Abstract
Lyme borreliosis, the most common vector-borne illness in Europe and the United States, is caused by spirochetes of the Borrelia burgdorferi sensu lato complex and transmitted by Ixodes ticks. In humans, the spirochetes disseminate from the tick bite site to multiple tissues, leading to serious clinical manifestations. The ability of spirochetes to survive in ticks during blood feeding is thought to be essential for Lyme borreliae to be transmitted to different vertebrate hosts. This ability is partly attributed to several B. burgdorferi proteins, including BBA52 and Lp6.6, which promote spirochete survival in nymphal ticks feeding on mice. One of the strategies to identify such proteins without using live animals is to feed B. burgdorferi-infected ticks on blood via artificial feeding chambers. In previous studies, ticks were only fed on bovine blood in the feeding chambers. In this study, we used this chamber model and showed that I. scapularis ticks will not only acquire bovine blood but human and quail blood as well. The latter two are the incidental host and an avian host of Lyme borreliae, respectively. We also investigated the roles that BBA52 and Lp6.6 play in promoting spirochete survival in nymphal ticks fed on human or quail blood. After feeding on human blood, spirochete burdens in ticks infected with an lp6.6-deficient B. burgdorferi were significantly reduced, while bba52-deficient spirochete burdens in ticks remained unchanged, similar to the wild-type strain. No strain showed a change in spirochete burdens in ticks fed on quail blood. These results indicate that Lp6.6 plays a role for B. burgdorferi in nymphs fed on human but not quail blood. Such information also demonstrates that the artificial feeding chamber is a powerful tool to identify B. burgdorferi proteins that promote vertebrate host blood-specific spirochete survival in I. scapularis ticks.
Keywords: Lyme borreliae, Artificial feeding chamber, BBA52, Lp6.6, Ixodes ticks
1. Introduction
The spirochete Borrelia burgdorferi sensu lato is the causative agent of Lyme borreliosis, the most common vector-borne disease in the U.S. and Europe (Steere et al., 2016). These spirochetes, including the three most prevalent species (B. garinii, B. afzelii, and B. burgdorferi sensu stricto), are transmitted by hard ticks of the genus Ixodes (e.g., blacklegged tick, Ixodes scapularis) (Radolf et al., 2012; Steere et al., 2016). Spirochetes are transmitted from ticks to vertebrate animals when ticks are taking blood meals (Radolf et al., 2012). In humans, the spirochete is then able to spread to tissues and organs via the bloodstream, leading to multiple disease manifestations, including carditis, neuroborreliosis, and arthritis (Steere et al., 2016). In addition to humans, Lyme borreliae are also transmitted to reservoir hosts, such as birds and small mammals, through tick feeding (Steere et al., 2016). Thus, the ability to survive in feeding ticks is essential for successful tick-to-vertebrate transmission. In fact, Lyme borreliae produce numerous proteins that have been shown to be important for this survival (Kung et al., 2013; Radolf et al., 2012; Rosa et al., 2005). Some of these proteins allow the spirochetes to acquire and metabolize nutrients in the ingested host blood in the feeding tick (Eggers et al., 2011; Jewett et al., 2009; Nogueira et al., 2012; Ouyang et al., 2009; Purser et al., 2003; Strother and de Silva, 2005), and others globally regulate genes required for spirochete survival in response to the influx of blood in feeding ticks (Caimano et al., 2007; Caimano et al., 2011; Fisher et al., 2005; He et al., 2011; Hubner et al., 2001; Kostick et al., 2011). Other Lyme borreliae-produced proteins have been shown to confer survival in feeding ticks, though their functions remain unclear (Pal et al., 2008; Xu et al., 2010; Zhang et al., 2009). These functionally unknown proteins include BBA52 and BBA62 (Lp6.6) (Kumar et al., 2010; Promnares et al., 2009).
Typically, the importance of a protein for spirochete survival in feeding nymphal ticks (and/or transmission to vertebrates) is identified by demonstrating that a mutant spirochete strain, lacking the protein of interest, exhibits a survival defect in ticks feeding on mice (Kung et al., 2013; Radolf et al., 2012; Rosa et al., 2005). However, a host-spirochete species association has previously been observed (van Dam, 2002). B. garinii and B. afzelii are commonly isolated from ticks feeding on birds or mammals, respectively, whereas B. burgdorferi sensu stricto can be found in ticks feeding on both types of vertebrate animals. These findings raise the possibility that the B. burgdorferi proteins that promote spirochete survival in ticks feeding on one animal may not necessarily confer similar phenotypes in ticks feeding on another.
