Abstract
The scientific basis and the clinical application of monoclonal antibody therapies that target specific immunologic pathways for eosinophilic gastrointestinal diseases (EGIDs) are areas of active interest. There is a growing recognition of a subset of patients with eosinophilic esophagitis, or EoE, whose disease does not respond well to topical steroids or elimination diets. In addition, long-term use of corticosteroids presents risks. Systemic therapy with a biologic agent offers potential advantages as a global approach that could limit the need for multiple, locally active medical therapies and allergen avoidance. The identification of novel biologic strategies is ongoing, and the recent validation of instruments and outcome measures to assess disease activity has proved essential in demonstrating efficacy. Studies using biologics that target IL-13 pathways in the treatment of EoE have demonstrated substantial promise.
Keywords: Eosinophilic esophagitis, gastroesophageal reflux disease, dysphagia, food allergy, esophageal stricture, esophagitis
Introduction
At present, our therapeutic options for eosinophilic esophagitis (EoE) include medications, elimination diets, and esophageal dilation. Medical therapeutics consist primarily of orally administered topical corticosteroids. Several randomized, controlled trials support the efficacy of topical corticosteroids, although the histologic response rates have varied, ranging from 40 to 90%1–6. To date, however, topical corticosteroids are not approved by the Food & Drug Administration (FDA) for the treatment of EoE. As a result, many clinicians are using preparations designed for asthma. A limited number of studies regarding long-term topical corticosteroid use have raised concerns regarding its effectiveness, particularly in the setting of attempts at dose reduction,7–9 and long-term adverse effects such as adrenal insufficiency10. For EGIDs that affect the stomach, small intestine, and colon, systemic corticosteroids are widely utilized with well-known adverse effects associated with prolonged administration11. Biologic therapies introduce novel approaches that target specific immune pathways and potentially address several unmet needs in the management of EoE and EGIDs. This review summarizes the therapeutic potential, scientific rationale, and available clinical trial data regarding past, present, and future biologic treatments.
Therapeutic Endpoints in EoE Clinical Trials
The interpretation of clinical trials of novel therapeutics in EGIDs relies upon the application of appropriate and validated endpoints, and the lack of an accepted set of clinical outcome metrics (COMs) for defining successful response to therapy impedes progress. Currently, focus lies on the co-primary endpoint of symptom assessment using patient reported outcome (PROs) instruments and histologic assessment of peak mucosal eosinophil density (eosinophils per high power field). The tools used to assess symptoms and histopathology, however, have varied considerably and have been largely unvalidated. Over the past several years, several PRO instruments have been designed and validated for evaluation of symptoms and QOL in both pediatric and adult EoE. For adults, validated instruments include the Daily Symptom Questionnaire (DSQ) and EoE Activity Index (EEsAI)12. For children, the Pediatric EoE Symptom Score (PEESS) has been validated but it has not yet been evaluated in terms of patients’ responsiveness to therapy13. Unfortunately, most of these validated tools were not available during the design of many clinical trials under current discussion.
While symptom assessment is a logical endpoint for trials in EoE, it is important to emphasize limitations to this measurement of outcome. Prolonged mastication, extended meal times, and avoidance of harder textured foods (e.g., meat, bread) can mitigate the intensity of dysphagia and lead to inaccurate assessment of disease activity. Another major concern lies in the relationship between symptoms and esophageal remodeling. Esophageal remodeling related to chronic inflammation manifests as esophageal strictures that are a major determinant of symptom outcomes of dysphagia and food impaction14. The ability of anti-inflammatory or immune therapies to reverse fibrostenosis is unproven in EoE, requiring such therapeutics to relieve dysphagia may overlook therapeutic benefits in preventing other disease consequences. The clinical observation that symptoms of dysphagia can be effectively ameliorated in over 90% of patients with esophageal dilation, without altering the underlying inflammatory response, supports this view15.
Randomized controlled trials have demonstrated that measuring EoE activity using esophageal mucosal eosinophil density offers an objective and quantifiable measure with a high degree of inter-observer agreement and with minimal placebo response Outcomes are commonly defined by a reduction in mucosal eosinophilia, but the method used to calculate eosinophil density has varied considerably. Furthermore, a variety of target thresholds have been used including endpoints of <15, < 10, < 6, and <5 eosinophils per high power field (eos/hpf) in some studies and percent reduction in eosinophil density in others. The recent development and validation of an EoE Histologic Severity Score (EoE-HSS) that incorporates histopathology beyond eosinophil density including basal cell hyperplasia, dilation of intercellular spaces and subepithelial fibrosis provides a more comprehensive and accurate characterization of mucosal inflammation in EoE for clinical trials16. While it is tempting to consider use of histology as the primary determinant of therapeutic efficacy, a marked dissociation between symptoms and pathology is well-recognized. This dissociation is likely explained by modification of eating behavior, subepithelial remodeling that is poorly assessed with standard biopsy technique, and a symptom-placebo response.
Endoscopic outcomes serve as primary determinants of therapeutic efficacy in GERD and inflammatory bowel disease, and increasing data supports their use as an objective endpoint in clinical trials of EoE. The EoE Endoscopic REFerence Scoring system, or EREFS, is a classification and grading system that has been validated and shows a high degree of accuracy in the diagnosis of EoE in children and adults13, 17, 18. Recent clinical trials of topical steroids optimized for esophageal delivery as well as phase 2 trials of biologic therapies have demonstrated responsiveness of EREFS in assessment of mucosal healing19–21.
