Abstract
Purpose
A better understanding of the effect of aging on trunk musculature will have implications for physical function, disability, pain and risk of injury in older adults. Thus, we determined the age- and sex-related differences in muscle density and size of both thoracic and lumbar trunk muscles
Method
In this cross-sectional study muscle density and size were measured from quantitative computed tomography (QCT) scans for 10 trunk muscle groups at different vertebral levels in 250 community-based men and women aged 40 to 90 years from the Framingham Offspring and Third Generation cohorts.
Results
Trunk muscles in men were 20–67 % larger and had 5–68% higher density than in women. The relative age-related deficits in muscle size were similar in both sexes, and decreased on average by ~ 8 % per decade in both sexes. In contrast, women had greater age-related decreases in muscle density than men (−17% in women, and −11% in men, p<0.01). Age-related declines varied by specific muscle, tending to be greater for outer trunk muscles than for paraspinal muscles, but within a given muscle the age-related changes in muscle density and size were similar among spinal levels.
Conclusion
This comprehensive study of trunk muscle deficits with increasing age may have important implications for physical function, disability, pain and risk of injury in older adults. The greater levels of mobility impairments with aging in women may in part be explained by greater proportion of intramuscular fat tissue and greater age-related fat accumulation in trunk muscles in women than in men.
Keywords: muscle density, muscle size, aging, muscle groups, trunk muscle, QCT
Introduction
Trunk muscles, which act to stabilize the spine, maintain posture and assist in movements, are essential for activities of daily living [1]. Atrophy of trunk musculature impairs physical function and increases the risk of disability and injury in older people [2–4]. Also, lower trunk muscle density, reflecting greater amounts of fat content in muscle, is more strongly associated with reduced functional capacity than thigh muscle density among elderly people, highlighting the key role of trunk muscles in activities such as balance control [4]. Moreover, a variety of spinal conditions are associated with deficits in the composition, morphology and/or strength of trunk muscles [5–7], including lumbar disc herniation [6, 8], facet joint osteoarthritis [9, 10], hyperkyphosis [11] and vertebral fragility fractures [12–14].
Moreover, low back pain, the most commonly reported musculoskeletal problem in people older than 75 years, is associated with degeneration in trunk muscles [3, 4, 15]. The muscles surrounding the spine (“paraspinal muscles”) have received considerable attention, being implicated as both the cause and consequence of low back pain [4, 6, 16]. In particular, it has been suggested that the pattern of multifidus muscle atrophy in patients with low back pain is localized rather than generalized, and can be specific to both spinal level and side [16, 17]. However, to be able to identify a pathological deviation in trunk muscle density and/or size there is a need to establish a normal range for these muscle parameters in population-based cohorts of both men and women across different age groups.
Muscle strength and size decrease, whereas fatty infiltration of the muscle increases with advancing age [18, 19]. Accordingly, trunk muscle density, a reflection of fatty infiltration, is lower in older (75–87 yrs) than younger adults (35–50 yrs) and lower in people with decreased physical activity, though muscle density varies widely among muscle groups [20]. In addition, age-related declines in lumbar paraspinal muscle size vary across spinal regions, as well as by muscle groups [21]. Muscle size is smaller in women, older individuals, and those of lesser weight [22]. However, many of these prior studies had limitations, including small sample sizes [20, 22–24], inclusion of individuals < 65 years of age only [21, 23, 24] or comparisons of young vs. old subjects, but no evaluation of the pattern of age-related changes [20]. Moreover, no study has assessed age-related differences in both trunk muscle size and density concurrently. These elements of study design limit prior findings, as they may not be generalizable to other age groups and the small sample sizes are inadequate to serve as normative reference data. Furthermore, there are limited data on trunk muscle morphology for muscles in the thorax, an important region, given the high prevalence of hyperkyphosis as well as vertebral fractures in the mid-thoracic (T7–T8) and thoracolumbar (T12-L1) regions [25]. Thus, normal ranges for trunk muscle density and size for men and women in different age groups in both thoracic and lumbar spine are lacking.
A better understanding of the effect of age and sex on trunk musculature will have implications for physical function, disability, pain and risk of injury in older adults. Thus the aim of this cross-sectional study, in community-based sample of men and women aged 40 to 90 years, is to determine age-related differences in the density and size of both thoracic and lumbar trunk muscles and to assess whether these age-related differences differ between sexes. We hypothesized that muscle density and size would decline linearly with age, but that the degree of age-related deficits would vary with spinal region within a muscle. Furthermore, we hypothesized that age-related deficits in muscle density would be greater than for deficits in muscle size. We also hypothesized that age-related muscle atrophy would vary by muscle according to their function and that outer trunk muscles would demonstrate greater age-associated decreases than paraspinal muscles.
Methods
Study participants
We used an age- and sex-stratified sample of individuals from the Offspring and Third Generation cohorts of the community-based Framingham Heart study who were part of the Multidetector Computed Tomography (MDCT) Study (N=3,479) [26] and underwent abdominal and chest CT scans in the years 2008–2011 (N=2109). Participants were eligible for the current study if they were at least 40 years old at time of CT scan and all spine levels between T5 and L4 were measurable in their CT scans. Twenty-five men and 25 women were randomly selected from each of the five age-decades: 40–49, 50–59, 60–69, 70–79, and over 80 yrs, as we had previously shown that should be a sufficient sample size to detect age-related differences in trunk muscles [20]. A total of 250 men and women aged 40–90 years were included in the current study. Overall, the height, weight and BMI were similar for this subset group as in the MDCT cohort after adjustment for age (p>0.18), except the men in the current study weighted slightly less than the full cohort (on average their BMI was 1.01 kg/m2 lower, p=0.03).
