Abstract
This study reports the synthesis and testing of a family of rhodamine pro-fluorophores and an enzyme capable of converting pro-fluorophores to Rhodamine 110. We prepared a library of simple N,N′-diacyl rhodamines and investigated porcine liver esterase (PLE) as an enzyme to activate rhodamine-based pro-fluorophores. A PLE-expressing cell line generated an increase in fluorescence rapidly upon pro-fluorophore addition demonstrating the rhodamine pro-fluorophores are readily taken up and fluorescent upon PLE-mediated release. Rhodamine pro-fluorophore amides trifluoroacetamide (TFAm) and proponamide (PAm) appeared to be the best substrates using a cell-based assay using PLE expressing HEK293. Our pro-fluorophore series showed diffusion into live cells and resisted endogenous hydrolysis. The use of our engineered cell line containing the exogenous enzyme PLE demonstrated the rigorousness of amide masking when compared to cells not containing PLE. This simple and selective pro-fluorophore rhodamine pair with PLE offers the potential to be used in vitro and in vivo fluorescence based assays.
Keywords: cell imaging, porcine liver esterase, pro-fluorophore, rhodamines
N,N′-Diacyl rhodamines were investigated as pro-fluorophores and became fluorescent in the presence of Porcine Liver Esterase (PLE). We were delighted to find that N,N′-propionyl rhodamine pro-fluorophore showed bright, cytoplasmic fluorescence in PLE-expressing cells thus demonstrating suitability for cellular imaging. This selective pro-fluorophore rhodamine pair with PLE offers the potential to be used in vitro and in vivo fluorescence based assays.
For more than 40 years fluorescent molecules like fluorescein and rhodamine have played critical roles in both basic and clinical research.[1] Uses of fluorescent dyes range from fluorescently tagged antibodies useful for determining protein expression and localization, to reporters attached to sensor motifs (e.g. metal ion, reactive oxygen species, pH) to serving as substrates for a broad variety of enzymatic activities.[2]
In the case of enzyme substrates, a common approach is to generate a non-fluorescent pro-fluorophore that acts as a substrate of an enzyme. By the action of the enzyme on the pro-fluorophore substrate a fluorescent product is formed. This approach has been applied using a wide range of enzymes including non-specific esterases (e.g. fluorescein diacetate), peptidases (e.g. rhodamine amides), and glycosidases (e.g. bis-β-galactoside) and others.[3]
A commonly used approach for determining where and when proteins are expressed has been use of β-galactosidase combined with a pro-fluorophore substrate.[4] These non-fluorescent pro-fluorophores are unable to be activated by endogenously expressed enzymes in most animal cells. However, when β-galactosidase is expressed under the control of a cell-type-specific/developmentally specific promoter, the cells expressing the enzyme become competent to activate these dyes. Thus, enabling studies of temporal and spatial localization of the promoter of interest as a surrogate for the gene whose expression is normally driven by the promoter.[5] The most commonly used of these pro-fluorophores is 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, commonly known as X-gal.[6] Unfortunately the product of β-lactamase cleavage of X-gal is a colored but not fluorescent, thus the X-gal/β-galactosidase paired system suffers a lack of sensitivity. In order address the lack of sensitivity of colorimetric products, glycosylated fluorescein pro-fluorophores have been previously reported,[7] but have yet to find widespread use, possibly due to limitations of loading[8] or applied to fixed cells.[9] In addition, fluoresceins are notably pH-sensitive within the range of physiological pH,[10] further limiting their utility.
In recent years, use of fluorescent proteins such as GFP has to some degree replaced the use of β-galactosidase for localization of expression since years of directed evolution has resulted in the bright, largely pH-insensitive molecules that do not require the addition of exogenous substrates.[11] However, enzyme-based activation of fluorescent dyes offers the advantage of catalysis—many very bright fluorescent molecules can be produced by a single molecule of the activating enzyme. This can lead to dramatic improvements in sensitivity when expression levels of the protein of interest are low. Therefore, even with the advent of GFP-based approaches, there is still a need for enzyme-activated fluorescent dyes.
