Abstract
Purpose
Determination of microsatellite instability (MSI) by PCR is the gold standard; however, immunohistochemistry (IHC) of mismatch repair (MMR) proteins is frequently performed instead. The reliability of these methods on post-neoadjuvant-therapy specimens is unknown. We examined the effect of neoadjuvant therapy on MSI results by PCR and IHC.
Experimental design
A total of 239 colorectal cancers resected after neoadjuvant therapy were assessed for MSI with PCR and IHC. PCR and IHC results for matched paired pre- and post-treatment specimens were compared. In parallel, two isogenic cell lines conditioned for MMR functioning and two different patient-derived xenografts (PDX) were exposed to chemotherapy, radiation or both. We also examined whether establishment of PDXs induced MSI changes in five tumors. IHC and MSI were tested after treatment to assess for changes.
Results
We identified paired pre- and post-treatment specimens for 37 patients: 2 with PCR only, 34 with IHC only, and 1 with both. All three patients with PCR had microsatellite stable pre- and post-treatment specimens. Of the 35 patients with IHC, 30 had intact MMR proteins in pre- and post-treatment specimens, 1 had equivocal MLH1 staining in the pre-treatment and loss in the post-treatment specimen, and 4 had intact pre-treatment MSH6 but variable post-treatment staining. In the experimental setting, no changes in MSI status were detected after treatment or tumor implantation in animals.
Conclusions
Our findings show that expression of MMR proteins, commonly MSH6, can change after neoadjuvant therapy and confirm PCR as the gold-standard test for MSI after neoadjuvant therapy.
Keywords: Colorectal cancer, mismatch repair system, chemotherapy, radiotherapy, patient-derived xenografts
Introduction
A new wave of therapeutic intervention has impacted cancer therapy. The use of novel immune regulators has revolutionized cancer medicine and provided therapeutic options that previously did not exist. It is postulated that due to potentially heightened immune infiltration, a higher neoantigenic burden or other unknown factors, one subset of cancer that appears to obtain benefit from immunotherapy is microsatellite instable colorectal cancers (1, 2). Germ-line or sporadic genomic alterations in the mismatch repair (MMR) genes MLH1, MSH2/TACSTD1, MSH6 and PMS2 allow for replication errors or instability in repeated DNA sequences, thus leading to a condition termed microsatellite instability (MSI) (3). Germ-line mutations in these genes cause a hereditary cancer syndrome named Lynch syndrome that predisposes them to development of colorectal cancer (CRC) and multiple other cancers, including endometrial, ovarian, and urinary tract cancers. Sporadic deficiency occurs secondary to silencing of MLH1 via promoter hypermethylation (4). Testing of MSI is usually accomplished by either PCR to detect instability in mono- or di-nucleotide microsatellite repeats or immunohistochemistry (IHC) to directly assess the expression of the MMR proteins. Although PCR-based techniques may be slightly more sensitive than IHC, in the clinical setting the two techniques have been determined to be equally reliable methods to detect the presence of MSI, showing high concordance (5, 6). Nevertheless, IHC may be performed more commonly because of its feasibility and lower cost (6).
MSI status has been determined to be a prognostic marker in stage II CRC (3, 7, 8) and therefore it has been argued that universal MSI testing should be performed in all surgically resected CRC (9). Traditionally, MSI testing has been completed on the initial diagnostic patient biopsy sample and may even be used to guide initial treatment decisions and referral to genetic counseling to evaluate for Lynch syndrome. Given this strategy, the majority of tumors tested are treatement naïve. However, endoscopic biopsies may yield insufficient tissue to permit both MSI-PCR and IHC, in which case additional testing must be performed on the resection specimen. Additionally, biopsies may not contain matched normal mucosal specimens for comparison. This scenario is particularly relevant to rectal cancers receiving neoadjuvant chemoradiation, for which the effect of chemotherapy and radiation on MSI status is yet unknown. We sought to determine the effect of neoadjuvant therapy on MSI analysis by PCR and IHC using in vitro, in vivo and clinical data. Available matched paired tumor samples (pre- and post-treatment) were reviewed to assess for the effect of neoadjuvant therapy on MSI status. Concurrently, in vitro and in vivo studies were conducted using isogenic cell lines conditioned for MMR functioning and with patient-derived xenografts (PDX) to determine the effect of neoadjuvant therapy on MSI tumor status (10). In addition, we tested if tissue engraftment in PDX models induces changes in MSI status secondary to an increase in genomic instability. In this modern era of treatment with both significant prognostic and predictive information of MSI status, it is imperative to properly define this subset of CRC patients.
Materials and Methods
Patient selection
Between September 2009 and August 2011, 608 patients who underwent surgical resection for CRC at The University of Texas MD Anderson Cancer Center (MDACC) were queried. Of these cases, 239 were surgically resected after having received prior neoadjuvant chemoradiation (n=192) or chemotherapy (n=47). All tumors were tested by both IHC and PCR-based MSI testing in either the pre- and/or post-treatment specimen. All specimens that had matched paired tumor samples pre- and post-treatment were re-reviewed by an expert gastrointestinal pathologist (M.W.T.)
Data collection
Demographic data, tumor characteristics, treatment types, treatment responses and survival rates were collected on all 239 patients identified from the electronic medical record. Response evaluation was based on the treating physician’s assessment. This study was approved by the MDACC Institutional Review Board.
MSH6 gene sequencing
A patient blood sample was collected, and DNA was extracted and tested using Sanger sequencing technology and multiplex ligation-dependent probe amplification to evaluate for pathogenic mutations in all exons of MSH6 and for the presence of large deletions and duplications in MSH6 and EPCAM (11).