CT acquisition
As previously described [26], CT scans were acquired using a General Electric Discovery VCT 64-slice PET/CT scanner (GE Healthcare), with the following scan settings: a tube voltage of 120 kVp, tube current of 300/350 mA (≤220/>220 lb body weight), and gantry rotation of 350 ms. The thoracic acquisition covered the entire chest from the lung base to the apices during a single inspirational breath hold and typically corresponds to T4 to L1 vertebral levels (slice thickness 0.625 mm, field of view [FOV] 35 cm). The abdominal acquisition began approximately 2cm above the S1 vertebra and 60 contiguous CT slices (slice thickness 2.5 mm, FOV 35 cm) were acquired cranially to this point. Height and weight were measured at the time of the CT exam and physical activity level estimated. Physical activity level was reported using the physical activity index (PAI), which was designed to evaluate general history of daily activity based on self-report and provide an estimate of overall energy expenditure. In short, a questionnaire was administered by an interviewer to determine the average number of hours per day a participant spends in each of five levels of physical activity (basal, sedentary, slight, moderate, and heavy). The hours for each activity level were multiplied by corresponding weighting factors (1.0, 1.1, 1.5, 2.4, and 5.0 respectively), and the results summed to determine PAI [27]. This study utilized previously collected, de-identi ed data and was approved by the institutional review boards of Boston University, Beth Israel Deaconess Medical Center, and Hebrew SeniorLife.
Muscle measurements
Muscle density and size were measured for 10 trunk muscle groups at different vertebral levels as noted in Supplementary Table 1. Each CT scan was spatially filtered using a sigma filter to reduce noise, and each muscle was contoured at the mid-vertebral slice for each level (T5-L4) using an image processing program (Analyze, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) [28]. Muscle size was assessed as the cross-sectional area (CSA; mm2) within the muscle contour, and muscle density as the mean of voxel attenuation in Hounsfield units (HU) within each muscle, averaging the right and left sides. Density measurements were standardized based on a hydroxyapatite phantom (Image Analysis, Inc., Lexington, KY, USA) scanned with each patient, and voxels outside the range of 50 to 150 HU were excluded before CSA and density were calculated to remove voxels of pure fat, tendon and bone along the periphery of the muscle contours. The muscle size and density measurements for each muscle were averaged across vertebral levels and measurements for four spinal regions were reported: upper thorax (T5–T6), mid thorax (T7–T10), thoracolumbar (T11-L2) and lower lumbar (L3–L4). Some individuals had missing values for the outer trunk muscles (e.g., rectus abdominus, serratus anterior, external oblique, latissimus dorsi and pectoralis major) (Supp. Table 1a & b) because a part of their trunk was outside of the CT scan field of view. All muscle measurements were performed by one observer and the intra-reader intra-class correlation coefficients were excellent, ranging from 0.941 to 0.997 for muscle CSA and ranged from 0.892 to 0.999 for muscle density. The inter-reader ICCs ranged from 0.847–0.990 for muscle CSA and range from 0.868–0.998 for muscle density. The intra and inter-reader reliability of the measurements was assessed by having two observers analyze a set of scans from 16 participants twice with a minimum of 2 weeks between the analyses.
Statistical analysis
Mean values (±SD) for all variables were calculated in men and women within various age groups. We used general linear mixed regression models to assess associations, with either muscle density or muscle CSA as the dependent variable, and age, sex and spinal region as the independent variables. Subject was included as a random effect, spinal region as within-subject factor and height and weight as covariates. Differences in changes with age between men and women were tested using an age–sex interaction term in the regression model and differences in effect of age in spinal regions were tested using an age-region interaction term, with post hoc Bonferroni correction. Age-related differences in muscle CSA and density in each spinal region were estimated from general linear regression models with adjustment for height and weight. Lowess curve fitting, essentially a type of moving average, was used to assess whether the age-related differences in muscle size and density were linear or nonlinear. We modeled all associations of muscle variables with age separately for men and women. Age-related differences were reported both as absolute and percent differences, with the age group 40–49 years (youngest) chosen as the reference group. The sex-related differences in muscle density and size were assessed with mixed regression models with adjustment for height weight and age and the differences were reported as percent differences, with the female age group 40–49 years (youngest age group) chosen as the reference group. We also further adjusted models for physical activity, but results were broadly similar, thus to use the most parsimonious model, we did not present this in the final results. To examine the overall age-related differences in muscle density and mass by muscle group and sex, we averaged muscle density across spinal regions and summed muscle CSA across spinal regions. Age-related differences were then estimated as previously described and p-values adjusted for multiple comparisons using the Bonferroni method. To visualize the changes in muscle morphology with age, we plotted the mean values for each age group by sex after adjusting for height and weight using a linear regression model. All statistical analyses were performed using R software (R-3.1.3, www.r-project.org) and p-values less than 0.05 were considered statistically significant.
Results
The average weight was 70.7 kg in women and 85.2 kg in men and independent of age for both sexes (p>0.22). The average height was 160 cm in women and 174 cm in men but height was negatively associated with age among women (p<0.0001) though not in men (p=0.08) (see Supplementary Table 2). Mean values for muscle density and CSA by sex and age group are found in Supplementary Table 3a & b, respectively.
Sex-related differences in muscle density and size
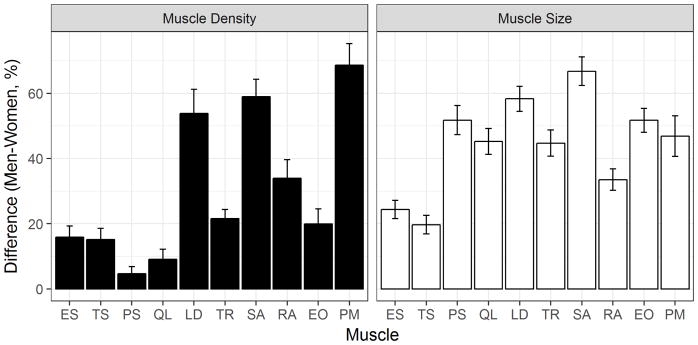
Overall men had 20–67% greater muscle CSA across all trunk muscles after adjustment for height, weight and age (Fig. 1, p < 0.0001). Men also had significantly higher muscle density than women in all muscle groups (p < 0.03). Specifically, men had 5–22 % higher muscle density than women for psoas major, quadratus lumborum, erector spinae, transversospinialis, external oblique and trapezius, while we observed a somewhat greater sex-related difference in muscle density, 34–68 %, for rectus abdominis, latissimus dorsi, serratus anterior and pectoralis major.