To investigate opportunities to produce bright, pH-insensitive fluorescent pro-fluorophores that are freely cell penetrant and easy and inexpensive to make, we first considered the use of rhodamine-based pro-fluorophores. Like fluorescein, Rhodamine 110 is bright and readily available,[12] but unlike fluorescein, their fluorescence is considerably less pH sensitive in the physiological range.[10,13] Prior work describing the activation of a non-fluorescent pro-fluorophore methylcyclopropylcarboxylate methyl ester derivative of fluorescein by porcine liver esterase (PLE) led us to investigate this enzyme more closely.[14] Interestingly, though the name PLE identifies the enzyme as an esterase, PLE is known to also exhibit amidase activity.[15] Therefore, we chose to investigate PLE as possible enzyme to activate rhodamine-based pro-fluorophores. Previously the compound 1 b had been reported as non-fluorescent].[12b] Thus we reasoned that similar simple rhodamine amides would be likewise non-fluorescent. To explore the use of simple amide-protected rhodamines as pro-fluorophores which could be activated by PLE, we prepared a library of simple rhodamine amides (Figure 1). Commercial Rhodamine 110 hydrochloride was acylated using either the corresponding anhydride or acid chloride under standard reaction conditions to afford the N,N′-diacyl rhodamine product in high yield (42–91%).[12a]
Figure 1.
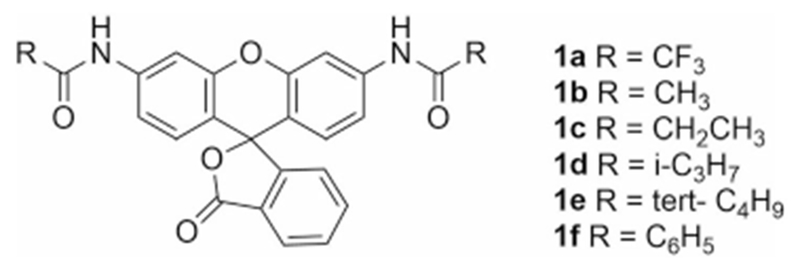
N,N′-Diacyl rhodamines prepared for this study.
As predicted, the N,N-diacyl rhodamines were colorless, non-fluorescent (Figure 2a), and very stable in aqueous solution in the absence of PLE (t1/2=≈2 days at 22° C for 1 a, and > than 1 week for the other species 1 b–1 f), consistent with the corresponding spirocyclic structure.[16] However, in the presence of PLE the pro-fluorophores were converted to the highly fluorescent Rhodamine 110 (Figure 2a).[17] When we evaluated the library of rhodamine-amide pro-fluorophores in vitro we found that all of the pro-fluorophores could be activated by PLE but that some appeared to be better substrates than others with compounds 1 a and 1 c showing the most effective conversion to the fluorescent Rhodamine 110 (Figure 2b). Calculated kinetic parameters (Table S1) range from a KM 2.06 μm and kcat/KM 2.95 × 104m−1 S−1 for 1 a to KM 7.45 μm and kcat/KM 3.81 × 101 m−1 S−1 for 1 f. The more effective conversion of 1 a by PLE may be due in part to the strongly electron-withdrawing nature of the trifluoromethyl moiety. We were somewhat surprised to find, based on their steric and electronic similarity, that 1 b was a less preferred substrate when compared to 1 c.
Figure 2.
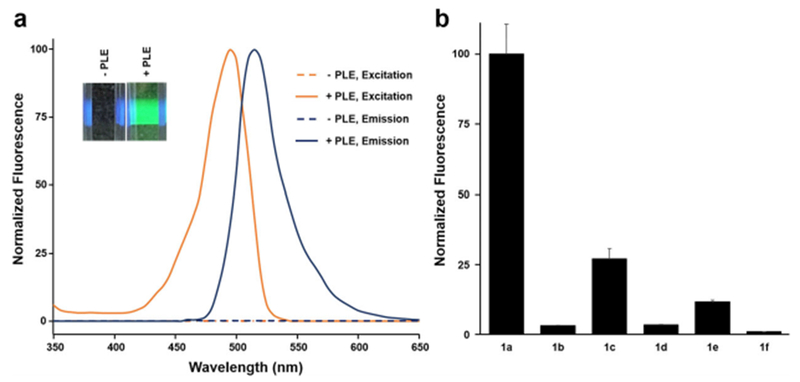
In vitro activation of amidated rhodamine pro-fluorophores by PLE. [a] Absorption and emission spectra of 1 c in the presence and absence of PLE. The inset shows a cuvette containing 1 c in the presence or absence of PLE illuminated with a violet laser. [b] Comparison of various amidated rhodamines (10 μm) for 2 min at 22°C in the presence of 30 nm PLE (excitation 480±20 nm, emission 540±20 nm). Y axis are normalized to the maximum observed fluorescence under the experimental conditions. Error bars represent the standard error of the mean (SEM) of 4 independent replicates.