Molecular testing
MSI PCR and IHC testing was performed on pre- and/or post-treatment resection specimens from the 239 patient tumors. Exclusion and inclusion criteria were applied to exclude non-adenocarcinoma histology, known cases of Familial Adenomatous Polyposis, cases where no neoadjuvant chemoradiation or chemotherapy was given, and cases where there was no IHC or MSI PCR on both the original biopsy and surgical specimens. Cases were included for analysis when there was IHC performed on paired biopsy and surgery specimen (regardless of MSI testing) or when MSI was done on paired biopsy and surgery specimen (regardless of IHC testing). Sections of paraffin embedded formalin fixed tissue from blocks containing the most viable tumor were utilized in the analysis. Representative 5-micron section(s) from block(s) containing tumor (and normal tissue for MSI analysis) were stained with hematoxylin and eosin.
For MSI analysis, DNA was obtained by manual microdissection. If viable (non-necrotic) tumor represented less than 30% of the designated tissue, microdissection was performed per standard of care. MSI-PCR analysis was performed using an expanded NCI panel of 7 markers (BAT25, BAT26, BAT40, D2S123, D5S346, D17S250 and TGFBR2) (12, 13). MSI-high (MSI-H) was defined as the presence of 2 or more (or >30%) loci showing instability, MSI-low (MSI-L) as the presence of one (or <30%), and MSI-stable (MSS) as no loci (14).
IHC was performed to detect the level of expression in Ki67 (MIB-1, 1:100; Dako, Carpenteria, CA) and DNA MMR proteins MLH1 (G168-15, 1:25; BD Biosciences Pharmingen, San Diego, CA), MSH2 (FE11, 1:100; Calbiochem, La Jolla, CA), MSH6 (44, 1:300; BD Biosciences Pharmingen), and PMS2 (Alb-4, 1:125; BD Biosciences Pharmingen). In patients with MLH1 loss, a methylation-specific PCR of the MLH1 promoter was conducted. All of these analyses were performed as per standard of care in Clinical Laboratory Improvement Amendments (CLIA) approved laboratories (15).
Cell line experiments
HCT116 cells were purchased from American Type Culture Collection and HCT116+Ch3 CRC cells were generously provided by Dr. Alan Clark (NIEHS). HCT116 cells harbor a hemizygous mutation in MLH1 (c.755C>A, p.S255*) and therefore are MMR deficient(16). MMR functioning has been restored in HCT116+Ch3 cells via transfer of chromosome 3 and hence transfer of a functional MLH1 gene (Supplementary Figure 1A and B) (10). Cultured cells were maintained in Dulbecco's modified Eagle's medium, DMEM (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY). Cell lines were authenticated using Short Tandem Repeat fingerprinting service provided by the Characterized Cell Line Core Facility of MD Anderson Cancer Center. In addition, Mycoplasma contamination was ruled out using a PCR-based method.
In vitro studies
Treatment consisted of three cycles of single agent chemotherapy (5-fluouracil, oxaliplatin, or irinotecan), radiation, or 5-fluouracil in combination with radiation (Supplementary Figure 2). Drug dosage used was based on tested IC50 concentration (50% inhibitory concentration) in the cell line model (F6627, 5-fluorouracil, 25µM; O9512, oxaliplatin, 3.5µM; I1406, irinotecan, 25µM, Sigma Aldrich, St. Louis, MO). Cells were treated with radiation at 2Gy for 60 seconds on two consecutive days using the RS-2000 Biological system. After each round of treatment pools of cells were assessed for MSI-PCR and IHC of MSH6.
MSI-PCR analysis in the context of in vitro studies was performed using a panel of 10 microsatellite markers. Microsatellite loci were amplified by three multiplex PCRs as following: 1) D10S197, BAT26, beta-catenin; 2) D18S58, BAT40, D2S123; 3) D17S250, BAT25, TGFBR2, D5S346. All three multiplex PCRs were performed under the same conditions (available upon request). The PCR fragments were detected by capillary electrophoresis on ABI370 at MDACC Sequencing Core and were analyzed using the software Peak Scanner v1.0 (Applied BioSystems, Foster City, CA). The patterns of the microsatellite markers before and after treatment were compared to identify changes as previously described (17). Once cell lines were treated, MSH6 staining (primary antibody, L990, Cell Signaling Technology, Danvers, MA) of the cells was performed using Vectostain ABC Elite kit from Vector Laboratories (Burlingame, CA) using standard procedures. MSH6 IHC was performed in vitro to assess for variable loss in post-treatment MSH6 seen in clinical data. IHC specimens were scored based on robustness of staining with 0 meaning no stain, 1+ weak staining, 2+ moderate staining, and 3+ strong staining by an expert gastrointestinal pathologist (M.W.T)
Genomic instability analysis
To evaluate the induction of genomic instability by treatment, 3 additional coding microsatellites in ATR, BLM and CHK1 were assessed by fragment analysis. These microsatellites are known to be stable in MMR proficient and deficient cell systems. Analysis was performed using the same methodology used for MSI analysis. Primer sequences and PCR conditions are available upon request.
PDXs studies
Primary human-tumor xenograft in vivo models were established as described previously (18). Tumors were obtained from specimens of 7 patients with metastatic colorectal cancer at MDACC and collaborating institutions (Supplementary Table 4). All patients provided written informed consent for their tumors to be used for research purposes including the creation of xenografts. Specimens were obtained with approval of the Institutional Review Board. A total of 7 PDXs were propagated in NU/J 6-week old female mice (Jackson Laboratory, Bar Harbor, ME). Animal experiments using PDXs were performed according to the protocol approved by the IACUC at MD Anderson Cancer Center.
