Abstract
The incidence and prevalence of eosinophilic esophagitis (EoE) have markedly increased over the past two decades, outpacing increased detection of the disease. While genetic susceptibility markers for EoE have begun to be elucidated, the rate at which EoE has increased in incidence suggests environmental factors predominate. Despite many advances in the understanding of the pathogenesis of EoE, the etiology of EoE is unknown. This paper reviews the emerging data related to environmental risk factors for EoE. Many of these environmental factors are rooted in the theoretical framework of the hygiene hypothesis, specifically mediation of disease development through dysbiosis. Other hypotheses are based on associations that have been observed in studies of non-EoE allergic disease. We describe the evidence that early life exposures, including antibiotic use, acid suppression, and cesarean delivery may increase risk of disease. We also describe the evidence that infectious agents, such as Helicobacter pylori, are inversely associated with disease. Current evidence on geographic risk factors, such as population density, climate zone, and seasonality is reviewed. We also describe behavioral factors that have been evaluated. Limitations of the existing research are discussed and recommendations for future areas of research, including assessment of gene-environment interaction, are presented.
Keywords: environment, early life, microbiome, epigenetics, gene-environment
Introduction
Eosinophilic esophagitis (EoE) is an immune-mediated,1–6 chronic disease associated with significant morbidity, including dysphagia, food impactions, and in the pediatric population in particular, food intolerance and faltering growth.7–13 Disease management can be challenging. No pharmacologic therapies have been approved for the treatment of EoE, and current treatments necessitate dietary elimination strategies, topical steroids, or elemental formula diets. Most patients with EoE have evidence of concomitant atopic illness. However, while specific foods elicit clinical and histologic manifestations of disease for many patients, EoE is not believed to be an IgE-mediated disease.14, 15
The incidence and prevalence of EoE have increased dramatically since its initial recognition as a unique disease entity just two decades ago.16–20 In the 1990’s when EoE was first described, disease incidence was estimated at just 0.4 cases/100,000/year. Current estimates of disease incidence and prevalence vary, but are generally described to be ~10 cases/100,000/year and a prevalence of 50–100 cases/100,000.17, 20–23. The economic burden of EoE is substantial. In the United States, where as many as ~400,000 people are affected,24 the estimated annual health-care costs associated with EoE are $1.4 billion.25
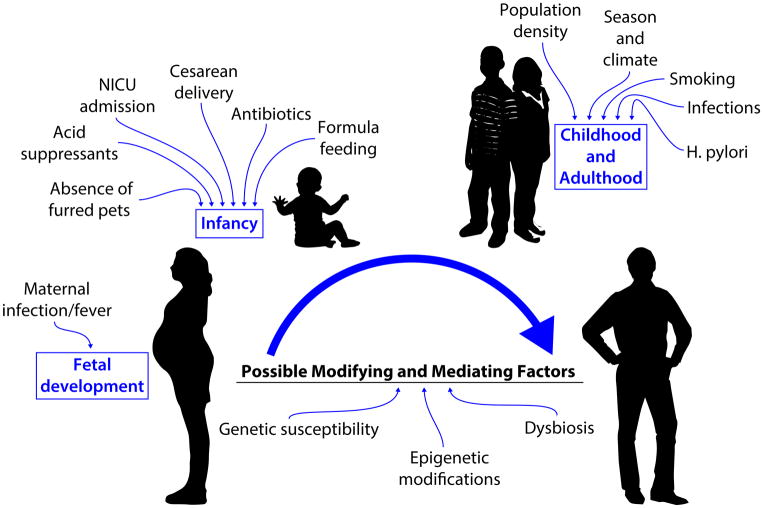
While some of this increase may be attributable to increased awareness and surveillance of the disease, incident diagnoses have outpaced the increase in upper endoscopies. 17, 20, 26 Candidate and genome-wide association studies have identified possible susceptibility genes associated with disease development; 27–30 however, given the rate at which the disease incidence has increased, environmental factors are likely implicated in disease pathogenesis. Furthermore, a twin and family study of EoE identified a stronger concordance for EoE between dizygotic twins than in siblings, suggesting that not only do environmental factors contribute, but that shared environmental factors experienced in early life may be important to disease etiology.31 To date, the body of evidence to support the contribution of environmental factors in EoE disease is still under development, with considerable gaps in knowledge. Much of the existing evidence has focused on early life factors implicated in other allergic diseases, infectious disease factors, geographical factors, and behavioral factors, with limited data on the contribution of genetic and epigenetic factors in relation to these environmental factors (figure 1). This paper describes the evidence thus far, and provides recommendations for future directions to address these gaps in knowledge.
Figure 1.
Candidate risk factors for development of Eosinophilic Esophagitis
Allergic diseases, the hygiene hypothesis, and the microbiome
Given the high proportion of EoE patients with concomitant atopic disease, it is not surprising that the focus of much of the research on environmental factors and EoE has focused on factors implicated in other atopic diseases. As with EoE, the incidence and prevalence of other atopic diseases have also increased in recent decades. One of the prevailing theories to explain this increase, the hygiene hypothesis, asserts that an overly hygienic environment, while important in the reduction of infectious disease, may have untoward effects on the host-microbiome balance necessary for immune system development. However, this theory has been met with scrutiny and has been recently adapted with advances in our ability to characterize the human gut microbiome.32–35 Evidence supports the role of the microbiome in establishing immune function health, but it is not necessarily an aseptic environment that is to blame, but rather the absence of certain necessary commensal bacteria. While the microbiome research field is still relatively underdeveloped (technology for characterizing species continues to evolve, and our capacity to analyze the complexity of the microbiota remains relatively crude),36–42 numerous studies have identified differences in microbiota diversity and patterns of relative abundance in association with atopic disease.43 A challenge in the literature is establishing the temporality of the association, specifically whether the differences observed are attributable to the disease process itself, or whether differences in the microbiota lead to the cascade of events that elicit disease development (e.g. microbiota-host interactions, alterations in the barrier function that contribute to aberrant immune response and loss of tolerance to antigens, etc.).44–47
While much of microbiota research initially focused on gut microbiota, the field has expanded to include assessment of the entire human microbiome. Differences in the esophageal microbiome have been described between patients with EoE, patients with gastroesophageal reflux disease (GERD), and healthy controls,48 but again, it is unknown whether these differences are driven by disease or whether they preceded disease development. With treatment, the differences between EoE cases and healthy controls has been suggested to diminish, although not completely.37 Studies evaluating the use of synbiotics to prevent atopic disease have yielded varied results.49–51 Likely, because our understanding of the microbiome and microbiota interactions is relatively immature, establishing which synbiotic(s) confers protection remains elusive.52 For EoE, a single study conducted in a murine model identified a beneficial effect of the probiotic Lactococcus lactis NCC 2287 on esophageal inflammation.53 Because only a few studies of the esophageal microbiome have been conducted, this is an area where further research is needed to establish the significance, if any, of the esophageal microbiome in disease pathogenesis.
