Abstract
Background
Patient-reported outcome (PRO) metrics for eosinophilic esophagitis (EoE) have been developed and validated but not used in a multicenter pediatric population or systematically aligned with histology.
Objective
We sought to understand 1) the potential of care-giver report to predict patient self-reported symptoms and 2) the correlation of PRO domains with histology.
Methods
Subjects with EoE (n=310) and their parents participating in the Consortium of Gastrointestinal Eosinophilic Disease Researchers (CEGIR) observational clinical trial were queried for baseline patient symptoms and quality of life (QOL) using the Pediatric Eosinophilic Esophagitis Symptom Score version 2 (PEESSv2.0), Pediatric QOL EoE module (PedsQL-EoE) and biopsies were analyzed using the EoE histology scoring system (EoEHSS).
Results
PEESSv2.0 parent and child report aligned across all domains (r=0.68-0.73, p<0.001). PedsQL-EoE reports correlated between parents and children across ages and multiple domains (r=0.48-0.79, p<0.001). There was a tight correlation between symptoms on PEESS2.0 and their impacts QOL both on self and parent report (p<0.001). Self-reported symptoms on PEESSv2.0 (positively) and PedsQL-EoE (inversely) showed a weak correlation with proximal, but not distal, peak eosinophil counts and features and architectural tissue changes on EoEHSS (p<0.05).
Conclusions
Parents of children with EoE aged 3-18 years old accurately reflected their children’s disease symptoms and QOL. Self- and parent-reported symptoms correlate with proximal esophageal histology. Our data suggest that parent report in young children can function as an adequate marker for self-reported symptoms and that self-reported symptoms may reflect changes in tissue histology in the proximal esophagus. These findings should be considered during clinical trials for drug development.
Clinical Implications
Parent report provides an accurate surrogate marker for self-reported symptoms and QOL in pediatric EoE. Self-reported symptoms may gauge proximal esophageal histology. This should be considered in clinical t design.
Keywords: Eosinophil, Eosinophilic esophagitis, CEGIR, Patient Reported Outcomes, PEESSv2.0, PedsQL-EoE, Symptoms, Quality of Life
Introduction
Eosinophilic esophagitis (EoE), a chronic, antigen-mediated disorder of children and adults, is diagnosed and monitored histologically, results in symptoms reflective of esophageal dysfunction, and does not currently have validated surrogate disease markers.1, 2 One barrier to drug development and clinical trials in children is the lack of self-reported patient-reported outcome (PRO) metrics and the assumption that child report is unreliable and that parent report cannot serve as an adequate surrogate for a child’s symptoms.3 Due to these challenges, young children, often the population most in need of novel therapies to halt disease progression and/or alter natural history, are excluded from clinical therapeutic trials.
There are two EoE-specific validated outcome metrics for children and their parents, namely the Pediatric Eosinophilic Esophagitis Symptom Scores version 2.0 (PEESSv2.0) and the age-specific Pediatric Quality of Life Inventory-EoE module version 3.0 (PedsQL-EoE).4–11 However, it is currently not clear how well these outcome metrics perform in terms of an accurate reflection of child symptoms by parent report.5 In addition, it is not clear whether parent-reported or self-reported symptom and/or quality of life metrics can perform as surrogate markers of tissue histology in children.4, 12 The EoE histology scoring system (EoEHSS) is a validated module that reports the grade and stage of features of tissue damage and eosinophilia in the esophagus.13 Eosinophil parameters include features of eosinophilic inflammation, eosinophil abscesses and surface layering, and surface epithelial alteration as well as the architectural changes of basal zone hyperplasia, dilated intercellular spaces, dyskeratotic epithelial cells and lamina propria fibrosis. The lack of validated indexes for symptoms/quality of life and histology was a prior barrier to completing a systematic assessment of PROs in relation to histology.
The use of multicenter consortia for rare diseases has a number of advantages over single-center studies including data obtained from multiple centers from across the U.S. and the ability to gather larger amounts of information in a shorter period. The Consortium for Eosinophilic Gastrointestinal Disease Researchers (CEGIR) is a national collaborative network of 14 academic centers and patient advocacy groups caring for adults and children with eosinophilic gastrointestinal disorders.14 The CEGIR clinical trial Outcomes Measures in Eosinophilic Gastrointestinal disorders Across the ages (OMEGA) is a longitudinal cohort study aimed at understanding the natural history of EoE, eosinophilic gastritis, and eosinophilic colitis during routine clinical care.15 Using baseline PEESSv2.0 and PedsQL-EoE data generated from CEGIR subjects, we aimed to understand the alignment between parent and child reported symptoms and the correlation of symptoms with esophageal histology in a multi-center study.
Methods
Patient-reported Outcomes
Three-hundred ten children aged 3-18 years old and their parents completed the PEESSv2.0 child self-report, PEESSv2.0 parent proxy report, age-specific parent-report, and/or self-report PedsQL-EoE questionnaires upon entry into CEGIR OMEGA in 8 pediatric centers across the continental United States (NCT02523118). PedsQL-EoE self-report was used in children aged 5-18 years old. PEESSv2.0 self-report was obtained from children aged 8-18 years old. Parents of children aged 3-18 years old completed the PEESSv2.0 parent report and age-specific PedsQL-EoE. Patients were consented/assented into the central (Cincinnati) and local Institutional Review Board (IRB) and the National Institutes of Health (NIH) approved protocol. The PEESSv2.0 is a content-validated metric for EoE-specific symptoms in children and assesses items within four domains: dysphagia, gastroesophageal reflux disease (GERD), nausea/vomiting, and pain.4–6 PEESSv2.0 is scored 0-100, with higher scores indicating more severe symptoms. The PedsQL-EoE version 3.0 is a content-validated index that measures the effect of various disease domains on quality of life. PedsQL-EoE assesses the domains of symptoms I (chest pain, heartburn, stomach aches, vomiting, nausea, and food regurgitation), symptoms II (trouble swallowing, food stuck in the throat/chest, drinking to aid swallowing, and prolonged eating time), problems with treatment (difficulty with medications, doctor visits, endoscopies, and allergy testing), worry (regarding illness, doctor visits, endoscopies, and allergy testing), feelings, communication (parents, adults, friend, practitioners), and food/eating (difficulties with food elimination).4, 8, 9 The PedsQL-EoE young child, child, and adolescent are self-reported metrics for children aged 5-7, 8-12, and 13-18 years old, respectively. The PedsQL-EoE for parents are metrics for parents of toddlers (2-4 years old), young children (5-7 years), children (8-12 years), and adolescents (13-18 years). It is scored from 0-100 with higher scores reflecting better quality of life. Data were stored in the data management and coordinating center (DMCC) centralized database.