Other investigations are actively evaluating biomarkers of EoE disease activity beyond mucosal healing and symptoms. mRNA expression provides a molecular fingerprint of key upregulated and downregulated genes in esophageal biopsies of EoE that is distinct from the signature identified in control subjects and patients with GERD22. The Eosinophil Diagnostic Panel (EDP) includes clusters of genes that depict TH2 inflammatory response, mast cell activation, and fibrosis pathways. Reversal of the EoE pattern has been demonstrated in the setting of randomized controlled trials using topical fluticasone in children and anti-IL-13 therapy in adults. The EDP offers potential for examining molecular pathways that may provide insights into EoE pathogenesis, inform a personalized approach to therapy, and improve diagnostic accuracy. For whole organ assessment of esophageal remodeling, the functional lumen imaging probe (FLIP) is a catheter-based technology performed during an endoscopic examination that applies impedance planimetry to measure of esophageal biomechanical properties in EoE23. Initial studies demonstrated reduction in esophageal mural distensibility that was associated with an increased risk of food impaction24, 25. Preliminary studies using FLIP have demonstrated a significant improvement in esophageal distensibility following administration of topical corticosteroids and anti-IL-4 receptor antibody therapy21, 26.
Targets without published trials
Siglec-8
While published clinical trials do not exist for Sialic acid-binding Ig-like lectin 8 (Siglec-8), its exclusive expression by eosinophils and mast cells makes it an interesting therapeutic target for EGIDs. Blockade of Siglec-F, the murine homolog in experimental murine eosinophilic disease, led to a reduction of eosinophils in the esophagus. This was associated with decreased angiogenesis, deposition of fibronectin, and basal zone hyperplasia, which are key aspects of EoE pathogenesis27. More importantly, engagement of Siglec-8 induces eosinophil cell death and decreases mediator release by mast cells28. Recent abstracts presented at the 2018 AAAAI meeting demonstrated that in a mouse model of eosinophilic gastritis, a novel antibody that targets Siglec-8 (AK002) resulted in selective depletion of tissue and blood eosinophils and a reduction in mast cells.29, 30. A clinical trial is further investigating the direct role of Siglec-8 in EGIDs.
TSLP
Two genomics studies initially identified TSLP single nucleotide polymorphisms in patients with EoE31, 32. More recent studies have confirmed this risk factor TSLP expression is increased in EoE patients as compared to controls in the differentiated supra-basal layer of the epithelium. Noti et al. examined TSLP function in murine experimental EoE associated with food impaction, and their data suggest that TSLP recruits basophils with downstream effects on IL-4 and ultimately eosinophils33. TSLP enhances the migration of eosinophils, likely in combination with IL-33, which enhances IL-5 and IL-13 production34. Importantly, blockade of TSLP pathways abated the eosinophilic inflammation and food impaction in the murine model. Gauvreau et al. reported on a double-blind placebo-controlled study of Tezepelumab (AMG 157), a human monoclonal IgG2 antibody against TSLP, for use in the treatment of allergic asthma35. The authors observed a reduction in early and late asthmatic responses, although the potential utility for EGIDs is unclear due to limited data regarding the role of TSLP.
Integrins
While they have not been well-studied in EGIDs, integrins have offered a therapeutic target in inflammatory diseases such as inflammatory bowel disease and rheumatoid arthritis. Erie et al described the alpha4beta7 integrin on leukocytes in 1994.36 This integrin plays a role in eosinophil recruitment to the intestine36, 37 and in intestinal mast cell hyperplasia38, 39. Forbes et al. reported that the beta2 integrin factors in colonic eosinophil recruitment40, suggesting that a role in lower EGIDs may also be a consideration. Cadherin 26 was recently found to be increased in pathologic allergic inflammation including that of EoE, and data suggested it enhances cellular adhesion to alpha4beta7 integrin and binds directly to alphaE and alpha441. This modulated CD4 T-cell activation, which is critical in EGIDs, underscores potential for integrin targeting. A recent retrospective series described improved histopathology in 5 patients with EGID following therapy with vedolizumab, but exposure to corticosteroids may have affected the responses42. The results of further studies are nonetheless awaited.
Eotaxins
Eotaxins, produced in large part by epithelial cells, play a crucial role in the chemotaxis of eosinophils to tissue. Activated eosinophils, mast cells, and fibroblasts are also capable of producing eotaxins43, although the relative contribution of each cell type is unclear. Eotaxins can be modulated by mast cell proteases44, and their production depends on STAT6 in response to IL-4 and IL-1345. Of the eotaxins (1–3), CCL26/eotaxin-3 is among those that are most highly expressed in the EoE transcriptome46, and glucocorticoids downregulate it.47, 48. Mice that are deficient in the eotaxin receptor have been observed to be protected from experimental EoE46. Interestingly, omeprazole blocks STAT6 binding to the promotor of eotaxin-3 in epithelial cells49, 50.
TGF-β1
TGF-β1 has long been known to hold a critical profibrotic role in the pathogenesis of EoE. Muir et al. examined fibroblasts that were treated with TGF-β1 and observed increased markers of fibrosis51. Notably, the stiffness of the matrix affected response to TGF-β1, suggesting that increased rigidity with disease chronicity may exacerbate fibrosis development. Aceves et al. identified TGF-β1+ mast cells in the smooth muscle layer52. Notably here, TGF-β1 enhanced smooth muscle contraction, likely via phospholamban53, which supported a contribution to disease symptoms. Blockade of TGF-β1 signaling in human esophageal fibroblasts and muscle cells led to reduced fibronectin and collagen.54 TGF-β1 is also active in the epithelial layer of the esophagus. Nguyen et al. recently reported that TGF-β1 alters epithelial barrier function via claudin-755, and Rawson et al. found a role in PAI-1 signaling56. Together, the data suggest that TGF-β1 may prove a useful target in patients with persistent symptoms that are associated with fibrotic disease. A multicenter, proof-of-concept study is currently investigating the effectiveness of losartan, an anti-hypertensive agent that has been demonstrated to inhibit the effects of TGF-β in experimental models57.
