Abstract
The regioselective and enantioselective intermolecular sp3 C–H functionalization of silicon-substituted alkanes was accomplished using Rh2(S-NTTL)4 with readily available 1-sulfonyl-1,2,3-triazoles as carbene precursors. These reactions generate a diverse array of stereodefined substituted silaalkanes.
Graphical abstract

Organosilicon compounds (Figure 1) represent an important area of chemical space for such diverse fields as agrochemistry1, materials science2, and pharmaceutical chemistry3, and hence, novel reactions that explore this rich and largely untapped chemical space are desirable. These compounds benefit from the unique atomic properties of silicon which include larger covalent radius, decreased electronegativity, and ability to engage hypervalent interactions with electron donors via availability of 3d orbitals. Moreover, the specific exploitation of silicon in medicinal chemistry has matured into the development of the “sila-substitution” strategy, which seeks to improve stability, reactivity, and lipophilicity of medicinal agents by generating silicon analogues of known drugs. Examples include silperisone (a muscle relaxant),4 the spirocyclic σ-receptor ligands,6 the silicon analogue of linezolid,6 and M2 proton channel probe of influenza A virus.7
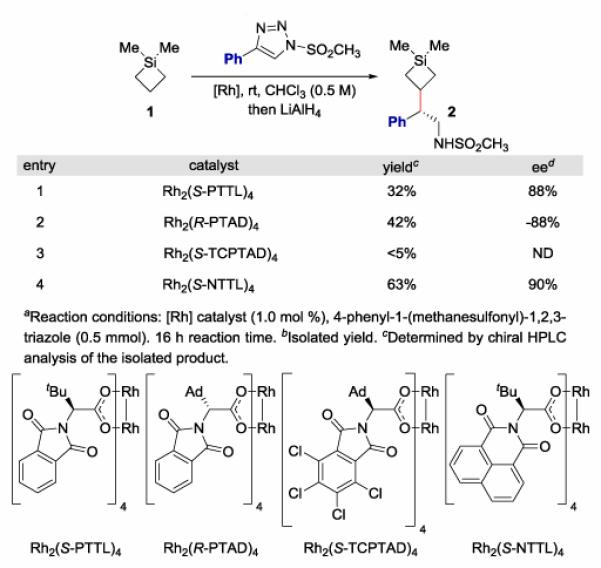
Figure 1. Catalyst Optimization for Intermolecular C-H Functionalization of Silacycloalkanesa.
Organosilicon compounds are often generated by appending the silicon functional group either by polar reactions (e.g., Grignard attack to Si-C1 bonds) or through organometallic transformations (e.g., addition of Si-H bonds using Pt, Rh, or Ir).8 While these strategies are important because they establish new carbon-silicon connectivites, an alternative approach resulting from the C-H functionalization of silicon-substituted alkanes could provide a diverse set of stereodefined functionalized organosilicon compounds from simple and readily available precursors. Selective functionalization of C-H bonds has rapidly evolved as a viable synthetic disconnection, and has transformed the way chemists strategize during chemical synthesis.9
One approach to C-H functionalization is the selective intermolecular insertion of rhodium-stabilized donor/acceptor carbenes, which are generated from either aryldiazoacetates or 1-sulfonyl-1,2,3-triazoles. These reactions often employ rhodium tetracarboxylate catalysts to accomplish regio- and stereoselective C-H functionalization reactions. In general, donor/acceptor metallocarbene intermediates insert into C-H bonds where a developing positive charge is most readily stabilized. As such, C-H bonds α to π systems (e.g., benzylic or allylic systems) or protected oxygen or protected nitrogen atoms readily undergo insertion.10 More recently, regioselective activation of aliphatic C-H bonds distal to stabilizing interactions has been achieved by deploying bulky catalysts.11 All of these investigations have provided a map for C-H functionalization reactivity, and yet, C-H bonds in the β position of silicon have been understudied, even though the lability of these hydrogen atoms is enhanced by the so-called β-silicon effect.12
Prior efforts directed towards the intermolecular functionalization of organosilicon compounds by C-H insertion reactions with carbenes focused on establishing highly regioselective reactions (Scheme 1). Seyferth and coworkers first showed that phenyl(bromo-dichloromethyl)mercury-derived dichlorocarbene inserts regioselectively into the β position of a variety of tetraalkylsilanes (Scheme 1a).13 This work unveiled the delicate balance of sterics and electronics around the silicon which can favorably dispose carbene insertion into the β position of silicon. Migita and coworkers later showed that photolysis of acceptor/acceptor carbenes in the presence of tetraalkylsilanes could also achieve β C-H functionalization, although the regioselectivity was poor (Scheme 1b).14 Danilkina and Sidorenko first implemented a dirhodium tetracarboxylate catalyst for β functionalization of tetraorganosilanes with ethyl diazoacetate, but the yields were low (10–26%);15 most recently, Sakurai and coworkers reported that silacycloalkanes can undergo regioselective Rh-catalyzed insertion reactions with α-diazo esters in high yields if long addition times were used (Scheme 1c).16 Despite all of the studies described above, no effort was made to explore the combined regio- and stereoselective functionalization at the β position of organosilicon compounds.