After tumors were established, when median tumor volume exceeding 300 mm3 treatment was initiated in 2 PDXs. One mouse received FOLFOX (5-fluouracil 100 mg/kg and oxaliplatin 10 mg/kg via intraperitoneal administration) on day 1 for one dose. One mouse received FOLFIRI (5-fluouracil 25mg weekly and irinotecan 15 mg/kg via intraperitoneal injection) for four constitutive weeks. Tumors were then harvested and DNA extracted. MSI-PCR analysis in the context of in vivo studies was performed using the panel of 10 microsatellite markers as described above. The patterns of the microsatellite markers before and after treatment and also before (original tumor) and after implantation (established PDX) were compared to find changes as previously described (17).
Statistical analysis
All the statistical analyses were performed using Microsoft Excel software. The significance of difference was obtained by performing the Chi-squared test, and the level was set at P<0.05. For comparison of two grading methods Cohen’s kappa statistical test was performed. Simple t-test and Fisher’s exact test were used to compare paired and unpaired patient cohorts.
Results
Patient characteristics
We identified 239 patients with resectable CRC having received prior neoadjuvant chemotherapy or chemoradiation. Patients were included in the study who had matched paired pre- and post-treatment specimens subjected to IHC only (34 patients), MSI-PCR analysis only (2 patients), or both (1 patient). Of these 37 patients, median age at diagnosis was 55 years (range: 31–85), 57% were male, 89% received chemoradiation prior to resection with the remaining receiving chemotherapy alone. Patient characteristics are displayed in Supplementary Table 1 with comparison between paired and unpaired patient cohorts. Both cohorts were similar with more patients with poorly differentiated tumors in the unpaired cohort.
Patient pre- and post-treatment IHC comparison
Of the 239 patients treated with neoadjuvant chemoradiation or chemotherapy alone, 35 tumors had both pre-treatment and post-treatment IHC performed. Thirty of the 35 tumors (86%) showed intact protein for all 4 MMR proteins in both pre- and post-treatment specimens. One patient, a 35-year-old woman with a strong family history, had equivocal MLH1 staining on pre-treatment IHC and definitive loss of MLH1 and PMS2 on post-treatment IHC. Post-treatment MSI showed allelic shift in 7 of 7 markers, thus being consistent with maintenance of MSI-H status despite treatment. The remaining 4 patients all showed completely intact MMR proteins in the pre- treatment samples but isolated changes in MSH6 expression in the post-treatment samples that was interpreted as loss of staining. One sample showed complete MSH6 loss in post-treatment resection specimens and three showed patchy staining of MSH6. No change in status was seen in the post-treatment specimens of the other 3 mismatch repair proteins tested (Figure 1). Of note, based on MSI-PCR analysis, also performed on either pre- or post- treatment samples, all tumors were MSS (Supplementary Table 2). Therefore, based on the observed changes in this retrospective review of the clinicopathologic data we decided to perform further in vitro MSH6 analysis as described below.
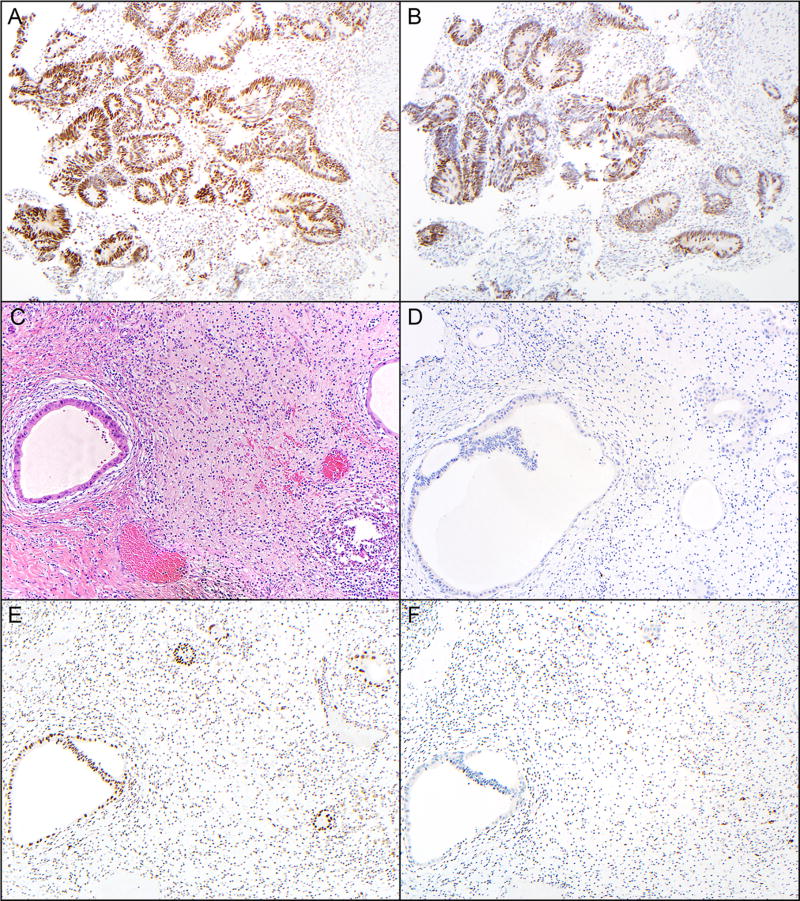
Figure 1.