One strategy to test this possibility is to allow ticks carrying these mutant spirochetes to feed on blood from different animals in vitro using an established artificial feeding chamber model (Bullard et al., 2016; de Moura et al., 1997; Perner et al., 2016; Stone et al., 1983; Tajeri et al., 2016). This chamber allows ticks to feed on blood in vitro and thus avoids ethical issues and reduces the space and cost typically associated with performing experiments on live animals. To date, only bovine blood has been utilized in this model (Andrade et al., 2014; Kröber and Guerin, 2007a; Oliver et al., 2016; Stone et al., 1983; Tajeri et al., 2016). There has been serological evidence supporting that Lyme borreliae may be infectious in cattle (Burgess et al., 1987; Burgess et al., 1993; Isogai et al., 1992). However, the fact that the constituents in blood vary among animals (Evans and Wiederanders, 1967; Mestas and Hughes, 2004; Turner et al., 1958) implies that the phenotype of spirochetes in ticks feeding on bovine blood may not reflect that in ticks feeding on the blood from other animals, such as humans (the incidental host) and birds (one of the reservoir hosts). In this study, we showed that I. scapularis nymphal ticks are capable of feeding on human and quail blood through an artificial feeding chamber model. Using this methodology, we further identified that the role the B. burgdorferi surface protein Lp6.6 plays in spirochete survival in feeding ticks is dependent on the source of host blood. Our findings emphasize a new application of this in vitro feeding model to identify the Lyme borreliae proteins that promote spirochete survival in feeding ticks in a vertebrate host blood-specific manner. Such information will facilitate the understanding of the mechanisms by which Lyme borreliae are transmitted between ticks and vertebrate animals including humans and different reservoir hosts.
2. Material and Methods
2.1 Tick and bacterial strains
Ixodes scapularis nymphal ticks were obtained from BEI Resources (Manassas, VA). B. burgdorferi-infected nymphs were generated by allowing larval ticks to feed on mice previously infected with spirochetes and permitting them to molt into nymphs. This procedure is described in section 2.6 (Generating I. scapularis nymphal ticks carrying B. burgdorferi). The B. burgdorferi strains used in this study are described in Supplemental Table 1. All B. burgdorferi strains were grown in BSK-II complete medium (Barbour et al., 1983) supplemented with kanamycin (200 μg/mL) and/or streptomycin (50 μg/mL) or no antibiotics, as appropriate (Supplemental Table 1).
2.2 Preparation of artificial feeding chamber
Artificial feeding chambers were prepared by modifying the chamber model reported previously (Andrade et al., 2014; Burkot et al., 2001; Kröber and Guerin, 2007a; Oliver et al., 2016). I. scapularis nymphal ticks were allowed to feed on blood through a silicone rubber-saturated rayon membrane, which mimics the hardness and elasticity of skin. Single-sided tape was used to adhere a 10-cm by 15-cm sheet of Kleensite brand lens cleaning paper (Kleensite, Cranston, RI) to plastic wrap (Fisher Scientific, Hampton, NH). Three milliliters of silicone rubber were made with Smooth-On ECOFLEX 00-30-Supersoft Platinum Silicone Kit (Smooth-On, Inc., East Texas, PA) per the manufacturer’s instructions. The silicone rubber was then poured over the lens cleaning paper and spread with a plastic squeegee to completely cover the paper. All excess silicone was removed, and the silicone-saturated lens cleaning paper was cured on a flat surface for a minimum of 24 hours. After 24 hours, the membrane was peeled from the plastic wrap, and cut into six 3-cm squares. Their thickness was measured with Traceable Digital Calipers (Fisher Scientific, Hampton, NH). To better facilitate nymph feeding, only membranes with a thickness of 40 to 75 μm were used, in order to accommodate the length of the nymphal ticks’ hypostomes (e.g., 170 μm for nymphs of Ixodes ticks). More silicone rubber was used to glue the membranes to one end of a 2-cm length of polycarbonate tubing (hereafter called the chamber; inner diameter: 2.5 cm; outer diameter: 3.2 cm; (Amazon Inc., Seattle, WA)), so that the side with the silicone rubber was facing the chamber (i.e., the side previously facing the plastic wrap was facing away from the chamber). The membrane chamber was then allowed to air dry for 24 hours.