Early life factors and EoE
EoE can develop in infancy, but more frequently is observed later in childhood and sometimes into adulthood. Thus, it may not be readily apparent how factors experienced in early life could contribute to disease development later in life. However, early life is a period of unique developmental susceptibility, and immune maturation may be sensitive to early life experiences.54, 55 Furthermore, it has been suggested that EoE may be part of the atopic march continuum, appearing later in cascade of atopic illnesses frequently co-existing in childhood.56
Antibiotic use, cesarean delivery, and other microbiome-altering factors
Colonization of the microbiome occurs in early life and after the age of 2 or 3 becomes relatively stable.57 Changes in the microbiome may be observed at older ages with dietary changes, use of probiotics, antibiotics, illness, and other exposures, but these changes have been generally characterized as transient and self-limited. Because early life is important in the development of the microbiome, and, consequently, development of the immune system,44–47 numerous studies have examined early life experiences that may shape microbiota colonization.58 Many factors are now described to alter the diversity and/or relative abundance of microbiota in early life; factors including cesarean delivery, preterm delivery, neonatal intensive care unit (NICU) admission, choice of infant feeding, maternal and infant use of antibiotics, and others.39, 59–73 It is this body of literature that has informed studies evaluating the contribution of early life factors in relation to EoE.
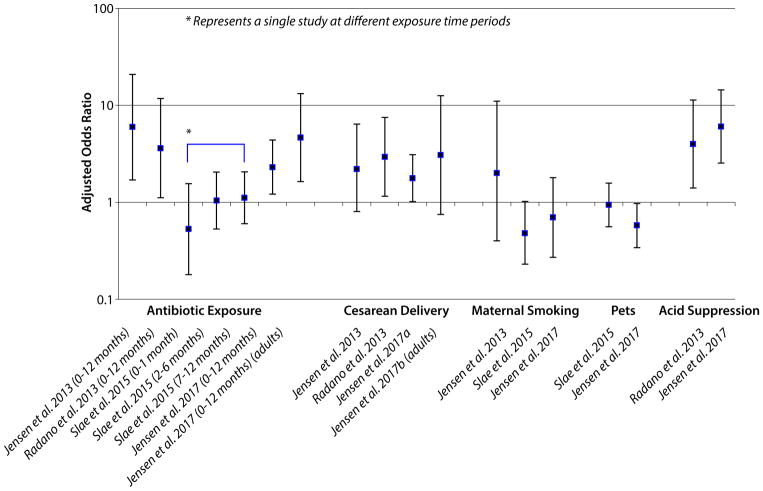
To date, five case-control, single center studies have been conducted examining the contribution of early life factors and the development of EoE. Four of these included pediatric patients only,74–77 one study examined adult patients.78 With the exception of one study, which observed only a weak inverse association between postnatal, environmental tobacco smoke exposure and EoE in the pediatric population,76 all four other studies, conducted at three different centers, identified associations between early life factors and the development of EoE.74, 75, 77, 78 For the pediatric studies, while not all of the studies examined the same factors and differences in the associations were observed between studies, factors identified were consistent with those factors that have been demonstrated to alter microbiota colonization in the gut, including supplemented breastfeeding or formula feeding (possible protective effect for breastfeeding observed), NICU admission, antibiotic use in infancy, cesarean delivery, ownership of a furred pet in the home in infancy (protective association observed), and infant use of acid suppressants (further described below). Perhaps the strongest and most consistent evidence of an association (positive association indicated in 4 of the 5 studies) has been observed for antibiotic use in infancy (figure 2).
Figure 2.
Early life factors evaluated in association with Eosinophilic Esophagitis
Acid suppressants
Acid suppressants, specifically proton pump inhibitors (PPIs), are routinely used to aid in the diagnosis and treatment of EoE as patients with clinical symptoms and histologic evidence consistent with EoE diagnosis (≥15 eosinophils/high power field on biopsy) may experience clinical and histologic improvement following treatment with a PPI. Paradoxically, acid suppressants, including PPIs, have been demonstrated to alter gut permeability.79–81 This increased permeability may compromise oral tolerance and in both animal model and human studies, acid suppressants have led to inhibition of dietary protein digestion and development of IgE antibodies in response to the inhibited protein(s).82, 83 In observational studies, acid suppressants, when used during pregnancy, have been associated with increased risk of atopy in offspring.84
The association between acid suppressant use and development of EoE has only been minimally evaluated. One study examined EoE among patients prescribed a PPI after an initial upper endoscopy and described that following repeat endoscopy, there was no evidence of an increase in absolute EoE cases nor evidence that increasing PPI dose was associated with increased proportion of EoE diagnoses on repeat endoscopy.85 Conversely, a small case series of three patients described development of EoE following initial diagnoses of reflux esophagitis or infectious esophagitis treated with a PPI.86 In the most recent case-control study of early life factors and EoE, a positive association was observed between reported use of an acid suppressant in infancy and EoE diagnosis at age 3 or older.77 While intriguing, the association observed could be attributable to protopathic bias, or symptoms of EoE leading to use of a PPI in infancy, with delayed diagnosis at age 3. Thus, additional mechanistic research is needed to evaluate these associations further.
Infectious risk factors for EoE
Helicobacter pylori
Perhaps providing support for the hypothesis that the increase in prevalence described for atopic conditions may be driven, in part, by changes in the environment that decrease infectious disease, Helicobacter pylori has been inversely associated with atopic conditions including allergic rhinitis, atopic dermatitis and asthma.87 This same inverse association has also been observed for EoE, in both pediatric and adult studies, with reduction in EoE risk in the absence of H. pylori.88–91 This relationship also fits the temporality noted for the increase in EoE over the past two decades, and is supported by a possible mechanism. Specifically, H. pylori is thought to polarize towards more of Th1 immune response, and absence of H. pylori may polarize towards a Th2 response.88 However, this mechanism has yet to be tested experimentally.