Scheme 1. C–H Functionalization Reactions of Tetraalkylsilanes.
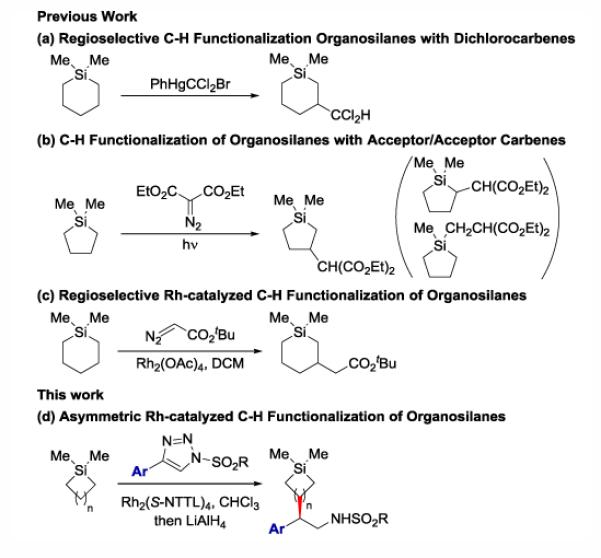
Herein, we report the first enantioselective C-H functionalization of sila-substituted alkanes using donor/acceptor carbenes derived from 1-sulfonyl-1,2,3-triazoles (Scheme 1d). The implementation of 1-sulfonyl-1,2,3-triazoles as carbenoid precursors is attractive because they are easily handled and they provide unique opportunities for incorporating aldehyde or amine functionalities; however, 1,2,3-triazoles have found limited use in C-H functionalization reactions, even though these carbenoid precursors have been effectively utilized for a plethora of other reactions.17 To date, only two reports delineate effective intermolecular C-H functionalization reactions with these carbenoid precursors.18 Fokin and coworkers showcased the first enantioselective C-H functionalization reaction of simple unsubstituted alkanes with 1,2,3-triazoles.19 Later, our group demonstrated that 1,2,3-triazoles could be utilized in the enantioselective C-H functionalization of activated allylic and benzylic C-H bonds.20 Other efforts towards exploring the intermolecular reactivity of 1,2,3-N-sulfonyl triazoles with heteroatom containing compounds, such as tetrahydrofuran or oxetane generated products derived from ylide intermediates, which may be the reason why intermolecular C-H functionalization reactions with these carbene precursors has been slow to develop.21
Our study began by investigation of commercially available silacyclobutane 1.22 Optimization reactions were conducted by reacting 1 with 4-phenyl-l-(methanesulfonyl)-1,2,3-triazole in the presence of a variety of dirhodium tetracarboxylate catalysts in chloroform, a solvent that has been shown to be particularly effective for the triazole reactions (Figure 1). The reaction was regioselective with exclusive functionalization at the β position to silicon. Four of the standard chiral dirhodium catalysts were examined (entries 1–4), and the highest levels of yield and enantioselectivity were obtained with RH2(S-NTTL)4.