Pretreatment endoscopic biopsies stained with the MSH2 (A) and MSH6 (B) from one patient demonstrates intact nuclear immunopositivity in both neoplastic cells (glands) as well as background stromal cells (magnification, 40×). Sections from the same tumor in the post-treatment resection specimen stained with hematoxylin and eosin (C), Ki67 (D), MSH2 (E) and MSH6 (F), show scant residual dilated neoplastic glands, no expression of Ki-67 in the remaining neoplastic glands, retained expression of MSH2 in the neoplastic glands and background non-neoplastic cells (predominantly inflammatory cells and some fibroblasts), and rare neoplastic cells with weak expression of MSH6 and retained expression (with variable intensity) in many of the non-neoplastic cells (magnification, 40×).
Patient pre- and post-treatment MSI comparison
Of the 239 patients treated with neoadjuvant chemoradiation or chemotherapy alone, 3 tumors had both pre-treatment and post-treatment MSI PCR analysis performed. All 3 tumors from pre-treatment samples displayed MSS phenotype with 0/7 markers showing MSI. Two of three post-treatment resected specimens again showed 0/7 markers with MSI with one tumor confirmed 1/7 markers consistent with an MSI-L phenotype (Supplementary Table 3). Based on PCR, there was no evidence of change in MSI status.
Finally, we combined the pre- and post-treatment results of the IHC and MSI comparisons and made a formal statistical analysis. Our observed Cohen’s Kappa was 0.3, thus indicating a fair consistency between pre- and post-ratings of MSI status. Then, we assessed statistical significance by resampling MSI and MSS calls, pre- and post-, given their marginal probabilities from the observed data. More than 97% of the time, the resulting Kappa statistic was less than our observed value of 0.3, which rendered a P-value of 0.03.
Patient post-treatment Ki67 IHC
Of the four patients with intact pre-treatment MSH6 expression, but variable post-treatment MSH6 IHC staining, two had received 8 cycles of neoadjuvant FOLFOX and bevacizumab chemotherapy and two patients received neoadjuvant chemoradiation with either 5-FU or capecitabine. Two samples were evaluable for Ki67 expression. One sample, pre-treated with FOLFOX plus bevacizumab, showed 5% Ki67 positivity and one sample pre-treated with neoadjuvant 5-fluorouracil chemoradiation showed no Ki67 positivity in tumor cells, which is consistent with a decreased expression of MSH6 secondary to a reduction in the cellular division rate (Figure 1E).
Tumor viability
All 3 tumor samples tested for MSI showed post-treatment tumor viability between 10–20%. Of the 4 tumors tested via IHC for MMR protein loss, with variable MSH6 staining on post resection specimens, post-treatment tumor viability ranged from 0–20% (Supplementary Table 2).
MSH6 germline sequencing
One of four patients who showed loss of MSH6 expression by IHC after treatment had undergone germline sequencing of DNA extracted from a peripheral blood sample. The germline blood sample was negative for mutations or large deletion/duplications MSH6, thus ruling out Lynch syndrome.
In vitro treatment and MSI-PCR analysis
MMR deficient (HCT116) and proficient (HCT116+Ch3) isogenic cell lines were chosen due to their clinical relevance and ability to recapitulate MMR deficient tumor biology (19). Cell lines were exposed to treatment conditions described above. HCT116 cells displayed MSI in all 10 markers tested. Conversely, all HCT116+Ch3 showed stability in all 10 markers tested. This remained consistent in both cell line models through all 3 cycles of treatment and no change in MSI status was detected in either cell line (Table 1).
Table 1.
Assessment of in vitro treatment and MSI analysis with reference to fragment analysis. Markers that display MSI are presented by white cells and HCT116 is MMR deficient and HCT116+3 is an MMR proficient. MMR, mismatch repair; XRT, radiation therapy; 5FU, 5-flurouracil; OXL, oxaliplatin; IRI, irinotecan.
| MSI ANALYSIS | GENOMIC INSTABILITY | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line | Treatment | Round | BAT26 | D10S197 | b catenin | BAT40 | D18S58 | D2S123 | TGFBR2 | BAT25 | D17S250 | D5S346 | ATR | BLM | CHK-1 | |
| MMRd | HCT116 | XRT | 1 | |||||||||||||
| HCT116 | XRT | 2 | ||||||||||||||
| HCT116 | XRT | 3 | ||||||||||||||
| HCT116 | XRT+5FU | 1 | ||||||||||||||
| HCT116 | XRT+5FU | 2 | ||||||||||||||
| HCT116 | XRT+5FU | 3 | ||||||||||||||
| HCT116 | 5FU | 1 | ||||||||||||||
| HCT116 | 5FU | 2 | ||||||||||||||
| HCT116 | 5FU | 3 | ||||||||||||||
| HCT116 | OXA | 1 | ||||||||||||||
| HCT116 | OXA | 2 | ||||||||||||||
| HCT116 | OXA | 3 | ||||||||||||||
| HCT116 | IRI | 1 | ||||||||||||||
| HCT116 | IRI | 2 | ||||||||||||||
| HCT116 | IRI | 3 | ||||||||||||||
| MMRp | HCT116+3 | XRT | 1 | |||||||||||||
| HCT116+3 | XRT | 2 | ||||||||||||||
| HCT116+3 | XRT | 3 | ||||||||||||||
| HCT116+3 | XRT+5FU | 1 | ||||||||||||||
| HCT116+3 | XRT+5FU | 2 | ||||||||||||||
| HCT116+3 | XRT+5FU | 3 | ||||||||||||||
| HCT116+3 | 5-FU | 1 | ||||||||||||||
| HCT116+3 | 5-FU | 2 | ||||||||||||||
| HCT116+3 | 5-FU | 3 | ||||||||||||||
| HCT116+3 | OXA | 1 | ||||||||||||||
| HCT116+3 | OXA | 2 | ||||||||||||||
| HCT116+3 | OXA | 3 | ||||||||||||||
| HCT116+3 | IRI | 1 | ||||||||||||||
| HCT116+3 | IRI | 2 | ||||||||||||||
| HCT116+3 | IRI | 3 | ||||||||||||||
Fragment analysis in alternative microsatellite markers for the assessment of the maintenance of genomic instability was performed on all cells lines tested in vitro. There was no increase in genomic instability post-treatment in cell lines in all three microsatellites tested (Table 1).