Histologic evaluation
Whole slide images of esophageal biopsies (400X magnification) obtained within ± 30 days of PRO completion were reviewed by pathologists comprising the CEGIR pathology core (MHC, KEC, G-YY). Pathologists were blinded to treatment status and therapy at the time the biopsies were procured. Peak eosinophil counts were obtained and the features of the EoEHSS were scored for both grade (severity) and stage (extent) of the features and were entered by the pathologists into the DMCC database. For purposes of this analysis, the EoEHSS features were grouped into those that relate directly to eosinophilic inflammation (eosinophilic inflammation which is a score of the peak eosinophil count, eosinophil abscesses, eosinophil surface layering and surface epithelial alteration) and those that relate to architectural aspects (basal zone hyperplasia, dilated intercellular spaces, dyskeratotic epithelial cells, lamina propria fibrosis).13
Statistics
Demographic and clinical characteristics of EoE patients were summarized using frequency and percent for categorical variables and median (interquartile range) for the continuous variables. Self-report for PEESSv2.0 and PedsQL-EoE were matched to parent reports by date to assess the correlation between these measures. Self-reports for PEESSv2.0 and PedsQL-EoE also were matched by date for each participant. The same approach was used for parent reports. The earliest visit date that resulted in a match was used for each participant. PROs were matched with EoEHSS within ± 30 days for associations between the metrics. The earliest endoscopy/HSS date that met the matching criteria was used for each patient in the analyses. Missing data was most commonly due to the lack of an endoscopy done within 30 days of the PROs; the subjects were not required to have a new diagnosis or an accompanying endoscopy at the time of enrollment. Missing data also was due to families not completing portions of a form or not completing forms in their entirety. For child self-report, not all age groups had PROs to fill out (<8 years old for PEESSv2.0 and <5 years old for PedsQL-EoE).
Spearman’s nonparametric correlations (Spearman’s r) are reported to assess the relationship between the following pairs of measurements: 1) parent versus child reports for PEESSv2.0 and PedsQL-EoE; 2) PEESSv2.0 versus PedsQL-EoE for self-reports and for parent’s reports; and 3) EoEHSS versus PEESSv2.0 and PedsQL-EoE. For assessing associations between self-reports and parent reports and between PEESSv2.0 and PedsQL-EOE for self-report and parent reports, Spearman’s correlations with p-values <0.001 were considered significant to control for multiple testing using the Bonferroni adjustment and assuming a correlation of 0.5 among the endpoints tested. For correlation analyses of PROs with HSS, p-values <0.01 were considered significant to control for multiple testing using the Bonferroni adjustment with a correlation of 0.5 among the endpoints tested. Data in the graphs also includes p-values <0.05 to show trends. Statistical analyses were done using SAS version 9.3 (Cary, NC) and Sigmaplot version 13.0.
Results
Baseline population
We analyzed cross-sectional data from 310 pediatric patients with EoE who were aged 3-18 years and recruited from 8 pediatric CEGIR centers. The mean (±SD) age was 10 (± 4.1) years old, and 223 (73%) were male and 262 (89%) were Caucasian, indicating that this population aligns with the reported demographics for the U.S. EoE population. On the basis of the presence of allergic rhinitis, food allergy, eczema, or aeroallergen/food-specific IgE, 189 (81%) of subjects were classified as atopic. At the time of their initial evaluation, more than 40% of subjects were on EoE-directed therapy with elimination diets and/or swallowed topical steroids. Seven percent and 38% were on proton pump inhibitor (PPI) alone or in combination with another therapy, respectively (Table 1).
Table 1.
Demographic Characteristics of Children in OMEGA
| Numbers | |
|---|---|
| Recruited (N) | 310 |
| Age (mean ± SD) (range: 3 - 18 years) | 10.1 (4.1) |
| Gender (N, %) | |
| Male | 223 (73.4) |
| Female | 81(26.6) |
| Race (N, %) | |
| White | 262 (88.8) |
| African American | 16 (5.4) |
| Native American | 1 (0.3) |
| Asian | 4 (1.4) |
| Mixed | 12 (4.1) |
| Ethnicity (N, %) | |
| Hispanic, Latino, or Spanish origin | 20 (6.6) |
| Not Hispanic, Latino, or Spanish origin | 277 (91.1) |
| Sites (N, %) | |
| Cincinnati Children’s Hospital | 70 (22.6) |
| Children’s Hospital of Colorado | 69 (22.3) |
| Children’s Hospital of Philadelphia | 74 (23.9) |
| Lurie Children’s Hospital of Chicago | 17 (5.5) |
| Saint Francis Medical Center | 9 (2.9) |
| Riley Children’s Hospital | 12 (3.9) |
| Rady Children’s Hospital, San Diego | 53 (17.1) |
| Tufts Medical Center | 6 (1.9) |
| Atopic* (N, %) | |
| Yes | 189 (81.1) |
| No | 44 (18.9) |
| Therapy (N=Yes, %) | |
| Elimination Diet | 148 (47.8) |
| Swallowed topical steroids | 135 (43.6) |
| Oral Systemic Steroids | 3 (1.0) |
| PPI alone | 23 (7.4) |
| PPI in combination | 119 (38.4) |
| Anti-IL5 | 0 (0) |
| Anti-IL13 | 0 (0) |
| Esophageal Dilation | 0 (0) |
Atopy was defined as the presence of allergic rhinitis, eczema, or aeroallergen/food specific IgE
Of the 310 children enrolled in CEGIR OMEGA, 254 (82%) and 290 (94%) had self-reported and parent-reported PedsQL-EoE, respectively. One-hundred eighty-four (59%) children had self-report, and 263 (85%) parents had utilizable baseline PEESSv2.0 metrics. Subjects were required to have at least one complete self-reported or parent-reported metric for inclusion in the analysis.