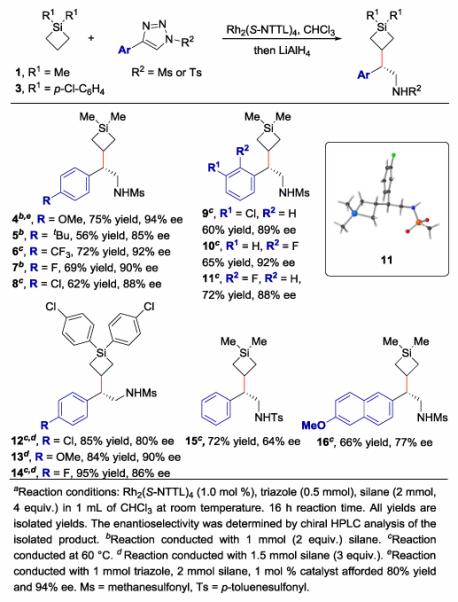
With an optimal catalyst in hand, the substrate scope was evaluated by reacting a variety of different substituted triazoles with 2–4 equivalents of silacyclobutanes (Figure 2). A variety of substitution patterns on the phenyl ring of the triazole were well tolerated, including electron-rich and electron-poor triazoles, as well as triazoles bearing ortho, meta, and para substitution.23 The mesyl group could be replaced by a tosyl group although the enantiopurity was diminished (92% ee for Ms and 64% ee for Ts). When substrates bearing p-Cl-Ph groups rather than methyl groups on the silicon were evaluated, the enantioselectivity dropped slightly to 80–90% ee, but the reaction yield was improved (84–95% yield) as shown for 12–14. The absolute configuration of 11 was determined to be S by X-ray crystallography, and the other products are tentatively assigned as S by analogy.
Figure 2. Selective C-H Functionalization of Silacyclobutanesa.
Having demonstrated that high levels of enantioselectivity can be obtained by C-H functionalization of silacyclobutanes, larger silacycles, such as 17 were considered as plausible targets for C-H functionalization (eq 1). Larger silacycles introduce higher orders of complexity because multiple stereoisomers can be generated by desymmetrization arising from the silicon heterocycle.
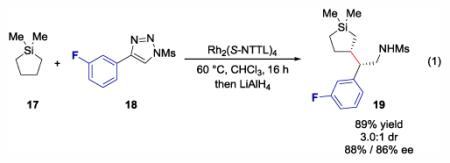
When 17 was tested, the functionalization product 19 was generated in 89% combined yield with a 3:1 d. r. and 88% ee and 86% ee for the major and minor diastereomer, respectively.
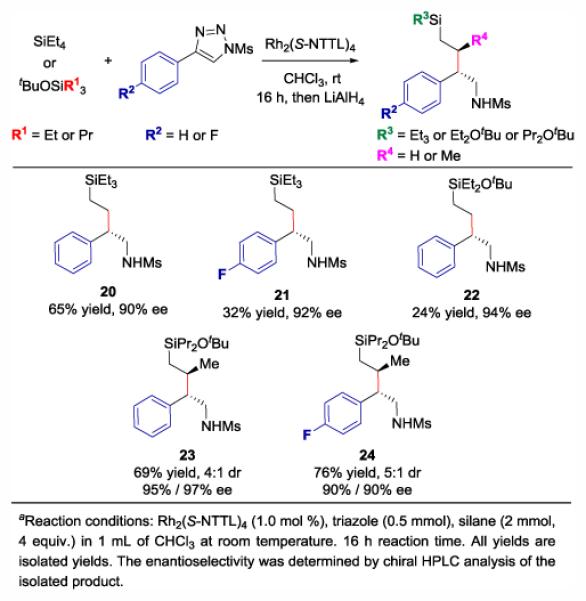
The initial success with silacycles prompted inquiries into the reactivity of aliphatic silaalkanes as illustrated in Figure 3. This study commenced with the investigation of commercially available tetraethylsilane. Tetraethylsilane represents a unique opportunity for C-H functionalization as the targeted C-H bond is primary which has been historically less accessible because the buildup of positive charge at a primary carbon is less stabilized. As shown for 26, successful C-H functionalization was achieved in 65% yield and 90% ee, representing the first example of primary C-H functionalization with 1-sulfonyl-1,2,3-triazoles. Unfortunately, the synthesis of 21 and 22 suffered from lower yields even though the enantioselectivity was high. The lower yield of 21 likely derives from the p-fluoro triazole which serves as a poorer source of the requisite carbene intermediate while the lower yield of 22 is probably due to a less effective β-silicon effect influenced by the tBuO-substituent.24 In contrast, propyl groups on the silane served as excellent substrates for achieving site-selective C-H functionalization at the β position of silicon. Products 23 and 24 were generated in good yields (69%−76%) and diastereoselectivity (4:1–5:1 dr) and great enantioselectivity (90–97% ee).
Figure 3. Selective C-H Functionalization of Acyclic Organosilanes.
In conclusion, the regio- and stereoselective C-H functionalization reaction of silicon-substituted alkanes was accomplished. These reactions show high stereoselectivity at the β position of both silacycloalkanes and aliphatic sila-akanes. We hope our studies herein will inspire others toward the C-H functionalization of organosilanes as a mode for accessing new chemical space.
