Abstract
Although classically characterized by chronic airway inflammation with eosinophil infiltration, asthma is a complex and multifactorial condition with numerous clinical phenotypes. Epidemiological studies strongly support the link between obesity and asthma and suggest that obesity precedes and promotes asthma development, increases asthma severity, and reduces steroid responsivity. Using a house dust mite (HDM) model of airway hyperresponsiveness in C57BL/6 mice, we examined the effects of diet-induced obesity on allergic airway inflammation and its treatment with dexamethasone. When compared to lean mice treated with HDM, obese-HDM mice had reduced plasma adiponectin, an anti-inflammatory adipokine, lower eosinophil and higher macrophage infiltration into the lungs and bronchoalveolar lavage (BAL) fluid, increased expression of total, M1 and M2 macrophage markers in the lungs, and enhanced Th2 and non-Th2 cytokine expression in the lungs. While Th2-associated responses in obese-HDM mice were suppressed by systemic dexamethasone, several Th2-independent responses, including total and M1 macrophage markers in the lungs, and lung CXC-motif ligand 1 (CXCL1) levels, were not improved following dexamethasone treatment. Thus, HDM combined with obesity promotes mixed localized inflammatory responses (e.g. M1, M2, Th1, and Th2) and shifts the cellular infiltration from eosinophils to macrophages, which are less sensitive to dexamethasone regulation. Because obese asthmatics exhibit more severe symptoms, lack a predominance of Th2 biomarkers and are predicted to experience more steroid resistance when compared to lean asthmatics, this model could be used to study blunted steroid responses in obese-HDM mice and to define the macrophages found in the lungs.
Keywords: asthma, steroid resistance, airway inflammation, diet-induced obesity
Introduction
In the US and many countries around the world, obesity has become an epidemic. Based on the 2007-2012 National Health and Nutrition Examination Survey (NHANES) more than two-thirds of Americans are overweight or obese [1]. Obesity is characterized by the addition of excess adipose tissue with enhanced immune cell infiltration, which is accompanied by increased production of pro-inflammatory mediators, altered glucose and lipid metabolism, and insulin resistance [2]. This dysfunctional metabolic state has been associated with an increased risk for asthma [3], a complex and heterogeneous condition defined by airway inflammation, remodeling, and hyperreactivity.
Similar to the increased prevalence of obesity, there has been a rise in the number of asthmatics in the US. Approximately 7% of adults and 8% of children in the US have been diagnosed with asthma [4] and the link between obesity and asthma is well-established [5–9]. The chronic low grade inflammation accompanying obesity is proposed to precede and predict the development of asthma [10]. Obese asthmatics typically experience a later onset, are highly symptomatic, lack a predominance of Th2 biomarkers and are expected to have worse prognoses compared to lean asthmatics [11]. Furthermore, they are less likely to maintain ‘asthma control’ with inhaled corticosteroids (with and without β2 agonists) when compared to non-obese asthmatics [12;13].
Based on the hypothesis that the low grade pro-inflammatory state accompanying obesity promotes more severe asthma and steroid resistance in asthmatics, we explored the effects of diet-induced obesity on airway inflammation and the regulation of airway inflammation by steroids using a mouse model of house dust mite (HDM)-mediated asthma in the presence and absence of dexamethasone treatment.
Materials and Methods
Mouse model of house dust mite (HDM)-induced airway hyperresponsiveness (± diet-induced obesity)
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Feinstein Institute prior to initiation (#2012-006). Nasal HDM challenge in C57BL/6 mice was performed as previously described [14;15]. Briefly, 5 wk old male C57BL/6 mice (Taconic, Germantown, NY) were separated into 2 groups, which were fed either regular chow (Rodent Diet 20 #5053, PicoLab, Fort Worth, TX) or high fat diet (HFD, Research Lab Diet D12492 [60% calories from fat], Research Diets, New Brunwick, NJ). Mice were housed according to diet, fed ad lib throughout the entire study (30 wks), and future treatment (n=6–10 per group; n=3–5 per cage). Weight gain was monitored weekly. For the last 6 wks (starting on wk 24), animals in each diet group received either 10µL of saline or house dust mite (HDM, 25µg protein/10µL, Greer Laboratories, Lenoir, NC) on the nares of their noses once daily (Monday through Friday). In addition, the lean-HDM and obese HDM-treated groups received either saline (i.p.) or dexamethasone (Dex, 1 mg/kg, i.p., Sigma Aldrich, St Louis, MO) during the last 2 wks (Monday through Friday). At the end of 30 wks all mice were euthanized with Euthasol (phenobarbital/ phenytoin, Virbac, Fort Worth, TX), followed by cardiac puncture using a heparin-coated syringe. Plasma was collected and frozen at −80°C until analyses.
Bronchoalveolar lavage and lung histopathology
Bronchoalveolar lavage (BAL) was performed twice on the right lung using 0.8mL PBS/1%fetal bovine serum. Pooled BAL samples (from each lung) were centrifuged and BAL fluid was collected and stored at −80°C until analyses. The BAL cells were resuspended in PBS, counted using an automated cell counter (Countess, Invitrogen, Carlsbad, CA), and subsequently cytospun onto slides and stained with Diff-Quik for differential cell counting.
The left lung was tied off at the main bronchus, filled with 10%formalin, removed and fixed in 10% formalin. Paraffin-embedded sagittal lung sections (4–5µM) were prepared by AML Laboratories (Baltimore, MD). For inflammation scoring, hematoxylin & eosin (H&E) stained tissue sections were scored by a board certified pathologist, blinded to the experimental conditions, based on the amount of inflammatory cells near conducting airways present ranging from 0, representing no inflammation, to 5, representing a thick layer of inflammatory cells (>5 cells), as previously described [16].
Assessment of eosinophils and macrophages in lung tissues
Eosinophil infiltration
To determine eosinophilic infiltration, lung tissue sections were stained using a modified Congo Red staining method, as previously described [17]. Briefly, slides were stained with Gill’s double strength hematoxylin for 5 min and 0.5% Congo Red for 15 min. Eosinophils, recognized by their pink cytoplasmic granules and fragmented nucleus, were counted (2 sections per mouse) from random high powered fields by an investigator blinded to the experimental conditions.