We then used the hair and hair extract from white-tailed deer (Odocoileus virginianus) as feeding stimuli, based on a previous study (Kröber and Guerin, 2007a). In brief, a 3-cm layer of deer hair was added to a 125-mL Wheaton bottle (Wheaton, Millville, NJ), and then incubated with 25 mL of dichloromethane for 45 minutes at 40°C. All dichloromethane was subsequently removed using vacuum centrifugation (Eppendorf, Hamburg, Germany) at 45°C for 60 minutes. The dry hair extract was stored at 4°C. When used, 0.5 mg of the hair extract was dissolved in 80 μL of dichloromethane. The dissolved hair extract was then added to the chamber around the inner surface of the membrane. The membrane chambers were subsequently placed into an oven at 40°C for 20 minutes to allow the dichloromethane to evaporate. The membranes were then cut flush with the chamber, and 2 mL of silicone rubber was smeared over the edge of the membrane to seal any pores formed from cutting it. Thus, the rubber would cover the outer surface of the chamber to avoid leakage when placed in the blood. One hundred microliters of silicone rubber were also spread across the outer surface of the membrane to fill any holes created by the dichloromethane when adding the hair extract. The chambers were then air dried for 24 hours prior to use for blood feeding.
2.3 I. scapularis nymphal ticks feeding on the blood from vertebrate animals via feeding chambers
To prevent coagulation, blood from the common quail (Coturnix coturnix; Canola Poultry Market, Brooklyn, NY) was treated with heparin (150 U/mL) at a 1:10 dilution rate. Bovine (HemoStat Laboratories, Dixon, CA) or human (New York Blood Center, New York, NY) blood was defibrinated by mixing with citrate-phosphate-dextrose solution (final concentration as 12.28%; Sigma, St. Louis, MO). Prior to nymph feeding, the blood was supplemented with a cocktail of antibiotics (final concentration: 50 μg/mL rifampicin, 20 μg/mL phosphomycin and 2.5 μg/mL amphotericin; Sigma) to avoid potential bacterial and fungal contamination. ATP (final concentration as 1μM; Sigma) and glucose (final concentration as 2 g/mL; Sigma) were also added to the blood to increase the efficiency of tick blood feeding (Andrade et al., 2014; Burkot et al., 2001; Kröber and Guerin, 2007a; Oliver et al., 2016). This blood was then placed into each well of a six-well cell culture plate (VWR, Inc, Radnor, PA) and warmed to 37°C. Membrane chambers were prepared by adding a 1.5-cm square of fiberglass mesh tape (3-mm pore) (Lowe’s Inc., Mooresville, NC) and a 1.5-cm plastic tile spacer (Lowe’s Inc., Mooresville, NC) onto the membrane. I. scapularis nymphal ticks were then placed into the chambers using Dumont fine-tipped forceps (Electron Microscopy Sciences, Hatfield, PA). Further, a 1-cm thick layer of deer hair was added, followed by a nickel coin to weigh the hair down. Membrane chambers were sealed with two layers of parafilm (Fisher Scientific), which were aerated to allow air flow. A rubber O-ring (inner diameter: 3 cm; outer diameter: 3.5 cm; Beckman Coulter Inc., Indianapolis, IN) was placed around the top portion of the chamber to adjust the depth at which the membrane chamber sat in the blood. The membrane chambers were then placed into the wells of blood. The six-well plate was placed into a tray filled with sterilized water and incubated in an incubator with 80% relative humidity. The water in the tray was used as moat. The feeding was performed in an incubator at 37°C, 5% CO2, and 80% relative humidity. Every 12 hours, the membrane chamber was removed from the six-well plates to change the blood to prevent clotting and mold growth. After removing the blood, the wells were rinsed with saline buffer (0.9% NaCl in Millipore water), and fresh blood was added. After five days of feeding, all nymphal ticks were removed from the chamber, placed into 1.5 mL Eppendorf tubes, and stored in a −20°C freezer until subsequent use.