Supplementary Material
ACKNOWLEDGMENT
Financial support was provided by NIH (GM099142) and AbbVie. Instrumentation used in this work was supported by the National Science Foundation (CHE 1531620 and CHE 1626172). We thank Dr. Eric Voight, Dr. Mark Matulenko and colleagues in the NSF Center for Selective C-H Functionalization (CHE-1700982) for helpful discussions regarding this work. We thank Dr. John Bacsa and Mr. Thomas Pickel at the Emory X-ray Crystallography Center for X-ray crystallographic analysis.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: XX.XXXX/XXX
REFERENCES
- (1).Cash GG Pestic. Sci 1997, 49, 29. [Google Scholar]; (e) Moberg WK; Basarab GS; Cuomo J; Liang PH Biologically Active Organosilicon Compounds: Fungicidal Silylmethyltriazoles. In Synthesis and Chemistry of Agrochemicals; ACS Symposium Series 355; American Chemical Society: Washington, DC, 1987; pp 288–301. [Google Scholar]
- (2).(a) Jones RG; Ando W; Chojnowski J Silicon-Containing Polymers, Springer: Berlin, 2000. [Google Scholar]; (b) Kumagai T; Itsuno S Macromolecules, 2002, 35, 5323. [Google Scholar]; (c) Bai D; Han S; Lu Z-H; Wang S Can. J. Chem 2008, 86, 230. [Google Scholar]; (d) Su TA; Widawsky JR; Li H; Klausen RS; Leighton JL; Steigerwald ML; Venkataraman L; Nuckolls C J. Am. Chem. Soc 2013,135, 18331. [DOI] [PubMed] [Google Scholar]
- (3).(a) Tacke R; Zilch H Endeavor, 1986, 10, 191. [DOI] [PubMed] [Google Scholar]; (b) Tacke R; Linoh H. Bioorganosilicon Chemistry. In Organic Silicon Compounds; John Wiley & Sons, Ltd: New York, 1989; Vol. 2, 1143. [Google Scholar]; (c) Showell GA; Mills JS Drug Discovery Today 2003, 8, 551. [DOI] [PubMed] [Google Scholar]; (d) Mills JS; Showell GA Expert Opin. Invest. Drugs 2004, 13, 1149. [DOI] [PubMed] [Google Scholar]; (e) Franz AK; Wilson SO J. Med Chem 2013, 56, 388. [DOI] [PubMed] [Google Scholar]; (f) Franz AK Curr. Opin. Drug Discovery Dev 2007, 10, 654. [PubMed] [Google Scholar]; (g) Fujii S; Hashimoto Y Future Med. Chem 2017, 9, 485. [DOI] [PubMed] [Google Scholar]; (h) Ramesh R; Reddy DS J. Med. Chem 2017, DOI: 10.1021/acs.jmedchem.7b00718. [DOI] [PubMed] [Google Scholar]
- (4).Farkas S CNS Drug Reviews, 2006, 12, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Tacke R; Handmann VI; Bertermann R; Burschka C; Penka M; Seyfried C Organometallics, 2003, 22, 916. [Google Scholar]
- (6).Seetharamsingh B; Ramesh R; Dange SS; Khaimar PV; Singhal S; Upadhyay D; Veeraraghavan S; Viswanadha S; Vakkalanka S; Reddy DS ACS Medicinal Chemistry Letters, 2015, 6, 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wang J; Ma C; Wu Y; Lamb RA; Pinto LH; DeGrado WF J. Am. Chem. Soc 2011,133, 13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Cheng C; Hartwig JF Chem. Rev 2015, 115, 8946. [DOI] [PubMed] [Google Scholar]; (b) Komiyama T; Minami Y; Hiyama T ACS Catal 2017, 7, 631. [Google Scholar]
- (9).(a) Gutekunst WR; Baran PS Chem. Soc. Rev 2011, 40, 1976. [DOI] [PubMed] [Google Scholar]; (b) Newhouse T; Baran PS Angew. Chem. Int. Ed 2011, 50, 3362. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yamaguchi J; Yamaguchi AD; Itami K Angew. Chem. Int. Ed 2012, 51, 8960. [DOI] [PubMed] [Google Scholar]
- (10).For a review, see: Davies HML; Morton D Chem. Soc. Rev 2011, 40, 1857. [DOI] [PubMed] [Google Scholar]