Macrophage infiltration
Formalin-fixed, paraffin-embedded lung tissue sections (4–5µM) were deparaffinized and rehydrated. Following antigen retrieval (20 min in a pressure cooker at 95°C with citrate buffer, pH 6.0) and blocking endogenous peroxidase (using freshly prepared 3% H2O2 for 30 min), sections were blocked and incubated with rat monoclonal IgG anti mouse F4/80 antibody (1:50 Clone C1:A3–1, Serotec, Cambridge, MA) overnight at 4°C in a humidified chamber. Staining was revealed following incubation with ImmPRESS anti-rat IgG secondary antibody (Vector Laboratories, Burlingame, CA) and developed using ImmPACT DAB Peroxidase Substrate (Vector Laboratories). Prior to dehydration and mounting with Permount, sections were lightly stained with hematoxylin. F4/80-positive macrophages per high powered field were counted in tissue sections (2 per mouse) by an investigator blinded to experimental conditions.
Quantitative RT-PCR
Frozen lung tissues were homogenized using the Bullet Blender® (Next Advance, Averill, NY). Total RNA was isolated using the RNeasy Plus Universal kit (Qiagen, Valencia,CA). Expression of F480, Fizz1, Arg1 and Nos2 mRNA were determined using a reverse transcription-based quantitative PCR (qPCR) using the Roche Lightcycler® 480 with target-specific primers and the Roche UniversalProbe library (See Table 1), as previously described [18]. Using Power SYBR® Green (ThermoFisherScientific, Waltham, MA) technology, mouse Gra (forward: 5’AAAGAGCTAGGAAAAGCCATTGTC3’; reverse: 5’TCAGCTAACATCTCTGGGAATTCA3’) and Grb (forward: 5’AAAGAGCTAGGAAAAGCCATTGTC 3’; reverse: 5’CTGTCTTTGGGCTTTTGAGATAGG3’) mRNA expression were determined by qPCR, as previously described [19] using the ABI 7900HT (7900 emulation mode) with a thermocycling protocol recommended by the manufacturer and mouse Actb as the housekeeping gene (forward: 5’CGGTTCCGATGCCCTGAGGCTCTT3’; reverse: 5’CGTCACACTTCATGATGGAATTGA3’). Relative changes in gene expression were calculated using the comparative Ct (ΔΔCt) method using a housekeeping gene with a similar level of expression. Data are presented as relative mRNA expression with the untreated lean control group set to 1.
Table 1. qPCR primers used to assess gene expression using the Roche UniversaProbe Library.
| Gene | Primer | Sequence 5’ _ 3’ | Accession NumberA (Probe Number) |
|---|---|---|---|
|
Arg1 |
Forward Reverse |
GAATCTGCATGGGCAACC GAATCCTGGTACATCTGGGAAC |
NM_007482.3 (2) |
|
F480/Emr1 |
Forward Reverse |
GGAGGACTTCTCCAAGCCTATT AGGCCTCTCAGACTTCTGCTT |
NM_010130.4 (42) |
|
Fizz1 |
Forward Reverse |
CACACCCAGTAGCAGTCATCC CCCTCCACTGTAACGAAGACTC |
NM_020509 (51) |
|
Gapdh |
Forward Reverse |
GAGCCAAACGGGTCATCA CATATTTCTCGTGGTTCACACC |
NM_001289726 (29) |
|
Hprt |
Forward Reverse |
TCCTCCTCAGACCGCTTTT CCTGGTTCATCATCGCTAATC |
NM_013556.2 (95) |
|
Nos2
|
Forward Reverse |
CTTTGCCACGGACGAGAC TCATTGTACTCTGAGGGCTGAC |
NM_010927.3 (13) |
Forward and reverse primers used for assessing mRNA expression in mouse lungs using the Roche Univeral ProbeLibrary.
A National Center for Biotechnology Information (NCBI) EntrezGene (http://www.ncbi.nlm.nih.gov/gene) with GenBank Accession numbers and specific Roche Universal Probe numbers in parentheses.
Plasma IgE and BAL fluid cytokine/chemokine analyses
Plasma was analyzed for IgE concentrations using the OptEIA kit (BD Biosciences Pharmingen, San Diego, CA) and adiponectin using ELISA (R&D Systems, Minneapolis, MN). IL-17 levels in BAL fluids and plasma were assessed by ELISA (R&D Systems). Other cytokines and chemokines were assessed in the BAL fluids using the MSD platform. The raw data were measured as electrochemiluminescence signals with the MSD Sector Imager 2400 plate reader (Meso Scale Diagnostics, Rockville, MD) and analyzed using the Discovery Workbench 3.0 software (MSD). Analyte concentrations in BAL fluids were corrected for total protein concentration (determined by Bio-Rad protein assay, Hercules, CA).
Statistics
All data are expressed as mean ± SD, unless indicated. Weight gain by HFD vs. control diet-fed mice was analyzed using a two group (HFD vs. control diet) x time (weekly measures, weeks 1–30) analysis of variance (ANOVA) using repeated measures on the second factor. All other data were analyzed using one-way ANOVAs for multiple comparisons followed by Dunnett’s post-hoc testing using GraphPad Prism 5.03 (GraphPad Software, San Diego, CA). P values <0.05 were considered significant.
Results
High fat diet (HFD) promotes weight gain in mice and weight is unaffected by house dust mite (HDM) or dexamethasone (Dex) treatments
Both groups of mice (lean vs. HFD-fed) gained weight over the study period, with the HFD-fed group gaining significantly more weight than the lean group (Fig. 1A). After 5 wks, the obese group weighed 9g more than the lean group (p<0.001) and after 30 wks, the obese group weighed almost 25g more than the lean group (Fig. 1A and 1B, p<0.001). In addition, within each diet group there were no observed differences in body weights between treatment groups (i.e. ±HDM and ±Dex, Fig. 1B).
Figure 1. High fat diet induces obesity in C57BL/6 mice irrespective of HDM and Dex treatments.
The average weight gain (in grams, g) over the course of the study among lean and obese mice (A) and final average weights among lean and obese groups (±house dust mite, HDM, ±dexamethasone, Dex) (B). ***=p<0.001, comparing high fat-fed obese vs. lean mice subgroups (±HDM, ±Dex).