Herpes simplex virus
Case report and case series have suggested a possible association between herpes simplex virus (HSV) esophagitis and EoE.92–94 In one series of three pediatric patients with atopy, HSV esophagitis was initially diagnosed and there was no evidence of EoE, but EoE developed within 2 months of the HSV diagnosis.93 A case series of 5 HSV esophagitis adult patients histologic and clinical symptoms consistent with EoE diagnosis95 Similarly, a retrospective assessment of 11 immunocompetent patients with HSV esophagitis identified 5 patients with eosinophilic infiltrate consistent with EoE at follow-up biopsy.96 While these reports suggest HSV esophagitis may co-occur with EoE in some patients, observational studies are needed to evaluate the potential for a temporal association.
Galactose-alpha,1,3-galactose
In a case control design study, IgE sensitization to tick-borne galactose-alpha,1,3-galactose (alpha gal) was evaluated in relation to EoE, as this is an infectious vector that causes a food allergy. Sera biobanked from adult EoE cases (n=50) and controls (n=50) were evaluated for IgE sensitization to alpha gal. While a high proportion of cases and controls were observed to have evidence of sensitization, no differences were observed between cases and controls.97
Mycoplasma pneumonia
A case series of 12 EoE patients found a high proportion (83%) of IgG positivity for Mycoplasma pneumonia with serological testing.98 However, without characterization of seroprevalence of M. pneumonia in controls, the association with EoE cannot be determined.
Geographic risk factors for EoE
Geographic factors, while likely not directly causal for EoE, could offer insight into other environmental factors that may be implicated in disease development.
Population density/geographic differences
To date, 4 studies have examined population density or rural versus urban residence in relation to EoE. The first, a single center, case-control study of 508 cases, 508 gastroenterology specialty clinic controls and 508 allergy controls observed a higher proportion of EoE cases arising from suburban areas, as compared to allergy controls (aOR: 2.1; 95% CI: 1.2, 3.5). However, when comparing EoE cases to GI controls, no association between residence was observed. Another study surveyed gastroenterologists and allergists on eosinophilic gastrointestinal disease patients, and found that EoE was more common in patients with a rural residence.22 In a study of 14,381 cases and 89,754 controls identified in a pathology database containing patients from throughout the United States, a dose-response, inverse relationship was observed between population density and increased risk of EoE. As compared to the most populous residence, there was a 40% increase in risk of EoE observed for the least populous area of residence (aOR: 1.4; 95% CI: 1.1, 1.8). Another study examined EoE incidence and clinical symptoms of EoE according to rural versus urban residence in 57 patients with EoE in Iowa. While no difference was observed in the incidence of EoE diagnoses, differences in symptoms were reported, with a higher proportion of urban residents reporting dysphagia (p=0.047) and a higher proportion of rural residents reporting heartburn or reflux (p=0.04).99
Climate zone/seasonality
The same national pathology database described above was also used to examine the association between climate zone and EoE. Relative to the temperate climate zone, this study reported an increased risk for EoE for patients residing in a cold climate zone (aOR: 1.4, 95% CI: 1.3, 1.5).100 Climate zones can be closed linked to local vegetation patterns and might implicate certain aeroallergens, but this requires further study.
Numerous single center studies have examined seasonality in relation to EoE, although a challenge in such studies is determining symptom onset, as diagnosis is known to lag far behind initial onset of symptoms. While some of these studies suggest an association between season and EoE,20, 101–105 some studies have indicated no association.106–108 Diagnostic delay may contribute to some of these inconsistencies, but geographic and climate differences may also contribute as aeroallergans (type, count, and temporal variability) are known to vary across climate zones. A recent study of seasonality and pollen counts conducted in 36 EoE patients in the New York City area identified increased patient reporting of symptoms in summer months (July-September) and increased diagnoses in the Fall (October-December). Counts from 11 different pollen taxa were examined, including Acer (maple), Betula (birch), Populus (poplar), Ulmus (elm), Quercus (oak), Carya (hickory), Fraxinus (ash), Platanus (sycamore, London planetree), Fagus (beech), Poaceae (grass pollen family), and Ambrosia (ragweed). Symptoms of EoE correlated with peak levels of grass pollen.109 Another study examined seasonality and EoE, taking into account climate zone, again using the national pathology data described above. As expected, this study identified differences in the relationship between seasonality and EoE by climate zone, with strongest evidence of seasonal variation in EoE diagnoses in temperate and cold climates. Summer months were associated with higher EoE diagnose, however peak diagnoses by month differed according to climate zone.110
Behavioral risk factors for EoE
Smoking and alcohol have been associated with GERD and non-steroidal anti-inflammatory drugs (NSAID) use has been associated with atopic illnesses111–113 and other inflammatory gastrointestinal illnesses including microscopic colitis.114 Only one study has thus far examined these factors in EoE. In a single center case-control study (n=115 incident cases and 225 controls) who had undergone upper endoscopy for symptoms of esophageal dysfunction, data on smoking behaviors, alcohol use and NSAID use was collected through patient questionnaire, administered prior to endoscopy and diagnosis. This study observed a decreased risk of EoE among those who had ever smoked (aOR: 0.5 95% CI: 0.2, 0.9), and a decreased risk of EoE for current NSAID use (aOR 0.4; 95% CI: 0.2, 0.8). Current alcohol use was moderately associated with EoE, but the estimate attenuated with adjustment for age, sex, race, education level, smoking, and atopy (aOR: 1.6; 95% CI: 0.8, 3.1).115 Other potential confounders, specifically factors that could be associated with smoking behaviors and diagnosis of EoE were not assessed.
Genetic and epigenetics and the environment
Gene-environment interaction
Studies of gene-environment interaction offer the potential to identify novel genes, exposures or both, whereby risk is only conferred in the presence or absence of the other. These studies could offer increased mechanistic understanding of disease, and also help identify modifiable environmental factors for disease prevention in those with underlying genetic susceptibility for disease (e.g. siblings of EoE patients). To date, only one study has investigated genetic susceptibility markers in relation to environmental factors. This study, while small (n=248), observed that breastfeeding conferred a protective effect for EoE, among those with the susceptibility gene variant at rs6736278 (CAPN14).116 More studies are needed to examine how environmental factors may interact with underlying genetic susceptibility to increase or decrease risk of disease.