2.4 Identification of the blood-acquired nymphal ticks by qPCR
Glass beads were added to tubes of frozen ticks for homogenization with a Precellys 24 High Powered Bead Mill Homogenizer (Bertin, Rockville, MD). The DNA from ticks was extracted using the Bio Basic EZ-10 Spin Column Genomic DNA Minipreps Kit for animal samples following the manufacturer’s instructions (Bio Basic, Markham, ON, Canada). DNA quality and quantity for each sample was assessed using a Nanodrop 1000 UV/Vis spectrophotometer (ThermoFisher, Waltham, MA) by determining the concentration of DNA and the ratio of UV adsorption at 260 nm to 280 nm. The A260:A280 ratios were between 1.75 to 1.85, which indicates the lack of RNA or proteins. Quantitative PCR (qPCR) was then performed using an Applied Biosystems 7500 Real-Time PCR system (ThermoFisher) to determine the presence of blood in ticks. Each qPCR reaction contained 1× SYBR Green (ThermoFisher), 100 ng of DNA, 500 nmol of primers (Supplemental Table 2), and filtered and UV-sterilized water. All reactions were performed in duplicate. The primers amplified DNA of human or quail ß-actin (for human or quail blood, respectively) or the cytB gene (for bovine blood), as described in Supplemental Table 2 (Drummond, 2013; Lindsay, 2008; Suzuki et al., 2005; Uno et al., 2012). Cycling parameters were 50°C for 2 minutes, 90°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Water and DNA extracted from unfed nymphs were used as negative controls to establish a threshold of Ct values for blood-acquired ticks. The samples with Ct values lower than the determined threshold were considered as positive for blood, indicating that the particular ticks had acquired blood.
2.5 Determination of the number of blood cells in nymphal ticks by qPCR
The total cell counts of human, bovine, or quail blood were obtained using a Hemocytometer Counting Chamber (ThermoFisher) under a dark-field microscope. The DNA was then extracted from different numbers of blood cells. The quantity and quality of the DNA were determined as described in section 2.4 (Identification of the blood-acquired nymphal ticks by qPCR). Subsequently, qPCR was performed using the DNA from different numbers of blood cells or from blood-fed ticks and the primers of human or quail ß-actin (for human or quail blood, respectively) or cytB gene (for bovine blood). The Ct values derived from blood cells were then correlated with the number of cells to create a standard curve. The number of cells in the blood ingested by ticks was calculated by comparing the Ct derived from blood-fed ticks to that standard curve.
2.6 Generating I. scapularis nymphal ticks carrying B. burgdorferi
Four-week-old female C3H/HeN mice were infected by subcutaneous injection as previously described (Lin et al., 2014) with 105 cells of B. burgdorferi strain B31-A3, B31-A3Δbba52, B31-A3Δlp6.6, or B31-A3Δlp6.6/lp6.6com. The plasmid profiles and the presence of the shuttle vector for each B. burgdorferi strain were verified prior to infection to ensure the stability of the vector and no loss of plasmids, as described previously (Purser and Norris, 2000). Ear punches were collected from mice and placed into BSK-II medium at 7 days post-infection. The growth of spirochetes was then evaluated to verify the infection of these mice, and only infected mice were used in the study. At 14 days post-infection, uninfected larvae were allowed to feed to repletion on the B. burgdorferi-infected C3H/HeN mice as described (Kern et al., 2016). Approximately 100 to 200 larvae were allowed to feed on each mouse. The replete larvae were collected and allowed to molt into nymphs in 4 to 6 weeks in a desiccator at room temperature and 95% relative humidity in a room with light dark control (light to dark, 12: 12 hours). DNA was extracted from post-molting flat nymphs to examine the plasmid profiles and the presence of the antibiotic resistant gene marker of the B. burgdorferi strains carried by these ticks to ensure no loss of plasmids during acquisition and molting, as described (Purser and Norris, 2000).
2.7 Quantification of B. burgdorferi in infected nymphal ticks
DNA was extracted from ticks, and its quantity and quality were determined as described in section 2.4 (Identification of the blood-acquired nymphal ticks by qPCR). qPCR was then performed to verify whether the ticks had acquired blood. B. burgdorferi genomic equivalents were calculated based on amplification of recA gene DNA (Supplemental Table 2), as previously described (Lin et al., 2014). The copy number of recA for experimental reactions was calculated by comparing the Ct to a standard curve of B. burgdorferi strain B31. The spirochete burdens were presented as the resulting copy number of recA per 100 ng of DNA extracted from those ticks.
2.8 Statistical analysis
Data sets were first applied to the D’Agostino-Pearson Omnibus normality test to examine for the normal distribution. As all data sets in this study were not in a normal distribution, significant differences between samples were determined using the non-parametric Kruskal-Wallis test and the post hoc Dunn’s multiple comparison test. p-values between samples were obtained. p < 0.05 was considered to be significant.
