC-SMCs). They transplanted two EHT patches into the pig heart under immunosuppression immediately after I/R injury. They showed improved contractile function and infarct size compared to the cell-free patch, with ∼10% of transplanted cells surviving 1 month after surgery. These inconsistent results may reflect differences in the animal models (rat versus guinea pig versus pig), injury model (I/R versus cryoinjury), timing of treatment (acute versus chronic), and/or properties of the utilized EHT.
Cell Sheets
Three-dimensional engineered cardiac tissue can be generated by stacking detachable monolayer cell sheets formed on temperature-responsive culture dishes.63 This technology has an advantage because the resulting EHT is constructed using a relatively simple method without specialized scaffolds. Masumoto et al.64 showed that transplantation of three cell sheets containing 2 million human iPSC-derived cells (∼70% CMs and ∼25% vascular cells) into an athymic nude rat sub-acute MI model significantly improved cardiac contraction compared to sham animals, during the first 2 weeks after transplantation. However, the lack of supply of oxygen and nutrients in a vascular system limits the stacking of iPSC-CMs sheets to three layers.65 Recently, Kawamura et al.66 solved this problem by a surgical procedure with an omental flap that is frequently used in clinical practice. One month following MI, seven iPSC-CM sheets prepared on 10-cm culture dishes were transplanted into infarcted pig hearts and covered with an omental flap under immunosuppressive treatment. Three months after transplantation, cardiac contractile function was significantly improved in the cell-sheet-transplanted group with omental flap (LVEF; from 36.8% to 52.1%) compared with the cell sheet only group (LVEF; from 35.7% to 44.9%) and the omental flap only group (LVEF; from 35.9% to 33.9%).
Novel Approaches for Heart Tissue Generation
In addition to conventional methods, newer approaches are being explored to generate three-dimensional (3D) cardiac tissue with a complex ECM structure similar to that of the native human myocardium tissue.67 The perfusion decellularization method has been successfully applied to create acellular scaffolds with a preserved ECM and a vascular network from human cadaveric whole hearts.68 The decellularized human cardiac matrix can support the engraftment of human iPSC-CMs and create force-generating myocardial tissue-like constructs. Gao et al.69 applied high-resolution 3D printing to generate EHT-containing human iPSCs-CMs, iPSC-ECs, and iPSC-SMCs. The scaffold was generated with photoactive gelatin polymer guided by a template based on the distribution of fibronectin in the native adult mouse heart tissue. Synchronous beating of the cell-seeded scaffold was established within 1 day, and increased contraction speed and calcium handling were observed during extended in vitro culture over 7 days. After transplantation of the cardiac patch into the infarcted immunodeficient mouse, graft survival was confirmed after 1 month as well as improved cardiac function.
Role of Paracrine Factors in Cardiac Regenerative Therapies Using PSC-CMs
There are many preclinical and clinical studies reporting that cell-based transplant therapy promotes functional recovery in MI models despite suboptimal engraftment of transplanted cells.55 These findings led to the concept that the observed beneficial effects on the damaged myocardium are mediated through paracrine factors released by the transplanted cells. Ong et al.70 reported that the injection of 2 million human iPSC-CMs improved cardiac function (LVEF; from 19.2% to 24.5%) 1 month after permanent LAD artery ligation in immunodeficient mice despite the limited engraftment of transplanted iPSC-CMs. They found increased neo-angiogenesis and reduced apoptosis in peri-infarcted myocardium after detecting proangiogenic and anti-apoptotic cytokines released from the transplanted iPSC-CMs in the hypoxic environment of the ischemic myocardium. Similarly, Tachibana et al.71 demonstrated a significant volumetric discrepancy between the engrafted cells and the increased viable myocardium 1 month after transplantation of iPSC-CMs or ESC-CMs into infarcted severe combined immunodeficient (SCID) mice. They also found that cardio-protective paracrine factors as well as anti-apoptotic (tumor necrosis factor alpha [TNF-α]), pro-angiogenic (interleukin-8 [IL-8], placental growth factor-1 [PIGF-1], granulocyte colony-stimulating factor [GCSF], and vascular endothelial growth factor [VEGF]), and pro-cell migration factors (stromal cell-derived factor-1 a [SDF-1α], TNF-α, vascular cell adhesion protein 1 [VCAM-1], and plasminogen activator inhibitor-1 [PAI-1]) were abundantly released from iPSC-CMs or ESC-CMs in the host myocardium.
First Clinical Trial Using PSC-Cardiac Progenitor Cells
Recently, Menasche et al.72, 73 reported the results of a clinical trial that evaluated the potential therapeutic effects of PSC-CMs in severe heart failure patients. The authors generated a fibrin patch containing ISL-1-positive cells isolated through immunomagnetic sorting of stage-specific embryonic antigen 1 (SSEA-1) (CD15) from differentiated human ESCs that were exposed to bone morphogenetic protein 2 (BMP-2) and fibroblast growth factor receptor (FGFR)-specific tyrosine kinase inhibitor for 4 days. A 20 cm2 piece of the patch containing 5–10 million SSEA-1 progenitor cells was implanted onto the epicardium of 6 ischemic cardiomyopathy patients with advanced congestive heart failure. The procedure was performed with concomitant coronary artery bypass graft (CABG) surgery after implantation of an internal cardioverter defibrillator, and immunosuppressive treatment was given until 1 to 2 months after the procedure. Although one patient died shortly after the procedure from treatment-unrelated comorbidities, no tumor formation or episodes of ventricular arrhythmias were detected after 6 months of follow-up. Except for the last recruited patient, the other 4 patients assessed at the 1-year follow-up showed improved LVEF (median 26% to 38.5%) and symptoms (New York Heart Association [NYHA] functional class III to class I/II), with new-onset contractility in the treated area. However, it is not clear whether the improved LVEF and symptoms were due to the fibrin patch or concomitant CABG procedure.