Targets with published trials
IgE
Classic TH2 pathology such as that underlying allergic asthma involves production of antigen-specific IgE, which binds to mast cells and basophils and degranulates upon cross-linking from antigen binding58. IgE holds a clearly defined role in immediate hypersensitivity reactions that yield acute or sub-acute respiratory, dermatologic, and intestinal symptoms and can be life-threatening, but its function in EoE or EGIDs is less clear. Numerous studies have examined the presence of food-specific IgE, which is not universal among those with EoE and often does not correlate with food triggers59. For many patients, elevated IgE represents allergic sensitization that is associated with immediate hypersensitivity responses60, 61. Pelz et al. found that food-specific IgE levels were increased in EoE patients with a clinical history that was consistent with immediate hypersensitivity as compared to EoE patients without such symptoms60. Notably, EoE dietary triggers were low as compared to triggers of immediate hypersensitivity, suggesting that systemic IgE responses are not a driving factor of eosinophilia. Not surprisingly, several groups have found no change in phenotype of murine experimental EoE when either B-cells or the IgE heavy chain was deficient33, 62. Vicario et al. did find evidence of esophageal plasma cells that produce IgE, and local IgE effects may contribute to EoE pathogenesis63. Patients with EoE are also commonly sensitized to aero-allergens. This may trigger esophageal inflammation directly and drive food sensitivity through cross-sensitization64, 65.
There is limited data to support the clinical use of anti-IgE therapy for EGIDs. A single-center, randomized, double-blind, placebo-controlled trial in which an anti-IgE antibody (omalizumab) was delivered for 16 weeks failed to demonstrate improvement in either symptoms or esophageal eosinophilia in 30 adults with EoE66. The authors took previous data demonstrating the poor sensitivity and specificity of IgE-based testing in predicting specific food triggers to EoE into account and concluded that EoE is not primarily an IgE-induced allergic response. A smaller proof-of-concept study examined the effectiveness of omalizumab treatment of 9 subjects with EGID67. While eosinophil counts decreased in both the stomach and duodenum (69% and 59% respectively), the differences were not significant. Symptoms, basophil expression and free serum IgE levels significantly improved, however, raising the possibility that such treatment may be more effective for patients with EGID than for those with EoE.
CRTH2
Chemoattractant receptor-homologous molecule on TH2 cells (CRTH2) is a receptor for prostaglandin D2 (PGD2)., PGD2 is produced by mast cells, and elevated levels have been observed in the plasma of EoE patients as compared to control subjects.68 A variety of inflammatory cells including eosinophils, basophils, type 2 innate lymphoid (ILC2) and T-helper type 2 (Th2) cells express CRTH269. It has a well-described role in cell chemotaxis and activation28, and it has been appreciated to promote inflammatory severity in atopic animal models70, 71.
A RDBPCT was conducted in 26 adults with EoE using OC000459, a selective, orally administered CRTH2 antagonist72. At the conclusion of an 8-week treatment period, the study achieved its primary endpoint of reduced esophageal eosinophil counts. OC000459 treatment significantly reduced mean eosinophil density (114.83 to 73.26 eos/hpf; p=0.0256), an effect that did not similarly transpire with placebo. The histologic improvement, however, was modest compared to other clinical trials in EoE. Furthermore, the degree of symptom improvement was similar between active drug and placebo, and endoscopic features did not improve.
IL-5
The cytokine IL-5 has a recognized central role in chronic TH2 inflammation that occurs in EGIDs. Desreumaux et al. initially appreciated such a TH2 response in lower EGIDs such as EGE in 199673, and Straumann et al later described its role in EoE.74 Studies since have confirmed these findings75–79. IL-5 is predominant in the GI tract amongst patients with EGIDs as compared to those with immediate hypersensitivity80. It is a well-characterized element of the EoE tissue cytokine profile that is reduced with treatment such as corticosteroids.47, 48 Studies utilizing transgenic over-expression and deficiency of IL-5 in mice have identified effects on eosinophil progenitor maturation, priming for chemokine stimulation to eotaxins CCL11, CCL24, and CCL26, and tracking to tissue81–84. IL-5 may also have role in remodeling when it is chronically overexpressed, as CD2-IL5 mice have increased collagen accumulation in the lamina propria and extended stromal papillae.82 Sources of IL-5 include TH2 cells, innate lymphoid cells (ILC2), mast cells, and eosinophils. ILC2 cells have been suggested to interact with mast cells85, and T-cells express IL-5 along with the activation marker CD154 in response to EoE food triggers such as milk75. IL-5 thus functions to increase eosinophil cell density in the tissue, making it an important potential target in the treatment of EGIDs.
Biologic therapies targeting interleukin-5 (IL-5) have demonstrated efficacy in hypereosinophilic syndromes and eosinophilic asthma. The use of mepolizumab, a humanized, anti-IL-5 monoclonal immunoglobulin G1 antibody, in EoE treatment was first reported in an open label, phase 1–2 study of 4 adults86. The study demonstrated significant reductions in peripheral and esophageal eosinophilia and improvement in clinical outcomes. A randomized, placebo-controlled, double blind trial of mepolizumab in 11 adults with EoE for 8 to 16 weeks followed.87 The authors reported histologic efficacy, with a 54% reduction in mean esophageal eosinophil counts, but no patient achieved the primary endpoint of reduction of peak eosinophil counts to less than 5 eos/hpf. Moreover, no patient achieved the threshold of < 15 eos/hpf, and findings did not demonstrate symptom improvement.
An international, multicenter, double-blind randomized trial investigated the effect ofmepolizumab therapy in pediatric EoE.88 Patients received one of three different dosing arms of mepolizumab every 4 weeks, for a total of three infusions with assessment. While a placebo arm was not used, the lowest dose was chosen to be minimally effective and to serve as a comparator. The primary endpoint was the proportion of patients with peak eosinophil counts of < 5 eos/hpf at week 12. The symptom endpoint was based on a non-validated daily PRO. While peak eosinophil counts did significantly decrease from 122.5 to 40.2 eos/hpf, the primary endpoint was achieved in only 8.8% of patients. Furthermore, significant improvement in symptoms was not detected across dosing arms.
In one of the largest randomized, placebo-controlled trials of EoE treatment, 226 children with the condition received reslizumab, a humanized monoclonal antibody to IL-5.89 The study compared three different dosing arms that were delivered every 4 weeks to placebo. The co-primary endpoint consisted of a reduction in eosinophil counts and physician global assessment at week 15. Significant reductions in peak eosinophil counts were demonstrated, ranging from 59% to 67% compared with 24% for placebo. However, fewer than 25% of subjects achieved the threshold of < 15 eos/hpf and no change was observed in physician global assessment of those who had received the drug as compared to placebo.