Epigenetic modifications and environmental factors
Epigenetic assessments have elucidated novel, mechanistic pathways in the development of childhood asthma and allergy,117–119 and environmental factors have been associated with changes in epigenetic methylation and histone modification patterns.120, 121 Epigenetic modifications in EoE have been minimally explored in EoE,122 yet offer the potential to improve our understanding of how environmental factors infer increased (or decreased) risk of disease. These evaluations could provide mechanistic insights that are important in the development of therapeutic targets for disease treatment.
EoE phenotypic heterogeneity
It should be noted that while most of the research on environmental factors in the development of EoE has been informed primarily by risk factors demonstrated to be associated with atopic disease, there is heterogeneity in the comorbid conditions that EoE patients experience, and the disease, in a proportion of patients (~30%), does not appear to be associated with having other atopic conditions. Indeed, for some patients with EoE, there appears to be increased co-occurrence of autoimmune conditions, including celiac disease, Crohn’s ulcerative colitis, rheumatoid arthritis, IgA deficiency, multiple sclerosis, CVID, and autoimmune thyroid disease.123–125
EoE has also been associated with tracheo-esophageal fistula (TEF), although even in those with co-occurring EoE and TEF, 70% were indicated to have at least one or more additional atopic conditions. Additionally, EoE has been associated with inherited connective tissue disorders (CTD), with 3.3% of EoE patients having a CTD (Marfan, Marfanoid-related syndrome, Ehlers-Danlos and related syndromes, and Loeys-Dietz syndrome) at one center (compared to a prevalence of ~0.02% in the general population).126–128 Again, the co-occurrence of atopy was similar in those with and without presence of a co-existing CTD. Environmental, etiologic studies of EoE conducted thus far have not differentiated EoE based on atopic co-occurrence or presence of other comorbid conditions.
Conclusions and future directions
While numerous studies have been conducted on environmental factors and EoE, this body of research remains relatively undeveloped. Consistent evidence has supported possible associations between antibiotics in infancy and development of EoE, but the studies conducted thus far have the potential for bias given the fact that use of antibiotics has been collected retrospectively, through recall. Furthermore, there is a potential that these associations could reflect confounding by indication, specifically some other factor associated with early life antibiotic use (e.g. asthma), may also be associated with EoE. None of the studies examining antibiotic use examined infection as a possible contributing factor, and antecedent for antibiotic use, although there are studies suggesting infections may increase risk for atopy.129–131 Thus, it is unknown whether antibiotics are the true causal agent in the associations observed for EoE, or whether they are simply intermediates in some other mechanistic pathway.
Mechanistic and observational studies support a possible role for acid suppressants, particularly early life use, in the development of EoE, however, this too must be explored more fully, ideally in a prospectively designed study where temporality of the association can be firmly established. A prospective assessment would also provide the opportunity to assess whether certain individuals (i.e. atopic) are at increased susceptibility to EoE given exposure to acid suppressants.
Clear evidence supports an inverse association between H. pylori and EoE, but this relationship has only been described through cross-sectional data, and it is unknown if this is a correlative or causative relationship. Other infectious factors, including HSV esophagitis and M. pneumonia, warrant investigation in robustly designed, case-control or case-cohort studies from which appropriately selected controls can provide a comparison for evaluation.
Studies on geographic factors have described the association between season and climate, and generally suggest the potential that aeroallergens contribute to disease development. However, seasonality and climate are relatively crude, proxy measures for aeroallergens and associations observed do not preclude the possibility that other factors associated with climate and season (e.g. particulate matter, pollutants, seasonal agricultural factors) could contribute. Studies of population density provide mixed evidence, although the largest of the studies conducted to date suggests risk is higher in rural areas. Population density is certainly a proxy for some other contributory factor, and thus additional studies are needed to evaluate what these other factors may include.
A single center study has been conducted on behavioral factors in adults, but this study suggests that there may be opportunities to mitigate risk even in adulthood. This is an area of research that merits additional development, however an on-going challenge will be establishing disease duration and whether exposures preceded disease development. Likely, adult patients presenting with long-standing fibrostenotic disease may be less suitable for studying exposures experienced in adulthood. The challenge of establishing temporality is pervasive in the literature evaluating environmental factors and EoE.
One approach to addressing this issue establishing temporality would be to assemble a prospective, longitudinal cohort for study of EoE. However, despite increasing incidence and prevalence, assembling a prospective cohort for evaluation of risk factors leading to development of EoE would be extremely challenging. Existing, population-based databases may be used, although often with concomitant loss in detailed exposure data. Consortia, specifically assembling cases across multiple sites into a shared resource for study, may be critical to building the sample sizes needed for developing this body of evidence further. Potentially, an existing cohort of children with atopy could be leveraged, but the relative uncommonness of EoE may prove challenging to study even in this higher risk population. Large sample sizes will be needed to investigate whether there are differences in the observed risk factors according to EoE phenotype or comorbid disease presentation. Another approach to evaluating the contribution of environmental factors in EoE would be designing a sibling pair study, specifically enrolling index cases and their siblings and evaluating differences in the exposures experienced. This design would, potentially, offer improved control for possible confounders I the associations observed. Hypotheses and associations generated by epidemiologic studies will need to be evaluated in in vivo an in vitro models, and in experimental animal models, to dissect disease mechanisms and confirm causality.
In conclusion, there is much to be learned about environmental factors and EoE. As this area of research continues to mature, more robustly designed studies, with appropriately selected comparator groups and well-characterized exposure and phenotypic data will continue to advance our capacity to identify exposures that are implicated in disease development. Integration of environmental factors data, with omics-based data sources, offers the potential to provide mechanistic insights and opportunities for disease mitigation through behavior or novel therapeutics.
Table 1.
What is unknown?
| Potential for interaction between environmental factors and EoE |
| Epigenetic modifications and the environment in relation to EoE |
| Factors that may contribute to dysbiosis of the gut microbiota, such as diet, in relation to EoE |
| Examination between early life factors and EoE and whether associations are mediated by dysbiosis |
| Temporal association between esophageal microbiome in association with EoE |
| Association between acid suppressant use in early life and EoE |
| Improved understanding of how geographical factors may contribute to disease pathogenesis |
Acknowledgments
Funding Support: This work was supported in part by U54AI117804 (CEGIR), which is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Disease Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED CURED and EFC. This work was also supported by R01DK101856 (ESD).