Heart Regeneration Using HLA Haplotype Matched iPSC Lines
In theory, differentiated human iPSCs are an ideal resource for autologous cell transplantation, but the production of adequate quantities of autologous CMs remains costly as well as time consuming and labor intensive.74 Therefore, allogeneic iPSC-based cell therapy has emerged as a more realistic strategy mainly because the iPSC bank system enables selection of HLA-matched iPSC lines based on recipient HLA haplotype. Recently, Shiba et al.75 demonstrated that allogeneic transplantation of nonhuman primate iPSC-CMs improved cardiac contractile function in a nonhuman primate model of MI. Two weeks after I/R injury, ∼400 million major histocompatibility complex (MHC)-matched primate iPSC-CMs were injected into the heart of adult cynomolgus monkeys (Macaca fascicularis). The investigators found that a substantial number of transplanted cells were vascularized by host vessels and electrically coupled with the host myocardium 3 months after cell transplantation. By contrast, after MHC-mismatched iPSC-CM injection, only a small number of transplanted cells were detected, with a massive infiltration of inflammatory cells. In addition, the LVEF was higher in the MHC-matched iPSC-CM group than in the sham group at 3 months after cell transplantation (62.0% ± 4.1% versus 48.8% ± 5.6%), whereas the incidence of ventricular arrhythmia was transiently but significantly increased in the MHC-matched iPSC-CM group. This proof-of-concept study provided encouraging results to justify continued effort toward establishing iPSC banks for allogeneic regenerative cell therapy.
Current Challenges for PSC-CM Therapy
Successful application of PSC-CMs in the clinical setting requires preparation of highly pure and mature CMs that can sufficiently engraft in the host myocardium and exhibit electrical and mechanical coupling with existing myocytes. A number of concerns exist regarding the application of PSC-CMs in cardiac disease (Figure 2).
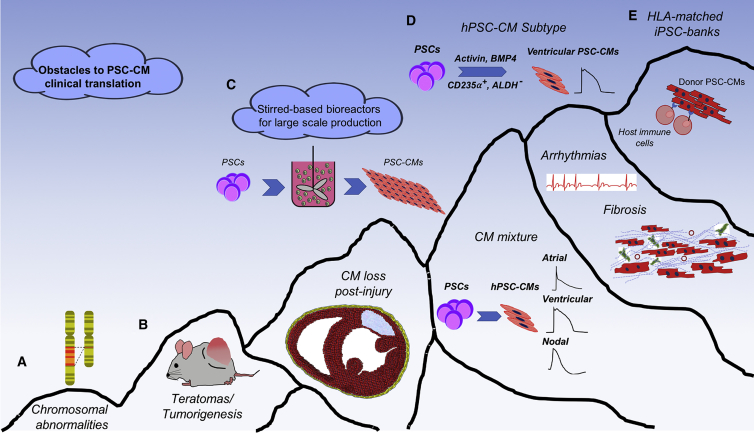
Figure 2.
Illustration of the “Mountain” of Obstacles to Overcome in Clinical Translation of PSC-CMs in Heart Disease
(A) Careful screening of PSC lines and state-of-the-art methodologies for the expansion and maintenance of PSCs are required to avoid genomic instability. (B) Engineering novel ways to eliminate undifferentiated PSCs from PSC derivatives can prevent teratoma formation. (C) To produce clinically relevant numbers of PSC-CMs, novel ways of PSC-CM manufacturing need to be developed. Advances in stirred-based bioreactors have allowed the maintenance and expansion of PSCs as well as the generation of PSC-CMs in a large scale that is sufficient to meet clinical demands. (D) The latest improvements in cardiac differentiation protocols toward specific cardiac subtypes will tremendously minimize the risk of arrhythmias caused by injection of mixed pools of PSC-CMs in fibrotic areas of the myocardium. (E) Creation of HLA-matched iPSC banks and engineering of immunoprivileged PSC-CMs will greatly reduce the risk of immune rejection of the grafted cells. HLA, human leukocyte antigen.
Stem Cell Genomic Stability
A critical requirement that needs to be fulfilled prior to any translational application of PSCs and their derivatives is the assessment of their karyotype.76 For example, genetic mutations were found in the iPSCs that were intended to be administered in the second patient with age-related macular degeneration, prompting a temporary halt of the RIKEN trial.77 Prolonged expansion periods, pre-existing genetic variations in parental cell lines, and reprogramming-induced mutations can all lead to chromosomal abnormalities that affect gene expression, cell function, teratoma formation, tumorigenicity, and efficacy of transplanted cells.78 Taapken et al.79 found chromosomal irregularities in ∼13% of 1,715 cultures analyzed that were derived from 259 different human PSC and ESC lines. Using next-generation sequencing, a low incidence of protein-coding mutations in every human iPSC line (∼6–12/line) was also reported.80 Thus, karyotypic instability is highly dependent on the specific cell line under investigation as well as the expansion conditions, thus necessitating systematic efforts to ensure genomic stability prior to any clinical application of stem-cell-derived CMs.
Teratoma Formation
A primary concern and a major unwanted side effect of PSC transplantation is the generation of teratomas in the recipients. Undifferentiated stem cells (present due to inefficient differentiation protocols or purification methodologies or genomic instability) may survive the transplantation process and generate highly undesirable tumors and teratomas. However, as shown by a large number of studies, only an extremely low risk of teratoma or tumor formation exists following transplantation of PSC-CMs in failing or injured hearts.57, 81, 82, 83, 84 Nevertheless, novel methods that can either identify or eradicate the residual undifferentiated PSCs while sparing their differentiated derivatives are required for safe translational application of PSCs.85, 86 Recent progress with the establishment of highly efficient CM differentiation and purification protocols has minimized the undifferentiated PSCs to undetectable levels, preventing teratoma or tumor formation.19 Various strategies have also been employed over the last several years to selectively eliminate PSCs, including the use of suicide genes,87, 88, 89, 90 tumor suppression genes,91 cytotoxic antibodies against the PSC-specific marker Claudin-6,92 SSEA-5,93 and expression of cell-specific microRNA molecules.94, 95 Additional studies have used chemical inhibitors to selectively eliminate PSCs by targeting different cellular processes or properties of these cells, such as oleate synthesis,96 cyclin-dependent kinase 1 (CDK-1)-dependent cell cycle progression, p53-induced cell death,97 and expression of ABC transporters ABCG-2 and MDR-1.98 Despite significant steps toward the thorough and robust elimination of PSCs, many of these methods are still limited by their relatively low effectiveness, specificity, and clinical applicability.