Fig. 1.
Percent differences in muscle density and size between men and women with adjustment for height, weight and age (mean difference ± Standard error). Men had greater muscle size and higher muscle density than women across all trunk muscles (p<0.03). ES, erector spinae; TS, transversospinalis; PS, psoas major; QL, quadratus lumborum; LD, latissimus dorsi; TR, trapezius; SA, serratus anterior; RA, rectus abdominis; EO, external oblique; PM, pectoralis major.
Age-related differences in muscle density
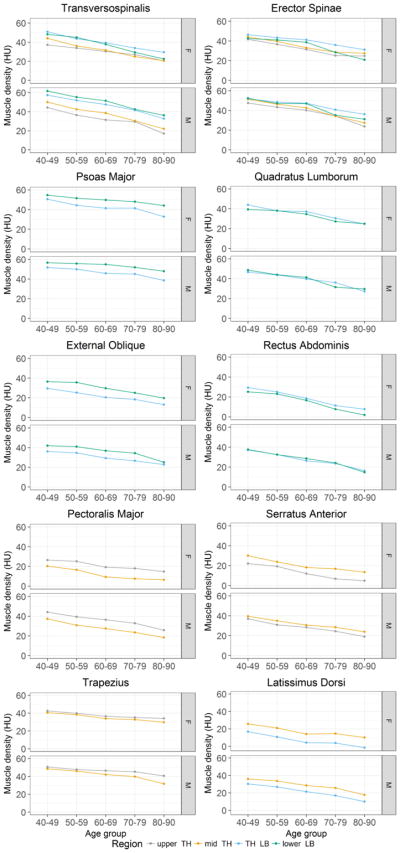
In both men and women, muscle density was negatively associated with age for all muscles in all spinal regions measured (r = − 0.40 to − 0.70, p < 0.001 for all). The lowess curve fitting indicated that the association between muscle density and age was linear. The decline in muscle density with age, expressed in HU, was similar among women (−2.7 to −8.6 HU per 10 years of age) and men (−2.5 to −7.1 HU per 10 per years of age) (Table 1, p > 0.07). Among women, the effect of age on muscle density depended on spinal region for erector spinae and transversospinalis, such that the age-related decline was greatest in the lower lumbar region compared to other regions (p < 0.01, Fig. 2). Further, the age-related decline in muscle density was greater at the thoracolumbar region than the lower lumbar region for psoas major (p < 0.001). In men, the interaction between age and spinal region was only significant for erector spinae (thoracolumbar region showing the smallest age-related decline, p < 0.01) and trapezius (mid thorax region showing the greatest age-related decline, p< 0.01).
Table 1.
Age- and sex-related differences in trunk muscle density with adjustment for height and weight according to spinal region
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean±SD (40–49 years) | Difference per 10 yrs of age | Age corr | Mean±SD (40–49 years) | Difference per 10 yrs of age | Age corr | |||
| % | Absolute | % | Absolute | |||||
| Pectoralis major | ||||||||
| Upper TH | 26.5 (8.9)§ | −16 (−21, −11)§ | −4.2 (−5.6, −2.8)† | −0.40† | 44.2 (7.5) | −10 (−13, −8) | −4.6 (−5.8, −3.5)† | −0.59† |
| Mid TH | 20.4 (12.0)§ | −24 (−31, −17)§ | −4.9 (−6.4, −3.4)† | −0.50† | 37.4 (7.8) | −13 (−17, −10) | −5.0 (−6.2, −3.8)† | −0.56† |
| Rectus abdominis | ||||||||
| TH LB | 29.3 (13.1)§ | −25 (−30, −20)§ | −7.3 (−8.7, −5.8)† | −0.65† | 37.2 (7.5) | −15 (−18, −12) | −5.6 (−6.6, −4.6)† | −0.70† |
| Lower LB | 25.2 (13.6)§ | −32 (−38, −26)§ | −8.0 (−9.5, −6.5)† | −0.70† | 37.7 (9.6) | −16 (−20, −12) | −6.0 (−7.4, −4.5)† | −0.61† |
| Serratus anterior | ||||||||
| Upper TH | 22.1 (8.3)§ | −27 (−33, −21)§ | −5.9 (−7.2, −4.6)† | −0.57† | 37.0 (8.9) | −12 (−16, −9) | −4.6 (−5.8, −3.4)† | −0.54† |
| Mid TH | 30.0 (6.7)§ | −17 (−20, −13)§ | −5.0 (−6.0, −4.0)† | −0.60† | 39.6 (6.7) | −10 (−13, −8) | −4.1 (−5.0, −3.2)† | −0.62† |
| Latissimus dorsi | ||||||||
| Mid TH | 25.9 (9.3)§ | −19 (−24, −14) | −5.0 (−6.3, −3.7)† | −0.54† | 36.0 (8.4) | −13 (−17, −10) | −4.8 (−6.0, −3.5)† | −0.60† |
| TH LB | 17.0 (12.2)§ | −34 (−42, −25)§ | −5.7 (−7.1, −4.2)† | −0.57† | 30.3 (8.6) | −17 (−21, −14) | −5.3 (−6.4, −4.1)† | −0.62† |
| Trapezius | ||||||||
| Upper TH | 42.6 (5.0)§ | −6 (−8, −4) | −2.7 (−3.6, −1.7)† | −0.43† | 50.7 (5.4) | −5 (−7, −3) | −2.5 (−3.3, −1.7)† | −0.48† |
| Mid TH | 40.6 (6.06)§ | −9 (−11, −6) | −3.5 (−4.4, −2.5)† | −0.52† | 48.7 (6.5) | −9 (−11, −7) | −4.3 (−5.3, −3.2)† | −0.59† |
| External oblique | ||||||||