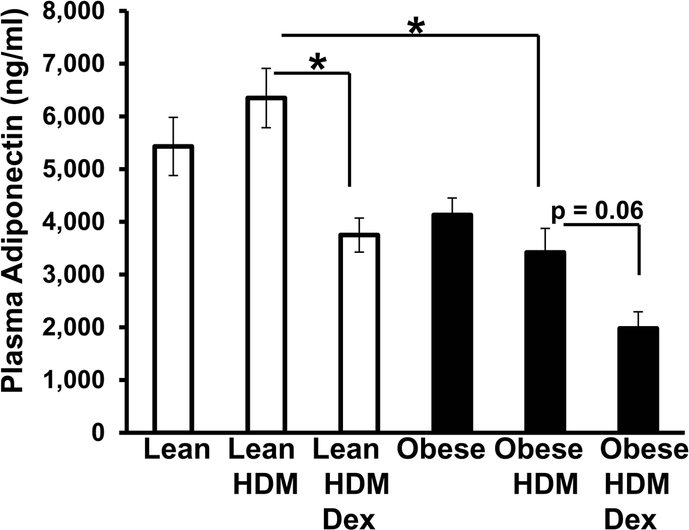
Circulating adiponectin is reduced in obese-HDM mice compared to lean-HDM mice
Plasma adiponectin, an anti-inflammatory adipokine proposed to play a beneficial role in asthma,[20] is inversely correlated with body weight [21]. Under all respective conditions, plasma adiponectin levels in the lean mice tended to be higher than levels in the obese mice (Fig. 2). When compared to obese-HDM mice, lean-HDM mice had significantly higher circulating adiponectin levels (Fig. 2). Interestingly, Dex treatment significantly lowered adiponectin levels in lean-HDM-treated mice and slightly (p=0.06) reduced adiponectin levels in obese-HDM-treated mice (Fig. 2).
Figure 2. Obesity reduces HDM-induced plasma adiponectin levels in obese mice.
Plasma adiponectin levels in lean and obese mice (±house dust mite, HDM, ±dexamethasone, Dex) were determined by ELISA. *=p<0.05 compared to obese-HDM mice.
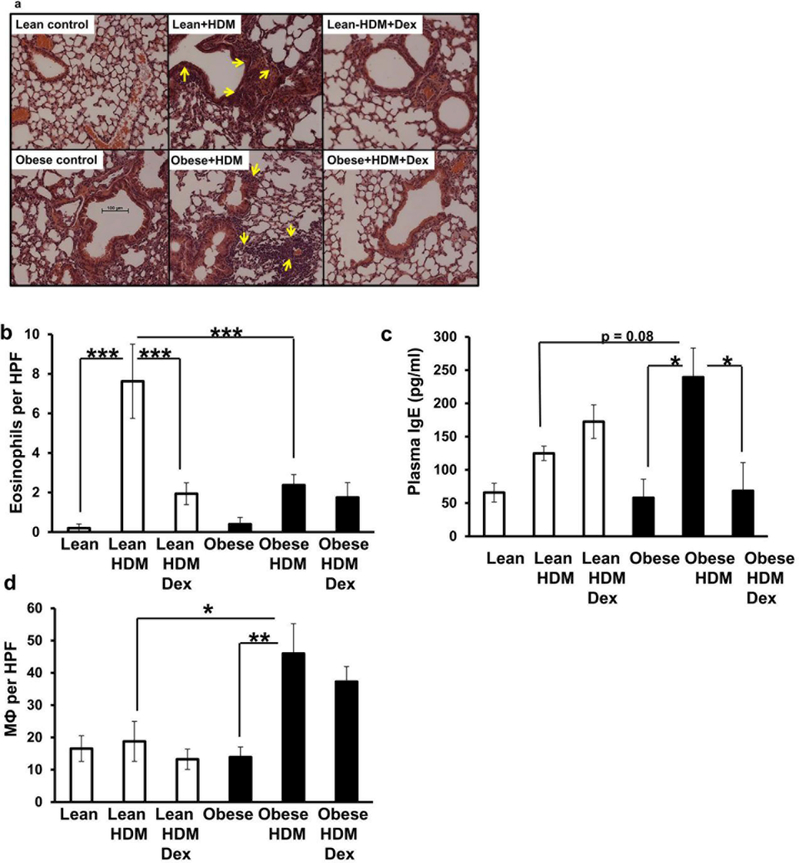
Obesity shifts the cellular infiltrates found in the lungs from eosinophils to macrophages following HDM: Dex does not suppress HDM-induced macrophage accumulation
Next, we examined the lung tissues for patterns of inflammation following HDM (±Dex). Both lean and obese mice exposed to HDM had inflammation around the airways and vasculature when compared to their respective vehicle controls (Fig. 3A). Obese mice showed more lung inflammation following HDM exposure when compared to lean HDM-exposed mice (inflammatory scores: 3.4±0.0.6 [obese-HDM] vs. 1.3±0.6 [lean-HDM], average±SD , p<0.05). Using Congo Red staining, we assessed the eosinophil content of the lung tissues. Lean mice treated with HDM showed significantly enhanced eosinophil infiltration into the lung tissues when compared to lean controls and this was reduced by Dex (Fig. 3B). In contrast, obese mice treated with HDM showed significantly less eosinophil infiltration when compared to the lean HDM-treated mice or saline treated obese mice (Fig. 3B). Consistent with these observations, lean mice had a slight (but not significant) increase in plasma IgE levels following HDM-exposure when compared to lean controls, whereas obese animals exposed to HDM showed significantly elevated plasma IgE levels compared to obese controls (Fig. 3C). Dex treatment significantly reduced plasma IgE levels in the obese-HDM-treated mice (Fig. 3C).
Figure 3. Obesity shifts the cellular infiltrate into lung tissue from eosinophils to macrophages following HDM: Dex does not suppress macrophage accumulation.
Representative H&E sections of lungs obtained from lean and obese mice (±house dust mite, HDM, ± dexamethasone, Dex) (A). Yellow arrows indicate areas of inflammation. Enumeration of Congo Red+ eosinophils per high power field (HPF) in lung tissues obtained from lean and obese mice following (±HDM, ±Dex) (B) and assessment of plasma IgE concentrations (C). Enumeration of F4/80+ lung macrophages (MФ) per HPF in lung tissue sections (D). *=p<0.05, **=p<0.01; ***=p<0.001, as indicated.
Using an F4/80-specific antibody to stain macrophages, we observed that HDM did not significantly affect the macrophage content of the lung tissues in the lean mice (Fig. 3D), but significantly increased the number of macrophages found in the lungs of the obese mice following HDM vs. obese controls (Fig. 3D). The number of macrophages in the obese-HDM lungs did not significantly decrease with Dex treatment (Fig. 3D).