In vitro treatment and MSH6 IHC analysis
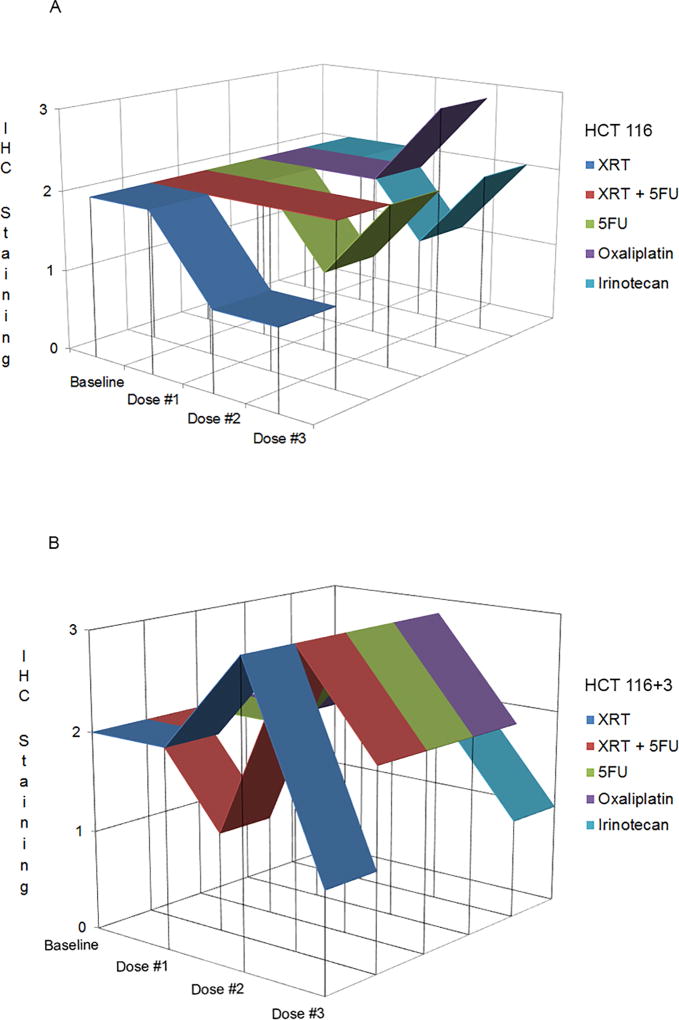
HCT116 and HCT116+Ch3 cells were exposed to three consecutive cycles of monotherapy chemotherapy, radiation, or combined radiation with 5-flurouracil. The MMR deficient cell line HCT116 showed 2+ moderate staining of MSH6 in all conditions after the first cycle of treatment. Following the second and third cycle of treatment with radiation, MSH6 staining remained 1+ but was never completely lost. MSH6 staining remained 2+ throughout all cycles of treatment with combined chemotherapy and radiation. After treatment with the second and third cycle of chemotherapy monotherapy, MSH6 staining was variable, however was never completely lost (Figure 2A). Similarly, the MMR proficient HCT116+Ch3 cells showed 2+ moderate staining of MSH6 in all conditions after the first cycle of treatment except chemoradiation with MSH6 stained 1+. Following the second cycle of treatment in all conditions, staining increased in intensity to 3+, although staining remained 2+ in the irinotecan treated cells. By cycle 3, MSH6 staining again decreased in all conditions but was never completely lost (Figure 2B). Overall, MSH6 expression was never lost neither in MMR-deficient nor in proficient cell lines.
Figure 2.
Analysis of immunohistochemistry after In vitro treatment. HCT116 (A) and HCT116+3 (B)
PDX characteristics and MSI-PCR analysis
We decided to test if genomic instability generated from tumor implantation and engrafting with influence MSI status in PDXs. We completed our in vitro assessment using 5 PDX mouse models derived from patients who had not been exposed to prior neoadjuvant chemotherapy or chemoradiation. These 5 PDXs were derived from patients with a median age at diagnosis of 55 years (range: 38–74), 60% were male, 40% were MSS. Of the 3 MSI-H PDXs, one was derived from a patient with MLH1 methylation, one with MLH1 loss, and one from MSH6 loss. Patient characteristics are displayed in Supplementary Table 4. After xenograft creation MSI status did not change post-implantation in all 5 animals (Figure 3).