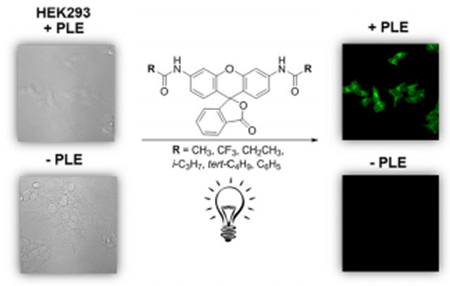
Encouraged by the in vitro results we sought to determine whether these acylated rhodamine pro-fluorophores were substrates for enzymes natively expressed in a commonly used mammalian cells line, HEK293 cells. For these studies, we constructed a HEK293 cell line which stably expresses PLE. We then tested our collection of acyl rhodamine pro-fluorophores with both untransfected and PLE-expressing HEK293 cells.[18] As shown in Figure 3a, addition of 3 μm 1 c to PLE-expressing HEK293 cells in a 384-well assay plate produced a rapid increase in fluorescence demonstrating that the pro-fluorophore readily enters into the cells. After the 6 min time point a modest decrease in fluorescence is noted, possibly as a result of photobleaching. However, when wild-type HEK293 cells were treated with 3 μm 1 c, no increase in fluorescence was observed. Figure 3b shows an image 384-plate wells containing ≈20000 cells per well treated with 10 μm of amidated rhodamine pro-fluorophores. In all cases where amidated rhodamine pro-fluorophores were exposed to wild-type HEK293 cells, fluorescence was not observed after 15 minutes of incubation at 22°C. However, when the rhodamine pro-fluorophores were exposed to PLE-expressing HEK cells, fluorescence was observed. The degree of fluorescence observed after 2 min for each of the samples was similar but not identical to the in vitro data with 1 a and 1 c producing the largest fold increase in fluorescence (Table S2). In these studies acetylated fluorescein pro-fluorophore, fluorescein diacetate (FDA), was included to demonstrate the ability of non-PLE-expressing HEK-293 cells to activate a simple ester-protected fluorescein.
Figure 3.
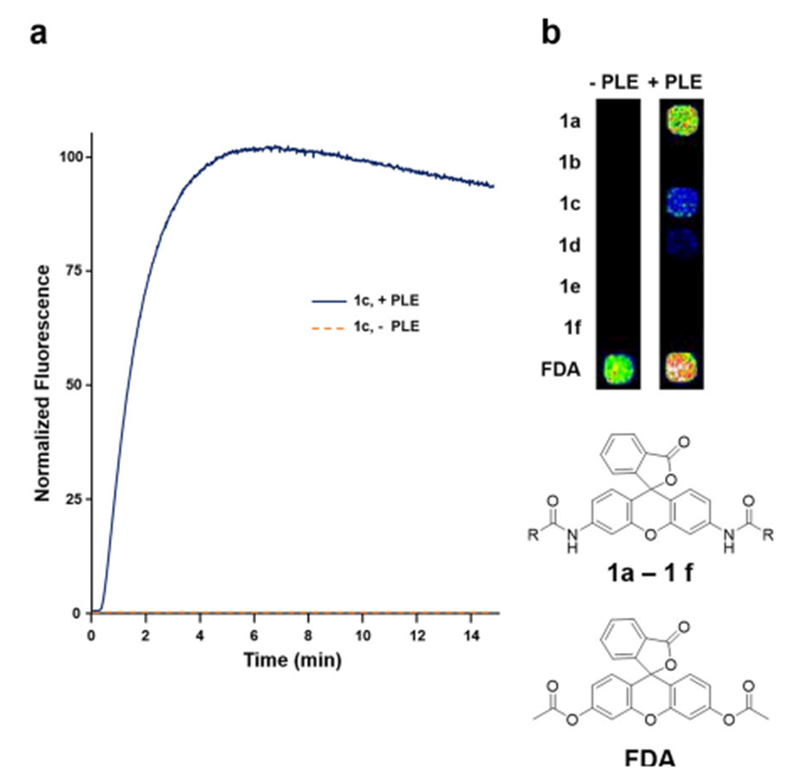
Activation of amidated rhodamine pro-fluorophores in HEK293 cells [a] Time-dependent increase in fluorescence in wild-type and PLE-expressing HEK293 cells upon addition of 3 μm 1 c (excitation 480±20 nm, emission 540±20 nm). The Y axis is normalized to the maximum observed fluorescence under the experimental conditions. [b] Fluorescence observed after treatment of wild-type or PLE-expressing HEK293 for 2 min at 22° C with 10 μm 1 a–1 f as well as 300 nm fluorescein diacetate (FDA), a substrate for esterases expressed in both wild-type and PLE-expressing HEK293 cells. All data are representative of 4 independent replicates.