3. Results
3.1 I. scapularis nymphal ticks are capable of acquiring human or quail blood via artificial feeding chambers
To determine if ticks are able to ingest blood from different animals via artificial feeding chambers, we used a previously reported silicone membrane chamber model (Kröber and Guerin, 2007a) with slight modifications of the device and the time point at which feeding ticks were collected (Fig. 1 and Material and Methods). Rather than using immovable beveled washers, we placed O-rings around the chamber to adjust the depth at which the chamber was immersed in blood (Fig. 1A). In addition, we aimed to eventually use this methodology to identify the Lyme borreliae proteins that promote spirochete survival in feeding ticks. Because the blood meal survival phenotypes conferred by several spirochete proteins have been shown to be most apparent shortly after feeding (Kumar et al., 2010; Promnares et al., 2009), we sought to collect ticks at earlier time points. Therefore, six I. scapularis nymphs were placed in each feeding chamber and allowed to feed on bovine blood for different durations. Quantitative PCR (qPCR) was used to differentiate the fed and unfed ticks (see Material and Methods) to determine the time point by which the majority of the ticks had begun to acquire blood. We observed that approximately 70% of ticks had acquired bovine blood by 5 days post-incubation. This percentage was significantly higher than the percentage of fed ticks in groups incubated for less than 5 days (approximately 20% and 30% for 2 and 4 days, respectively, p < 0.05) but no different from groups incubated for more than 5 days (approximately 70% for 6 days, p > 0.05) (data not shown). The ticks were thus allowed to feed in chambers for 5 days in the remainder of the study.
Figure 1. Schematic diagram and pictures of the artificial feeding chamber.
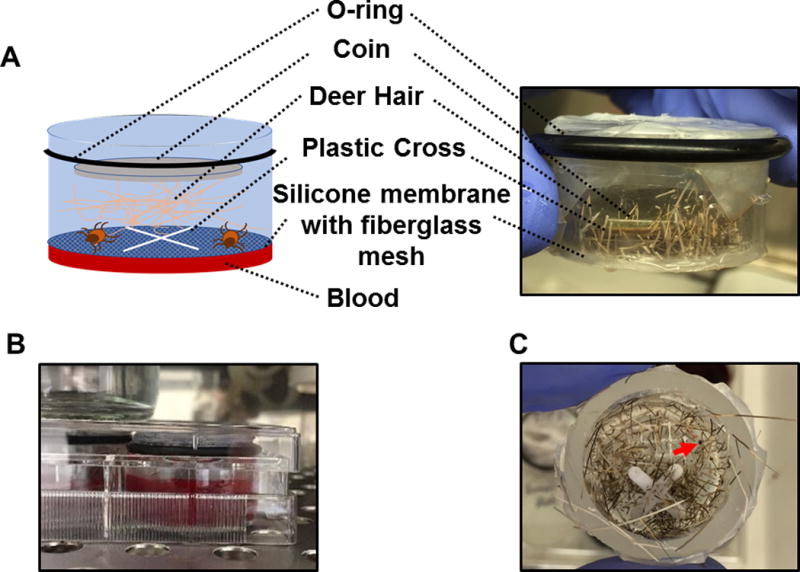
(A) A schematic diagram and picture of the artificial feeding chamber used in this study. (B) Picture showing that artificial feeding chambers were placed into a six-well cell culture plate with blood. (C) Top views of the artificial feeding chamber with the coin and deer hair removed after I. scapularis nymphal ticks were allowed to feed for 5 days. A partially engorged nymph is indicated by the arrow.
We then tested whether ticks are capable of obtaining, via feeding chambers, blood from various sources: bovine (the previously reported source of blood as a positive control [Andrade et al., 2014; Kröber and Guerin, 2007a; Oliver et al., 2016; Stone et al., 1983; Tajeri et al., 2016]), human (the incidental host); or Coturnix quail (an avian host that could be infected with Lyme borreliae [Isogai et al., 1994]). Flat I. scapularis nymphal ticks were permitted to feed on human or quail blood for 5 days. Approximately 72% of ticks ingested bovine blood (Table 1), which is consistent with previous studies using an artificial feeding chamber (Andrade et al., 2014; Kröber and Guerin, 2007a; Oliver et al., 2016). Similarly, approximately 83 and 89% of ticks consumed human and quail blood, respectively (Table 1), and there were no significant differences in the proportions of fed ticks between these blood sources and bovine blood (Fig. 2, top panel, Table 1 and Supplemental Table 3). Additionally, to quantitatively determine the amount of blood from each of these animals in fed ticks, the number of blood cells in these ticks was determined using qPCR (see Material and Methods). Though the number of blood cells ingested by each tick varies, no statistical differences were observed in the average number of blood cells in ticks feeding on human, quail, or bovine blood (Fig. 2, bottom panel and Supplemental Table 3). These results imply that there are no differences in feeding efficiency among ticks feeding on human, quail or bovine blood using the artificial feeding chamber.