| TH LB | 29.5 (9.8) | −17 (−22, −13)§ | −5.1 (−6.4, −3.7)† | −0.52† | 36.1 (7.2) | −10 (−13, −7) | −3.7 (−4.6, −2.7)† | −0.58† |
| Lower LB | 36.5 (9.2) | −16 (−19, −12)§ | −5.8 (−7.1, −4.5)† | −0.60† | 42.0 (8.8) | −10 (−13, −7) | −4.2 (−5.4, −3.0)† | −0.53† |
| Erector spinae | ||||||||
| Upper TH | 41.7 (9.0) | −14 (−17, −10) | −5.7 (−7.1, −4.2)† | −0.52† | 47.5 (9.5) | −13 (−16, −10) | −6.2 (−7.6, −4.8)† | −0.61† |
| Mid TH | 44.1 (7.8)§ | −12 (−15, −10) | −5.5 (−6.7, −4.3)† | −0.58† | 51.3 (7.6) | −13 (−15, −10) | −6.6 (−7.8, −5.3)† | −0.68† |
| TH LB | 46.4 (8.0) | −10 (−13, −7) | −4.7 (−6.0, −3.4)† | −0.55† | 51.9 (5.1) | −8 (−10, −6) | −4.3 (−5.3, −3.3)† | −0.62† |
| Lower LB | 42.5 (11.7)§ | −17 (−20, −13)§ | −7.1 (−8.6, −5.6)† | −0.65† | 52.5 (8.8) | −12 (−14, −9) | −6.2 (−7.6, −4.8)† | −0.62† |
| Transversospinalis | ||||||||
| Upper TH | 37.3 (11.1) | −14 (−18, −10) | −5.4 (−6.8, −3.9)† | −0.56† | 44.3 (12.3) | −15 (−19, −12) | −6.7 (−8.2, −5.2)† | −0.61† |
| Mid TH | 44.2 (11.2)§ | −17 (−20, −13) | −7.3 (−8.7, −5.9)† | −0.66† | 50.1 (9.4) | −15 (−18, −12) | −7.4 (−8.9, −5.9)† | −0.66† |
| TH LB | 51.1 (10.7) | −13 (−16, −10) | −6.6 (−8.0, −5.2)† | −0.67† | 57.3 (7.9) | −11 (−13, −9) | −6.4 (−7.6, −5.2)† | −0.69† |
| Lower LB | 48.4 (12.2)§ | −18 (−21, −15)§ | −8.6 (−10.1, −7.2)† | −0.75† | 61.7 (9.5) | −12 (−14, −9) | −7.1 (−8.5, −5.6)† | −0.66† |
| Psoas major | ||||||||
| TH LB | 50.6 (7.0) | −9 (−11, −7)§ | −4.8 (−5.8, −3.7)† | −0.66† | 51.8 (6.5) | −6 (−8, −4) | −3.3 (−4.3, −2.3)† | −0.51† |
| Lower LB | 54.6 (6.0) | −6 (−7, −4) | −3.2 (−3.9, −2.4)† | −0.65† | 56.6 (6.0) | −4 (−6, −3) | −2.3 (−3.2, −1.5)† | −0.44† |
| Quadratus lumborum | ||||||||
| TH LB | 43.9 (8.1) | −13 (−15, −10) | −5.6 (−6.8, −4.4)† | −0.67† | 46.6 (6.5) | −10 (−13, −8) | −4.8 (−6.0, −3.7)† | −0.62† |
| Lower LB | 39.4 (9.8) § | −13 (−16, −10) | −5.2 (−6.4, -4.1)† | −0.63† | 48.7 (9.2) | −12 (−14, −9) | −5.7 (−6.8, −4.5)† | −0.68† |
P < 0.05
P < 0.001
Sex difference
Age corr: pearson correlation coefficient between muscle density and age
Fig. 2.
Average muscle density (HU) by spinal level and age group with adjustment for height and weight. upper TH: T5–T6, mid TH: T7–T10, TH LB: T11–L2 and lower LB: L3–L4.
The average age-related difference in density across all trunk muscles was greater in women than men, −17 % per 10 yrs versus −11 % per 10 yrs (p = 0.02). The age-related decline in muscle density, expressed in percent, ranged from −6% to −32% per decade in women, compared to −4% to −17% per decade in men (Table 1 & Fig. 2). Notably, women had a greater percent decrease in muscle density with age than men for pectoralis major, rectus abdominis, serratus anterior and external oblique in all spinal regions (p<0.05). The density of the latissimus dorsi and psoas major showed a greater age-related decline in women than men at the thoracolumbar region only (p<0.04). The percent decline in muscle density with age did not differ between the sexes for erector spinae and transversospinalis, except at the lower lumbar region where women had greater diminution than men (p<0.007). Among men, there was no interaction between age and spinal regions except for trapezius (mid thorax > upper thorax, p < 0.01) and erector spinae (thoracolumbar lowest difference, p<0.01). In women, the percent age-related decline differed by spinal region for the serratus anterior (upper thorax > mid thorax, p < 0.01), latissimus dorsi (thoracolumbar > mid thorax, p < 0.01), trapezius (mid thorax > upper thorax, p < 0.01) and psoas major (thoracolumbar > lower lumber, p = 0.02).