Baseline PedsQL-EoE and PEESSv2.0 in children and parents
PedsQL-EoE
Self-reported PedsQL-EoE were reviewed from 70, 104, and 80 children aged 5-7 (young child), 8-12 years (child), and 13-18 years old (adolescent), respectively. The highest self-reported quality of life was in the communication domain, regardless of age (median, interquartile range) young child 75 (50, 100); child 85 (60, 100); adolescent 93 (70, 100). All child self-report groups had the poorest quality of life in the domain of food and eating with median scores (interquartile range) of 50 (25, 63), 59 (44, 88), 63 (38, 88) in young child, child, and adolescent groups, respectively. Overall, younger children tended to have poorer quality of life over multiple self-reported domains of PedsQL-EoE than did older children (Table 2). Parent-reported PedsQL-EoE were reviewed from 29, 72, 106, and 83 parents of toddler, young child, child, and adolescent subjects, respectively. Parental report for the best quality of life domain varied by child age with high scores in multiple domains for children ages 5-7 years old (median scores ranged from 75 to 87.5), the communication domain for 8-12 years old with median score (interquartile range) 80 (55, 100) and the symptom I and symptom II domains for 13-18 years old with median scores 87.5 (67, 00) and 87.5 (63, 100) respectively (Table 2).
Table 2.
PedsQL-EoE Self and Parent Report by Age
| Self | Parent | Correlation | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | Median (IQR) | N | Median (IQR) | N | Spearman’s R | p-value |
| 2-4 years old | |||||||
| Symptoms I | 29 | 75.0 (58, 92) | |||||
| Symptoms II | 29 | 75.0 (63, 88) | |||||
| Treatment | 27 | 87.5 (63, 100) | |||||
| Worry | 28 | 75.0 (66,100) | |||||
| Communication | NA | NA | |||||
| Food and eating | 25 | 75.0 (50, 100) | |||||
| Feelings | 24 | 75.0 (50, 83) | |||||
| 5-7 years old | |||||||
| Symptoms I | 69 | 66.7 (50, 83) | 72 | 75.0 (58, 83) | 64 | 0.75 | <.001 |
| Symptoms II | 70 | 75.0 (50, 88) | 72 | 75.0 (53, 100) | 64 | 0.83 | <.001 |
| Treatment | 70 | 50.0 (25, 75) | 72 | 75.0 (63, 94) | 64 | 0.07 | 0.585 |
| Worry | 69 | 62.5 (50, 88) | 72 | 75.0 (59, 100) | 63 | 0.68 | <.001 |
| Communication | 70 | 75.0 (50, 100) | 70 | 75.0 (50, 100) | 64 | 0.57 | <.001 |
| Food and eating | 63 | 50.0 (25, 63) | 67 | 68.8 (50, 94) | 58 | 0.62 | <.001 |
| Feelings | 62 | 50.0 (17, 67) | 67 | 58.3 (33, 75) | 58 | 0.79 | <.001 |
| 8-12 years old | |||||||
| Symptoms I | 104 | 75.0 (58, 92) | 106 | 75.0 (63, 92) | 101 | 0.70 | <.001 |
| Symptoms II | 104 | 81.3 (69, 100) | 106 | 75.0 (56, 94) | 101 | 0.69 | <.001 |
| Treatment | 104 | 70.0 (50, 85) | 105 | 80.0 (65, 95) | 100 | 0.57 | <.001 |
| Worry | 104 | 79.2 (58, 96) | 106 | 75.0 (58, 92) | 101 | 0.52 | <.001 |
| Communication | 104 | 85.0 (60, 100) | 106 | 80.0 (55, 100) | 101 | 0.56 | <.001 |
| Food and eating | 88 | 59.4 (44, 88) | 85 | 75.0 (56, 94) | 81 | 0.48 | <.001 |
| Feelings | 88 | 66.7 (42, 83) | 85 | 66.7 (33, 75) | 81 | 0.55 | <.001 |
| 13-18 years old | |||||||
| Symptoms I | 80 | 83.3 (67, 96) | 83 | 87.5 (67, 100) | 73 | 0.61 | <.001 |
| Symptoms II | 80 | 84.4 (59, 100) | 83 | 87.5 (63, 100) | 73 | 0.62 | <.001 |
| Treatment | 80 | 70.0 (50, 90) | 83 | 85.0 (70, 95) | 73 | 0.48 | <.001 |
| Worry | 80 | 87.5 (73, 100) | 83 | 83.3 (67, 100) | 73 | 0.40 | <.001 |
| Communication | 80 | 92.5 (70,100) | 83 | 80.0 (60, 100) | 73 | 0.34 | 0.003 |
| Food and eating | 59 | 62.5 (38, 88) | 66 | 68.8 (50, 94) | 51 | 0.54 | <.001 |
| Feelings | 59 | 66.7 (50, 92) | 66 | 66.7 (50, 83) | 51 | 0.50 | <.001 |
PEESSv2.0
With the PEESSv2.0, children and their parents reported symptoms to be slightly more frequent rather than severe (Table 3). Children reported the highest symptom frequency scores in the pain frequency domain median scores (interquartile range) 25 (13, 50)) and lowest symptom severity scores in the GERD and nausea/vomiting severity domain 13 (0, 38). In contrast, parents reported the highest symptom frequency scores for their children in the dysphagia frequency domain (30 (10, 50)).
Table 3.