Immune Rejection
A major concern in the design of translational PSC therapy studies is immune rejection, particularly with allogeneic transplantation.99, 100, 101 To that end, immunocompromised or immunodeficient animals and various cocktails of pharmacological immunosuppressants have been employed to study and prevent unwanted immune reactions.102, 103 However, this approach can increase risks of opportunistic infections as well as the development of malignancies. Recent studies have shed some light on the molecular mechanisms involved in the immune rejection of allogeneic human ESCs and their derivatives. For instance, Gornalusse et al.104 attempted to generate a universal donor ESCs by genetically engineering them to overexpress HLA-E single-chain peptides. They showed that HLA-E-engineered stem cells and their differentiated counterparts were immunoprivileged against all major immune rejection mechanisms, such as cytotoxic CD8+ T cells, anti-HLA antibodies, and natural killer (NK)-cell-mediated cell lysis. In addition, the identification of suitable donors for iPSC generation requires the establishment of stem cell banks with a wide diversity of HLA types. It is estimated that more than 150 human iPSC lines are required to provide a beneficial effect for HLA-matched lines, with a minimum immunosuppression requirement for more than 90% of the patients in the UK population.105 Other studies have revealed that ethnic composition largely drives the percentage of haplotype-compatible individuals in the general population and particularly in more diverse ethnic groups.106 To facilitate such efforts, international consortia have been established to assure the quality and clinical characteristics of haplotype screening.107, 108, 109
Arrhythmogenicity due to the Injection of Mixed CM Pools
One of the most challenging adverse effects of cell therapy for cardiovascular disease is the arrhythmogenic phenotype of “graft-derived” myocardium. Although transplanted cells manage to functionally integrate into the injured “host myocardium,” they do not operate in a synchronous manner with the residual CMs, which significantly endangers the health of the recipient due to episodes of arrhythmia. Initial studies showed that human ESC-CM grafts not only improved cardiac function, but intriguingly also reduced spontaneous and induced arrhythmias in guinea pigs.81, 110, 111 However, the same group also reported that injection of human ESC-CMs was accompanied by significant ventricular arrhythmias in NHP models.57 The reason for this inconsistency is unclear, but may stem from species differences between small and large animals, such as in their heart sizes or beating rates. Alternatively, injection of iPSC-CMs in large numbers may lead to arrhythmias due to the generation of dead cells that might affect propagation of the electrical signal in cardiac muscle. Although an implanted cardiac defibrillator may prevent the life-threatening event in these patients, further studies evaluating the optimal cell dosage in relationship to occurrence of post-transplant arrhythmias are warranted to address safety.
Development of Cardiomyocyte Subtype-Specific Protocols
Current protocols of stem cell cardiogenic differentiation produce a heterogeneous mixture of CMs of different subtypes, primarily ventricular CMs, but also substantial quantities of atrial and nodal CMs.112 Such cells can maintain an autonomous electrical stimulation capacity representing potential sources of arrhythmogenic episodes. In addition to reducing this risk, the development of subtype-specific cardiac differentiation protocols would tremendously assist cardiac disease modeling, drug discovery, and particularly cardiac cell therapy efforts. A large number of transcription factors are implicated in subtype specification, including Nkx2.5, Isl-1, Hand-1/2, Irx-4, Cited-1, COUP transcription factor-2, CHF-1/Hey-2, and the T-box family, among many others.113, 114 Similarly, various growth factors are associated with the regulation of cardiac lineage and subtype specification, including neuregulin, endothelin, Wnt ligands, and retinoic acid (RA), among others. Neuregulin and endothelin are implicated in the generation of nodal cells and Purkinje fibers, respectively.115, 116, 117 Recently, Lee et al.118 reported a detailed molecular network that dictates cardiac subtype specification. Using spontaneously differentiating embryoid bodies, they first found that the effects of RA are mediated during a narrow 2-day temporal window. During this period, the addition of RA in embryoid bodies reduced the specification of ventricular-like CMs and increased that of atrial-like CMs, as measured by a panel of respective markers. Next, they identified a specific RA-synthesizing subpopulation of ALDH+platelet-derived growth factor receptor (PDGFR)+ cells that might represent a source of atrial RA-responsive CMs. Following a step-by-step path of careful titration of Activin A and BMP-4 as well as cell surface marker screening, they found two distinct mesodermal cell populations, CD235a+ and ALDH+CD235a−, that have the potential to generate RA-non-responsive, ventricular-like CMs and RA-responsive, atrial-like CMs, respectively.
Besides ventricular- and atrial-like CMs, extensive efforts have been devoted to generate pacemaker cells from PSCs. The generation of pure populations of pacemaker cells would enable further investigations into advanced biological pacemaking devices. Birket et al.119 developed a methodology to generate pacemaker cells through the regulated expression of c-Myc in an Nkx-2.5eGFP/W ESC reporter line. Doxycycline-induced expression of c-Myc in combination with manipulation of the BMP, combined with fibroblast growth factor (FGF) signaling in human ESCs, resulted in a highly enriched population of functionally competent pacemaker cells.119 More recently, Protze et al.120 established another methodology for the generation of sinoatrial node (SAN)-like pacemaker cells by manipulating the BMP, transforming growth factor (TGF), FGF, and RA signaling pathways. Functional analysis as well as in vivo testing revealed that their properties closely resemble those of pacemaker cells and that they can function as biological pacemakers.
Improving Scalability of Human PSC-CM Production
The primary goal of cardiac cell therapy is the replacement of lost cardiac muscle tissue due to severe cardiac injury such as MI. Previous calculations estimated that following a typical MI episode, up to one-quarter of the existing CMs can be lost, leading to various pathologies and heart failure.121 Current conventional methodologies for PSC expansion and CM differentiation do not meet the standards for effective high-yield, clinical-grade, and cost-effective PSC expansion and CM differentiation, which are required for therapeutic applications. Therefore, novel methodologies are needed to achieve this goal. An example of such technological advancement is the employment of stirred tank bioreactor culture platforms that can accommodate the expansion of PSCs on micro-carriers or function as floating aggregates.122 They also allow homogeneous mixing, distribution of cells, nutrients, and gases, as well as a constant assessment of key parameters, such as levels of oxygen, pH, and key metabolites.123
Recent efforts have established long-term, cost-effective, scalable, GMP-compatible, expansion of PSCs using xeno-free, chemically defined culture media.124, 125 The next challenge is to adapt the highly specific and effective differentiation protocols19 for the aforementioned “industrial-scale” cell culture methodologies to produce large amounts of CMs. Recently, Kempf et al.126 described the robust formation of highly enriched human PSC-CMs using a suspension-culture-based method. The authors also demonstrated efficient applicability of their method in a 100-mL stirred-based bioreactor culture system, which resulted in the generation of PSC-CMs, with purities reaching above 80%.126 Similarly, Chen et al.127 used a matrix-free aggregate suspension system in 1 L spinner flasks to produce large quantities of human PSC-CMs under chemically defined, GMP-compatible culture conditions. This method produced CMs with over 90% purity that demonstrated typical morphological and electrophysiological characteristics. Additional efforts to standardize large-scale production of human PSC-CMs in a cost effective, highly specific, and reproducible manner are needed to meet the demands of pre-clinical and clinical studies.