As shown (Figure 3a) fluorescence increased rapidly upon pro-fluorophore addition demonstrating the rhodamine pro-fluorophores are readily taken up into HEK293 cells. We found that constitutive overexpression of PLE was well tolerated by the cells with no discernable difference in growth rate or morphology compared to untransfected cells.
Although the N,N′-ditrifluoroacetyl rhodamine produced the largest increase in fluorescence, we chose to continue our studies using the N,N′-dipropanoyl rhodamine since the product of PLE’s action on the rhodamine-trifluoroacetamide is trifluoroacetate which is more likely to be toxic to cells[19] than is propionic acid which naturally occurs in cells and is readily metabolized by them.
Following on from our observation that 1 c was rapidly activated in HEK293 cells, we set out to determine the suitability of the 1 c for cellular imaging. When we used confocal fluorescence microscopy to visualize HEK293 cells treated with 1 c for 1 h at 22°C, we failed to observe fluorescence in wild-type cells. However, HEK293 cells expressing PLE showed bright, predominantly cytoplasmic, fluorescence (Figure 4). These data clearly demonstrate the utility of this rhodamine-propanamide pro-fluorophore/PLE enzyme pair for cellular imaging of PLE-expressing cells.
Figure 4.

Confocal Imaging of HEK293 cells treated with amidated rhodamine pro-fluorophore, 1 c. [a–c] Images of a set of PLE-expressing HEK293 cells treated with 1 c and the DNA-specific fluorescent dye, Hoechst. [d–f] Images of a set of wild-type HEK293 cells treated under the same conditions as panels a–c. The scale bar shown in the lower right of each image is 20 μm. Images are representative of 4 independent replicates.
In summary, we have used standard per-acylation of commercial Rhodamine 110 to provide easy access to rhodamine pro-fluorophores, released upon exposure to PLE in vitro and in cells. We demonstrated that these pro-fluorophores were very stable in aqueous solution and in wild-type HEK293 cells but that they could be converted to the fluorescent Rhodamine 110 by PLE in vitro as well as in HEK293 cells engineered to express PLE. We found that the N,N′-di-trifluoroacetyl and propionyl rhodamine pro-fluorophores were better substrates that the others that we synthesized. Finally, we found that N,N′-dipropionyl rhodamine pro-fluorophore showed bright, cytoplasmic fluorescence in PLE-expressing cells thus demonstrating suitability for cellular imaging. Recently, Lavis[12b] and others have demonstrated the utility of PLE for activating rhodamine-based pro-fluorophores using the tri-methyl lock approach and recently re-evaluated prior work[20] with rhodamines released by serine hydrolase with varying peptide pro-fluorophore scaffolds.[21] Although compound 1 b had been previously prepared[12b] testing of 1 b as a substrate for PLE was not reported. Interestingly, although 1 b appears to be a poor substrate for PLE, the closely related and equally easy to prepare 1 a and 1 c are good PLE substrates suitable for in vitro and cell-based use. It is important to note that in Lavis[12b] the action of PLE on an ester indirectly results in the liberation of the Rhodamine 110 in contrast to the direct, amidase mechanism we observe with the pro-fluorophores in the present study. Thus, our study extends the use of PLE as a pro-fluorophore activating enzyme for Rhodamine 110 via a different mechanism which takes advantage of PLE’s amidase activity to catalyze the release of Rhodamine 110 from simple rhodamine amide pro-fluorophores. Future studies will focus on the cell-type specific expression of PLE to enable discrimination of PLE-expressing cells from non-expressing cells in cell culture as well as in tissues and whole organisms. While we did not observe any measurable level of conversion of our pro-fluorophores to Rhodamine 110 in wild-type HEK293 cells, in future studies with other cell types it will be important to determine whether any natively expressed activities in the cells of interest interfere with the use of the pro-fluorophores. Furthermore, the extension of this simple rhodamine-amide chemistry to carbo- and silyl-rhodamines[22] with redshifted absorption and emission spectra is desirable to minimize contributions of auto-fluorescence in high-throughput screening and when imaging tissues and organisms.
Supplementary Material
Acknowledgements
The authors acknowledge the Vanderbilt High-throughput Screening Facility and the Vanderbilt Chemical Synthesis Facility. Funding for CDW through the Department of Pharmacology and the Vanderbilt Institute of Chemical Biology. K.K.A. is supported by funds from NIH grant 2 R25 GM059994 18. S.J.R. acknowledges the support of the Vanderbilt Chemical Biology Interface (CBI) training program (T32 GM065086) and Fisk-Vanderbilt Master to Ph.D. Bridge program. Meharry Medical College Morphology Core is supported by NIH grants U54 MD007593, G12 MD007586, U54 CA163069, R24 DA036420, and S10 RR0254970.