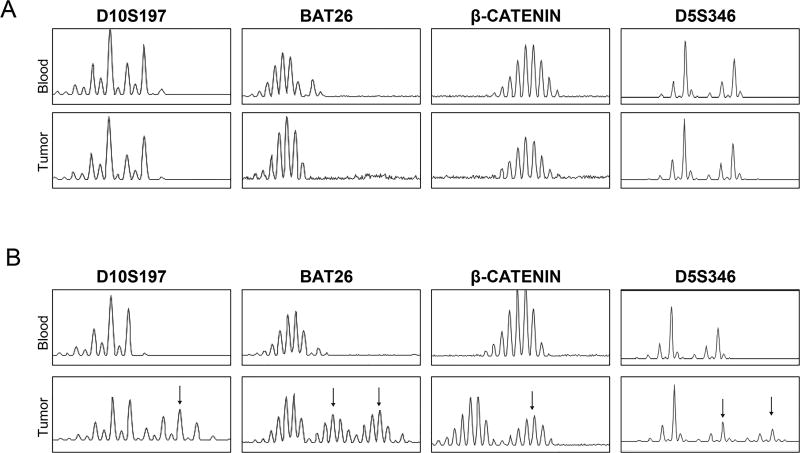
Figure 3.
Detection of MSI by fragment analysis of microsatellite markers in xenografts and in corresponding human blood normal. The figure shows four representative microsatellites: D10S197, BAT26, β-Catenin and D5S346. Arrows highlight those microsatellite markers that display instability. A. Tumor with MSS pattern. B. Tumor with MSI-H pattern.
PDX pre- and post-treatment MSI comparisons
Two additional MSS PDX mice were treated with chemotherapy using FOLFOX (Figure 4) and FOLFIRI. MSI-PCR status was assessed pre- and post-treatment and MSI status remained stable pre- and post-treatment in both models.
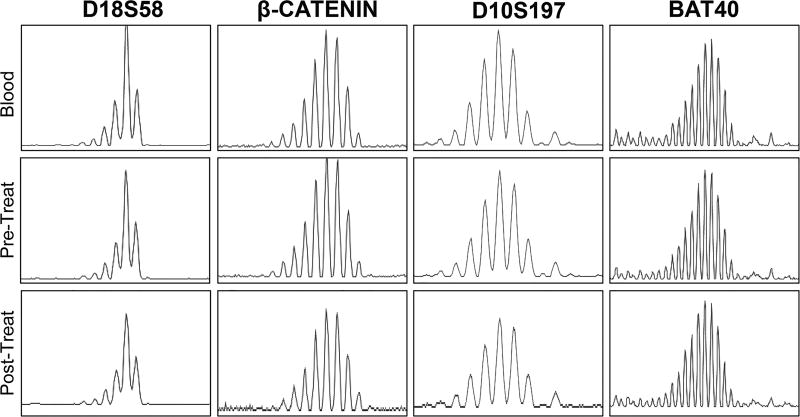
Figure 4.
Detection of MSI by analysis of microsatellite markers in a tumor sample from PDX model pre- and post-chemotherapy (FOLFOX) treatment. Matched blood was used as a reference. Abbreviations: Treat; Treatment.
Discussion
Immunotherapy has revolutionized cancer care in the last five years with promising impact of checkpoint inhibitors in MSI-high CRC. Additionally, in recent years it has been argued that universal MSI testing should be performed on all CRC patients due to its relatively common incidence, prognostic effect and predictive implications for treatment (9, 20). The majority of this testing occurs in the pre-resection biopsy sample. Once treated in the neoadjuvant setting with chemoradiation, MSI status is not necessarily retested. Despite the lower incidence of MSI-high rectal tumors (21), there is currently no clear understanding of the effect of chemotherapy and radiation on genomic instability and the impact of treatment on MSI status. It is also unclear whether a resistant MSS subpopulation may be selected for over the more chemosensitive MSI-high population within the tumor.
In our study we looked at in vitro and in vivo modeling systems to assess change in MSI status pre- and post-treatment. In the experimental setting, we focused on MSH6 staining as this was the only MMR protein seen to change in the clinical data set. Based on this data, it appears that neither chemotherapy, radiation, nor the combination of the two led to significant changes in MSI status based on microsatellite testing. In patients, testing showed variable post-treatment expression of MSH6 by IHC, however, MSI status by PCR did not change. In vitro testing of proficient and deficient cell line models also shows variability of expression of MSH6 by IHC after serial rounds of treatment; however, complete loss of expression was not observed in any condition.
Based on other studies and consistent with our own results; immunohistochemistry for MSH6 may render conflicting results. In 2010, Bao et al published a study where 51 patients who received neoadjuvant therapy and resection underwent post-treatment IHC testing. Any post-treatment loss of IHC markers, was confirmed by MSI PCR as well as MMR IHC protein expression, in the pre-treatment tumor samples. All of the 51 post-treatment tumor samples showed preserved MLH1, MSH2, and PMS2, however 10 post-treatment samples (20%) showed decreased MSH6 staining (22). In our cohort of 34 patients with pre and post-treatment IHC, we saw a similar loss of 11.7% despite remaining MSI stable by PCR. It has therefore been argued that before moving forward with genetic testing for Lynch syndrome, patients whose tumors show diminished MSH6 staining in treated tumors should prompt IHC testing of pretreatment biopsy samples (22). In at least 1 of 4 patients whose specimens showed patchy loss of MSH6, no germline mutations were detected in MSH6.
One could postulate that the loss in MSH6 protein post-neoadjuvant treatment may be secondary to subclonal mutational changes in the MSH6 gene or mutations in intronic regions (e.g. the gene promoter) that may be responsible for altered gene expression. IHC results of other MMR proteins did not show changes in the clinical data set. As described by Kondo et al, hypoxia and low tissue pH caused by chemoradiation therapy may also select for cells that are MMR deficient and cause focal or patchy loss of MSH6 (23). Additionally, in MSS patients, loss or decreased MSH6 expression may be related to decreased cellular division rates and induction of a resting state in response to chemoradiation therapy. We believe that this is the most likely explanation as we have also observed concurrent post-treatment decreases in Ki67 and MMR protein expression as recently reported by Kuan et al (24).