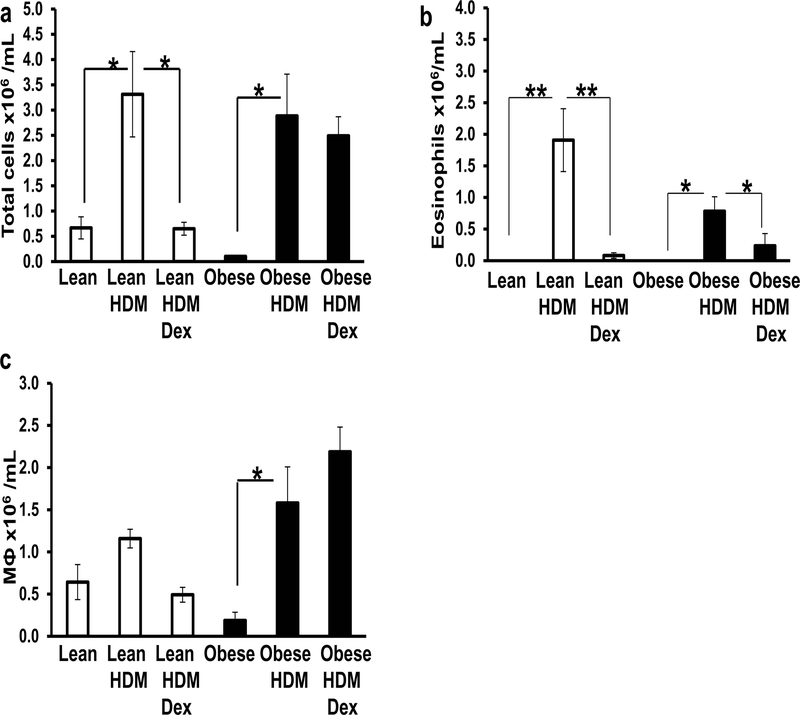
To further assess the inflammatory profiles of the lungs, we examined the cellular content of the BAL fluid. When the total numbers of cells in the BAL fluid were enumerated we found that HDM exposure increased the total cell count, irrespective of diet (Fig. 4A). Dex treatment of lean HDM-exposed mice reduced the accumulation of total cells found in the BAL fluids (Fig. 4A). By contrast, Dex did not reduce the total number of cells found in the obese BAL fluids (Fig. 4A). To compare the inflammatory responses across the groups we examined the types of immune cells present in the BAL fluid. There were no eosinophils present in the BAL fluid of lean or obese mice in the absence of HDM (Fig. 4B), but following HDM exposure BAL fluids from both lean and obese mice had increased numbers of eosinophils (Fig. 4B). The obese mice had lower eosinophil counts (54% less) in their BAL fluid following HDM exposure when compared to HDM-lean mice, although this difference was not significant (Fig 4B). In both lean and obese mice, Dex treatment significantly reduced the number of BAL fluid eosinophils (Fig. 4B). Too few neutrophils and lymphocytes were found in any BAL fluids to enumerate. By contrast, all BAL fluid samples contained macrophages. The numbers of macrophages in the BAL fluids of lean mice did not significantly change following HDM exposure (±Dex) (Fig. 4C). However, HDM-exposure of obese mice significantly increased the number of macrophages found in the lung fluid when compared to obese controls and this increase was not reversed by Dex treatment (Fig. 4C).
Figure 4. BAL fluid shows increased macrophage accumulation in obese-HDM mice: No effect of Dex.
The total number of cells (A) and number of eosinophils (B) and macrophages (MФ) (C) found in the BAL fluid of lean and obese mice following house dust mite (HDM) administration in the presence and absence of dexamethasone (Dex). *=p<0.05 and **=p<0.01, as indicated.
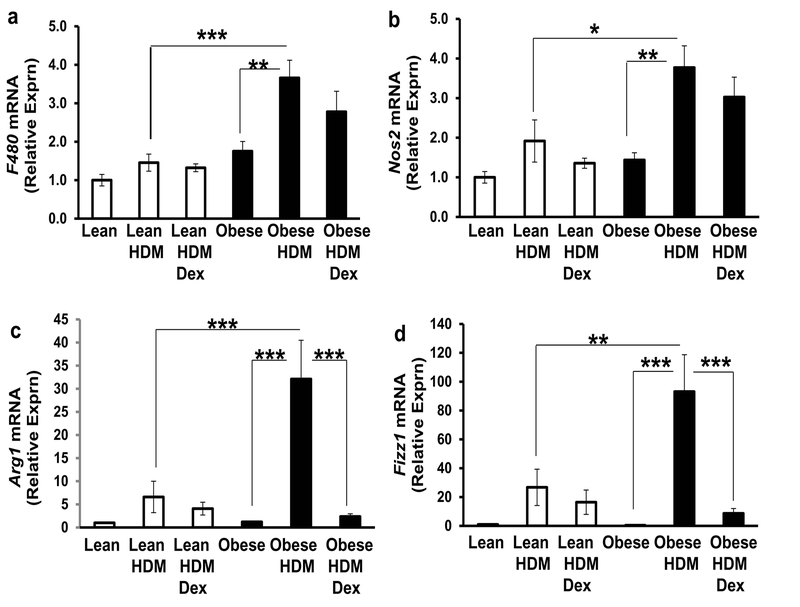
Assessment of macrophage marker expression in the lungs of lean and obese mice following HDM ± Dex
We employed qPCR methods to confirm total macrophage counts and to assess markers of M1 (proinflammatory) and M2 (immunosuppressive, repair) macrophages. Consistent with our previous results, the general (total) macrophage mRNA marker, F480, was relatively unchanged among the lean groups and was significantly increased in the lungs of obese mice following HDM vs. lean-HDM mice and obese controls (Fig. 5A); this expression was not significantly reduced by Dex (Fig. 5A). Lung Nos2 mRNA expression, indicative of M1 macrophages, was significantly enhanced by HDM in obese mice vs. leanHDM mice and vs. obese controls and its expression was not reversed by Dex treatment (Fig. 5B). Fizz1 and Arg1 mRNA expression in the lungs, reflective of M2 macrophages, was enhanced by HDM in the setting of obesity (when compared to lean-HDM mice and obese controls) and decreased by Dex (Figs. 5C and 5D, respectively).
Figure 5. Obesity differentially regulates gene expression markers for macrophages in the lungs following HDM (±Dex).
The effect of house dust mite (HDM) administration (± dexamethasone, Dex) on the expression of genes indicative of total (F480 mRNA, A), as well as M1 (Nos2 mRNA, B) and M2 (Arg1 mRNA, C and Fizz1 mRNA, D) macrophages in lung tissues obtained from lean and obese mice. Data are shown as relative mRNA expression with the untreated lean control group set to 1. *=p<0.05, **=p<0.01; ***=p<0.001, as indicated.