Footnotes
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/chem.201801409.
Conflict of interest
C.D.W. is an owner of WaveFront Biosciences, the manufacturer of the Panoptic plate reader used in these studies
References
- [1].Tsien RY. Nature. 1981;290:527. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- [2]. Lavis LD Raines RT ACS Chem. Biol 2014. 9 855–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Zimmerman M Ashe B Yurewicz EC Patel G Anal. Biochem 1977. 78 47–51 [DOI] [PubMed] [Google Scholar]; b) Liu J Bhalgat M Zhang C Zhenjun D Hoyland B Klaubert DH Bioorg. Med. Chem. Lett 1999. 9 3231–3236 [DOI] [PubMed] [Google Scholar]; c) Leytus SP Melhado LL Mangel WF Biochem. J 1983. 209 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Yokoo T Ohashi T Shen JS Sakurai K Miyazaki Y Utsunomiya Y Takahashi M Terada Y Eto Y Kawamura T Osumi N Hosoya T Proc. Natl. Acad. Sci. USA 2005. 102 3296–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Forss-Petter S Danielson PE Catsicas S Battenberg E Price J Nerenberg M Sutcliffe G Neuron 1990. 5 187–197 [DOI] [PubMed] [Google Scholar]
- [6]. Horwitz JP Chua J Curby RJ Tomson AJ Da Rooge MA Fisher BE Mauricio J Klundt I J. Med. Chem 1964. 7 574–575 [DOI] [PubMed] [Google Scholar]
- [7]. Hofmann J Sernetz M Anal. Biochem 1983. 131 180–186 [DOI] [PubMed] [Google Scholar]
- [8]. Nolan GP Fiering S Nicolas JF Herzenberg LA Proc. Natl. Acad. Sci. USA 1988. 85 2603–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Rakhmanova VA MacDonald RC Anal. Biochem 1998. 257 234–237 [DOI] [PubMed] [Google Scholar]
- [10]. Arbeloa FL Arbeloa TL Estevez MJT Arbeloa IL J. Phys. Chem 1991. 95 2203–2208 [Google Scholar]
- [11]. Tsien RY Annu. Rev. Biochem 1998. 67 509–544 [DOI] [PubMed] [Google Scholar]
- [12].a) Beija M Afonso CAM Martinho JMG Chem. Soc. Rev 2009. 38 2410–2433 [DOI] [PubMed] [Google Scholar]; b) Lavis LD Chao T-Y Raines RT ACS Chem. Biol 2006. 1 252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. El Baraka M Deumié M Viallet P Lampidis TJ J. Photochem. Photobiol. A 1991. 62 195–216 [Google Scholar]
- [14].a) Tian L Yang Y Wysocki LM Arnold AC Hu A Ravichandran B Sternson SM Looger LL Lavis LD Proc. Natl. Acad. Sci. USA 2012. 109 4756–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Musidlowska-Persson A Bornscheuer UT Protein Eng 2003. 16 1139–1145 [DOI] [PubMed] [Google Scholar]
- [15]. Junge W Heymann E Eur. J. Biochem 1979. 95 519–525 [DOI] [PubMed] [Google Scholar]
- [16].Grimm JB, English BP, Chen J, Slaughter JP, Zhang Z, Revyakin A, Patel R, Macklin JJ, Normanno D, Singer RH, Lionnet T, Lavis LD. Nat. Methods. 2015;12:244. doi: 10.1038/nmeth.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Sabnis RW Handbook of Fluorescent Dyes and Probes 2015. 380–383 Wiley; Hoboken: [Google Scholar]
- [18]. Jiang S-H Cheng Y-Y Huo T-I Tsai T-H J. Agric. Food Chem 2017. 65 7797–7804 [DOI] [PubMed] [Google Scholar]
- [19]. Cornish J Callon KE Lin CQ Xiao CL Mulvey TB Cooper GJ Reid IR Am. J. Physiol 1999. 277 E779–E783 [DOI] [PubMed] [Google Scholar]
- [20]. Sueyoshi K Nogawa Y Sugawara K Endo T Hisamoto H Anal. Sci 2015. 31 1155–1161 [DOI] [PubMed] [Google Scholar]
- [21]. MacDonald MJ Lavis LD Hilvert D Gellman SH Org. Lett 2016. 18 3518–3521 [DOI] [PubMed] [Google Scholar]
- [22]. Grimm JB Sung AJ Legant WR Hulamm P Matlosz SM Betzig E Lavis LD ACS Chem. Biol 2013. 8 1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