PEESSv2.0 Self and Parent Report
| Self | Parent | Correlation | |||||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Spearman’s R | p | |
| Overall frequency | 182 | 22.7 (10, 35) | 262 | 25.0 (11, 39) | 174 | 0.72 | <.001 |
| Overall severity | 180 | 18.1 (6, 33) | 257 | 16.7 (6, 28) | 172 | 0.70 | <.001 |
| Overall frequency and severity | 182 | 20.0 (9, 33) | 261 | 21.3 (10, 34) | 174 | 0.72 | <.001 |
| Frequency | |||||||
| GERD | 184 | 12.5 (0, 38) | 263 | 12.5 (0, 38) | 176 | 0.65 | <.001 |
| Nausea and vomiting | 182 | 12.5 (0, 38) | 262 | 12.5 (0, 38) | 174 | 0.76 | <.001 |
| Pain | 182 | 25.0 (13, 50) | 262 | 25.0 (13, 50) | 174 | 0.70 | <.001 |
| Dysphagia | 182 | 20.0 (5, 40) | 262 | 30.0 (10, 50) | 174 | 0.69 | <.001 |
| Severity | |||||||
| GERD | 182 | 12.5 (0, 25) | 258 | 12.5 (0, 25) | 174 | 0.65 | <.001 |
| Nausea and vomiting | 181 | 12.5 (0, 38) | 259 | 12.5 (0, 38) | 173 | 0.66 | <.001 |
| Pain | 180 | 25.0 (0, 44) | 260 | 25.0 (6, 38) | 172 | 0.69 | <.001 |
| Dysphagia | 180 | 8.3 (0, 33) | 257 | 8.3 (0, 25) | 172 | 0.61 | <.001 |
| Problems | |||||||
| Chest pain | 182 | 12.5 (0, 38) | 262 | 0.0 (0, 38) | 174 | 0.65 | <.001 |
| Heart burn | 182 | 12.5 (0, 38) | 261 | 12.5 (0, 38) | 174 | 0.66 | <.001 |
| Stomach aches | 182 | 37.5 (0, 50) | 262 | 37.5 (13, 50) | 174 | 0.73 | <.001 |
| Trouble swallowing | 182 | 0.0 (0, 25) | 262 | 12.5 (0, 25) | 174 | 0.61 | <.001 |
| Food gets stuck | 182 | 6.3 (0, 38) | 261 | 0.0 (0, 25) | 173 | 0.60 | <.001 |
| Drink to swallow | 182 | 6.3 (0, 38) | 262 | 12.5 (0, 38) | 174 | 0.58 | <.001 |
| Throw up | 182 | 0.0 (0, 25) | 262 | 0.0 (0, 25) | 174 | 0.67 | <.001 |
| Nauseous | 182 | 25.0 (0, 50) | 262 | 25.0 (0, 38) | 174 | 0.69 | <.001 |
| Reflux | 182 | 0.0 (0, 38) | 261 | 0.0 (0, 25) | 174 | 0.64 | <.001 |
| Eat less | 181 | 25.0 (0, 75) | 259 | 50.0 (0, 100) | 171 | 0.67 | <.001 |
| Need more time to eat | 182 | 25.0 (0, 75) | 260 | 50.0 (0, 100) | 173 | 0.67 | <.001 |
| Overall Domains | |||||||
| Overall GERD - heart burn and reflux | 184 | 12.5 (0, 31) | 262 | 12.5 (0, 31) | 176 | 0.68 | <.001 |
| Overall nausea and vomiting - throw up and nauseous | 182 | 17.7 (0, 38) | 262 | 12.5 (0, 31) | 174 | 0.73 | <.001 |
| Overall pain - chest pain and stomach aches | 182 | 25.0 (6, 44) | 262 | 25.0 (13, 44) | 174 | 0.72 | <.001 |
| Overall dysphagia - trouble swallowing, food gets stuck, drink to swallow, eat less and need more time to eat | 182 | 15.6 (6, 38) | 261 | 21.9 (9, 41) | 174 | 0.68 | <.001 |
Association between parent and child PROs—PedsQL-EoE and PEESSv2.0
Parent to age-specific PedsQL-EoE
In order to understand whether parent report associated with child self-report, we assessed the relationship between the parent and child PROs by child age group. Matched parent-age group PedsQL-EoE PROs were available from 64, 101, and 73 parents and children in the young child, child, and adolescent subject groups, respectively. PedsQL-EoE gauges the impact on quality of life in 7 domains. Symptoms I assess pain, vomiting/nausea, and heartburn/regurgitation symptoms, whereas symptoms II assess dysphagia symptoms. The remaining domains assess the impacts of medications, testing, procedures, and doctors’ visits (“treatment”), worry, communication about their disease (“communication”), restricted diets (“food/eating”), and feelings about food restriction (“feelings”). Parent reports and age-specific child self-reports of quality of life correlated strongly over almost all domains in children up to 12 years old (Table 2). In the 5-7–year-old children, the domains of symptoms I, symptoms II, worry, communication, food/eating, and feelings correlated significantly (r=0.57-0.83, p<0.001 for all) between parents and their children. In children 8-12 years old, the domains of symptoms I and II, treatment, worry, communication, food/eating, and feelings all correlated strongly and significantly (r=0.52-0.70, p<0.001 for all) between children and their parents (Table 2). In the 13-18–year-old group, symptoms I and II and food/eating correlated the most strongly (r=0.54-0.62, p<0.001 for all) between children and their parents (Table 2). These data suggest that parent and child quality of life assessments align more strongly in children under the age of 13 years. Although symptom domains align tightly, other domains, such as communication, worry, food/eating, treatment and feelings are variable. The treatment domain had variable correlation by age group.
Parent to child PEESSv2.0
There were up to 176 matched child and parent PEESSv2.0 available to evaluate. There were strikingly significant correlations among all the overall and specific domains in PEESSv2.0 between children and their parents (Table 3). The total PEESSv2.0 score for all frequency and severity questions correlated strongly between children and their parents (r=0.70-0.72, p<0.001). Indeed, this overall positive association held true over all of the PEESSv2.0 domains, including GERD (r=0.65, p<0.001), nausea/vomiting (r=0.66 (severity), 0.76 (frequency), p<0.001), pain (r=0.70 (frequency), 0.69 (severity), p<0.001), and dysphagia (r=0.69 (frequency), 0.61 (severity), p<0.001) (Table 3). Within the dysphagia domain items, for which both parents and children reported the highest symptom scores (eats less and needs more time to eat), there were strong correlations (r=0.67, p<0.001) (Table 3). These data suggest that the parent report of symptoms as assessed by the PEESSv2.0 can effectively reflect the child’s symptoms in all domains and support the hypothesis that parent-reported symptoms accurately reflect the child self-report.