The current literature indicates that therapeutic agents targeting IL-5 have demonstrated statistically significant although relatively modest reductions in esophageal eosinophilic inflammation. These agents have been well tolerated in all four short-term induction studies. The inability to demonstrate improvement in symptom outcomes may reflect the heterogeneous symptoms in pediatric EoE, lack of validated PRO instruments in pediatric EoE, issues with a higher than anticipated placebo response rate, limited reversibility of fibrotic remodeling, and involvement of cell types other than eosinophils in disease pathogenesis. The limitations of mepolizumab and reslizumab to achieve < 15 eosinophils per high-powered field in the majority of patients may be due to insufficient ability to deplete existing tissue eosinophils; additionally, longer duration of therapy may be needed. Benralizumab, which binds to IL-5 Receptor alpha on eosinophils, is afucosylated and results in more marked depletion of eosinophils by enhanced antibody-dependent cell-mediated toxicity.90 It was recently approved for add-on maintenance therapy for severe eosinophilic asthma. Preliminary data demonstrated clinical and histologic efficacy in a small series of patients with EGID and hypereosinophilic syndrome91. We await future studies regarding the clinical efficacy of benralizumab in the treatment of EGIDs.
IL-13
IL-13 has many functions as an activator of the esophageal TH2 inflammatory response, and it is produced by both TH2 cells and activated eosinophils74, 76, 92. The IL-13 signature of the EoE transcriptome is largely replicated in IL-13-treated epithelial cells, and it reverses with corticosteroid therapy.93 IL-13-mediated epithelial stimulation induces eotaxin-1, eotaxin-2, and eotaxin-3 expression via STAT6, thus contributing to eosinophil recruitment94. This essential role of IL-13 in eosinophil recruitment, via eotaxin production, has been confirmed in experimental murine EoE models using IL-13 deficient mice. Additionally, intra-tracheal IL-13 can induce esophageal eosinophilia in mice95, and stimulation of fibroblasts facilitates eosinophil recruitment via periostin, which enhances adhesion to fibronectin96. Beyond cell recruitment, IL-13 has a role in barrier function. It reduces epithelial differentiation including down regulation of desmosomal cadherin desmoglein-1 (DSG1),97 and increases expression of the intracellular calcium-dependent protease calpain-14 (CAPN14)98, leucine-rich repeat-containing protein 31 (LRRC31)99, and the cytoskeletal protein synaptopodin (SYNPO)100. A role in esophageal remodeling has also been described via enhancement of collagen deposition101, along with epithelial autophagy in a ROS-dependent manner102, and induction of IgE expression103. Thus IL-13 has a pleotropic role in eosinophil recruitment, barrier dysfunction, and esophageal remodeling, and it may offer an appreciable target for biologic therapy.
Three clinical trials have evaluated the efficacy of anti-IL-13 antibody therapy. Two trials utilized monoclonal antibodies targeting IL-13 while the third targeted both IL-13 and IL-4 signaling. QAX576, a monoclonal IgG1 antibody to IL-13, was reported in a randomized double blind, placebo-controlled trial of 23 adults with EoE104. The proportion of patients who achieved the study’s primary endpoint of a greater than 75% reduction in peak esophageal eosinophil counts at week 12 with QAX576 treatment was not significantly different from those who had received placebo. It should be noted that the study did not use validated endpoints, as it was one of the earliest trials of a biologic agent in treating EoE. In support of a key role for IL-13, the mean eosinophil counts significantly decreased by 60% with QAX576 treatment as compared with a 23% decrease with placebo, and expression of a series of relevant esophageal gene transcripts improved.
More recently, a randomized, double-blind, placebo controlled, 16-week trial in 99 adults with EoE reported the efficacy of RPC4046, a humanized monoclonal IgG1kappa anti-IL-13 antibody.105 The primary endpoint of reduction of mean esophageal eosinophil counts was over 90 eos/hpf with active drug as compared to 4 eos/hpf with placebo (p≤0·0001). The study noted significant improvement in endoscopically identified esophageal features of EoE and a trend for improvement in symptoms of dysphagia using validated instruments (EREFS, EEsAI). Of note, greater symptom improvement was found in patients whose disease was identified as steroid-refractory.
IL-4 receptor
IL-4 is a well-described TH2 cytokine that has been observed at increased levels in EoE patients.106 It is an allergic disease genetic risk locus107, although its exact role in EGIDs is not yet clear. In allergic disorders, IL-4 coaxes naïve T-cells to become TH2 cells and facilitate B-cell class switching to IgE. A variety of cells produce it, including TH2 cells, activated mast cells, and eosinophils. IL-4 stimulation of the epithelium leads to production of eotaxin-3 via STAT6, thus contributing to eosinophil recruitment. The mechanism is similar to that of IL-13 and it is blocked by omeprazole94. This may explain the anti-inflammatory effect of PPIs in some patients. As IL-4 shares a common heterodimeric receptor with IL-13, therapy that targets IL-4 signaling through IL4Ralpha will affect both IL-4 and IL-13 pathways and may prove efficacious.
Dupilumab, a fully human, anti-IL-4 receptor alpha monoclonal antibody that inhibits signaling of IL-4 and IL-13, was recently approved for the treatment of adults with moderate to severe atopic dermatitis. The results of a phase 2, multicenter, double-blind, randomized, placebo-controlled trial in adults with EoE were recently reported21. The study met the primary endpoint of significant improvement in symptoms of dysphagia as well as secondary endpoints regarding esophageal eosinophil counts, endoscopic features (EREFS), and comprehensive histologic scoring (EoE-HSS). The authors reported that 82.6% of patients achieved the threshold of < 15 eos/hpf at week 12 with dupilumab as compared to none with placebo.