HDM exposure increases cytokines in BAL fluid of obese mice: Effect of Dex
Allergic asthma is linked to Th2 immune processes, whereas low level chronic inflammation accompanying obesity is associated with Th1 responses. Next, we examined the effect of diet and Dex treatment on Th1 and Th2 inflammatory mediators in the BAL fluids. IL-2, a cytokine secreted by Th1 cells, was not detected in any of the BAL fluids obtained from lean mice, regardless of treatment (Fig. 6A). By contrast, IL-2 was detected in the BAL fluids obtained from obese mice ±HDM exposure (Fig. 6A); IL-2 levels in the obese-HDM BAL fluids were decreased by Dex, although not significantly (Fig. 6A). IL-4 and IL-5, Th2 cytokines elevated in asthmatics and experimental models of asthma [22], were slightly (but not significantly) increased in the BAL fluid of lean-HDM mice (Fig. 6B and 6C). However, both IL-4 and IL-5 levels were substantially higher following HDM in the setting of obesity (Fig. 6B and 6C). Treatment of obese-HDM mice with Dex significantly reduced both IL-4 and IL-5 concentrations in their BAL fluids (Fig. 6B and 6C).
Figure 6. HDM increases IL-4 and IL-5 levels in BAL fluid obtained from obese mice: Reversed by Dex.
IL-2 (A), IL-4 (B), and IL-5 (C) protein levels in bronchoalveolar lavage (BAL) fluids in lean and obese mice following house dust mite (HDM) ±dexamethasone (Dex). *=p<0.05 and **=p<0.01, as indicated.
HDM with obesity alter BAL levels of CXCL1, a marker of macrophage infiltration in the lung
IL-17 has been implicated in obesity [23], asthma severity [24], and steroid resistance in asthma [25], as well as chemokine production (e.g. CXCL1 [26]) and leukocyte infiltration [27]. IL-17A was not detected in any of the BAL samples (data not shown). Exposure of both lean and obese mice to HDM significantly increased CXCL1 levels in the BAL fluids when compared to vehicle-treated controls (Fig. 7) and this increase was not significantly reduced by Dex (Fig. 7).
Figure 7. CXCL1 protein levels in the lungs of obese and lean asthmatics.
The effect of house dust mite (HDM) (±dexamethasone, Dex) on CXCL1 protein levels in the BAL fluids obtained from lean and obese mice were determined by ELISA. Data are shown as pg CXCL1 per gram (g) lung tissue. *=p<0.05 and **=p<0.01, as indicated.
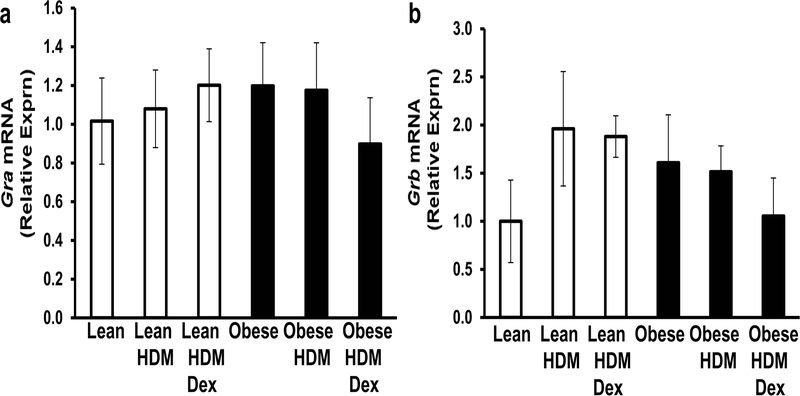
The effect of HDM (±Dex) on glucocorticoid receptor mRNA expression in lean and obese mouse lungs
Next, we assessed glucocorticoid receptor (GR) mRNA expression in the lungs. The Gr gene encodes two splice variants, GRα and GRβ. GRα, the classic receptor, binds glucocorticoids and promotes the activation of genes encoding anti-inflammatory factors and prevents the activation of genes that encode pro-inflammatory factors [28]. In our model, Gra mRNA expression was not significantly altered by diet, HDM, or Dex treatment (Fig. 8A). GRβ is a dominant negative inhibitor of GRα and thus, inhibits its function [28]. Although not statistically significant, obese control mice showed slightly increased Grb mRNA expression in the lungs when compared to lean controls (Fig. 8B) and this expression was not significantly affected by HDM (±Dex) treatment (Fig. 8B).
Figure 8. Glucocorticoid receptor mRNA expression in the lungs of lean and obese mice.
The effect of house dust mite (HDM) (±dexamethasone, Dex) on Gra (A) and Grb (B) mRNA expression in the lungs of lean and obese mice were determined by qPCR. Data are shown as relative mRNA expression with the untreated lean control group set to 1.
Discussion
Approximately 7–8% of the American population suffers with asthma [4] and approximately 5% of asthmatics have severe asthmatic disease, that in many cases, is refractory to traditional therapies [29]. Asthma is not a single disease, but rather a heterogeneous condition characterized by numerous and sometimes ‘mixed’ phenotypes [11;30;31]. Many factors contributing to the pathogenesis of asthma have been described, including obesity, which has been increasing at an alarming rate [32], with approximately one third of all men, women, and children in the US currently obese [33]. Several reports support that obesity promotes the development of asthma and when combined with asthma creates a phenotype that is more severe and more difficult to manage [3;30;31]. While obesity does not influence steroid absorption, clearance or metabolism [34], it has been proposed to promote a non-eosinophilic inflammatory infiltration into the lungs and induce the expression of the antagonistic GRβ [11;31]. Therefore, we used a mouse model of diet-induced obesity and HDM-induced airway disease, where chronic and severe obesity preceded HDM-induced airway inflammation, to examine the effect of obesity on lung inflammation and steroid responsiveness.
Adiponectin, an adipokine that regulates glucose metabolism and insulin sensitivity, exerts antiinflammatory effects and has been implicated in asthma. More specifically, adiponectin−/− and adiponectin receptor−/− mice show significantly increased allergic airway inflammation, with more eosinophilia and monocyte infiltration into the lung [35;36]. By contrast, adiponectin promotes an M2 ‘anti-inflammatory’ macrophage phenotype [37] and improved outcomes in mouse models of allergic airway hyperresponsiveness [36;38]. As predicted based on previous reports [21], we found that the lean-HDM mice had significantly higher plasma adiponectin levels than obese-HDM mice (Fig. 2). Adiponectin levels in lean-HDM mice were reduced by Dex, whereas in obese-HDM mice adiponectin levels were lowered by Dex, but not significantly (Fig. 2). These findings are consistent with previous studies describing the negative regulation of adiponectin gene expression and protein release by glucocorticoids in vitro [39] and in vivo [40] and thus, highlight an important question: if adiponectin is beneficial in asthma, would preserving its expression during treatment improve outcomes?