Abbreviations
- EoE
Eosinophilic esophagitis
- GERD
Gastroesophageal reflux disease
- PPIs
Proton pump inhibitors
- NICU
Neonatal intensive care unit
- HSV
Herpes simplex virus
- NSAID
Non-steroidal anti-inflammatory drugs
- CTD
Connective tissue disorders
- TEF
Tracheo-esophageal fistulae
- CAPN14
Calpain-14
Footnotes
Potential competing interests: Neither author reports any potential conflicts of interest with this study. Dr. Dellon is a consultant for Adare, Allakos, Alvio, Banner, Enumeral, GSK, Celgene/Receptos, Regeneron, and Shire; receives research funding from Adare, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; and has received educational grants from Banner and Holoclara.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of Eosinophilic Gastritis, Gastroenteritis, and Colitis: Estimates From a National Administrative Database. J Pediatr Gastroenterol Nutr. 2015 doi: 10.1097/MPG.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assa’ad A. Eosinophilic esophagitis: association with allergic disorders. Gastrointest Endosc Clin N Am. 2008;18:119–32. x. doi: 10.1016/j.giec.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Chehade M, Sampson HA. Epidemiology and etiology of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:33–44. viii. doi: 10.1016/j.giec.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 5.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–5. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 6.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–6. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 7.Abonia JP, Rothenberg ME. Eosinophilic esophagitis: rapidly advancing insights. Annual review of medicine. 2012;63:421–34. doi: 10.1146/annurev-med-041610-134138. [DOI] [PubMed] [Google Scholar]
- 8.Aceves SS, Newbury RO, Dohil R, Schwimmer J, Bastian JF. Distinguishing eosinophilic esophagitis in pediatric patients: clinical, endoscopic, and histologic features of an emerging disorder. J Clin Gastroenterol. 2007;41:252–6. doi: 10.1097/01.mcg.0000212639.52359.f1. [DOI] [PubMed] [Google Scholar]
- 9.Attwood SE. Mechanisms underlying dysphagia in eosinophilic oesophagitis. Gut. 2009;58:1041–2. doi: 10.1136/gut.2008.175612. [DOI] [PubMed] [Google Scholar]
- 10.Basavaraju KP, Wong T. Eosinophilic oesophagitis: a common cause of dysphagia in young adults? Int J Clin Pract. 2008;62:1096–107. doi: 10.1111/j.1742-1241.2008.01782.x. [DOI] [PubMed] [Google Scholar]
- 11.Baxi S, Gupta SK, Swigonski N, Fitzgerald JF. Clinical presentation of patients with eosinophilic inflammation of the esophagus. Gastrointest Endosc. 2006;64:473–8. doi: 10.1016/j.gie.2006.03.931. [DOI] [PubMed] [Google Scholar]
- 12.Dauer EH, Freese DK, El-Youssef M, Thompson DM. Clinical characteristics of eosinophilic esophagitis in children. Ann Otol Rhinol Laryngol. 2005;114:827–33. doi: 10.1177/000348940511401103. [DOI] [PubMed] [Google Scholar]
- 13.Liacouras CA. Clinical presentation and treatment of pediatric patients with eosinophilic esophagitis. Gastroenterol Hepatol (N Y) 2011;7:264–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–9. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71:611–20. doi: 10.1111/all.12846. [DOI] [PubMed] [Google Scholar]
- 16.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 17.Hruz P, Bussmann C, Heer P, Simon HU, Zwahlen M, Beglinger C, et al. Escalating Epidemiology of Eosinophilic Esophagitis: 21 Years of Prospective Population-Based Documentation in Olten County. Gastroenterology. 2011;140(Suppl 1):S238–9. [Google Scholar]
- 18.Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128:1349–50. e5. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 20.Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:201–18. doi: 10.1016/j.gtc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a nationwide cohort. Gastroenterology. 2012;142(Suppl 1):Su 1138. doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12:589–96. e1. doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-Care Utilization, Costs, and the Burden of Disease Related to Eosinophilic Esophagitis in the United States. Am J Gastroenterol. 2015;110:626–32. doi: 10.1038/ajg.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–13. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherrill JD, Rothenberg ME. Genetic dissection of eosinophilic esophagitis provides insight into disease pathogenesis and treatment strategies. J Allergy Clin Immunol. 2011;128:23–32. doi: 10.1016/j.jaci.2011.03.046. quiz 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins MH, Blanchard C, Abonia JP, Kirby C, Akers R, Wang N, et al. Clinical, pathologic, and molecular characterization of familial eosinophilic esophagitis compared with sporadic cases. Clin Gastroenterol Hepatol. 2008;6:621–9. doi: 10.1016/j.cgh.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–91. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134:1084–92. e1. doi: 10.1016/j.jaci.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. 2015;136:860–5. doi: 10.1016/j.jaci.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Weber J, Illi S, Nowak D, Schierl R, Holst O, von Mutius E, et al. Asthma and the hygiene hypothesis. Does cleanliness matter? Am J Respir Crit Care Med. 2015;191:522–9. doi: 10.1164/rccm.201410-1899OC. [DOI] [PubMed] [Google Scholar]
- 34.Ellermann M, Carr JS, Fodor AA, Arthur JC, Carroll IM. Chapter 2 -Characterizing and Functionally Defining the Gut Microbiota: Methodology and Implications A2 -Floch, Martin H. In: Ringel Y, Walker WA, editors. The Microbiota in Gastrointestinal Pathophysiology. Boston: Academic Press; 2017. pp. 15–25. [Google Scholar]
- 35.Bendiks M, Kopp MV. The relationship between advances in understanding the microbiome and the maturing hygiene hypothesis. Curr Allergy Asthma Rep. 2013;13:487–94. doi: 10.1007/s11882-013-0382-8. [DOI] [PubMed] [Google Scholar]