Collectively, the recent trials of biologics directed at IL-13 and IL-4 have demonstrated significant reductions in esophageal eosinophilia and improvement in symptoms of dysphagia. Although direct comparisons are not possible due to differences in methodology, the reported histologic improvements appear more substantial than those that have been reported in previous trials of biologic agents with IL-5-directed therapy. The use of validated symptom, endoscopic, and histologic endpoints substantiates IL-4- and IL-13-directed therapy efficacy. Preliminary results suggesting effectiveness in steroid refractory patients are intriguing, as such patients may prove to be ideal candidates for this type of treatment.
Conclusions
The scientific basis and clinical application of therapies targeting specific immunologic pathways in EoE and EGIDs are areas of active interest and growing relevance. An important subset of patients with EoE show limited response to topical steroids and elimination diet therapies, bringing an important unmet therapeutic need to light. Furthermore, the data are inconclusive regarding the long-term effectiveness and safety of topical steroids and diet therapies. For EGIDs, the use of systemic steroids present unacceptable long-term risks. Dietary therapy for both EoE and EGIDs adversely affect quality of life by necessitating avoidance of many commonly ingested table foods. Several of the biologic agents this review discusses target mechanisms common to multiple manifestations of atopy. Since patients with EoE and EGIDs typically manifest “extra-esophageal” and “extra-intestinal” forms of allergic disease, systemic therapy with a biologic offers potential advantages as a global treatment approach that could limit the need for multiple, locally active medical therapies and allergen avoidance. Of the agents that have been evaluated, biologics targeting IL-4 and IL-13 have demonstrated the most robust treatment benefits using validated outcome assessments. Ongoing clinical trials utilizing clinical outcome metrics and investigative work will hopefully lead to novel, effective, and safe biologic treatment strategies for gastrointestinal eosinophilic disorders that are increasingly recognized worldwide.
Supplementary Material
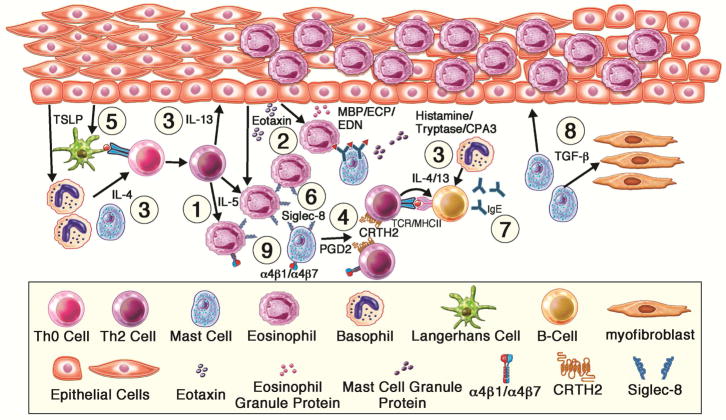
Figure.
Therapeutic targets for current and future biologics in eosinophilic esophagitis. Basophils and antigen-presenting cells mediate dietary antigen presentation to naïve T-cells (Th0), which through TSLP and IL-4 drives T-helper cell Type 2 (Th2) cell expansion. Th2 are recruited to the esophagus via integrins and prostaglandins and drive B-cell production of immunoglobulins, along with mast cell hyperplasia. Th2 cells secrete IL-5 which further enhances eosinophil recruitment via release of eotaxins and eosinophil survival. Th2 cells secrete IL-13, which dysregulates the epithelium to recruit Th2 inflammatory cells and promotes remodeling. Eosinophils and mast cells are effector cells that are activated to secrete proteases, cytokines and histamine which drive mucosal inflammatory changes and symptoms. TGF-β1 has a key role in fibrosis. Specific targets discussed include: 1) IL-5R, 2) Eotaxins, 3) IL4R/IL13, 4) CRTH2, 5) TSLP, 6) Siglec-8, 7) IgE, 8) TGF-β1, 9) Integrin α4β1/7
Table 1.
Targets of therapy, role in pathogenesis, and associated pharmaceuticals
| Target | Role in pathogenesis | Clinical Trials in EGIDs | Clinical Trials (Atopic Disease) | Clinical Trials (non-Atopic Disease) |
|---|---|---|---|---|
| IL-5/IL5-R | Activation and Recruitment of Eosinophils | Mepolizumab, Reslizumab, Benralizumab | Mepolizumab, Reslizumab, Benralizumab | Mepolizumab |
| IL-13 | Promote eosinophil recruitments, barrier dysfunction, remodeling | QAX576, RPC4046 | Tralokinumab | Tralokinumab |
| IL-4RA | Maintenance of Th2 inflammatory process | Dupilumab | Dupilumab, AMG 317 | |
| CRTH2 | Recruitment of T-cells | OC000459 | AZD1981, OC459, QAV680 | |
| Siglec-8 |
induction of eosinophil cell death inhibition of mast cell activation |
AK002 | AK001, AK002 | |
| TSLP | Recruitment of basophils, stimulation of IL-4 to promote Th2 | Tezepelumab (AMG 157) | ||
| Integrin alpha4beta7 | Recruitment of T-cells, eosinophils and mast cells | Vedolizumab | ||
| Eotaxin-1, -2, -3 | Recruitment of eosinophils | GW766994 | ||
| TGF-β1 |
Enhance collagen production to promote fibrosis Promote smooth muscle contraction Worsen barrier integrity |
Losartan | Fresolimumab, Losartan |
Acknowledgments
Financial support:
The authors acknowledge grant support from the NIH Consortium of Eosinophilic Gastrointestinal disease Researchers (CEGIR, U54 AI117804) which is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED CURED and EFC. Dr. Wechsler receives grant support from K08DK097721.