Challenges of Improving Survival and Engraftment of Transplanted Cells
The various approaches for cell delivery have undergone intensive scrutiny and debate. Following intramyocardial delivery, most of the cells are washed out rapidly.128 In addition, most of the injected cells die acutely within the first week after injection.55 The problem of acute donor cell death exists not only in cardiac stem cell therapy, but also in other fields, such as neurologic, skeletal, and hepatic stem cell transplantation. Notably, the exception involves hematopoietic stem cell transplantation, which can effectively repopulate the patient’s bone marrow but also requires total body irradiation prior to the procedure.129, 130, 131 To overcome the problem of acute donor cell death, heat shock pretreatment and a cocktail of pro-survival factors, including insulin-like growth factor 1 (IGF-1) and cyclosporine A, have been used to enhance survival of transplanted cells via the anti-apoptotic effect.110 However, the short biological half-lives of these factors limit their effect on graft cell survival following delivery into the ischemic myocardium. To enable the slow release of pro-survival factors, Lee et al.132 recently developed a novel collagen-dendrimer biomaterial crosslinked with pro-survival peptide analogs, and demonstrated that the peptides can enhance cell survival after transplantation into the mouse ischemic heart.
Conclusions
The central premise of stem cell therapy for heart disease is to induce remuscularization of lost myocardium following injury. PSCs are prime candidates for the generation of new CMs that can be utilized in cell therapy protocols. Recent years have seen various technological advancements, and bioengineering platforms are being employed to improve the engraftment, survival, and effectiveness of the transplanted cells. New developments to establish differentiation protocols for cardiac-specific subtypes hold great promise for preventing arrhythmias caused by administration of mixed CM pools. However, multiple additional hurdles, such as tumorigenicity, immunogenicity, arrhythmogenicity, and donor cell death, still remain to be addressed. Given that CVD and HF represent the leading causes of morbidity and mortality worldwide, additional multidisciplinary efforts are needed to overcome current challenges and make cell therapy a routine clinical reality.
Conflicts of Interest
The authors have no disclosures.
Acknowledgments
This publication was supported in part by research grants from the NIH (R01 HL133272 and R01 HL113006), the American Heart Association (17MERIT33610009), the California Institute of Regenerative Medicine (RT3-07798 and DR2A-05394) to J.C.W., the American Heart Association Scientist Development Grant (17SDG33660794) to A.O., and the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad to T.K. The authors would also like to acknowledge the image database https://ebsco.smartimagebase.com/ for providing part of Figure 2, which was further modified. Because of space constraints, the authors apologize in advance for not including all relevant citations on the subject matter.
References
- 1.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., de Ferranti S., Després J.P., Fullerton H.J., Howard V.J., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 4.American Heart Association. (2017). Cardiovascular disease costs will exceed $1 trillion by 2035, warns the American Heart Association. http://newsroom.heart.org/news/cardiovascular-disease-costs-will-exceed-1-trillion-by-2035-warns-the-american-heart-association.
- 5.McMurray J.J., Adamopoulos S., Anker S.D., Auricchio A., Böhm M., Dickstein K., Falk V., Filippatos G., Fonseca C., Gomez-Sanchez M.A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 6.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabé-Heider F., Walsh S., Zupicich J., Alkass K., Buchholz B.A., Druid H. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai C.-L., Molkentin J.D. The elusive progenitor cell in cardiac regeneration: slip slidin’ away. Circ. Res. 2017;120:400–406. doi: 10.1161/CIRCRESAHA.116.309710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen P.K., Rhee J.-W., Wu J.C. Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. 2016;1:831–841. doi: 10.1001/jamacardio.2016.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C., Termglinchan V., Karakikes I. Concise review: mending a broken heart: the evolution of biological therapeutics. Stem Cells. 2017;35:1131–1140. doi: 10.1002/stem.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Avilés F., Sanz-Ruiz R., Climent A.M., Badimon L., Bolli R., Charron D., Fuster V., Janssens S., Kastrup J., Kim H.S., TACTICS (Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes) Writing Group. Authors/Task Force Members. Chairpersons. Basic Research Subcommittee. Translational Research Subcommittee. Challenges of Cardiovascular Regenerative Medicine Subcommittee. Tissue Engineering Subcommittee. Delivery, Navigation, Tracking and Assessment Subcommittee. Clinical Trials Subcommittee. Regulatory and funding strategies subcommittee. Delivery, Navigation, Tracking and Assessment Subcommittee Global position paper on cardiovascular regenerative medicine. Eur. Heart J. 2017;38:2532–2546. doi: 10.1093/eurheartj/ehx248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koudstaal S., Jansen Of Lorkeers S.J., Gaetani R., Gho J.M., van Slochteren F.J., Sluijter J.P., Doevendans P.A., Ellison G.M., Chamuleau S.A. Concise review: heart regeneration and the role of cardiac stem cells. Stem Cells Transl. Med. 2013;2:434–443. doi: 10.5966/sctm.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ieda M., Fu J.-D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G., Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burridge P.W., Keller G., Gold J.D., Wu J.C. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebert A.D., Diecke S., Chen I.Y., Wu J.C. Reprogramming and transdifferentiation for cardiovascular development and regenerative medicine: where do we stand? EMBO Mol. Med. 2015;7:1090–1103. doi: 10.15252/emmm.201504395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida Y., Yamanaka S. Induced pluripotent stem cells 10 years later: for cardiac applications. Circ. Res. 2017;120:1958–1968. doi: 10.1161/CIRCRESAHA.117.311080. [DOI] [PubMed] [Google Scholar]
- 17.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 18.Cyranoski, D. Japanese man is first to receive ‘reprogrammed’ stem cells from another person. Nature. Published online March 29, 2017. 10.1038/nature.2017.21730.