- 36.Sankar SA, Lagier JC, Pontarotti P, Raoult D, Fournier PE. The human gut microbiome, a taxonomic conundrum. Syst Appl Microbiol. 2015;38:276–86. doi: 10.1016/j.syapm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Benitez AJ, Hoffmann C, Muir AB, Dods KK, Spergel JM, Bushman FD, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome. 2015;3:23. doi: 10.1186/s40168-015-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–91. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muir AB, Benitez AJ, Dods K, Spergel JM, Fillon SA. Microbiome and its impact on gastrointestinal atopy. Allergy. 2016;71:1256–63. doi: 10.1111/all.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Statovci D, Aguilera M, MacSharry J, Melgar S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front Immunol. 2017;8:838. doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart CJ, Marrs EC, Nelson A, Lanyon C, Perry JD, Embleton ND, et al. Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS One. 2013;8:e73465. doi: 10.1371/journal.pone.0073465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy. 2015;45:43–53. doi: 10.1111/cea.12332. [DOI] [PubMed] [Google Scholar]
- 43.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–40. 40.e1–2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Walker A. Intestinal colonization and programming of the intestinal immune response. J Clin Gastroenterol. 2014;48(Suppl 1):S8–11. doi: 10.1097/MCG.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res. 2015;77:220–8. doi: 10.1038/pr.2014.160. [DOI] [PubMed] [Google Scholar]
- 46.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–50. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 47.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris JK, Fang R, Wagner BD, Choe HN, Kelly CJ, Schroeder S, et al. Esophageal microbiome in eosinophilic esophagitis. PLoS One. 2015;10:e0128346. doi: 10.1371/journal.pone.0128346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013:Cd006474. doi: 10.1002/14651858.CD006474.pub3. [DOI] [PubMed] [Google Scholar]
- 50.Cabana MD, McKean M, Caughey AB, Fong L, Lynch S, Wong A, et al. Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. Pediatrics. 2017:140. doi: 10.1542/peds.2016-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008;122:788–94. doi: 10.1016/j.jaci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Ferreiro A, Crook N, Gasparrini AJ, Dantas G. Multiscale Evolutionary Dynamics of Host- Associated Microbiomes. Cell. 2018;172:1216–27. doi: 10.1016/j.cell.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holvoet S, Doucet-Ladeveze R, Perrot M, Barretto C, Nutten S, Blanchard C. Beneficial effect of Lactococcus lactis NCC 2287 in a murine model of eosinophilic esophagitis. Allergy. 2016;71:1753–61. doi: 10.1111/all.12951. [DOI] [PubMed] [Google Scholar]
- 54.Georgountzou A, Papadopoulos NG. Postnatal Innate Immune Development: From Birth to Adulthood. Front Immunol. 2017;8:957. doi: 10.3389/fimmu.2017.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butel MJ, Waligora-Dupriet AJ, Wydau-Dematteis S. The developing gut microbiota and its consequences for health. J Dev Orig Health Dis. 2018:1–8. doi: 10.1017/S2040174418000119. [DOI] [PubMed] [Google Scholar]
- 56.Hill DA, Spergel JM. Is eosinophilic esophagitis a member of the atopic march? Annals of Allergy, Asthma & Immunology. 2018;120:113–4. doi: 10.1016/j.anai.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen ET, Bertelsen RJ, Ringel-Kulka T. The Microbiota in Gastrointestinal Pathophysiology. Boston: Academic Press; 2017. Chapter 3 - Microbiota of the Gastrointestinal Tract in Infancy; pp. 27–35. [Google Scholar]
- 58.Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, et al. Factors influencing the infant gut microbiome at age 3–6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART) J Allergy Clin Immunol. 2017;139:482–91. e14. doi: 10.1016/j.jaci.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arboleya S, Sanchez B, Milani C, Duranti S, Solis G, Fernandez N, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166:538–44. doi: 10.1016/j.jpeds.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 60.Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45:632–43. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 61.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Cmaj. 2013;185:385–94. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. Bjog. 2015 doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 63.Bager P, Melbye M, Rostgaard K, Benn CS, Westergaard T. Mode of delivery and risk of allergic rhinitis and asthma. J Allergy Clin Immunol. 2003;111:51–6. doi: 10.1067/mai.2003.34. [DOI] [PubMed] [Google Scholar]
- 64.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–5. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–52. e1–5. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 66.Cassidy-Bushrow AE, Sitarik A, Levin AM, Lynch SV, Havstad S, Ownby DR, et al. Maternal group B Streptococcus and the infant gut microbiota. J Dev Orig Health Dis. 2016;7:45–53. doi: 10.1017/S2040174415001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Celedon JC, Fuhlbrigge A, Rifas-Shiman S, Weiss ST, Finkelstein JA. Antibiotic use in the first year of life and asthma in early childhood. Clin Exp Allergy. 2004;34:1011–6. doi: 10.1111/j.1365-2222.2004.01994.x. [DOI] [PubMed] [Google Scholar]
- 68.Chernikova DA, Koestler DC, Hoen AG, Housman ML, Hibberd PL, Moore JH, et al. Fetal exposures and perinatal influences on the stool microbiota of premature infants. J Matern Fetal Neonatal Med. 2015:1–7. doi: 10.3109/14767058.2014.987748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corvaglia L, Tonti G, Martini S, Aceti A, Mazzola G, Aloisio I, et al. Influence of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on Gut Microbiota in the First Month of Life. J Pediatr Gastroenterol Nutr. 2016;62:304–8. doi: 10.1097/MPG.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 70.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51:77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 72.Kozyrskyj AL, Ernst P, Becker AB. Increased Risk of Childhood Asthma From Antibiotic Use in Early Life*. CHEST Journal. 2007;131:1753–9. doi: 10.1378/chest.06-3008. [DOI] [PubMed] [Google Scholar]
- 73.Mulder IE, Schmidt B, Lewis M, Delday M, Stokes CR, Bailey M, et al. Restricting microbial exposure in early life negates the immune benefits associated with gut colonization in environments of high microbial diversity. PLoS One. 2011;6:e28279. doi: 10.1371/journal.pone.0028279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2013;57:67–71. doi: 10.1097/MPG.0b013e318290d15a. [DOI] [PubMed] [Google Scholar]