Age-related differences in muscle size
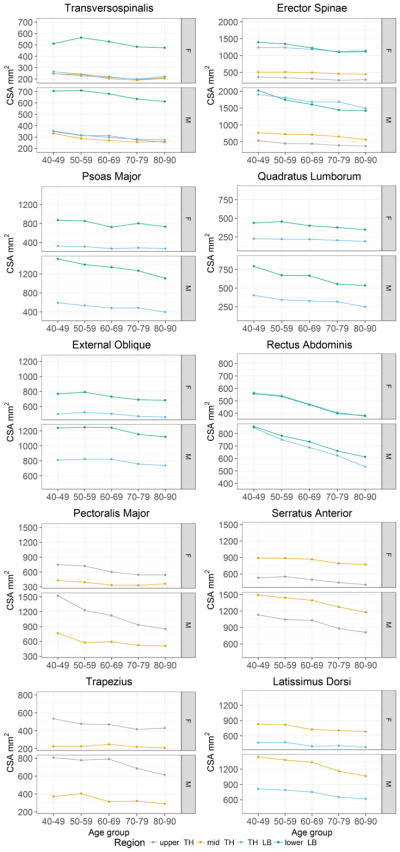
In men, muscle size was negatively associated with age for all muscles in all spinal regions measured (r = −0.20 to −0.68, p < 0.05 for all). Among women, muscle size was negatively associated with age for all muscles in all regions, except the trapezius at the mid thorax (p = 0.35). The lowess curve fitting indicated that the decline in muscle size with age was linear. Expressed as absolute values, the age-related decrements in muscle CSA were greater in men than women (Table 2 & Fig. 3). Specifically, pectoralis major, serratus anterior, latissimus dorsi, trapezius, erector spinae, psoas major and one spinal region for transversospinalis showed more diminution with age in men than women (p<0.01 for all). However, when expressed as a percent, the age-related decline in muscle CSA was similar between the sexes (Table 2) with the exception of the rectus abdominis, for which the age-related decline was greater among women than men at the lower lumbar region (p<0.01). The average age-related difference in muscle size was, −8 % per 10 yrs and −7 % per 10 yrs in men and women, respectively (p=0.16).
Table 2.
Age- and sex-related differences in trunk muscle cross-sectional area with adjustment for height and weight according to spinal region
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean±SD (40–49 years) | Difference per 10 yrs of age | Age corr | Mean±SD (40–49 years) | Difference per 10 yrs of age | Age corr | |||
| % | Absolute | % | Absolute | |||||
| Pectoralis major | ||||||||
| Upper TH | 749 (213)§ | −11 (−14, −8) | −81 (−103, −59)†§ | −0.60† | 1524 (457) | −12 (−14, −9) | −176 (−212, −140)† | −0.66† |
| Mid TH | 430 (153)§ | −7 (−11, −3) | −29 (−47, −11)*§ | −0.37† | 767 (330) | −8 (−11, −4) | −60 (−88, −32)† | −0.36† |
| Rectus abdominis | ||||||||
| TH LB | 566 (101)§ | −11 (−14, −10) | −65 (−78, −54)† | −0.72† | 847 (162) | −9 (−11, −7) | −78 (−94, −63)† | −0.68† |
| Lower LB | 557 (104)§ | −11 (−13, −9)§ | −63 (−75, −50)† | −0.68† | 856 (124) | −7 (−9, −5) | −62 (−80, −45)† | −0.55† |
| Serratus anterior | ||||||||
| Upper TH | 532 (191)§ | −8 (−14, −3) | −44 (−73, −14)* | −0.18* | 1131 (394) | −7 (−11, −4) | −84 (−126, −43)† | −0.31† |
| Mid TH | 888 (142)§ | −5 (−7, −3) | −43 (−62, −25)†§ | −0.33† | 1491 (189) | −6 (−7, −4) | −83 (−105, −60)† | −0.50† |
| Latissimus dorsi | ||||||||
| Mid TH | 832 (122)§ | −7 (−9, −5) | −56 (−74, −38)†§ | −0.44† | 1439 (283) | −7 (−9, −5) | −102 (−130, −73)† | −0.53† |
| TH LB | 470 (67)§ | −9 (−10, −5) | −36 (−47, −25)†§ | −0.48† | 816 (138) | −7 (−9, −5) | −55 (−71, −39)† | −0.50† |
| Trapezius | ||||||||
| Upper TH | 534 (84)§ | −6 (−8, −4) | −32 (−45, −20)†§ | −0.43† | 807 (125) | −6 (−8, −4) | −49 (−63, −34)† | −0.50† |
| Mid TH | 228 (67)§ | −2 (−7, 2) | −5 (−16, 5)§ | −0.08 | 372 (153) | −7 (−11, −3) | −25 (−40, −11)* | −0.29† |
| External oblique | ||||||||
| TH LB | 499 (70)§ | −3 (−6, −1) | −16 (−29, −3)* | −0.14 | 812 (157) | −3 (−5, −1) | −22 (−39, −6)* | −0.20* |
| Lower LB | 768 (119)§ | −4 (−7, −2) | −34 (−51, −17)† | −0.38† | 1239 (214) | −3 (−5, −1) | −37 (−63, −10)* | −0.29* |
| Erector spinae | ||||||||
| Upper TH | 364 (89)§ | −8 (−10, −5) | −29 (−38, −20)† | −0.53† | 538 (113) | −8 (−10 −6) | −42 (−53, −30)† | −0.55† |
| Mid TH | 507 (101)§ | −4 (−7, −2) | −21 (−33, −8)*§ | −0.31† | 769 (148) | −7 (−9, −5) | −51 (−68, −35)† | −0.46† |
| TH LB | 1239 (191)§ | −3 (−5, −1) | −40 (−64, −16)*§ | −0.28* | 1903 (291) | −5 (−7, −4) | −100 (−133, −67)† | −0.44† |
| Lower LB | 1395 (221)§ | −8 (−10, −6) | −109 (−138, −79)†§ | −0.59† | 2022 (264) | −8 (−10, −6) | −162 (−196, −128)† | −0.63† |
| Transversospinalis | ||||||||
| Upper TH | 265 (45)§ | −7 (−9, −5) | −18 (−24, −12)† | −0.50† | 349 (64) | −7 (−9, −5) | −25 (−31, −18)† | −0.58† |
| Mid TH | 246 (39)§ | −7 (−9, −4) | −16 (−22, −10)† | −0.49† | 332 (42) | −6 (−8, −4) | −19 (−26, −12)† | −0.44† |
| TH LB | 249 (38)§ | −4 (−6, −2) | −11 (−16, −5)†§ | −0.40† | 355 (58) | −6 (−8, −4) | −20 (−28, −13)† | −0.46† |
| Lower LB | 512 (128)§ | −4 (−8, −2) | −23 (−39, −8)* | −0.32† | 705 (163) | −4 (−6, −2) | −28 (−45, −11)* | −0.31† |
| Psoas major | ||||||||
| TH LB | 325 (88)§ | −5 (−8, −2) | −17 (−27, −7)†§ | −0.31† | 592 (190) | −8 (−10, −5) | −45 (−62, −28)† | −0.44† |
| Lower LB | 872 (148)§ | −5 (−7, −3) | −43 (−64, −22)†§ | −0.39† | 1516 (283) | −6 (−8, −4) | −97 (−126, −68)† | −0.51† |
| Quadratus lumborum | ||||||||
| TH LB | 227 (56)§ | −5 (−8, −2) | −11 (−18, −4)†§ | −0.29* | 404 (107) | −8 (−11, −6) | −33 (−43, −23)† | −0.51† |
| Lower LB | 439 (85)§ | −8 (−11, −5) | −35 (−47, −23)†§ | −0.50† | 795 (133) | −9 (−11, −7) | −70 (−84, −55)† | −0.64† |
P < 0.05
P <= 0.001
Sex difference
Age corr: pearson correlation coefficient between muscle CSA and age
Fig. 3.