Associations between PROs within groups
A number of statistically significant and revealing patterns existed upon comparison of the PROs to one another. Comparison of parent PEESSv2.0 and parent PedsQL-EoE revealed that among the 4 overall PEESSv2.0 domains of GERD, nausea/vomiting, pain, and dysphagia, there were significant inverse correlations to the PedsQL-EoE symptoms I and II domains for all of the age groups (Figure 1). An inverse correlation would be expected in the presence of worse symptoms; higher PEESSv2.0 scores are reflective of a greater impact and lower PedsQL-EoE scores represent lower quality of life. For all of the age groups of the parent reports, the PEESSv2.0 dysphagia domain correlated most strongly and inversely with the Symptom II domain on PedsQL-EoE (r= −0.80 to −0.88, p<0.001 for all) (Figure 1). For the PedsQL-EoE symptoms I domain, which represents GERD and nausea/vomiting symptom impact, the PEESSv2.0 domains of nausea/vomiting or pain correlated most strongly (−0.80 to −0.85, p<0.001 for all) in parent reports (Figure 1). In parents of toddlers, the PedsQL-EoE symptoms I domain inversely correlated the most strongly with each of the four PEESSv2.0 domains (r= −0.67 to −0.86, p<0.001 for all). There were inverse correlations (p<0.05) over multiple of the 7 domains queried in the parent PedsQL-EoE and the 4 domains queried in the PEESSv2.0 questionnaire, suggesting that a simple intake measure such as PEESSv2.0 might accurately reflect the loss of quality of life as perceived by parents, especially in children aged 5-18 years old (Figure 1).
Figure 1. Correlation between parent-reported PEESSv2.0 and PedsQL-EoE.
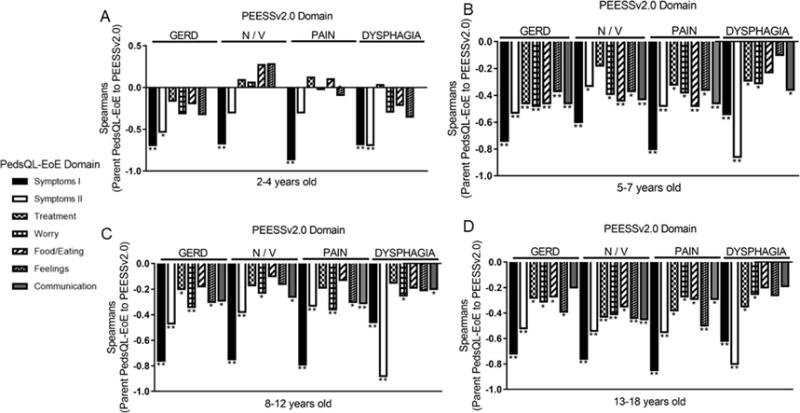
Correlation coefficients between PEESSv2.0 domains of GERD, nausea/vomiting (N/V), pain, and dysphagia and the 7 domains of PedsQL-EoE (symptoms I, symptoms II, treatment, worry, food/eating, feelings, communication) as reported by parents of children aged 2-4 (communication domain is not assessed) (A), 5-7 (B), 8-12 (C), and 13-18 (D) years old. **p<0.001, *p<0.05.
Among child self-reported PEESSv2.0 and PedsQL-EoE, there significant inverse correlations between the four PEESSv2.0 domains and the PedsQL-EoE symptoms I or II domains (r= −0.67 to −0.86, p<0.001) (Figure 2). Some of the lowest symptom correlations were in mismatched domains—PEESSv2.0 GERD and PedsQL-EoE symptoms II and PEESSv2.0 dysphagia and PedsQL-EoE symptoms I while some of the strongest correlations were between appropriately matched symptom domains (PEESSv2.0 GERD and PedsQL-EoE symptoms I; PEESSv2.0 dysphagia and PedsQL-EoE symptoms II) (r= −0.73 to −0.86, p<0.001). These data support the notion that among all the domains, symptoms significantly influence self-reported pediatric quality of life in EoE.
Figure 2. Correlation between child self-reported PEESSv2.0 and PedsQL-EoE.
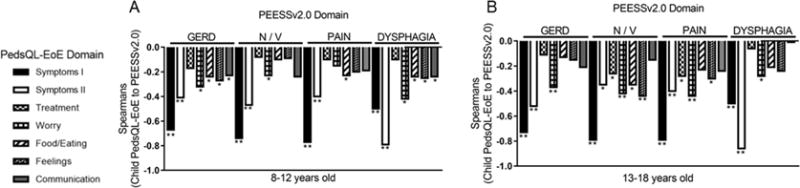
Correlation coefficients (Spearman’s r) between PEESSv2.0 domains of GERD, nausea/vomiting (N/V), pain, and dysphagia and the 7 domains of PedsQL-EoE (symptoms I, symptoms II, treatment, worry, food/eating, feelings, communication) as reported by children aged 8-12 (A), and 13-18 (B) years old. **p<0.001, *p<0.05.
Correlation of symptoms and quality of life with EoEHSS
Whole slide images of esophageal biopsies from 145 subjects were available for histopathology evaluation. The median (IQR) maximum peak eosinophil count in the esophagus in the cohort was 16 (2, 33) per HPF. The most severe histologic features were in the architectural features group of the proximal and distal esophagus with median scores of 0.25 and 0.42 (of 1.0 possible) for proximal and distal grade and median scores of 0.25 and 0.42 (of 1.0) for proximal and distal stage, respectively (Table 4).
Table 4.
Histologic Features on EoEHSS
| Feature | N | Mean ± SD | Median (IQR) |
|---|---|---|---|
| Maximum of peak eosinophils (proximal and distal) | 145 | 26.70 ± 33.30 | 16.00 (2.0, 33.0) |
| Proximal Peak Count | 129 | 14.16 ± 23.97 | 3.00 (0.0, 20.0) |
| Distal Peak Count | 142 | 24.68 ± 32.80 | 12.00 (1.0, 31.0) |
| Eosinophil features Distal Grade | 142 | 0.18 ± 0.17 | 0.17 (0.08, 0.25) |
| Eosinophil features Distal Stage | 142 | 0.15 ± 0.16 | 0.08 (0.00, 0.25) |
| Eosinophil features Proximal Grade | 129 | 0.14 ± 0.17 | 0.08 (0.00, 0.17) |
| Eosinophil features Proximal Stage | 129 | 0.10 ± 0.14 | 0.00 (0.00, 0.17) |
| Structural features Distal Grade | 143 | 0.39 ± 0.24 | 0.42 (0.17, 0.58) |
| Structural features Distal Stage | 143 | 0.40 ± 0.24 | 0.42 (0.25, 0.67) |
| Structural features Proximal Grade | 129 | 0.33 ± 0.25 | 0.25 (0.17, 0.50) |
| Structural features Proximal Stage | 130 | 0.34 ± 0.26 | 0.25 (0.11, 0.56) |
PEESSv2.0 Correlations with Peak Eosinophils
We correlated self-reported PEESSv2.0 domains with peak eosinophil counts. The scores for “all frequency” questions and GERD frequency showed a weak correlation with peak eosinophil counts in the proximal esophagus (r=0.26-0.30, p<0.05) but did not reach statistical significance after adjusting for multiple testing (Figure 3a). There were no significant correlations with the distal eosinophil counts.