- 75.Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2014;2:475–7. e1. doi: 10.1016/j.jaip.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 76.Slae M, Persad R, Leung A-T, Gabr R, Brocks D, Huynh H. Role of Environmental Factors in the Development of Pediatric Eosinophilic Esophagitis. Digestive Diseases and Sciences. 2015:1–9. doi: 10.1007/s10620-015-3740-7. [DOI] [PubMed] [Google Scholar]
- 77.Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen ET, Shaheen O, Koutlas NT, Chang AO, Martin LJ, Rothenberg ME, et al. Early Life Factors are Associated with Risk for Eosinophilic Esophagitis Diagnosed in Adulthood. Gastroenterology. 152:S861. doi: 10.1093/dote/doaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hopkins AM, McDonnell C, Breslin NP, O’Morain CA, Baird AW. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. J Pharm Pharmacol. 2002;54:341–7. doi: 10.1211/0022357021778583. [DOI] [PubMed] [Google Scholar]
- 80.Mullin JM, Valenzano MC, Whitby M, Lurie D, Schmidt JD, Jain V, et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. 2008;28:1317–25. doi: 10.1111/j.1365-2036.2008.03824.x. [DOI] [PubMed] [Google Scholar]
- 81.Gabello M, Valenzano MC, Zurbach EP, Mullin JM. Omeprazole induces gastric transmucosal permeability to the peptide bradykinin. World J Gastroenterol. 2010;16:1097–103. doi: 10.3748/wjg.v16.i9.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–23. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 83.Trikha A, Baillargeon JG, Kuo YF, Tan A, Pierson K, Sharma G, et al. Development of food allergies in patients with gastroesophageal reflux disease treated with gastric acid suppressive medications. Pediatr Allergy Immunol. 2013;24:582–8. doi: 10.1111/pai.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andersen AB, Erichsen R, Farkas DK, Mehnert F, Ehrenstein V, Sorensen HT. Prenatal exposure to acid-suppressive drugs and the risk of childhood asthma: a population-based Danish cohort study. Aliment Pharmacol Ther. 2012;35:1190–8. doi: 10.1111/j.1365-2036.2012.05073.x. [DOI] [PubMed] [Google Scholar]
- 85.Moawad FJ, Maydonovitch CL, Lake JM, Veerappan GR. PPIs may not predispose to eosinophilic esophagitis. Am J Gastroenterol. 2010;105:468–9. doi: 10.1038/ajg.2009.617. author reply 9. [DOI] [PubMed] [Google Scholar]
- 86.Orel R, Turk H. Re: Might the use of acid-suppressive medications predispose to the development of eosinophilic esophagitis? Am J Gastroenterol. 2010;105:468. doi: 10.1038/ajg.2009.603. author reply 9. [DOI] [PubMed] [Google Scholar]
- 87.Taye B, Enquselassie F, Tsegaye A, Medhin G, Davey G, Venn A. Is Helicobacter Pylori infection inversely associated with atopy? A systematic review and meta-analysis. Clin Exp Allergy. 2015;45:882–90. doi: 10.1111/cea.12404. [DOI] [PubMed] [Google Scholar]
- 88.Dellon ES, Peery AF, Shaheen NJ, Morgan DR, Hurrell JM, Lash RH, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011;141:1586–92. doi: 10.1053/j.gastro.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.von Arnim U, Wex T, Link A, Messerschmidt M, Venerito M, Miehlke S, et al. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43:825–30. doi: 10.1111/apt.13560. [DOI] [PubMed] [Google Scholar]
- 90.Furuta K, Adachi K, Aimi M, Ishimura N, Sato S, Ishihara S, et al. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr. 2013;53:60–2. doi: 10.3164/jcbn.13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elitsur Y, Alrazzak BA, Preston D, Demetieva Y. Does Helicobacter pylori protect against eosinophilic esophagitis in children? Helicobacter. 2014;19:367–71. doi: 10.1111/hel.12129. [DOI] [PubMed] [Google Scholar]
- 92.Žaja Franulović O, Lesar T, Busic N, Tešović G. Herpes simplex primo-infection in an immunocompetent host with eosinophilic esophagitis. Pediatrics International. 2013;55:e38–e41. doi: 10.1111/ped.12027. [DOI] [PubMed] [Google Scholar]
- 93.Squires KA, Cameron DJ, Oliver M, da Fonseca Junqueira JC. Herpes simplex and eosinophilic oesophagitis: the chicken or the egg? J Pediatr Gastroenterol Nutr. 2009;49:246–50. doi: 10.1097/MPG.0b013e31817b5b73. [DOI] [PubMed] [Google Scholar]
- 94.Lindberg GM, Van Eldik R, Saboorian MH. A case of herpes esophagitis after fluticasone propionate for eosinophilic esophagitis. Nat Clin Pract Gastroenterol Hepatol. 2008;5:527–30. doi: 10.1038/ncpgasthep1225. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann D, Criblez DH, Dellon ES, Bussmann C, Pfeifer D, Froh M, et al. Acute Herpes Simplex Viral Esophagitis Occurring in 5 Immunocompetent Individuals With Eosinophilic Esophagitis. ACG Case Rep J. 2016;3:165–8. doi: 10.14309/crj.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fritz J, Lerner D, Suchi M. Herpes Simplex Virus Esophagitis in Immunocompetent Children: A Harbinger of Eosinophilic Esophagitis? J Pediatr Gastroenterol Nutr. 2017 doi: 10.1097/MPG.0000000000001748. [DOI] [PubMed] [Google Scholar]
- 97.Burk CM, Beitia R, Lund PK, Dellon ES. High rate of galactose-alpha-1,3-galactose sensitization in both eosinophilic esophagitis and patients undergoing upper endoscopy. Dis Esophagus. 2016;29:558–62. doi: 10.1111/dote.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srivastava MDM. Pneumoniae Is a Potential Trigger for Eosinophilic Esophagitis. Journal of Allergy and Clinical Immunology. 131:AB177. [Google Scholar]
- 99.Lee YJ, Redd M, Bayman L, Frederickson N, Valestin J, Schey R. Comparison of clinical features in patients with eosinophilic esophagitis living in an urban and rural environment. Diseases of the Esophagus. 2015;28:19–24. doi: 10.1111/dote.12164. [DOI] [PubMed] [Google Scholar]
- 100.Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107:698–706. doi: 10.1038/ajg.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moawad FJ, Veerappan GR, Lake JM, Maydonovitch CL, Haymore BR, Kosisky SE, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31:509–15. doi: 10.1111/j.1365-2036.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 102.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41:451–3. doi: 10.1097/01.mcg.0000248019.16139.67. [DOI] [PubMed] [Google Scholar]
- 103.Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104:828–33. doi: 10.1038/ajg.2008.169. [DOI] [PubMed] [Google Scholar]
- 104.Iwanczak B, Janczyk W, Ryzko J, Banaszkiewicz A, Radzikowski A, Jarocka-Cyrta E, et al. Eosinophilic esophagitis in children: frequency, clinical manifestations, endoscopic findings, and seasonal distribution. Adv Med Sci. 2011;56:151–7. doi: 10.2478/v10039-011-0038-7. [DOI] [PubMed] [Google Scholar]