We found reduced HDM-induced eosinophilia in our C57BL/6 mice than that previously described for BALB/c mice (vs. C57BL/6 mice) [41]. This is not surprising given that C57BL/6 and BALB/c mice are classical Th1 and Th2 strains, respectively [42]. However, we chose the C57BL/6 strain because of its proclivity towards diet-induced obesity, while BALB/c mice are obese-resistant. In our combined model of obesity and asthma, we found slightly higher IgE levels, fewer eosinophils in the obese-HDM mice when compared to lean-HDM mice. As expected, Dex suppressed the eosinophil counts in both lean-HDM and obese-HDM mice (Fig. 4B). By contrast, more macrophages were found in the lungs of the obese-HDM mice when compared to obese controls and lean (±HDM) mice.
The role of macrophages in asthma is not completely understood. Alveolar macrophages are the dominant immune cell type found in the lung under basal conditions and in asthma they regulate the contractility of the airway smooth muscle cells through their effects on histamine release, production of free radicals, stimulation of Th2 cytokine release by T cells and by promoting cholinergic signaling [43]. While macrophage counts in the BAL fluid and lung tissues, as well as F480 mRNA expression (reflecting total macrophages) in the lungs of lean groups were unaffected by HDM (±Dex), the lungs of obese mice showed a significant increase in F4/80-staining, F480 mRNA expression, and the numbers of macrophages in their BAL fluids following HDM exposure and these increases were not reversed by Dex (Figs. 3D, 4C and 5A). Similarly, in severe asthmatic patients, macrophages collected from BALs show decreased sensitivity to glucocorticoids [44]. The appearance of macrophages in the lungs of obese-HDM mice along with enhanced inflammation (and lower circulating adiponectin levels) is consistent with increased MCP-1/CCL2 levels in the lungs, monocyte infiltration and more severe airway inflammation reported in adiponectin−/− and adiponectin receptor−/− mice using allergic airway hyperresponsiveness models [35;36] and a less ‘Th2-dependent’ asthma phenotype observed in humans [45;46]. Although we were unable to detect IL-7 in the circulation of the mice, it has been shown to be upregulated in asthmatic lungs and proposed to contribute to disease progression by inducing the expression of numerous chemokines, including CXCL1, which is implicated in leukocyte recruitment [47;48]. Macrophages, including alveolar macrophages are a major source of CXCL1 and they may contribute to elevated CXCL1 levels found in the lungs of obese HDM-mice when compared to control obese mice (Fig. 7).
The link between obesity and low grade inflammation (characterized by increased M1 macrophages) has been proposed to underlie asthma pathogenesis and even severity in some cases [49]. More recent studies reveal increased M2 macrophages in the lung tissues and BAL fluids of asthma patients and in mouse models of allergic airway infiltration [50–52] and M2 macrophages have been associated with increased asthma/allergic airway inflammation severity [53]. Despite these reports, the role of M2 macrophages in allergic asthma is somewhat controversial and the M2 phenotype has been proposed to be a result of an increased Th2 response [54]. Consistent with our observation that Dex did not reduce HDM-induced Nos2 mRNA expression (M1 marker) in obese mice, Goleva and co-workers showed that steroid resistant asthmatics have more M1 than M2 macrophages in their BAL fluids [55]. Similarly, obesity can shift the adipose tissue macrophage phenotype towards M1 with increased Nos2 mRNA expression [56–58]. It is important to note that the assessment of distinct macrophage phenotypes (i.e. M1 vs. M2) in vivo has been criticized as the current view ignores the source and context of stimuli, that these stimuli do not exist alone in tissues, that macrophages are plastic, and that macrophages do not appear to form totally distinct activation subsets or expand clonally [59]. Our results show that M1 and M2 (or mixed M1/M2) macrophages are present in the lungs of obese-HDM mice based on gene expression markers, as previously described [60–62]. This method of assessment significantly limits our conclusions regarding macrophage subsets. While both types of macrophages are proposed to contribute to asthma development and progression [59], some types might be less sensitive to steroid regulation than others and these may be enhanced in the setting of obesity. Future studies are required to define the macrophage subsets in the lungs of obese and lean ‘asthmatic’ mice and humans and to assess their responsiveness to glucocorticoids.
HDM-induced airway inflammation in lean mice mediates significant inflammatory changes in the lung tissues and BAL fluid cellular content, which can be ameliorated by glucocorticoids [63]. In this study numerous aspects of obesity-related lung inflammation induced by HDM were either significantly or slightly reduced by glucocorticoids, including BAL adiponectin levels, plasma IgE levels, lung eosinophil numbers, BAL IL-2 levels, and M2 macrophage marker expression, while other macrophage-related markers were not (e.g. total cell count and macrophage count in BAL fluid, macrophages in lung tissues, as well as F480 mRNA expression (total macrophages), and Nos2 mRNA (M2 macrophage marker)). Glucocorticoids diffuse across cellular membranes, bind to GRα in the cytoplasm, and then the glucocorticoid-GRα complex is transported into the nucleus where it exerts anti-inflammatory effects via up-regulation of anti-inflammatory gene expression and down-regulation of pro-inflammatory gene expression [64]. In contrast, GRβ directly binds DNA to block glucocorticoid-GRα interactions and thus serves to inhibit glucocorticoid action [64]. In this study, we found no differences in either Gra or Grb mRNA expression among the various diet and HDM vs. saline groups (Figs. 8A and 8B). GRβ expression has been reported to be elevated in steroid resistant asthmatics [65;66] and highly expressed in the lungs of patients who suffered fatal asthma attacks [67]. We did not observe this effect at the level of mRNA expression. These results need further investigation because we were unable to assess GRα and GRβ protein expression due to the lack of specific GRα and GRβ reagents for mice. In addition, because we used whole lung homogenates, we did not assess GRα and GRβ expression by various lung cell types. Furthermore, we did not perform pulmonary function tests so we are unable to conclude that Dex affected the resolution of airway hyperresponsiveness in the mice. Clearly, more investigation is needed to further examine the effects of obesity on glucocorticoid sensitivity and the effects of glucocorticoids on specific cell types in obese asthma models.