Average cross-sectional area (mm2) by spinal level and age group with adjustment for height and weight. upper TH: T5–T6, mid TH: T7–T10, TH LB: T11–L2 and lower LB: L3–L4.
Age-related differences in muscle morphology by specific muscle
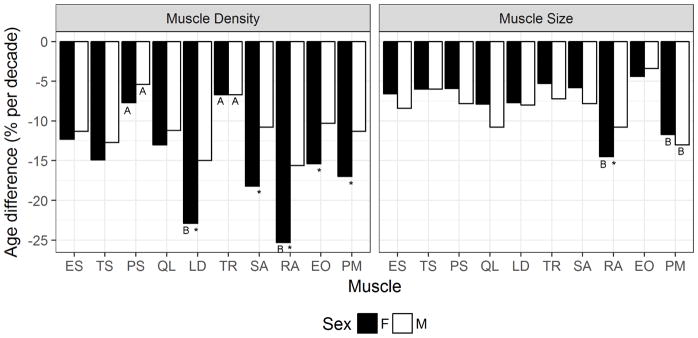
The percent difference in muscle size and density with age varied by muscle (Fig. 4). The psoas major and trapezius had the smallest percent differences in density with age in both men and women (p < 0.05). Rectus abdominis and latissimus dorsi had the greatest percent difference in density with age among women (p <0.05). The percent differences in density with age for the muscles that stabilize the spine (erector spine, transversospinalis, psoas major and quadratus lumborum) did not differ between the sexes whereas the chest, abdominal and lateral muscles (pectoralis major, rectus abdominis, serratus anterior, latissimus dorsi and external oblique) showed 1.5–1.7-fold greater percent differences with age in women. In men, the pectoralis major showed the largest percent difference in size with age (p<0.05) but in women the rectus abdominis and pectoralis major showed the largest percent differences in size with age (p<0.05).
Fig. 4.
Percent difference in trunk muscle density (left) and size (right) by age (% difference per decade). * Significantly different between men and women (p<0.05); A smallest percent difference by age within sex (p<0.05); B largest percent difference by within sex.
Discussion
In this cross-sectional community-based study, we determined the sex- and age-related differences in trunk muscle density and size at both the thoracic and lumbar regions. We found that men had greater muscle size and density across all trunk muscles. When expressed as an absolute difference, the diminution in muscle mass with age was greater in men than women, whereas there was no sex difference in the age-related decrement in muscle density. However, when age-related changes were expressed as a percent decrement compared to young adults, trunk muscle mass declined similarly in men and women, but muscle density declines were greater in women than men. These findings have implications for physical function, disability, pain and risk of injury in older adults. Notably, the greater relative age-related difference in trunk muscle density in women might contribute to the higher prevalence of certain musculoskeletal problems in women, including hyperkyphosis [11] and poorer balance control [29, 30].
Our results are consistent with those from prior studies showing that muscle size and density decrease with advancing age in people over 40 years old [5, 20, 22]. Moreover, similar to the current findings, prior studies of skeletal muscle atrophy also report that muscle cross-sectional area decreases about 1 %/yr [19, 31] and that women have higher fat content and lower muscle density than men [4, 20, 32, 33]. Alterations in both muscle cross-sectional area and muscle density contribute to the reduced strength and poorer physical function in old age [19, 20, 32, 34].
The effect of age on muscle size and density varied with specific muscles. Especially among women, age-related declines in muscle density were greater in the outer trunk muscles (e.g. rectus abdominis, serratus anterior, and latissimus dorsi) than the paraspinal muscles. Age-related deficits in density of the paraspinal muscles, which stabilize the spine, did not differ between the sexes, whereas the outer trunk trunk muscles showed greater percent age-related declines in women. As trunk muscles are important for stabilizing the spine, maintaining posture, and assisting in movements, more fat accumulation in these muscles with age in women compared to men, may explain, in part, greater functional deficits and higher risk of injury in older women than men [30, 35–37]. In fact, greater fatty infiltration has been shown to be associated with muscle weakness [32] and mobility limitations [38]. Moreover, low trunk muscle density is associated with diminished functional capacity in healthy older adults, with stronger association detected for individuals with moderate to extreme low back pain [3].
Our results indicate that the relative age-related decline in muscle density and size is relatively uniform across spinal levels in this community based study. This finding differs from a prior study in men which reported greater changes at L5–S1 than L3–L4 [21]. We only detected variation in muscle density with age by spinal level for serratus anterior, latissimus dorsi and psoas major among women and trapezius in both sexes. We found no obvious pattern, that is, no spinal level consistently showed more rate of loss or preservation in muscle density than others. These discrepant results may be due to methodologic differences as they assessed muscle size (total CSA and fat-free CSA) by magnetic resonance imaging (MRI) and measured at different spinal levels (L5–S1 and L3–L4).