- 105.Sorser SA, Barawi M, Hagglund K, Almojaned M, Lyons H. Eosinophilic esophagitis in children and adolescents: epidemiology, clinical presentation and seasonal variation. J Gastroenterol. 2013;48:81–5. doi: 10.1007/s00535-012-0608-x. [DOI] [PubMed] [Google Scholar]
- 106.Elitsur Y, Aswani R, Lund V, Dementieva Y. Seasonal distribution and eosinophilic esophagitis: the experience in children living in rural communities. Journal of clinical gastroenterology. 2013;47:287–8. doi: 10.1097/MCG.0b013e31826df861. [DOI] [PubMed] [Google Scholar]
- 107.Elias MK, Kopacova J, Arora AS, Dierkhising RA, Enders FT, Katzka DA, et al. The diagnosis of esophageal eosinophilia is not increased in the summer months. Dysphagia. 2015;30:67–73. doi: 10.1007/s00455-014-9574-1. [DOI] [PubMed] [Google Scholar]
- 108.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2013;25:47–52. e5. doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 109.Fahey L, Robinson G, Weinberger K, Giambrone AE, Solomon AB. Correlation between Aeroallergen Levels and New Diagnosis of Eosinophilic Esophagitis in NYC. Journal of pediatric gastroenterology and nutrition. 2017;64:22–5. doi: 10.1097/MPG.0000000000001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jensen ET, Shah ND, Hoffman K, Sonnenberg A, Genta RM, Dellon ES. Seasonal variation in detection of oesophageal eosinophilia and eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;42:461–9. doi: 10.1111/apt.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shaheen SO, Newson RB, Henderson AJ, Headley JE, Stratton FD, Jones RW, et al. Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood. Clin Exp Allergy. 2005;35:18–25. doi: 10.1111/j.1365-2222.2005.02151.x. [DOI] [PubMed] [Google Scholar]
- 112.Sordillo JE, Scirica CV, Rifas-Shiman SL, Gillman MW, Bunyavanich S, Camargo CA, Jr, et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol. 2015;135:441–8. doi: 10.1016/j.jaci.2014.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Magnus MC, Karlstad O, Haberg SE, Nafstad P, Davey Smith G, Nystad W. Prenatal and infant paracetamol exposure and development of asthma: the Norwegian Mother and Child Cohort Study. Int J Epidemiol. 2016;45:512–22. doi: 10.1093/ije/dyv366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masclee GM, Coloma PM, Kuipers EJ, Sturkenboom MC. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol. 2015;110:749–59. doi: 10.1038/ajg.2015.119. [DOI] [PubMed] [Google Scholar]
- 115.Koutlas NT, Eluri S, Rusin S, Perjar I, Hollyfield J, Woosley JT, et al. Impact of smoking, alcohol consumption, and NSAID use on risk for and phenotypes of eosinophilic esophagitis. Dis Esophagus. 2017 doi: 10.1093/dote/dox111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jensen ETKJ, Martin LJ, Langefeld CD, Dellon ES, Rothenberg ME. Early-life environmental exposures interact with genetic susceptibility variants in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaci.2017.07.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu CJ, Soderhall C, Bustamante M, Baiz N, Gruzieva O, Gehring U, et al. DNA methylation in childhood asthma: an epigenome-wide meta-analysis. Lancet Respir Med. 2018 doi: 10.1016/S2213-2600(18)30052-3. [DOI] [PubMed] [Google Scholar]
- 118.DeVries A, Wlasiuk G, Miller SJ, Bosco A, Stern DA, Lohman IC, et al. Epigenome-wide analysis links SMAD3 methylation at birth to asthma in children of asthmatic mothers. J Allergy Clin Immunol. 2017;140:534–42. doi: 10.1016/j.jaci.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Michel S, Busato F, Genuneit J, Pekkanen J, Dalphin JC, Riedler J, et al. Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy. 2013;68:355–64. doi: 10.1111/all.12097. [DOI] [PubMed] [Google Scholar]
- 120.Vercelli D. Are we what our mothers made us? Lessons from epigenetics. J Allergy Clin Immunol. 2018;141:525–6. doi: 10.1016/j.jaci.2017.12.973. [DOI] [PubMed] [Google Scholar]
- 121.Jahreis S, Trump S, Bauer M, Bauer T, Thurmann L, Feltens R, et al. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J Allergy Clin Immunol. 2018;141:741–53. doi: 10.1016/j.jaci.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 122.Jensen ETLC, Dellon ES. Genome-wide epigenetic scan of EoE patients with and without treatment response to topical steroids. Gastroenterology. 2018 [Google Scholar]
- 123.Peterson K, Firszt R, Fang J, Wong J, Smith KR, Brady KA. Risk of Autoimmunity in EoE and Families: A Population-Based Cohort Study. Am J Gastroenterol. 2016;111:926–32. doi: 10.1038/ajg.2016.185. [DOI] [PubMed] [Google Scholar]
- 124.Jensen ETMC, Shaheen NJ, Kappelman MD, Dellon ES. High prevalence of co-existing autoimmune conditions among patients with eosinophilic esophagitis. Gastroenterology. 2013;144(Suppl 1):S-491. (Su1852) [Google Scholar]
- 125.Jensen ET, Eluri S, Lebwohl B, Genta RM, Dellon ES. Increased Risk of Esophageal Eosinophilia and Eosinophilic Esophagitis in Patients With Active Celiac Disease on Biopsy. Clin Gastroenterol Hepatol. 2015;13:1426–31. doi: 10.1016/j.cgh.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013;132:378–86. doi: 10.1016/j.jaci.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Judge DP, Dietz HC. Marfan’s syndrome. The Lancet. 2005;366:1965–76. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Byers PH, Belmont J, Black J, De Backer J, Frank M, Jeunemaitre X, et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175:40–7. doi: 10.1002/ajmg.c.31553. [DOI] [PubMed] [Google Scholar]
- 129.Amberbir A, Medhin G, Abegaz WE, Hanlon C, Robinson K, Fogarty A, et al. Exposure to Helicobacter pylori infection in early childhood and the risk of allergic disease and atopic sensitization: a longitudinal birth cohort study. Clin Exp Allergy. 2014;44:563–71. doi: 10.1111/cea.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schwarze J, Gelfand EW. Respiratory viral infections as promoters of allergic sensitization and asthma in animal models. Eur Respir J. 2002;19:341–9. doi: 10.1183/09031936.02.00254302. [DOI] [PubMed] [Google Scholar]
- 131.Holt PG. Infection and the development of allergic disease. Allergy. 2011;66(Suppl 95):13–5. doi: 10.1111/j.1398-9995.2011.02623.x. [DOI] [PubMed] [Google Scholar]