Abbreviations
- EoE
Eosinophilic Esophagitis
- eos/hpf
eosinophils / high-power field
- GERD
Gastroesophageal reflux disease
- PPI
Proton pump inhibitor
- mAb
monoclonal antibody
- RDBPCT
Randomized, double blind, placebo-controlled trial
Footnotes
Specific author contributions: IH and JW contributed to manuscript drafting and revision
Conflict of Interest Disclosure: Dr. Wechsler has nothing to disclose. Dr. Hirano reports consulting fees and research funding from Regeneron, Receptos, Shire, Adare, Allakos outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Moawad FJ, Veerappan GR, Dias JA, Baker TP, Maydonovitch CL, Wong RK. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol. 2013;108:366–72. doi: 10.1038/ajg.2012.443. [DOI] [PubMed] [Google Scholar]
- 3.Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci. 2010;55:1313–9. doi: 10.1007/s10620-009-0859-4. [DOI] [PubMed] [Google Scholar]
- 4.Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. A prospective open clinical trial of a proton pump inhibitor, elimination diet and/or budesonide for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43:985–93. doi: 10.1111/apt.13576. [DOI] [PubMed] [Google Scholar]
- 5.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Teitelbaum JE, Fox VL, Twarog FJ, Nurko S, Antonioli D, Gleich G, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216–25. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 7.Andreae DA, Hanna MG, Magid MS, Malerba S, Andreae MH, Bagiella E, et al. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children With Eosinophilic Esophagitis. Am J Gastroenterol. 2016;111:1187–97. doi: 10.1038/ajg.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9 e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Greuter T, Bussmann C, Safroneeva E, Schoepfer AM, Biedermann L, Vavricka SR, et al. Long-Term Treatment of Eosinophilic Esophagitis With Swallowed Topical Corticosteroids: Development and Evaluation of a Therapeutic Concept. Am J Gastroenterol. 2017;112:1527–1535. doi: 10.1038/ajg.2017.202. [DOI] [PubMed] [Google Scholar]
- 10.Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, Putnam PE, Backeljauw PF. Adrenal Insufficiency after Chronic Swallowed Glucocorticoid Therapy for Eosinophilic Esophagitis. J Pediatr. 2016;170:240–5. doi: 10.1016/j.jpeds.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Reed C, Woosley JT, Dellon ES. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis. 2015;47:197–201. doi: 10.1016/j.dld.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safroneeva E, Straumann A, Coslovsky M, Zwahlen M, Kuehni CE, Panczak R, et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults With Eosinophilic Esophagitis. Gastroenterology. 2016;150:581–590 e4. doi: 10.1053/j.gastro.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano I, Spechler S, Furuta G, Dellon ES. White Paper AGA: Drug Development for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2017;15:1173–1183. doi: 10.1016/j.cgh.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:297–316. doi: 10.1016/j.gtc.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon HU, Straumann A, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–70. doi: 10.1038/ajg.2009.657. [DOI] [PubMed] [Google Scholar]
- 16.Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2017;30:1–8. doi: 10.1111/dote.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–95. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler JB, Bolton S, Amsden K, Wershil BK, Hirano I, Kagalwalla AF. Eosinophilic Esophagitis Reference Score Accurately Identifies Disease Activity and Treatment Effects in Children. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I, et al. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology. 2017;152:776–786 e5. doi: 10.1053/j.gastro.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta SK, Schoepfer A, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of a Novel Recombinant, Humanized, Anti-Interleukin-13 Monoclonal Antibody (RPC4046) in Patients with Active Eosinophilic Esophagitis: Results of the HEROES Study. United European Gastroenterology Week. 2016 [Google Scholar]
- 21.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson KA, Chehade M, et al. Dupilumab Efficacy and Safety in Adult Patients With Active Eosinophilic Esophagitis: A Randomized Double-Blind Placebo-Controlled Phase 2 Trial American College of Gastroenterology National Meeting. 2017 [Google Scholar]
- 22.Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology. 2013;145:1289–99. doi: 10.1053/j.gastro.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:325–334. doi: 10.1016/j.cgh.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicodeme F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101–1107 e1. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson DA, Hirano I, Zalewski A, Gonsalves N, Lin Z, Pandolfino JE. Improvement in Esophageal Distensibility in Response to Medical and Diet Therapy in Eosinophilic Esophagitis. Clin Transl Gastroenterol. 2017;8:e119. doi: 10.1038/ctg.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubinstein E, Cho JY, Rosenthal P, Chao J, Miller M, Pham A, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53:409–16. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radonjic-Hoesli S, Valent P, Klion AD, Wechsler ME, Simon HU. Novel targeted therapies for eosinophil-associated diseases and allergy. Annu Rev Pharmacol Toxicol. 2015;55:633–56. doi: 10.1146/annurev-pharmtox-010814-124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youngblood BB, Emily C, Leung John, Bebbington Christopher, Nenad Tomasevic. Anti-Siglec-8 Antibody Reduced Eosinophil and Mast Cell Infiltration In A Mouse Model of Eosinophilic Gastritis: A Novel Therapeutic Approach For Eosinophilic Gastrointestinal Diseases. J Allergy Clin Immunol. 2018:141. [Google Scholar]
- 30.Youngblood BB, Emily C, Leung John, Bebbington Christopher, Nenad Tomasevic. Ak002, A First-In-Class, Humanized, Non-Fucosylated IGG1 Siglec-8 Antibody Selectively Depletes Blood and Tissue Eosinophils. J Allergy Clin Immunol. 2018:141. [Google Scholar]
- 31.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–91. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:160–5 e3. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–13. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SG, Gugilla A, Mukherjee M, Merim K, Irshad A, Tang W, et al. Thymic stromal lymphopoietin and IL-33 modulate migration of hematopoietic progenitor cells in patients with allergic asthma. J Allergy Clin Immunol. 2015;135:1594–602. doi: 10.1016/j.jaci.2014.12.1918. [DOI] [PubMed] [Google Scholar]