Men had significantly larger trunk muscles than women and greater absolute loss of muscle cross-sectional area with age, which is in agreement what has been reported for skeletal muscle mass [39]. The mechanisms leading to greater age-related loss of muscle mass with age in men are incompletely understood but have been posited to be related to more profound related declines in sex steroids and growth hormones [35, 40–42]. Men had also significantly higher trunk muscle density than women, but similar absolute decline in muscle density than women which indicates that proportional accumulation of intramuscular fat is greater among women than men. The factors contributing to age-related accumulation of intramuscular fat are not well understood [43].
Our findings indicated that there might be a need to identify appropriate interventions to target specific muscles that we observed to demonstrate the greatest age-associated decreases. Interventions, such as aerobic exercise and/or resistance training, might reduce intramuscular fat accumulation in trunk muscles of healthy older adults. For example, Goodpaster et al. imply that physical activity mitigates the intramuscular fat accumulation in thigh muscles in older adults [44]. We showed that whereas advancing age negatively affects all trunk muscle groups, some muscles show greater declines than others. Thus, strategies to maintain trunk muscles may need to be targeted to specific muscles. To the best of our knowledge, the effectiveness of exercise programs to maintain trunk muscle density with advancing age in healthy older adults has not been established. Future studies investigating this could lead to interventions to preserve mobility and reduce injury older people.
This study has several limitations. First, the data come from a cross-sectional study that might introduce survival effect bias, such that healthier people may have been more likely to survive to old age and be examined in this cross-sectional study. Thus, we may have underestimated the true age-related declines in trunk muscle morphology. Moreover, although we adjusted for some of potential confounding variables, we could not exclude the possibility that other covariates may have influenced the age-related differences in muscle density and CSA. We described mean age-related differences in a population using a cross-sectional design. Future work should focus on studying the sources of variation between individuals to permit the evaluation of genetic influences and other confounding factors that were not considered in this study such as diet, lifestyle and diseases such as diabetes mellitus. Second, the measurements of this study are representative of lean muscle composition and did not examine the amount of adipose tissue present in and around the muscles (i.e., intermuscular fat depots), as has previously been reported for the thigh [18, 32, 34]. Finally, because the Framingham Study Offspring and Third Generation cohort were primarily white, extrapolations of our data to other racial and ethnic groups cannot be made. In spite of these limitations, this study has several important strengths. In particular, it draws upon a community-based cohort of individuals. Furthermore, our sample size was relatively large and represented a relevant age range, 40 to 90 years old, including 25 individuals per sex and decade, offering a comprehensive view of trunk muscle aging not previously available in the literature. In addition, in this study age-related differences in trunk muscle size and density were assessed both in the thoracic and lumbar spine, as data on age-related differences in muscles of the thoracic region are particularly lacking.
In conclusion, relative age-related declines in trunk muscle density were greater in women than men whereas the relative decline in muscle mass with age was similar in both sexes. Thus, the observation that women suffer more functional disabilities with advancing age than men might be attributable, in part, to their greater age-related loss in trunk muscle density. Our data assist in establishing a normal range in trunk muscle density and size in thoracic and lumbar spine by sex and age that potentially can serve as a comparative range for different spinal conditions. Future studies are required to identify interventions that could prevent or slow age-related decline in trunk muscle mass and density that would help older men and women to maintain physical function with aging.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 AR053986, K99 AG042458, R01 AR041398), and by the National Heart, Lung, and Blood Institute (NHLBI) Framingham Heart Study (NIH/NHLBI Contract N01-HC-25195). The contents are solely the responsibility of the authors, and do not necessarily represent the views of the NIH.
Footnotes
Conflict of Interest
Fjola Johannesdottir, Brett Allaire, Dennis E. Anderson, Elizabeth J. Samelson, Douglas P. Kiel and Mary L. Bouxsein declare that they have no conflict of interest.
Contributor Information
Fjola Johannesdottir, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA, Department of Orthopedic Surgery, Harvard Medical School, Boston, MA, USA.
Brett Allaire, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Dennis E. Anderson, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA. Department of Orthopedic Surgery, Harvard Medical School, Boston, MA, USA.
Elizabeth J. Samelson, Institute for Aging Research, Hebrew SeniorLife, Boston, MA, USA. Department of Medicine, Harvard Medical School, Boston, MA, USA. Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Douglas P. Kiel, Institute for Aging Research, Hebrew SeniorLife, Boston, MA, USA. Department of Medicine, Harvard Medical School, Boston, MA, USA. Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Mary L. Bouxsein, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA, USA. Department of Orthopedic Surgery, Harvard Medical School, Boston, MA, USA.