IHC is frequently performed on small pre-treatment biopsy specimens when there is little available tissue. IHC may therefore be more difficult to interpret after treatment. As seen in Table 2, patient 1 had patchy loss of MSH6 in the context of a pT0 tumor, this may have been due to MSH6 staining of stroma or lymphocytes, but negative in fibrotic tumor. Improvements in future treatment of CRC may also mean less viable and more necrotic post-treatment tumor specimens. Factors such as tumor viability and performance of MSH6 antibodies may account for the observed loss of expression in post-treatment specimens.
Previous studies have also documented variable MLH1 IHC staining patterns similar to the one in the patient in our series who had decreased MLH1 staining in the pre-treatment specimen and loss of MLH1 staining in the post-treatment specimen. Over one-third of all MLH1 mutations are missense mutations resulting in non-functional proteins that are antigenically intact (25). These mutant proteins may therefore result in a mildly positive or weak staining pattern. Such individuals could show different MLH1 IHC staining patterns as in our patient (26).
The main limitation inherent to our study include small numbers of patients with paired pre- and post-treatment specimen MSI and IHC analysis, despite of the fact that many samples in the original cohort were tested in either setting. Although, our observed correlation between the status of the samples pre- and post-treatment was indicative of a fair consistency, we decided to assess the statistical significance by resampling MSI and MSS calls, pre- and post-, given their marginal probabilities of our observed results. More than 97% of the time, the resulting Kappa statistic was less than our observed value of 0.3, which could be interpreted as a P-value of 0.03. Of course, the effect of inconsistencies in MSI determinations are not equal. For example, calling a tumor MSI when in fact it is MSS can involve a change of therapy that would be ineffective in the patient and with high toxicity. The reverse inconsistency will result on withholding a potentially very active therapy. In this statistical analysis we made no attempt to weigh these contrasts but rather scored them equally. Another limitation is that limited tumor viability (10–20%) in post-treatment specimens also made interpretation of IHC results difficult and unfortunately no other tissue was available for staining. Additionally, neoadjuvant treatment was relatively inconsistent. Whether a patient received chemotherapy alone versus chemoradiation was practitioner dependent upon tumor location and stage at diagnosis. In regards to in vitro and in vivo experiments, we are limited by experiments on two cell lines, one with an MSI-H, and one with MSS phenotype as well as two PDX models used to assess pre- and post-treatment status. Although these are the most commonly used pair of isogenic cell lines. In the future other colorectal cell lines may be tested to assess for variability.
The effect of neoadjuvant therapy on MSI status in rectal cancer cases has been a subject of debate in the CRC research community. In this study, we used both in vivo and in vitro modeling systems to assess this question. Our findings indicate that in the post-treatment setting, IHC testing is relatively unreliable and may lead to unnecessary work-up for suspicion of Lynch syndrome. In contrast, it appears on the basis of our study that MSI PCR status remains unchanged after chemotherapy or chemoradiation. In light of these new data, it appears that MSI PCR analysis is currently the most reliable test for evaluation of MMR in colorectal tumors and should be performed upon initial evaluation or to confirm a negative IHC result. In the future, as demonstrated by Le and colleagues, with the use of checkpoint inhibitors in MMR-deficient colorectal cancer patients, mutational load may replace IHC and MSI PCR analysis as a surrogate to identify treatment candidates and follow treatment response (1). We believe this information is imperative to define the MMR-deficient patient subset in the modern era of cancer immunotherapy.
Supplementary Material
Statement of Translation.
A new wave of immunotherapies has revolutionized cancer treatment to provide therapeutic options that previously did not exist. It is postulated that colorectal cancers (CRCs) with microsatellite instability (MSI), which may have heightened immune infiltration secondary to higher neoantigenic burden, will benefit from immunotherapy. Moreover, MSI status has also been determined to be a prognostic marker in stage II CRC. Therefore, it has been argued that universal MSI testing should be performed in all surgically resected CRCs. Traditionally, MSI testing has been completed on the diagnostic biopsy sample. With this strategy, the majority of tumors tested are treatment naïve. In rectal cancers treated with neoadjuvant chemoradiation, the effect of the neoadjuvant therapy on MSI status has been ignored. In this study, we demonstrated on the basis of in vitro, in vivo, and clinical data that neoadjuvant therapy has no effect on MSI analysis by polymerase chain reaction.
Acknowledgments
We thank the Sequencing and Microarray Facility at MD Anderson for their service.
Funding: This work was supported by part by the Conquer Cancer Foundation of the American Society of Clinical Oncology, Young Investigator Award to E.V.; the Janice Davis Gordon Memorial Postdoctoral Fellowship in Colorectal Cancer Prevention (Division of Cancer Prevention/The University of Texas MD Anderson Cancer Center) to E.B.; The University of Texas MD Anderson Cancer Center G.S. Hogan Award in Gastrointestinal Research to Y.N.Y.; and National Cancer Institute/National Institutes of Health Cancer Center Support Grant P30CA016672, which supports the Sequencing and Microarray Facility Core (P30 CA016672)
Abbreviations
- CLIA
Clinical Laboratory Improvement Amendments
- CRC
colorectal cancer
- IHC
immunohistochemistry
- MDACC
The University of Texas MD Anderson Cancer Center
- MMR
mismatch repair
- MSI
microsatellite instability
- MSI-H
MSI-high
- MSI-L
MSI-low
- PDX
patient-derived xenograft
Footnotes
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31–June 4, 2013.