- 19.Burridge P.W., Matsa E., Shukla P., Lin Z.C., Churko J.M., Ebert A.D., Lan F., Diecke S., Huber B., Mordwinkin N.M. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakikes I., Ameen M., Termglinchan V., Wu J.C. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ. Res. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C., Wong J., Wen J., Wang S., Wang C., Spiering S., Kan N.G., Forcales S., Puri P.L., Leone T.C. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494:105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asimaki A., Kapoor S., Plovie E., Arndt A.K., Adams E., Liu Z., James C.A., Judge D.P., Calkins H., Churko J. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2014;6:240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretti A., Bellin M., Welling A., Jung C.B., Lam J.T., Bott-Flügel L., Dorn T., Goedel A., Höhnke C., Hofmann F. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 24.Egashira T., Yuasa S., Suzuki T., Aizawa Y., Yamakawa H., Matsuhashi T., Ohno Y., Tohyama S., Okata S., Seki T. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovasc. Res. 2012;95:419–429. doi: 10.1093/cvr/cvs206. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Liang P., Lan F., Wu H., Lisowski L., Gu M., Hu S., Kay M.A., Urnov F.D., Shinnawi R. Genome editing of isogenic human induced pluripotent stem cells recapitulates long QT phenotype for drug testing. J. Am. Coll. Cardiol. 2014;64:451–459. doi: 10.1016/j.jacc.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahti A.L., Kujala V.J., Chapman H., Koivisto A.-P., Pekkanen-Mattila M., Kerkelä E., Hyttinen J., Kontula K., Swan H., Conklin B.R. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis. Model. Mech. 2012;5:220–230. doi: 10.1242/dmm.008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsa E., Rajamohan D., Dick E., Young L., Mellor I., Staniforth A., Denning C. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur. Heart J. 2011;32:952–962. doi: 10.1093/eurheartj/ehr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itzhaki I., Maizels L., Huber I., Zwi-Dantsis L., Caspi O., Winterstern A., Feldman O., Gepstein A., Arbel G., Hammerman H. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 29.Davis R.P., Casini S., van den Berg C.W., Hoekstra M., Remme C.A., Dambrot C., Salvatori D., Oostwaard D.W., Wilde A.A., Bezzina C.R. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation. 2012;125:3079–3091. doi: 10.1161/CIRCULATIONAHA.111.066092. [DOI] [PubMed] [Google Scholar]
- 30.Yazawa M., Hsueh B., Jia X., Pasca A.M., Bernstein J.A., Hallmayer J., Dolmetsch R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocchetti M., Sala L., Dreizehnter L., Crotti L., Sinnecker D., Mura M., Pane L.S., Altomare C., Torre E., Mostacciuolo G. Elucidating arrhythmogenic mechanisms of long-QT syndrome CALM1-F142L mutation in patient-specific induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc. Res. 2017;113:531–541. doi: 10.1093/cvr/cvx006. [DOI] [PubMed] [Google Scholar]
- 32.Limpitikul W.B., Dick I.E., Tester D.J., Boczek N.J., Limphong P., Yang W., Choi M.H., Babich J., DiSilvestre D., Kanter R.J. A precision medicine approach to the rescue of function on malignant calmodulinopathic long-qt syndrome. Circ. Res. 2017;120:39–48. doi: 10.1161/CIRCRESAHA.116.309283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatima A., Xu G., Shao K., Papadopoulos S., Lehmann M., Arnáiz-Cot J.J., Rosa A.O., Nguemo F., Matzkies M., Dittmann S. In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell. Physiol. Biochem. 2011;28:579–592. doi: 10.1159/000335753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung C.B., Moretti A., Mederos y Schnitzler M., Iop L., Storch U., Bellin M., Dorn T., Ruppenthal S., Pfeiffer S., Goedel A. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol. Med. 2012;4:180–191. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novak A., Barad L., Zeevi-Levin N., Shick R., Shtrichman R., Lorber A., Itskovitz-Eldor J., Binah O. Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation. J. Cell. Mol. Med. 2012;16:468–482. doi: 10.1111/j.1582-4934.2011.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan F., Lee A.S., Liang P., Sanchez-Freire V., Nguyen P.K., Wang L., Han L., Yen M., Wang Y., Sun N. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun N., Yazawa M., Liu J., Han L., Sanchez-Freire V., Abilez O.J., Navarrete E.G., Hu S., Wang L., Lee A. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med. 2012;4:130ra47. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H., Lee J., Vincent L.G., Wang Q., Gu M., Lan F., Churko J.M., Sallam K.I., Matsa E., Sharma A. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell. 2015;17:89–100. doi: 10.1016/j.stem.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drawnel F.M., Boccardo S., Prummer M., Delobel F., Graff A., Weber M., Gérard R., Badi L., Kam-Thong T., Bu L. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9:810–821. doi: 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 40.Hashem S.I., Perry C.N., Bauer M., Han S., Clegg S.D., Ouyang K., Deacon D.C., Spinharney M., Panopoulos A.D., Izpisua Belmonte J.C. Brief report: oxidative stress mediates cardiomyocyte apoptosis in a human model of Danon disease and heart failure. Stem Cells. 2015;33:2343–2350. doi: 10.1002/stem.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G., McCain M.L., Yang L., He A., Pasqualini F.S., Agarwal A., Yuan H., Jiang D., Zhang D., Zangi L. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebert A.D., Kodo K., Liang P., Wu H., Huber B.C., Riegler J., Churko J., Lee J., de Almeida P., Lan F. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci. Transl. Med. 2014;6:255ra130. doi: 10.1126/scitranslmed.3009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raval K.K., Tao R., White B.E., De Lange W.J., Koonce C.H., Yu J., Kishnani P.S., Thomson J.A., Mosher D.F., Ralphe J.C. Pompe disease results in a Golgi-based glycosylation deficit in human induced pluripotent stem cell-derived cardiomyocytes. J. Biol. Chem. 2015;290:3121–3136. doi: 10.1074/jbc.M114.628628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burridge P.W., Li Y.F., Matsa E., Wu H., Ong S.G., Sharma A., Holmström A., Chang A.C., Coronado M.J., Ebert A.D. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 2016;22:547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma A., Burridge P.W., McKeithan W.L., Serrano R., Shukla P., Sayed N., Churko J.M., Kitani T., Wu H., Holmström A. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med. 2017;9:eaaf2584. doi: 10.1126/scitranslmed.aaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasqualini F.S., Sheehy S.P., Agarwal A., Aratyn-Schaus Y., Parker K.K. Structural phenotyping of stem cell-derived cardiomyocytes. Stem Cell Reports. 2015;4:340–347. doi: 10.1016/j.stemcr.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott C.W., Zhang X., Abi-Gerges N., Lamore S.D., Abassi Y.A., Peters M.F. An impedance-based cellular assay using human iPSC-derived cardiomyocytes to quantify modulators of cardiac contractility. Toxicol. Sci. 2014;142:331–338. doi: 10.1093/toxsci/kfu186. [DOI] [PubMed] [Google Scholar]