- 35.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 36.Brandt EB, Zimmermann N, Muntel EE, Yamada Y, Pope SM, Mishra A, et al. The alpha4bbeta7-integrin is dynamically expressed on murine eosinophils and involved in eosinophil trafficking to the intestine. Clin Exp Allergy. 2006;36:543–53. doi: 10.1111/j.1365-2222.2006.02456.x. [DOI] [PubMed] [Google Scholar]
- 37.Oyoshi MK, Elkhal A, Scott JE, Wurbel MA, Hornick JL, Campbell JJ, et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest. 2011;121:2210–20. doi: 10.1172/JCI43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, et al. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J Exp Med. 2001;194:1243–52. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Issekutz TB, Palecanda A, Kadela-Stolarz U, Marshall JS. Blockade of either alpha-4 or beta-7 integrins selectively inhibits intestinal mast cell hyperplasia and worm expulsion in response to Nippostrongylus brasiliensis infection. Eur J Immunol. 2001;31:860–8. doi: 10.1002/1521-4141(200103)31:3<860::aid-immu860>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Forbes E, Hulett M, Ahrens R, Wagner N, Smart V, Matthaei KI, et al. ICAM-1-dependent pathways regulate colonic eosinophilic inflammation. J Leukoc Biol. 2006;80:330–41. doi: 10.1189/jlb.1105643. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell JM, Collins MH, Kemme KA, Sherrill JD, Wen T, Rochman M, et al. Cadherin 26 is an alpha integrin-binding epithelial receptor regulated during allergic inflammation. Mucosal Immunol. 2017;10:1190–1201. doi: 10.1038/mi.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HP, Reed CC, Herfarth HH, Dellon ES. Vedolizumab Treatment May Reduce Steroid Burden and Improve Histology in Patients With Eosinophilic Gastroenteritis. Clin Gastroenterol Hepatol. 2018 doi: 10.1016/j.cgh.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wechsler JB, Bryce PJ. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:281–96. doi: 10.1016/j.gtc.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gela A, Kasetty G, Jovic S, Ekoff M, Nilsson G, Morgelin M, et al. Eotaxin-3 (CCL26) exerts innate host defense activities that are modulated by mast cell proteases. Allergy. 2015;70:161–70. doi: 10.1111/all.12542. [DOI] [PubMed] [Google Scholar]
- 45.Cheng E, Zhang X, Wilson KS, Wang DH, Park JY, Huo X, et al. JAK-STAT6 Pathway Inhibitors Block Eotaxin-3 Secretion by Epithelial Cells and Fibroblasts from Esophageal Eosinophilia Patients: Promising Agents to Improve Inflammation and Prevent Fibrosis in EoE. PLoS One. 2016;11:e0157376. doi: 10.1371/journal.pone.0157376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucendo AJ, De Rezende L, Comas C, Caballero T, Bellon T. Treatment with topical steroids downregulates IL-5, eotaxin-1/CCL11, and eotaxin-3/CCL26 gene expression in eosinophilic esophagitis. Am J Gastroenterol. 2008;103:2184–93. doi: 10.1111/j.1572-0241.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- 48.van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, de Jonge WJ, et al. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol. 2015;110:1289–97. doi: 10.1038/ajg.2015.247. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. doi: 10.1371/journal.pone.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park JY, Zhang X, Nguyen N, Souza RF, Spechler SJ, Cheng E. Proton pump inhibitors decrease eotaxin-3 expression in the proximal esophagus of children with esophageal eosinophilia. PLoS One. 2014;9:e101391. doi: 10.1371/journal.pone.0101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muir AB, Dods K, Henry SJ, Benitez AJ, Lee D, Whelan KA, et al. Eosinophilic Esophagitis-Associated Chemical and Mechanical Microenvironment Shapes Esophageal Fibroblast Behavior. J Pediatr Gastroenterol Nutr. 2016;63:200–9. doi: 10.1097/MPG.0000000000001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–204 e4. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 53.Beppu LY, Anilkumar AA, Newbury RO, Dohil R, Broide DH, Aceves SS. TGF-beta1-induced phospholamban expression alters esophageal smooth muscle cell contraction in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134:1100–1107 e4. doi: 10.1016/j.jaci.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rieder F, Nonevski I, Ma J, Ouyang Z, West G, Protheroe C, et al. T-helper 2 cytokines, transforming growth factor beta1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology. 2014;146:1266–77. e1–9. doi: 10.1053/j.gastro.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen N, Fernando SD, Biette KA, Hammer JA, Capocelli KE, Kitzenberg DA, et al. TGF-beta1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rawson R, Yang T, Newbury RO, Aquino M, Doshi A, Bell B, et al. TGF-beta1-induced PAI-1 contributes to a profibrotic network in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2016;138:791–800 e4. doi: 10.1016/j.jaci.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wengrower D, Zanninelli G, Latella G, Necozione S, Metanes I, Israeli E, et al. Losartan reduces trinitrobenzene sulphonic acid-induced colorectal fibrosis in rats. Can J Gastroenterol. 2012;26:33–9. doi: 10.1155/2012/628268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fireman P. Understanding asthma pathophysiology. Allergy Asthma Proc. 2003;24:79–83. [PubMed] [Google Scholar]
- 59.Kagalwalla AF, Wechsler JB, Amsden K, Schwartz S, Makhija M, Olive A, et al. Efficacy of a 4-Food Elimination Diet for Children With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2017;15:1698–1707 e7. doi: 10.1016/j.cgh.2017.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelz BJ, Wechsler JB, Amsden K, Johnson K, Singh AM, Wershil BK, et al. IgE-associated food allergy alters the presentation of paediatric eosinophilic esophagitis. Clin Exp Allergy. 2016;46:1431–1440. doi: 10.1111/cea.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract. 2017;5:369–375. doi: 10.1016/j.jaip.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81:916–24. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 63.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guajardo JR, Zegarra-Bustamante MA, Brooks EG. Does Aeroallergen Sensitization Cause or Contribute to Eosinophilic Esophagitis? Clin Rev Allergy Immunol. 2018 doi: 10.1007/s12016-018-8671-6. [DOI] [PubMed] [Google Scholar]
- 65.Olson AA, Evans MD, Johansson MW, Kim CH, Manthei DM, Gaumnitz EA, et al. Role of food and aeroallergen sensitization in eosinophilic esophagitis in adults. Ann Allergy Asthma Immunol. 2016;117:387–393 e2. doi: 10.1016/j.anai.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–9. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 67.Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120:594–601. doi: 10.1016/j.jaci.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnsson M, Bove M, Bergquist H, Olsson M, Fornwall S, Hassel K, et al. Distinctive blood eosinophilic phenotypes and cytokine patterns in eosinophilic esophagitis, inflammatory bowel disease and airway allergy. J Innate Immun. 2011;3:594–604. doi: 10.1159/000331326. [DOI] [PubMed] [Google Scholar]