Figure 3. Correlation of proximal eosinophil counts and EoEHSS features with self-reported PEESSv2.0.
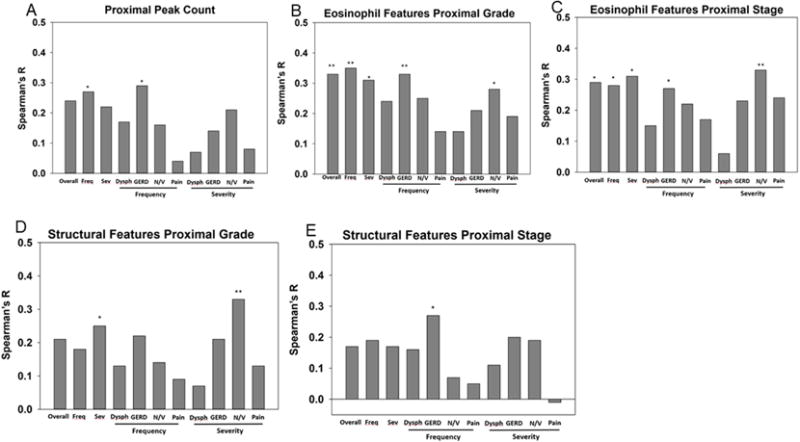
Spearman’s r for the correlations between self-reported child PEESS2.0 domains (overall, all frequency (freq), all severity (sev), and dysphagia (dys), GERD, nausea/vomiting (N/V), and pain for frequency and severity) with proximal peak eosinophil counts (A) and proximal EoEHSS eosinophil features for grade (B) and stage (C) and structural features grade (D) and stage (E). *p<0.05, **p<0.01.
PEESSv2.0 self-report and Correlations with EoEHSS Features
PEESSv2.0 self-report (overall frequency/severity, GERD frequency, and nausea/vomiting severity) correlated with grade or stage of the eosinophil features group of the EoEHSS (0.31-0.35, p<0.01 for all) (Figure 3b,c). Architectural changes in the proximal esophagus tended to correlate with the symptoms of overall severity and GERD frequency while nausea/vomiting frequency scores correlated significantly with structural features (r=0.33, p<0.01) (Figure 3d,e). It was striking that proximal eosinophil and structural features, as opposed to distal were correlated with symptoms. Unlike child self-reported PEESSv2.0 symptoms, parent-reported PEESSv2.0 symptoms did not well reflect tissue histology.
PedsQL-EoE Correlations with EoEHSS Features
The symptoms I and symptoms II domains of the self-reported PedsQL-EoE among 13-18 year olds had inverse correlations with proximal eosinophil features on EoEHSS (Table 5). In addition, proximal architectural features scores tended to correlated with the PedsQL-EoE symptom domain in 5-7–year-olds. Self-reported PedsQL-EoE for children 8-12 years old did not align with any EoEHSS domains.
Table 5.
Correlation of Self and Parent Reported PedsQL-EoE Domains with EoEHSS (for p<0.05)
| Age Group | PedsQL-EoE | EoEHSS Feature | r | p for Spearman r |
|---|---|---|---|---|
| Self-Report | ||||
| 5-7 years old | Symptoms II | Structural Features Proximal Stage | −0.61 | 0.020 |
| 13-18 years old | Symptoms I | Proximal Peak Eosinophil Count | −0.46 | 0.036 |
| Symptoms I | Eosinophil Features Proximal Grade | −0.61 | 0.003 | |
| Symptoms I | Eosinophil Features Proximal Stage | −0.66 | <.001 | |
| Symptoms I | Structural Features Proximal Grade | −0.48 | 0.023 | |
| Symptoms II | Maximum Peak Eosinophil Count of Proximal and Distal Score | −0.55 | 0.006 | |
| Symptoms II | Proximal Peak Eosinophil Count | −0.54 | 0.012 | |
| Symptoms II | Eosinophil Features Proximal Grade | −0.48 | 0.025 | |
| Treatment | Eosinophil Features Proximal Stage | −0.50 | 0.017 | |
| Parent Report | ||||
| 2-4 years old | Symptoms II | Structural Features Proximal Grade | −0.52 | 0.040 |
| Worry | Maximum Peak Eosinophil Count of Proximal and Distal Score | −0.50 | 0.041 | |
| Worry | Distal Peak Eosinophil Count | −0.60 | 0.014 | |
| Worry | Eosinophil Features Distal Grade | −0.62 | 0.014 | |
| Worry | Eosinophil Features Distal Stage | −0.58 | 0.018 | |
| 13-18 years old | Worry | Eosinophil Features Distal Stage | −0.40 | 0.036 |
| Communication | Distal Peak Eosinophil Count | −0.54 | 0.003 | |
| Communication | Eosinophil Features Distal Grade | −0.65 | <.001 | |
| Communication | Eosinophil Features Distal Stage | −0.60 | <.001 | |
| Communication | Structural Features Distal Grade | −0.62 | <.001 | |
| Communication | Structural Features Distal Stage | −0.47 | 0.011 | |
Parent report in the PedsQL-EoE worry domain of toddlers had a trend toward correlations, largely in the distal esophagus for eosinophil features (r= −0.40 to −0.65, p<0.05 for all) (Table 5). Parent report of communication on PedsQL-EoE significantly correlated with distal peak eosinophil count as well as eosinophil and structural features in the distal esophagus. These data suggest that symptoms can potentially positively correlate and quality of life can negatively correlate with proximal esophageal histology as assessed by the EoEHSS.