References
- 1.Granacher U, Gollhofer A, Hortobagyi T, Kressig RW, Muehlbauer T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: a systematic review. Sports Med. 2013;43:627–641. doi: 10.1007/s40279-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DE, Quinn E, Parker E, Allaire BT, Muir JW, Rubin CT, Magaziner J, Hannan MT, Bouxsein ML, Kiel DP. Associations of Computed Tomography-Based Trunk Muscle Size and Density With Balance and Falls in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71:811–816. doi: 10.1093/gerona/glv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, Tylavsky FA. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–1424. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 4.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, Tylavsky FA. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–887. doi: 10.1093/gerona/60.7.882. [DOI] [PubMed] [Google Scholar]
- 5.Cuellar WA, Wilson A, Blizzard CL, Otahal P, Callisaya ML, Jones G, Hides JA, Winzenberg TM. The assessment of abdominal and multifidus muscles and their role in physical function in older adults: a systematic review. Physiotherapy. 2017;103:21–39. doi: 10.1016/j.physio.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Kalichman L, Carmeli E, Been E. The Association between Imaging Parameters of the Paraspinal Muscles, Spinal Degeneration, and Low Back Pain. Biomed Res Int. 2017;2017:2562957. doi: 10.1155/2017/2562957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suri P, Fry AL, Gellhorn AC. Do Muscle Characteristics on Lumbar Spine Magnetic Resonance Imaging or Computed Tomography Predict Future Low Back Pain, Physical Function, or Performance? A Systematic Review. Pm r. 2015;7:1269–1281. doi: 10.1016/j.pmrj.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Kim WH, Lee SH, Lee DY. Changes in the cross-sectional area of multifidus and psoas in unilateral sciatica caused by lumbar disc herniation. J Korean Neurosurg Soc. 2011;50:201–204. doi: 10.3340/jkns.2011.50.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalichman L, Kim DH, Li L, Guermazi A, Hunter DJ. Computed tomography–evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. The Spine Journal. 2010;10:200–208. doi: 10.1016/j.spinee.2009.10.018. doi: https://doi.org/10.1016/j.spinee.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalichman L, Klindukhov A, Li L, Linov L. Indices of Paraspinal Muscles Degeneration: Reliability and Association With Facet Joint Osteoarthritis: Feasibility Study. Clinical Spine Surgery. 2016;29:465–470. doi: 10.1097/BSD.0b013e31828be943. [DOI] [PubMed] [Google Scholar]
- 11.Katzman W, Cawthon P, Hicks GE, et al. Association of spinal muscle composition and prevalence of hyperkyphosis in healthy community-dwelling older men and women. J Gerontol A Biol Sci Med Sci. 2012;67:191–195. doi: 10.1093/gerona/glr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha-Henriques S, Costa-Paiva L, Pinto-Neto AM, Fonsechi-Carvesan G, Nanni L, Morais SS. Postmenopausal women with osteoporosis and musculoskeletal status: a comparative cross-sectional study. J Clin Med Res. 2011;3:168–176. doi: 10.4021/jocmr537w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinaki M, Khosla S, Limburg PJ, Rogers JW, Murtaugh PA. Muscle strength in osteoporotic versus normal women. Osteoporos Int. 1993;3:8–12. doi: 10.1007/BF01623170. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Chae SU, Kim GD, Cha MS. Changes of paraspinal muscles in postmenopausal osteoporotic spinal compression fractures: magnetic resonance imaging study. J Bone Metab. 2013;20:75–81. doi: 10.11005/jbm.2013.20.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bressler HB, Keyes WJ, Rochon PA, Badley E. The prevalence of low back pain in the elderly. A systematic review of the literature. Spine (Phila Pa 1976) 1999;24:1813–1819. doi: 10.1097/00007632-199909010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008;13:43–49. doi: 10.1016/j.math.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Kamaz M, Kiresi D, Oguz H, Emlik D, Levendoglu F. CT measurement of trunk muscle areas in patients with chronic low back pain. Diagn Interv Radiol. 2007;13:144–148. [PubMed] [Google Scholar]
- 18.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 20.Anderson DE, D’Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci. 2013;68:317–323. doi: 10.1093/gerona/gls168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortin M, Videman T, Gibbons LE, Battie MC. Paraspinal muscle morphology and composition: a 15-yr longitudinal magnetic resonance imaging study. Med Sci Sports Exerc. 2014;46:893–901. doi: 10.1249/mss.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 22.Anderson DE, D’Agostino JM, Bruno AG, Manoharan RK, Bouxsein ML. Regressions for estimating muscle parameters in the thoracic and lumbar trunk for use in musculoskeletal modeling. J Biomech. 2012;45:66–75. doi: 10.1016/j.jbiomech.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, Ulbrich EJ. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am J Neuroradiol. 2016;37:742–748. doi: 10.3174/ajnr.A4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valentin S, Licka T, Elliott J. Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Man Ther. 2015;20:90–95. doi: 10.1016/j.math.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosman F, Krege JH, Looker AC, et al. Spine fracture prevalence in a nationally representative sample of US women and men aged >/=40 years: results from the National Health and Nutrition Examination Survey (NHANES) 2013–2014. Osteoporos Int. 2017;28:1857–1866. doi: 10.1007/s00198-017-3948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining Normal Distributions of Coronary Artery Calcium in Women and Men (from the Framingham Heart Study) The American Journal of Cardiology. 2008;102:1136–1141. e1131. doi: 10.1016/j.amjcard.2008.06.038. doi: https://doi.org/10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young DR, Sharp DS, Petrovitch H, Curb JD. Internal validity of the physical activity index over 26 years in middle-aged and older men. J Am Geriatr Soc. 1995;43:999–1006. doi: 10.1111/j.1532-5415.1995.tb05564.x. [DOI] [PubMed] [Google Scholar]
- 28.Robb RA. The biomedical imaging resource at Mayo Clinic. IEEE Transactions on Medical Imaging. 2001;20:854–867. doi: 10.1109/42.952724. [DOI] [PubMed] [Google Scholar]
- 29.Stevens J, Sogolow E. Gender differences for non-fatal unintentional fall related injuries among older adults. Injury Prevention. 2005;11:115–119. doi: 10.1136/ip.2004.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfson L, Whipple R, Derby CA, Amerman P, Nashner L. Gender differences in the balance of healthy elderly as demonstrated by dynamic posturography. J Gerontol. 1994;49:M160–167. doi: 10.1093/geronj/49.4.m160. [DOI] [PubMed] [Google Scholar]
- 31.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 32.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 33.Schafer AL, Vittinghoff E, Lang TF, et al. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. J Clin Endocrinol Metab. 2010;95:E368–372. doi: 10.1210/jc.2010-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Frontiers in Physiology. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 37.Katzman WB, Miller-Martinez D, Marshall LM, Lane NE, Kado DM. Kyphosis and paraspinal muscle composition in older men: a cross-sectional study for the Osteoporotic Fractures in Men (MrOS) research group. BMC Musculoskelet Disord. 2014;15:19. doi: 10.1186/1471-2474-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 39.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985) 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 40.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mechanisms of Ageing and Development. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. doi: https://doi.org/10.1016/S0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 41.Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The Role of Androgens and Estrogens on Healthy Aging and Longevity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012;67:1140–1152. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakuma K, Yamaguchi A. Sarcopenia and Age-Related Endocrine Function. International Journal of Endocrinology. 2012;2012:10. doi: 10.1155/2012/127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Frontiers in Endocrinology. 2016;7:69. doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.