- 69.Pettipher R, Vinall SL, Xue L, Speight G, Townsend ER, Gazi L, et al. Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. J Pharmacol Exp Ther. 2012;340:473–82. doi: 10.1124/jpet.111.187203. [DOI] [PubMed] [Google Scholar]
- 70.Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–9. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- 71.Shiraishi Y, Asano K, Niimi K, Fukunaga K, Wakaki M, Kagyo J, et al. Cyclooxygenase-2/prostaglandin D2/CRTH2 pathway mediates double-stranded RNA-induced enhancement of allergic airway inflammation. J Immunol. 2008;180:541–9. doi: 10.4049/jimmunol.180.1.541. [DOI] [PubMed] [Google Scholar]
- 72.Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375–85. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 73.Desreumaux P, Bloget F, Seguy D, Capron M, Cortot A, Colombel JF, et al. Interleukin 3, granulocyte-macrophage colony-stimulating factor, and interleukin 5 in eosinophilic gastroenteritis. Gastroenterology. 1996;110:768–74. doi: 10.1053/gast.1996.v110.pm8608886. [DOI] [PubMed] [Google Scholar]
- 74.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–61. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 75.Cianferoni A, Ruffner MA, Guzek R, Guan S, Brown-Whitehorn T, Muir A, et al. Elevated expression of activated TH2 cells and milk-specific TH2 cells in milk-induced eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2017 doi: 10.1016/j.anai.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Straumann A, Kristl J, Conus S, Vassina E, Spichtin HP, Beglinger C, et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11:720–6. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 77.Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:22–6. doi: 10.1097/01.mpg.0000188740.38757.d2. [DOI] [PubMed] [Google Scholar]
- 78.Bullock JZ, Villanueva JM, Blanchard C, Filipovich AH, Putnam PE, Collins MH, et al. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- 79.Caldwell JM, Collins MH, Stucke EM, Putnam PE, Franciosi JP, Kushner JP, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol. 2014;134:1114–24. doi: 10.1016/j.jaci.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(−) T(H)2 responses. J Allergy Clin Immunol. 2009;124:1326–32 e6. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masterson JC, McNamee EN, Hosford L, Capocelli KE, Ruybal J, Fillon SA, et al. Local hypersensitivity reaction in transgenic mice with squamous epithelial IL-5 overexpression provides a novel model of eosinophilic oesophagitis. Gut. 2014;63:43–53. doi: 10.1136/gutjnl-2012-303631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–14. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mavi P, Rajavelu P, Rayapudi M, Paul RJ, Mishra A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1347–55. doi: 10.1152/ajpgi.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fulkerson PC, Schollaert KL, Bouffi C, Rothenberg ME. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J Immunol. 2014;193:4043–52. doi: 10.4049/jimmunol.1400732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–794 e3. doi: 10.1016/j.jaci.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–9. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 87.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 88.Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 89.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456–63. 463 e1–3. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 90.Ghazi A, Trikha A, Calhoun WJ. Benralizumab--a humanized mAb to IL-5Ralpha with enhanced antibody-dependent cell-mediated cytotoxicity--a novel approach for the treatment of asthma. Expert Opin Biol Ther. 2012;12:113–8. doi: 10.1517/14712598.2012.642359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuang FLAH, Kumar S, Powers A, Quezado M, Wang Z, Ware JA, Wetzler L, Brown T, Paneez K, Klion AD. Benralizumab (anti-IL5Ra) depletes gut tissue eosinophilia and improves symptoms in hypereosionphilic syndrome with gastrointestinal involvement. J Allergy Clin Immunol. 2018;141:AB196. [Google Scholar]
- 92.Yamazaki K, Murray JA, Arora AS, Alexander JA, Smyrk TC, Butterfield JH, et al. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Dig Dis Sci. 2006;51:1934–41. doi: 10.1007/s10620-005-9048-2. [DOI] [PubMed] [Google Scholar]
- 93.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 94.Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–32. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–27. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 96.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–96. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7:718–29. doi: 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1:e86355. doi: 10.1172/jci.insight.86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Mello RJ, Caldwell JM, Azouz NP, Wen T, Sherrill JD, Hogan SP, et al. LRRC31 is induced by IL-13 and regulates kallikrein expression and barrier function in the esophageal epithelium. Mucosal Immunol. 2016;9:744–56. doi: 10.1038/mi.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rochman M, Travers J, Abonia JP, Caldwell JM, Rothenberg ME. Synaptopodin is upregulated by IL-13 in eosinophilic esophagitis and regulates esophageal epithelial cell motility and barrier integrity. JCI Insight. 2017:2. doi: 10.1172/jci.insight.96789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zuo L, Fulkerson PC, Finkelman FD, Mingler M, Fischetti CA, Blanchard C, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol. 2010;185:660–9. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Whelan KA, Merves JF, Giroux V, Tanaka K, Guo A, Chandramouleeswaran PM, et al. Autophagy mediates epithelial cytoprotection in eosinophilic oesophagitis. Gut. 2017;66:1197–1207. doi: 10.1136/gutjnl-2015-310341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135:500–7. doi: 10.1016/j.jaci.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 105.Dellon ES, Collins MH, Assouline-Dayan Y, Evans L, Gupta SK, Schoepfer A, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of a Novel Recombinant, Humanized, Anti-Interleukin-13 Monoclonal Antibody (RPC4046) in Patients with Active Eosinophilic Esophagitis: Results of the HEROES Study (Abstract) American College of Gastroenterology National Meeting. 2016 [Google Scholar]
- 106.Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–17. 217 e1–7. doi: 10.1016/j.jaci.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin LJ, He H, Collins MH, Abonia JP, Biagini Myers JM, Eby M, et al. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE-specific and general atopic disease loci. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.