Discussion
In this manuscript, we report the relationships between parent-reported and self-reported pediatric PROs and the correlations between PROs and a validated histologic scoring tool, the EoEHSS, in a large, multicenter study as part of the CEGIR network.14, 15 As these patients had varying disease activity, this cohort likely represents a “real life” reflection of chronic EoE symptoms and histology, which is a strength of this study.
We report a number of novel observations. First, we showed that parent-reported and child-reported symptoms gauged on the PEESSv2.0 align strongly across all domains, confirming a prior small study.5 This was true for symptom frequency and severity in the overall domains of nausea/vomiting, GERD, dysphagia, and pain, as well as for multiple items within each domain. This observation is particularly salient because it could alter the current design of acceptable PROs used for clinical therapeutic trials. Second, we demonstrated that the reported impact of EoE on quality of life as gauged in the age-specific PedsQL-EoE positively correlated between parents and their children. This was true especially in the younger population (5-12 years old) over symptoms, worry, communication, eating, and feelings. Third, we found statistically significant correlations between proximal EoEHSS eosinophil and architectural features with PEESSv2.0 domains by child self-report but not by parent PEESSv2.0 report. Fourth, we found that the PEESSv2.0 and PedsQL-EoE correlate inversely to one another, especially in the symptoms domain. This is logical because higher symptom scores on the PEESSv2.0 should translate to poorer quality of life, as reflected in lower PedsQL-EoE scores. Fifth, we report that eosinophil and architectural features in the proximal esophagus, correlated inversely with self-reported symptoms PedsQL-EoE scores in 2 of the 3 age groups, suggesting positive correlation of histology with lower quality of life. Further, parent-reported quality of life measures of symptoms and worry inversely correlated with esophageal pathology in toddlers, suggesting that in the youngest children more severe histology associated with poorer perceived life quality. These findings have a number of potential impacts in the field because they begin to point to 1) the ability of surrogate reports to reflect a child’s symptoms of EoE and 2) the possibility of symptoms to reflect histologic changes in EoE. It is notable that the EoEHSS had greater alignment with symptoms than isolated peak eosinophil counts and that there were stronger relationships between symptoms and histology in the proximal, rather than distal esophagus. From a statistical standpoint, we have attempted to correct for multiple testing by setting the statistical threshold at p<0.01, but it is notable that statistical trends (p<0.05) were nearly all observed in the proximal esophagus, rather than the distal esophagus, suggesting that these trends may well be relevant. Since the correlation between histology and symptoms/quality of life as reported by children and parents was relatively weak, this area requires additional study.
Children are, by definition, a vulnerable population who, under the age of 8 years, do not have PROs that are acceptable self-reported outcomes for drug development trials. This is due to the dogma that caregiver or practitioner-filtered symptom tools do not constitute an adequate surrogate marker for “self-report”.3 However, in this large, cross-sectional cohort of children with active and inactive EoE, parents of children aged 8-18 years old can accurately assess their children’s’ symptoms. Parents of children as young as 5 years can adequately gauge their child’s quality of life. Therefore, these data may provide reliable, validated measures for pediatric EoE, in which parent and child report can be used as acceptable PROs and reduce barriers for drug approval in children.16–19
Our population had relatively mild symptoms and relatively good quality of life on PEESSv2.0 and PedsQL-EoE. These findings likely reflect two distinct processes. First, the enrollment of some patients who were in histological remission likely had an impact on symptom metrics. Second, the use of coping skills acquired during the course of a chronic disease allows symptoms control through behavior modifications. It is likely that symptom and quality of life may be quite different in a newly diagnosed population of children who have active disease prior to any intervention, as compared to those with a longer duration of diagnosis. Comparative and longitudinal studies will yield insights into the alterations and potential disconnections between symptoms, quality of life, and histology. Such studies are best done using the same validated scoring tools over time. Indeed, the design of the CEGIR OMEGA trial is to gauge longitudinal symptoms, quality of life, and histology.14 Our future efforts focused on understanding symptom and histologic shifts over time in EoE will be essential measures for further understanding of this chronic disease.
The PEESSv2.0 and PedsQL-EoE aligned inversely and significantly with one another across both parents and their children, suggesting that greater symptoms on PEESS2.0 associates with poorer quality of life. This was most notable in the symptom domains of the PedsQL-EoE. In addition, the symptom domain of the PedsQL-EoE had stronger correlations with histology than those of the PEESSv2.0. These findings suggest that the future development of a hybrid symptom and quality of life tool may be able to accurately reflect histologic changes in the esophagus of children. Because children under the age of 5 years old cannot well describe their symptoms, these could be assessed using parent report on a scoring tool such as the PedsQL-EoE toddler, which aligned strongly with histology over symptoms and worry domains. Lastly, parent-reported PedsQL-EoE in the youngest and oldest age groups inversely correlated with distal histologic features. This may suggest that parent-report versus self-report could align differently with esophageal histology. These findings need to be verified and validated longitudinally and in larger cohorts.
In conclusion, we report a number of practical findings that could impact on pediatric EoE management and clinical trial design. Our data suggest that parent report can be considered an accurate surrogate marker for child self-reported symptoms and quality of life. In terms of clinical practice, the use of a simple metric such as PEESS2.0 can accurately reflect both child and parent assessments but it is unlikely that symptoms or quality of life can substitute for histologic assessment. Symptoms reflecting proximal esophageal histology may shift the focus of EoE to an area of the esophagus that is less influenced by acid. These data provide important insights for the development of future tools that could decrease the need for repeated invasive procedures in children. In addition, it is likely that parent report is an adequate PRO for use in interventional trials in children.
Capsule Summary.
This study shows that parent report correlates with pediatric self-report of EoE symptoms and quality of life and that self-reported symptoms correlate with histologic changes. Parent report should be considered as a self-report surrogate for future clinical trials.