Conflicts of Interest: The authors declare no potential conflicts of interest.
Statement of Author Contributions:
J.G., M.A.R-B, A.C., M.E.M., S.A.B., P.M.L., Y.N.Y., E.V. collected and analyzed clinical data.
W.W., G.B., E.B., E.V. designed, carried out, and analyzed in vitro experiments.
M.W.T. analyzed all pathologic specimens.
E.S.K, J.Y.W. designed and carried out in vivo experiments
P.S. provided statistical supervision.
J.G. and E.V. wrote the manuscript.
All authors: read, revised, and approved the final manuscript.
References
- 1.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–9. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, Burgart LJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58:3455–60. [PubMed] [Google Scholar]
- 5.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. The Journal of molecular diagnostics : JMD. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. The Journal of molecular diagnostics : JMD. 2008;10:301–7. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–52. [PubMed] [Google Scholar]
- 8.Sinicrope FA, Mahoney MR, Smyrk TC, Thibodeau SN, Warren RS, Bertagnolli MM, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664–72. doi: 10.1200/JCO.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beamer LC, Grant ML, Espenschied CR, Blazer KR, Hampel HL, Weitzel JN, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–63. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koi M, Umar A, Chauhan DP, Cherian SP, Carethers JM, Kunkel TA, et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N'-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 1994;54:4308–12. [PubMed] [Google Scholar]
- 11.Rumilla K, Schowalter KV, Lindor NM, Thomas BC, Mensink KA, Gallinger S, et al. Frequency of deletions of EPCAM (TACSTD1) in MSH2-associated Lynch syndrome cases. The Journal of molecular diagnostics : JMD. 2011;13:93–9. doi: 10.1016/j.jmoldx.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 13.Agostini M, Enzo MV, Morandi L, Bedin C, Pizzini S, Mason S, et al. A ten markers panel provides a more accurate and complete microsatellite instability analysis in mismatch repair-deficient colorectal tumors. Cancer biomarkers : section A of Disease markers. 2010;6:49–61. doi: 10.3233/CBM-2009-0118. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25:1032–8. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila) 2012;5:320–7. doi: 10.1158/1940-6207.CAPR-11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umar A, Boyer JC, Thomas DC, Nguyen DC, Risinger JI, Boyd J, et al. Defective mismatch repair in extracts of colorectal and endometrial cancer cell lines exhibiting microsatellite instability. J Biol Chem. 1994;269:14367–70. [PubMed] [Google Scholar]
- 17.Vilar E, Mukherjee B, Kuick R, Raskin L, Misek DE, Taylor JM, et al. Gene expression patterns in mismatch repair-deficient colorectal cancers highlight the potential therapeutic role of inhibitors of the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway. Clin Cancer Res. 2009;15:2829–39. doi: 10.1158/1078-0432.CCR-08-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiebig HH, Maier A, Burger AM. Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer. 2004;40:802–20. doi: 10.1016/j.ejca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Christensen J, El-Gebali S, Natoli M, Sengstag T, Delorenzi M, Bentz S, et al. Defining new criteria for selection of cell-based intestinal models using publicly available databases. BMC Genomics. 2012;13:274. doi: 10.1186/1471-2164-13-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward RL, Hicks S, Hawkins NJ. Population-based molecular screening for Lynch syndrome: implications for personalized medicine. J Clin Oncol. 2013;31:2554–62. doi: 10.1200/JCO.2012.46.8454. [DOI] [PubMed] [Google Scholar]
- 21.de Rosa N, Rodriguez-Bigas MA, Chang GJ, Veerapong J, Borras E, Krishnan S, et al. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J Clin Oncol. 2016;34:3039–46. doi: 10.1200/JCO.2016.66.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao F, Panarelli NC, Rennert H, Sherr DL, Yantiss RK. Neoadjuvant therapy induces loss of MSH6 expression in colorectal carcinoma. Am J Surg Pathol. 2010;34:1798–804. doi: 10.1097/PAS.0b013e3181f906cc. [DOI] [PubMed] [Google Scholar]
- 23.Kondo A, Safaei R, Mishima M, Niedner H, Lin X, Howell SB. Hypoxia-induced enrichment and mutagenesis of cells that have lost DNA mismatch repair. Cancer Res. 2001;61:7603–7. [PubMed] [Google Scholar]
- 24.Kuan SF, Ren B, Brand R, Dudley B, Pai RK. Neoadjuvant Therapy in Microsatellite Stable Colorectal Carcinoma Induces Concomitant Loss of MSH6 and Ki67 Expression. Hum Pathol. 2017 doi: 10.1016/j.humpath.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Salahshor S, Koelble K, Rubio C, Lindblom A. Microsatellite Instability and hMLH1 and hMSH2 expression analysis in familial and sporadic colorectal cancer. Lab Invest. 2001;81:535–41. doi: 10.1038/labinvest.3780262. [DOI] [PubMed] [Google Scholar]
- 26.Mangold E, Pagenstecher C, Friedl W, Fischer HP, Merkelbach-Bruse S, Ohlendorf M, et al. Tumours from MSH2 mutation carriers show loss of MSH2 expression but many tumours from MLH1 mutation carriers exhibit weak positive MLH1 staining. J Pathol. 2005;207:385–95. doi: 10.1002/path.1858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.