- 48.Spira M.E., Hai A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013;8:83–94. doi: 10.1038/nnano.2012.265. [DOI] [PubMed] [Google Scholar]
- 49.Gilchrist K.H., Lewis G.F., Gay E.A., Sellgren K.L., Grego S. High-throughput cardiac safety evaluation and multi-parameter arrhythmia profiling of cardiomyocytes using microelectrode arrays. Toxicol. Appl. Pharmacol. 2015;288:249–257. doi: 10.1016/j.taap.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Chang Liao M.-L., de Boer T.P., Mutoh H., Raad N., Richter C., Wagner E., Downie B.R., Unsöld B., Arooj I., Streckfuss-Bömeke K. Sensing cardiac electrical activity with a cardiac myocyte-targeted optogenetic voltage indicator. Circ. Res. 2015;117:401–412. doi: 10.1161/CIRCRESAHA.117.306143. [DOI] [PubMed] [Google Scholar]
- 51.Leyton-Mange J.S., Mills R.W., Macri V.S., Jang M.Y., Butte F.N., Ellinor P.T., Milan D.J. Rapid cellular phenotyping of human pluripotent stem cell-derived cardiomyocytes using a genetically encoded fluorescent voltage sensor. Stem Cell Reports. 2014;2:163–170. doi: 10.1016/j.stemcr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denning C., Borgdorff V., Crutchley J., Firth K.S.A., George V., Kalra S., Kondrashov A., Hoang M.D., Mosqueira D., Patel A. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta. 2016;1863(7 Pt B):1728–1748. doi: 10.1016/j.bbamcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Álamo J.C., Lemons D., Serrano R., Savchenko A., Cerignoli F., Bodmer R., Mercola M. High throughput physiological screening of iPSC-derived cardiomyocytes for drug development. Biochim. Biophys. Acta. 2016;1863:1717–1727. doi: 10.1016/j.bbamcr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee W.H., Chen W.-Y., Shao N.-Y., Xiao D., Qin X., Baker N., Bae H.R., Wei T.T., Wang Y., Shukla P. Comparison of non-coding RNAs in exosomes and functional efficacy of human embryonic stem cell- versus induced pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2017;35:2138–2149. doi: 10.1002/stem.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen P.K., Neofytou E., Rhee J.-W., Wu J.C. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol. 2016;1:953–962. doi: 10.1001/jamacardio.2016.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayed N., Liu C., Wu J.C. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J. Am. Coll. Cardiol. 2016;67:2161–2176. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chong J.J.H., Yang X., Don C.W., Minami E., Liu Y.-W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson M.E., Goldhaber J., Houser S.R., Puceat M., Sussman M.A. Embryonic Stem Cell–Derived Cardiac Myocytes Are Not Ready for Human Trials. Circ. Res. 2014;115:335–338. doi: 10.1161/CIRCRESAHA.114.304616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu K., Wu Q., Ni C., Zhang P., Zhong Z., Wu Y., Wang Y., Xu Y., Kong M., Cheng H. Lack of remuscularization following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ. Res. 2018 doi: 10.1161/CIRCRESAHA.117.311578. Published online January 17, 2018. [DOI] [PubMed] [Google Scholar]
- 60.Riegler J., Tiburcy M., Ebert A., Tzatzalos E., Raaz U., Abilez O.J., Shen Q., Kooreman N.G., Neofytou E., Chen V.C. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015;117:720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinberger F., Breckwoldt K., Pecha S., Kelly A., Geertz B., Starbatty J., Yorgan T., Cheng K.H., Lessmann K., Stolen T. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 2016;8:363ra148. doi: 10.1126/scitranslmed.aaf8781. [DOI] [PubMed] [Google Scholar]
- 62.Gao L., Gregorich Z.R., Zhu W., Mattapally S., Oduk Y., Lou X., Kannappan R., Borovjagin A.V., Walcott G.P., Pollard A.E. Large cardiac-muscle patches engineered from human induced-pluripotent stem-cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.030785. Published online December 12, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J. Biomed. Mater. Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 64.Masumoto H., Ikuno T., Takeda M., Fukushima H., Marui A., Katayama S., Shimizu T., Ikeda T., Okano T., Sakata R. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep. 2014;4:6716. doi: 10.1038/srep06716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu T., Sekine H., Yang J., Isoi Y., Yamato M., Kikuchi A., Kobayashi E., Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 66.Kawamura M., Miyagawa S., Fukushima S., Saito A., Miki K., Funakoshi S., Yoshida Y., Yamanaka S., Shimizu T., Okano T. Enhanced therapeutic effects of human ips cell derived-cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci. Rep. 2017;7:8824. doi: 10.1038/s41598-017-08869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tzatzalos E., Abilez O.J., Shukla P., Wu J.C. Engineered heart tissues and induced pluripotent stem cells: Macro- and microstructures for disease modeling, drug screening, and translational studies. Adv. Drug Deliv. Rev. 2016;96:234–244. doi: 10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guyette J.P., Charest J.M., Mills R.W., Jank B.J., Moser P.T., Gilpin S.E., Gershlak J.R., Okamoto T., Gonzalez G., Milan D.J. Bioengineering human myocardium on native extracellular matrix. Circ. Res. 2016;118:56–72. doi: 10.1161/CIRCRESAHA.115.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao L., Kupfer M.E., Jung J.P., Yang L., Zhang P., Da Sie Y., Tran Q., Ajeti V., Freeman B.T., Fast V.G. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ. Res. 2017;120:1318–1325. doi: 10.1161/CIRCRESAHA.116.310277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ong S.-G., Huber B.C., Lee W.H., Kodo K., Ebert A.D., Ma Y., Nguyen P.K., Diecke S., Chen W.Y., Wu J.C. Microfluidic single-cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes after acute myocardial infarction. Circulation. 2015;132:762–771. doi: 10.1161/CIRCULATIONAHA.114.015231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tachibana A., Santoso M.R., Mahmoudi M., Shukla P., Wang L., Bennett M., Goldstone A.B., Wang M., Fukushi M., Ebert A.D. Paracrine effects of the pluripotent stem cell-derived cardiac myocytes salvage the injured myocardium. Circ. Res. 2017;121:e22–e36. doi: 10.1161/CIRCRESAHA.117.310803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menasché P., Vanneaux V., Hagège A., Bel A., Cholley B., Cacciapuoti I., Parouchev A., Benhamouda N., Tachdjian G., Tosca L. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 73.Menasché P., Vanneaux V., Hagège A., Bel A., Cholley B., Parouchev A., Cacciapuoti I., Al-Daccak R., Benhamouda N., Blons H. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018;71:429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 74.Neofytou E., O’Brien C.G., Couture L.A., Wu J.C. Hurdles to clinical translation of human induced pluripotent stem cells. J. Clin. Invest. 2015;125:2551–2557. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y., Ogasawara T., Okada K., Shiba N., Sakamoto K. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 76.Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat. Biotechnol. 2015;33:890–891. doi: 10.1038/nbt0915-890. [DOI] [PubMed] [Google Scholar]