In summary, when compared to lean-HDM mice the obese-HDM mice had reduced ‘anti-inflammatory’ plasma adiponectin levels, enhanced lung inflammation and BAL fluid macrophage counts, as well as increased expression of markers for total, M1, and M2 macrophages in their lungs, elevated Th1 and Th2 cytokine levels (or mixed Th1, Th2, M1, and M2 response), and CXCL1 in their BAL fluid. Numerous outcomes (e.g. IL-2, IL-4, IL-5 levels, lung and BAL fluid eosinophils, plasma IgE levels, and M2 macrophage markers) were suppressed by Dex in lean and/or obese mice. However, many of these outcomes were not reduced by Dex in obese-HDM mice, including the number of lung tissue and BAL fluid macrophages, total and M1 macrophage mRNA markers, and BAL fluid CXCL1 levels. These findings clearly support previous reports suggesting that obesity modulates the development of allergic asthma and that when obesity accompanies asthma, it leads to a phenotype that is more severe and in some aspects, less sensitive to steroid treatment. Furthermore, the results of this study highlight lung macrophages as an important cellular component of the obese-asthmatic phenotype and show that lung macrophage infiltration appears to be less sensitive to regulation by steroids. Future studies should confirm the presence of macrophages in the lungs of obese vs. lean asthma patients and examine whether these differences correlate with steroid resistance. Furthermore, all immune cells found in the lungs of lean vs. obese asthmatics could be assessed for their specific sensitivity to steroids. Profiling the immune cells present in the lungs of severe asthmatics and their responses to steroids could be useful in choosing the best therapies for asthma patients. Finally, our results support investigating additional mechanisms that might contribute to blunted steroid responses in obese vs. lean asthmatics.
Financial support
JD and LH were Fellows supported by a T32 training grant (AI083223–06, V Bonagura PI), The Feinstein Institute for Medical Research, and Hofstra North Shore-LIJ School of Medicine (LW)
Abbreviations
- ANOVA
analysis of variance
- BAL
bronchoalveolar lavage
- CXCL1
CXC-motif ligand 1
- Dex
dexamethasone
- GRα
glucocorticoid receptor alpha
- GRβ
glucocorticoid receptor beta
- HDM
house dust mite
- H&E
hematoxylin and eosin
- HFD
high fat diet
- qPCR
quantitative polymerase chain reaction
Footnotes
The authors report no conflicts of interest.
References
- 1.Yang L, Colditz GA: Prevalence of Overweight and Obesity in the United States, 2007–2012. JAMA Intern Med 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RW, Stephens JM: Fat in flames: Influence of cytokines and pattern recognition receptors on adipocyte lipolysis. Am J Physiol Endocrinol Metab 2015;ajpendo. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B: Emerging interface between metabolic syndrome and asthma. Am J Respir Cell Mol Biol 2011;44:270–275. [DOI] [PubMed] [Google Scholar]
- 4.CDC Asthma Data and Surveillance. http://www.cdc.gov/asthma/asthmadata.htm . 2015. Ref Type: Electronic Citation
- 5.Delgado J, Barranco P, Quirce S: Obesity and asthma. J Investig Allergol Clin Immunol 2008;18:420–425. [PubMed] [Google Scholar]
- 6.Beuther DA: Recent insight into obesity and asthma. Curr Opin Pulm Med 2010;16:64–70. [DOI] [PubMed] [Google Scholar]
- 7.Beuther DA, Sutherland ER: Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007;175:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A: Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy 2009;39:700–707. [DOI] [PubMed] [Google Scholar]
- 9.Hjellvik V, Tverdal A, Furu K: Body mass index as predictor for asthma: a cohort study of 118,723 males and females. Eur Respir J 2010;35:1235–1242. [DOI] [PubMed] [Google Scholar]
- 10.Weiss ST: Obesity: insight into the origins of asthma. Nat Immunol 2005;6:537–539. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE: Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012;18:716–725. [DOI] [PubMed] [Google Scholar]
- 12.Boulet LP, Franssen E: Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med 2007;101:2240–2247. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM: Influence of body mass index on the response to asthma controller agents. Eur Respir J 2006;27:495–503. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M: Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 2004;169:378–385. [DOI] [PubMed] [Google Scholar]
- 15.Yao X, Dai C, Fredriksson K, Lam J, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, Jeffries N, Lin J, Kaler M, Shamburek R, Costello R, Csako G, Dahl M, Nordestgaard BG, Remaley AT, Levine SJ: Human apolipoprotein E genotypes differentially modify house dust mite-induced airway disease in mice. Am J Physiol Lung Cell Mol Physiol 2012;302:L206-L215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papapetropoulos A, Simoes DC, Xanthou G, Roussos C, Gratziou C: Soluble guanylyl cyclase expression is reduced in allergic asthma. Am J Physiol Lung Cell Mol Physiol 2006;290:L179-L184. [DOI] [PubMed] [Google Scholar]
- 17.Meyerholz DK, Griffin MA, Castilow EM, Varga SM: Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol 2009;37:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solanki MH, Chatterjee PK, Gupta M, Xue X, Plagov A, Metz MH, Mintz R, Singhal PC, Metz CN: Magnesium protects against cisplatin-induced acute kidney injury by regulating platinum accumulation. Am J Physiol Renal Physiol 2014;307:F369-F384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinds TD Jr., Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, Sanchez ER: Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol Endocrinol 2010;24:1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigro E, Daniele A, Scudiero O, Ludovica MM, Roviezzo F, D’Agostino B, Mazzarella G, Bianco A: Adiponectin in asthma: implications for phenotyping. Curr Protein Pept Sci 2015;16:182–187. [DOI] [PubMed] [Google Scholar]
- 21.Hossain MM, Mukheem A, Kamarul T: The prevention and treatment of hypoadiponectinemiaassociated human diseases by up-regulation of plasma adiponectin. Life Sci 2015;135:55–67. doi: 10.1016/j.lfs.2015.03.010. Epub;%2015 Mar 26.:55–67. [DOI] [PubMed] [Google Scholar]
- 22.Kips JC, Tournoy KG, Pauwels RA: New anti-asthma therapies: suppression of the effect of interleukin (IL)-4 and IL-5. Eur Respir J 2001;17:499–506. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed M, Gaffen SL: IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev 2010;21:449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YH, Wills-Karp M: The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep 2011;11:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK: TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008;181:4089–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujie H, Niu K, Ohba M, Tomioka Y, Kitazawa H, Nagashima K, Ohrui T, Numasaki M: A distinct regulatory role of Th17 cytokines IL-17A and IL-17F in chemokine secretion from lung microvascular endothelial cells. Inflammation 2012;35:1119–1131. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C: A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes PJ: Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 2010;120:76–85. [DOI] [PubMed] [Google Scholar]