Acknowledgments
The CEGIR is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Science (NCATS), and is co-funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Diabetes and Digestive Diseases (NIDDK) and NCATS. JPK is supported by the NIH grant U54TR001263. We thank Amanda Rudman-Spergel for guidance and input; Shawna Hottinger for editorial assistance; colleagues and clinical support staff in CEGIR for procuring biopsy specimens and clinical data; and families for participating in the trial.
Grant Funding: CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), and is co-funded by National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and NCATS. CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Disease (CURED), and Eosinophilic Family Coalition (EFC). Dr. Jeffrey Krischer is supported by NIH U54TR001263.
Abbreviations
- CEGIR
Consortium of Eosinophilic Gastrointestinal Disease Researchers
- EoE
eosinophilic esophagitis
- EoEHSS
EoE Histology Scoring System
- HPF
high power field
- OMEGA
Outcomes Measures in Eosinophilic Gastrointestinal disorders Across the ages
- PEESSv2.0
Pediatric Eosinophilic Esophagitis Symptom Score version 2
- PedsQL-EoE
Pediatric QOL EoE module
- PRO
Patient Reported Outcome
- QOL
Quality of Life
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Aceves reports a UCSD patented licensed to Shire Pharma and a consutant for Regeneron. Dr. King reports being a former employee of The Procter & Gamble Company and has stock and stock options in The Procter & Gamble Company. Dr. Collins reports grants from Shire and Regeneron. Dr. Dellon reports grants and/or personal fees from Adare, Banner, Celgene/Receptos, Regeneron, Shire; Alivio, Allakos, AstraZeneca, Enumeral, Robarts Clinical Trials, Meritage, Miraca, Nutricia, and Holoclara. Dr. Falk reports grants and/or personal fees from Shire, ADARE, Regeneron, Banner Bioscience and Celgene. Dr. Gupta reports personal fees from Allakos, Abbott, Receptos, QOL and Shire. Dr. Hirano reports grants and/or consultant fees from Adare, Shire, Regeneron, and Receptos. Dr. Mukkada reports grants and/or personal fees from Shire Pharmaceutical. Dr. Spergel reports grants and/or personal fees from DBV Technologies, End Allergy Together, Food Allergy Research Education, Aimmune Therapeutics, UpToDate, Regeneron and Shire. Dr. Rothenberg reports personal fees from Celgene, Genetech, Glaxo Smith Kline, Merck, Allakos, AstraZeneca, Celgene and Regeneron, as well as an equity in Immune Pharmaceuticals, PulmOne, and Spoon Guru, and receives royalties from UpToDate and reslizumab (Teva Pharmaceuticals). Dr. Furuta reports personal fees and/or grants from EnteroTrack, Shire, and UpToDate. Outside of NIH funding, all support was unrelated to this study. All other authors have nothing to disclose.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20 e6. doi: 10.1016/j.jaci.2011.02.040. quiz 1-2. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:1066–78. doi: 10.1016/j.cgh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang IC, Revicki DA, Schwartz CE. Measuring pediatric patient-reported outcomes: good progress but a long way to go. Qual Life Res. 2014;23:747–50. doi: 10.1007/s11136-013-0607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franciosi JP, Hommel KA, DeBrosse CW, Greenberg AB, Greenler AJ, Abonia JP, et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126. doi: 10.1186/1471-230X-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin LJ, Franciosi JP, Collins MH, Abonia JP, Lee JJ, Hommel KA, et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v20) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol. 2015;135:1519–28 e8. doi: 10.1016/j.jaci.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2009;48:152–60. doi: 10.1097/MPG.0b013e31817f0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos MFO, Barros CP, Silva C, Paro H. Translation and cultural adaptation of the Pediatric Eosinophilic Esophagitis Symptom Score (PEESS v2.0) J Pediatr (Rio J) 2017 doi: 10.1016/j.jped.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Franciosi JP, Hommel KA, Bendo CB, King EC, Collins MH, Eby MD, et al. PedsQL Eosinophilic Esophagitis Module: Feasibility, Reliability, and Validity. J Pediatr Gastroenterol Nutr. 2013;57:57–66. doi: 10.1097/MPG.0b013e31828f1fd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franciosi JP, Hommel KA, Greenberg AB, Debrosse CW, Greenler AJ, Abonia JP, et al. Development of the Pediatric Quality of Life InventoryTM Eosinophilic Esophagitis Module items: qualitative methods. BMC Gastroenterol. 2012;12:135. doi: 10.1186/1471-230X-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch MK, Barnes MJ, Dimmitt RA, Martin L, Rothenberg ME, Goodin BR. Disease-Related Predictors of Health-Related Quality of Life in Youth With Eosinophilic Esophagitis. J Pediatr Psychol. 2018;43:464–71. doi: 10.1093/jpepsy/jsx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukkada V, Falk GW, Eichinger CS, King D, Todorova L, Shaheen NJ. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin Gastroenterol Hepatol. 2018;16:495–503 e8. doi: 10.1016/j.cgh.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Aceves SS, Newbury RO, Dohil MA, Bastian JF, Dohil R. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Ann Allergy Asthma Immunol. 2009;103:401–6. doi: 10.1016/S1081-1206(10)60359-6. [DOI] [PubMed] [Google Scholar]
- 13.Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2017;30:1–8. doi: 10.1111/dote.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng K, Gupta SK, Kantor S, Kuhl JT, Aceves SS, Bonis PA, et al. Creating a multi-center rare disease consortium - the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) Transl Sci Rare Dis. 2017;2:141–55. doi: 10.3233/TRD-170016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoda T, Wen T, Aceves SS, Abonia JP, Atkins D, et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross sectional study. Lancet Gastroenterology Hepatol. 2018 doi: 10.1016/S2468-1253(18)30096-7. provide update. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aceves SS. Unmet therapeutic needs in eosinophilic esophagitis. Dig Dis. 2014;32:143–8. doi: 10.1159/000357131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–85 e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greuter T, Bussmann C, Safroneeva E, Schoepfer AM, Biedermann L, Vavricka SR, et al. Long-Term Treatment of Eosinophilic Esophagitis With Swallowed Topical Corticosteroids: Development and Evaluation of a Therapeutic Concept. Am J Gastroenterol. 2017;112:1527–35. doi: 10.1038/ajg.2017.202. [DOI] [PubMed] [Google Scholar]
- 19.Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–6e1-2. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]