- 78.Yoshihara M., Hayashizaki Y., Murakawa Y. Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev. 2017;13:7–16. doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taapken S.M., Nisler B.S., Newton M.A., Sampsell-Barron T.L., Leonhard K.A., McIntire E.M., Montgomery K.D. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat. Biotechnol. 2011;29:313–314. doi: 10.1038/nbt.1835. [DOI] [PubMed] [Google Scholar]
- 80.Cheng L., Hansen N.F., Zhao L., Du Y., Zou C., Donovan F.X., Chou B.K., Zhou G., Li S., Dowey S.N., NISC Comparative Sequencing Program Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10:337–344. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shiba Y., Fernandes S., Zhu W.-Z., Filice D., Muskheli V., Kim J., Palpant N.J., Gantz J., Moyes K.W., Reinecke H. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye L., Chang Y.-H., Xiong Q., Zhang P., Zhang L., Somasundaram P., Lepley M., Swingen C., Su L., Wendel J.S. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee A.S., Tang C., Cao F., Xie X., van der Bogt K., Hwang A., Connolly A.J., Robbins R.C., Wu J.C. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartman M.E., Dai D.-F., Laflamme M.A. Human pluripotent stem cells: prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv. Drug Deliv. Rev. 2016;96:3–17. doi: 10.1016/j.addr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riegler J., Ebert A., Qin X., Shen Q., Wang M., Ameen M., Kodo K., Ong S.G., Lee W.H., Lee G. Comparison of magnetic resonance imaging and serum biomarkers for detection of human pluripotent stem cell-derived teratomas. Stem Cell Reports. 2016;6:176–187. doi: 10.1016/j.stemcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee A.S., Tang C., Hong W.X., Park S., Bazalova-Carter M., Nelson G., Sanchez-Freire V., Bakerman I., Zhang W., Neofytou E. Brief report: external beam radiation therapy for the treatment of human pluripotent stem cell-derived teratomas. Stem Cells. 2017;35:1994–2000. doi: 10.1002/stem.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho S.-J., Kim S.-Y., Jeong H.-C., Cheong H., Kim D., Park S.-J., Choi J.J., Kim H., Chung H.M., Moon S.H. Repair of ischemic injury by pluripotent stem cell based cell therapy without teratoma through selective photosensitivity. Stem Cell Reports. 2015;5:1067–1080. doi: 10.1016/j.stemcr.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yagyu S., Hoyos V., Del Bufalo F., Brenner M.K. An inducible Caspase-9 suicide gene to improve the safety of therapy using human induced pluripotent stem cells. Mol. Ther. 2015;23:1475–1485. doi: 10.1038/mt.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao F., Lin S., Xie X., Ray P., Patel M., Zhang X., Drukker M., Dylla S.J., Connolly A.J., Chen X. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao F., Drukker M., Lin S., Sheikh A.Y., Xie X., Li Z., Connolly A.J., Weissman I.L., Wu J.C. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007;9:107–117. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- 91.Menendez S., Camus S., Herreria A., Paramonov I., Morera L.B., Collado M., Pekarik V., Maceda I., Edel M., Consiglio A. Increased dosage of tumor suppressors limits the tumorigenicity of iPS cells without affecting their pluripotency. Aging Cell. 2012;11:41–50. doi: 10.1111/j.1474-9726.2011.00754.x. [DOI] [PubMed] [Google Scholar]
- 92.Ben-David U., Nudel N., Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat. Commun. 2013;4:1992. doi: 10.1038/ncomms2992. [DOI] [PubMed] [Google Scholar]
- 93.Tang C., Lee A.S., Volkmer J.-P., Sahoo D., Nag D., Mosley A.R., Inlay M.A., Ardehali R., Chavez S.L., Pera R.R. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat. Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miki K., Endo K., Takahashi S., Funakoshi S., Takei I., Katayama S., Toyoda T., Kotaka M., Takaki T., Umeda M. Efficient detection and purification of cell populations using synthetic microRNA switches. Cell Stem Cell. 2015;16:699–711. doi: 10.1016/j.stem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Parr C.J.C., Katayama S., Miki K., Kuang Y., Yoshida Y., Morizane A., Takahashi J., Yamanaka S., Saito H. MicroRNA-302 switch to identify and eliminate undifferentiated human pluripotent stem cells. Sci. Rep. 2016;6:32532. doi: 10.1038/srep32532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ben-David U., Gan Q.-F., Golan-Lev T., Arora P., Yanuka O., Oren Y.S., Leikin-Frenkel A., Graf M., Garippa R., Boehringer M. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 97.Huskey N.E., Guo T., Evason K.J., Momcilovic O., Pardo D., Creasman K.J., Judson R.L., Blelloch R., Oakes S.A., Hebrok M. CDK1 inhibition targets the p53-NOXA-MCL1 axis, selectively kills embryonic stem cells, and prevents teratoma formation. Stem Cell Reports. 2015;4:374–389. doi: 10.1016/j.stemcr.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuo T.-F., Mao D., Hirata N., Khambu B., Kimura Y., Kawase E., Shimogawa H., Ojika M., Nakatsuji N., Ueda K. Selective elimination of human pluripotent stem cells by a marine natural product derivative. J. Am. Chem. Soc. 2014;136:9798–9801. doi: 10.1021/ja501795c. [DOI] [PubMed] [Google Scholar]