- (11).Liao K; Negretti S; Musaev DG; Bacsa J; Davies HML Nature, 2016, 533,230. [DOI] [PubMed] [Google Scholar]
- (12).(a) Lambert JB; Zhao Y; Emblidge RW; Salvador LA; Liu X ; So J-H; Chelius EC Ace. Chem. Res 1999, 32, 183. [Google Scholar]; (b) Lambert JB Tetrahedron, 1990, 46, 2677. [Google Scholar]
- (13).Seyferth D; Washburne SS; Attridge CJ; Yamamoto KJ Am. Chem. Soc 1970, 92, 4405. [Google Scholar]; (b) Seyferth D; Damrauer R; Andrews SB; Washbume SS J. Am. Chem. Soc 1971, 93, 3709. [Google Scholar]; (c) Seyferth D; Shih H-M; Dubac J; Mazerolles P; Serres B J. Organometal. Chem 1973, 50, 39. [Google Scholar]
- (14).Ando W; Konishi K; Migita TJ Organometal. Chem 1974, 67, C7. [Google Scholar]
- (15).Danilkina LP; Sidorenko GV J. Gen. Chem. USSR (Engl. Transl.), 1979, 49,2460. [Google Scholar]
- (16).Hatanaka Y; Watanabe M; Onozawa S; Tanaka M; Sakurai HJ Org. Chem 1998, 63, 422. [DOI] [PubMed] [Google Scholar]; For another example of the β-silicon effect related to carbenes, see: Hosomi A; Mikami M Bull. Chem. Soc. Jpn 1983, 56,2784. [Google Scholar]
- (17).(a) For reviews on triazole-based reactions, see Davies HML; Alford JS Chem. Soc. Rev 2014, 43, 5151. [DOI] [PubMed] [Google Scholar]; (b) Chattopadhyay B; Gevorgyan V Angew. Chem. Int. Ed 2012, 51, 862. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Anbarasan P; Yadagiri D; Rajasekar S Synthesis, 2014, 46, 3004. [Google Scholar]; (d) Jiang Y; Sun R; Tang X-Y; Shi M Chem. Eur. J 2016, 22, 17910. [DOI] [PubMed] [Google Scholar]
- (18).(a) For intramolecular C-H functionalization reactions with triazoles, see: Lindsay VNG; Viart HMF; Sarpong R J. Am. Chem. Soc 2015, 137, 8368. [DOI] [PubMed] [Google Scholar]; (b) Shen M-H; Pan Y-P; Jia Z-H; Ren X-T; Zhang P; Xu H-D Org. Biomol. Chem 2015, 13, 4851. [DOI] [PubMed] [Google Scholar]; (c) Ma X; Wu F; Yi X; Wang H; Chen W Chem. Commun 2015, 51, 6862. [DOI] [PubMed] [Google Scholar]; (d) Li L; Xia X-H; Wang Y; Bora PP; Kang Q Adv. Synth. Catal 2015, 357, 2089. [Google Scholar]; (e) Senoo M; Fumkawa A; Hata T; Urabe H Chem. Eur. J 2016, 22, 890. [DOI] [PubMed] [Google Scholar]; (f) Shen H; Fu J; Yuan H; Gong J; Yang Z J. Org. Chem 2016, 81, 10180. [DOI] [PubMed] [Google Scholar]
- (19).Chuprakov S; Malik JA; Zibinsky M; Fokin VV J. Am. Chem. Soc 2011,133, 103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kubiak II RW; Mighion JD; Wilkerson-Hill SM; Alford JS; Yoshidomi T; Davies HML Org. Lett 2016,18, 3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Homeff T; Chuprakov S; Chernyak N; Gevorgyan V; Fokin VV J. Am. Chem. Soc 2008, 130, 14972. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Medina F; Besnard C; Lacour J Org. Lett 2014, 16, 3232. [DOI] [PubMed] [Google Scholar]; (c) Guarnieri-Ibáñez A; Medina F; Besnard C; Kidd SL; Spring DR; Lacour J Chem. Sci 2017, 8, 5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hirano K, Yorimitsu H; Oshima K 20011,1 Dimethylsilacyclobutane. e-EROS Encyclopedia of Reagents for Organic Synthesis. Commercially available from Gelest or Sigma Aldrich [Google Scholar]
- (23). Attempted extension of the reaction to alkyl N sulfonyltriazoles failed to give any C-H functionalization product.
- (24).Shimizu N; Watanabe S-I; Hayakawa F; Yasuhara S; Tsuno Y; Inazu T Bull. Chem. Soc. Jpn 1994, 67, 500. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