- 29.Dennis RJ, Solarte I, Rodrigo G: Asthma in adults. BMJ Clin Evid 2011;2011. pii: 1512.:1512. [PMC free article] [PubMed] [Google Scholar]
- 30.Gibeon D, Batuwita K, Osmond M, Heaney LG, Brightling CE, Niven R, Mansur A, Chaudhuri R, Bucknall CE, Rowe A, Guo Y, Bhavsar PK, Chung KF, Menzies-Gow A: Obesity-associated severe asthma represents a distinct clinical phenotype: analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest 2013;143:406–414. [DOI] [PubMed] [Google Scholar]
- 31.Lugogo NL, Kraft M, Dixon AE: Does obesity produce a distinct asthma phenotype? J Appl Physiol (1985) 2010;108:729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL: The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378:804–814. [DOI] [PubMed] [Google Scholar]
- 33.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM: Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004;291:2847–2850. [DOI] [PubMed] [Google Scholar]
- 34.Davidson JM, Covar RA, Brown E, Spahn JD: Does Obesity Influence Steroid Absorption, Metabolism, and In Vitro Steroid Response in Adults with Severe Asthma. Journal of Allergy and Clinical Immunology 2009;123:156–159. [Google Scholar]
- 35.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD: Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol 2009;41:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AS, Kasahara DI, Verbout NG, Fedulov AV, Zhu M, Si H, Wurmbrand AP, Hug C, Ranscht B, Shore SA: Role of the adiponectin binding protein, T-cadherin (Cdh13), in allergic airways responses in mice. PLoS ONE 2012;7:e41088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S: Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol 2010;299:H656-H663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shore SA, Terry RD, Flynt L, Xu A, Hug C: Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006;118:389–395. [DOI] [PubMed] [Google Scholar]
- 39.gawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, Vidal-Puig A, Jones R, Considine RV: Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes Res 2005;13:662–669. [DOI] [PubMed] [Google Scholar]
- 40.Halleux CM, Takahashi M, Delporte ML, Detry R, Funahashi T, Matsuzawa Y, Brichard SM: Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem Biophys Res Commun 2001;288:1102–1107. [DOI] [PubMed] [Google Scholar]
- 41.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M: Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 2004;169:378–385. [DOI] [PubMed] [Google Scholar]
- 42.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van HC, Tournoy K, Louis R, Foidart JM, Noel A, Cataldo DD: Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res 2009;58:845–854. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Kumar RK, Hansbro PM, Foster PS: Emerging roles of pulmonary macrophages in driving the development of severe asthma. J Leukoc Biol 2012;91:557–569. [DOI] [PubMed] [Google Scholar]
- 44.Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, Chung KF: Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax 2008;63:784–790. [DOI] [PubMed] [Google Scholar]
- 45.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH: Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008;178:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leiria LO, Martins MA, Saad MJ: Obesity and asthma: beyond T(H)2 inflammation. Metabolism 2015;64:172–181. [DOI] [PubMed] [Google Scholar]
- 47.Traves SL, Donnelly LE: Th17 cells in airway diseases. Curr Mol Med 2008;8:416–426. [DOI] [PubMed] [Google Scholar]
- 48.Hong JY, Chung Y, Steenrod J, Chen Q, Lei J, Comstock AT, Goldsmith AM, Bentley JK, Sajjan US, Hershenson MB: Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma. Respir Res 2014;15:63 doi: 10.1186/1465-9921-15-63.:6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shore SA: Obesity and asthma: possible mechanisms. J Allergy Clin Immunol 2008;121:1087–1093. [DOI] [PubMed] [Google Scholar]
- 50.Hong JY, Chung Y, Steenrod J, Chen Q, Lei J, Comstock AT, Goldsmith AM, Bentley JK, Sajjan US, Hershenson MB: Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma. Respir Res 2014;15:63 doi: 10.1186/1465-9921-15-63.:6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melgert BN, Oriss TB, Qi Z, xon-McCarthy B, Geerlings M, Hylkema MN, Ray A: Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol 2010;42:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN: More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol 2011;127:831–833. [DOI] [PubMed] [Google Scholar]
- 53.Draijer C, Robbe P, Boorsma CE, Hylkema MN, Melgert BN: Characterization of macrophage phenotypes in three murine models of house-dust-mite-induced asthma. Mediators Inflamm 2013;2013:632049 doi: 10.1155/2013/632049. Epub;%2013 Feb 27.:632049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nieuwenhuizen NE, Kirstein F, Jayakumar J, Emedi B, Hurdayal R, Horsnell WG, Lopata AL, Brombacher F: Allergic airway disease is unaffected by the absence of IL-4Ralpha-dependent alternatively activated macrophages. J Allergy Clin Immunol 2012;130:743–750. [DOI] [PubMed] [Google Scholar]
- 55.Goleva E, Hauk PJ, Hall CF, Liu AH, Riches DW, Martin RJ, Leung DY: Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol 2008;122:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K: Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005;54:2277–2286. [DOI] [PubMed] [Google Scholar]
- 57.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr.: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H: Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez FO, Gordon S: The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13 doi: 10.12703/P6-13 eCollection;%2014.:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon S: Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 62.Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, Kindt A, Van den BR, Leenen PJ, De BP, Ghassabeh GH: Macrophage galactosetype C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol 2005;77:321–327. [DOI] [PubMed] [Google Scholar]
- 63.Ulrich K, Hincks JS, Walsh R, Wetterstrand EM, Fidock MD, Sreckovic S, Lamb DJ, Douglas GJ, Yeadon M, Perros-Huguet C, Evans SM: Anti-inflammatory modulation of chronic airway inflammation in the murine house dust mite model. Pulm Pharmacol Ther 2008;21:637–647. [DOI] [PubMed] [Google Scholar]
- 64.Rhen T, Cidlowski JA: Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med 2005;%20;353:1711–1723. [DOI] [PubMed] [Google Scholar]
- 65.Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, Chrousos GP, Szefler SJ, Leung DY: Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med 1999;159:1600–1604. [DOI] [PubMed] [Google Scholar]
- 66.Goleva E, Li LB, Eves PT, Strand MJ, Martin RJ, Leung DY: Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am J Respir Crit Care Med 2006;173:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christodoulopoulos P, Leung DY, Elliott MW, Hogg JC, Muro S, Toda M, Laberge S, Hamid QA: Increased number of glucocorticoid receptor-beta-expressing cells in the airways in fatal asthma. J Allergy Clin Immunol 2000;106:479–484. [DOI] [PubMed] [Google Scholar]