- 99.de Almeida P.E., Ransohoff J.D., Nahid A., Wu J.C. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013;112:549–561. doi: 10.1161/CIRCRESAHA.111.249243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X., Li W., Fu X., Xu Y. The immunogenicity and immune tolerance of pluripotent stem cell derivatives. Front. Immunol. 2017;8:645. doi: 10.3389/fimmu.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pearl J.I., Kean L.S., Davis M.M., Wu J.C. Pluripotent stem cells: immune to the immune system? Sci. Transl. Med. 2012;4:164ps25. doi: 10.1126/scitranslmed.3005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearl J.I., Lee A.S., Leveson-Gower D.B., Sun N., Ghosh Z., Lan F., Ransohoff J., Negrin R.S., Davis M.M., Wu J.C. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swijnenburg R.-J., Schrepfer S., Govaert J.A., Cao F., Ransohoff K., Sheikh A.Y., Haddad M., Connolly A.J., Davis M.M., Robbins R.C. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc. Natl. Acad. Sci. USA. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gornalusse G.G., Hirata R.K., Funk S.E., Riolobos L., Lopes V.S., Manske G., Prunkard D., Colunga A.G., Hanafi L.A., Clegg D.O. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017;35:765–772. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 106.Gourraud P.-A., Gilson L., Girard M., Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells. 2012;30:180–186. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- 107.Barry J., Hyllner J., Stacey G., Taylor C.J., Turner M. Setting up a Haplobank: issues and solutions. Curr. Stem Cell Rep. 2015;1:110–117. doi: 10.1007/s40778-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Sousa P.A., Steeg R., Wachter E., Bruce K., King J., Hoeve M., Khadun S., McConnachie G., Holder J., Kurtz A. Rapid establishment of the European Bank for Induced Pluripotent Stem Cells (EBiSC) - the hot start experience. Stem Cell Res. (Amst.) 2017;20:105–114. doi: 10.1016/j.scr.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 109.Kim J.-H., Kurtz A., Yuan B.-Z., Zeng F., Lomax G., Loring J.F., Crook J., Ju J.H., Clarke L., Inamdar M.S. Report of the international stem cell banking initiative workshop activity: current hurdles and progress in seed-stock banking of human pluripotent stem cells. Stem Cells Transl. Med. 2017;6:1956–1962. doi: 10.1002/sctm.17-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 111.Shiba Y., Filice D., Fernandes S., Minami E., Dupras S.K., Biber B.V., Trinh P., Hirota Y., Gold J.D., Viswanathan M. Electrical integration of human embryonic stem cell-derived cardiomyocytes in a guinea pig chronic infarct model. J. Cardiovasc. Pharmacol. Ther. 2014;19:368–381. doi: 10.1177/1074248413520344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burridge P.W., Sharma A., Wu J.C. Genetic and epigenetic regulation of human cardiac reprogramming and differentiation in regenerative medicine. Annu. Rev. Genet. 2015;49:461–484. doi: 10.1146/annurev-genet-112414-054911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paige S.L., Plonowska K., Xu A., Wu S.M. Molecular regulation of cardiomyocyte differentiation. Circ. Res. 2015;116:341–353. doi: 10.1161/CIRCRESAHA.116.302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rana M.S., Christoffels V.M., Moorman A.F.M. A molecular and genetic outline of cardiac morphogenesis. Acta Physiol. (Oxf.) 2013;207:588–615. doi: 10.1111/apha.12061. [DOI] [PubMed] [Google Scholar]
- 115.Rentschler S., Zander J., Meyers K., France D., Levine R., Porter G., Rivkees S.A., Morley G.E., Fishman G.I. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc. Natl. Acad. Sci. USA. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu W.-Z., Xie Y., Moyes K.W., Gold J.D., Askari B., Laflamme M.A. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ. Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gassanov N., Er F., Zagidullin N., Hoppe U.C. Endothelin induces differentiation of ANP-EGFP expressing embryonic stem cells towards a pacemaker phenotype. FASEB J. 2004;18:1710–1712. doi: 10.1096/fj.04-1619fje. [DOI] [PubMed] [Google Scholar]
- 118.Lee J.H., Protze S.I., Laksman Z., Backx P.H., Keller G.M. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell. 2017;21:179–194.e4. doi: 10.1016/j.stem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Birket M.J., Ribeiro M.C., Verkerk A.O., Ward D., Leitoguinho A.R., den Hartogh S.C., Orlova V.V., Devalla H.D., Schwach V., Bellin M. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat. Biotechnol. 2015;33:970–979. doi: 10.1038/nbt.3271. [DOI] [PubMed] [Google Scholar]
- 120.Protze S.I., Liu J., Nussinovitch U., Ohana L., Backx P.H., Gepstein L., Keller G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017;35:56–68. doi: 10.1038/nbt.3745. [DOI] [PubMed] [Google Scholar]
- 121.Caulfield J.B., Leinbach R., Gold H. The relationship of myocardial infarct size and prognosis. Circulation. 1976;53(Suppl):I141–I144. [PubMed] [Google Scholar]
- 122.Zweigerdt R. Large scale production of stem cells and their derivatives. Adv. Biochem. Eng. Biotechnol. 2009;114:201–235. doi: 10.1007/10_2008_27. [DOI] [PubMed] [Google Scholar]
- 123.dos Santos F.F., Andrade P.Z., da Silva C.L., Cabral J.M.S. Bioreactor design for clinical-grade expansion of stem cells. Biotechnol. J. 2013;8:644–654. doi: 10.1002/biot.201200373. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y., Chou B.-K., Dowey S., He C., Gerecht S., Cheng L. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res. (Amst.) 2013;11:1103–1116. doi: 10.1016/j.scr.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen V.C., Couture L.A. The suspension culture of undifferentiated human pluripotent stem cells using spinner flasks. Methods Mol. Biol. 2015;1283:13–21. doi: 10.1007/7651_2014_118. [DOI] [PubMed] [Google Scholar]
- 126.Kempf H., Kropp C., Olmer R., Martin U., Zweigerdt R. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat. Protoc. 2015;10:1345–1361. doi: 10.1038/nprot.2015.089. [DOI] [PubMed] [Google Scholar]
- 127.Chen V.C., Ye J., Shukla P., Hua G., Chen D., Lin Z., Liu J.C., Chai J., Gold J., Wu J. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. (Amst.) 2015;15:365–375. doi: 10.1016/j.scr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dow J., Simkhovich B.Z., Kedes L., Kloner R.A. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc. Res. 2005;67:301–307. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 129.Linkermann A. Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int. 2016;89:46–57. doi: 10.1016/j.kint.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 130.Fischer S., Cassivi S.D., Xavier A.M., Cardella J.A., Cutz E., Edwards V., Liu M., Keshavjee S. Cell death in human lung transplantation: apoptosis induction in human lungs during ischemia and after transplantation. Ann. Surg. 2000;231:424–431. doi: 10.1097/00000658-200003000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rock K.L., Latz E., Ontiveros F., Kono H. The sterile inflammatory response. Annu. Rev. Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee A.S., Inayathullah M., Lijkwan M.A., Zhao X., Sun W., Park S. Prolonged survival of transplanted stem cells after ischaemic injury via the slow release of pro-survival peptides from a collagen matrix. Nat. Biomed. Eng. 2018;2:104–113. doi: 10.1038/s41551-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]