Table 1.
The percentage of I. scapularis nymphal ticks acquiring blood of vertebrate animals using artificial feeding chambers
| Blood sourcea | nb | % positive for blood in ticksc |
|---|---|---|
| Plate 1 | ||
| Bovine | 6 | 50% (3/6) |
| Human | 6 | 66% (4/6) |
| Quail | 6 | 100% (6/6) |
| Plate 2 | ||
| Bovine | 6 | 83% (5/6) |
| Human | 6 | 100% (6/6) |
| Quail | 5 | 80% (4/5) |
| Plate 3 | ||
| Bovine | 6 | 83% (5/6) |
| Human | 6 | 83% (5/6) |
| Quail | 8 | 87% (7/8) |
I. scapularis nymphal ticks were fed on blood sourced from animals listed.
Number of ticks placed into the chamber.
Nymphal ticks were tested to determine their blood feeding using qPCR, following 5 days of incubation in the feeding chamber. The data are presented as the percentage of fed ticks (percent positive for blood in ticks), with the number of fed ticks/total number of ticks in parentheses.
Figure 2. I. scapularis nymphal ticks are capable of acquiring bovine, human, and quail blood.
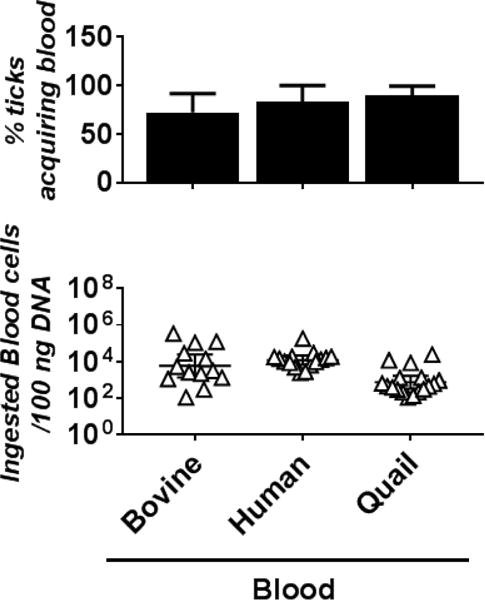
Naïve and flat I. scapularis nymphal ticks were placed into the artificial feeding chamber in six-well cell culture plates with the indicated bovine, human, or quail blood (six to eight nymphs per chamber [see Table 1]). After being placed in the chambers for 5 days, the nymphs were collected to determine whether they had acquired blood from these animals using qPCR (see Material and Methods). (Top panel) The percentage of blood-acquired nymphs was calculated using the number of nymphs determined positive with blood (see Material and Methods) divided by total number of the nymphs in each membrane chamber. Bars represent the mean of three independent membrane chambers ± standard deviation. The values of these determinations are also shown in Table 1. The data were subjected to a D’Agostino-Pearson Omnibus normality test to examine for the normal distribution. As all values were not in a normal distribution, statistical significance was determined using a non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparisons test. There is no significant difference between the percent of nymphs fed among the different blood types. The p-values between each group are shown in Supplemental Table 3. (Bottom panel) The number of blood cells in these blood-fed nymphs were also determined (see Material and Methods). Each triangle indicates the number of blood cells ingested by a single nymph. Shown are the geometric means of the numbers of ingested blood cells in nymphs per 100 ng of DNA ± 95% confidence interval of 13 nymphs per group. As all values were not in a normal distribution using a D’Agostino-Pearson Omnibus normality test, statistical significance was determined using a non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparisons test. No statistically significant difference in the number of blood cells was observed. The p-values between each group are shown in Supplemental Table 3.
3.2 B. burgdorferi Lp6.6 promotes different levels of spirochete survival in nymphal ticks feeding on human or quail blood
B. burgdorferi requires BBA52 and Lp6.6 to facilitate optimal survival in nymphal ticks feeding on mice (Kumar et al., 2010; Promnares et al., 2009). We aimed to test whether each of these proteins also promote spirochete survival in ticks feeding on the blood from humans or quail. Thus, we allowed I. scapularis larval ticks to feed on mice infected with wild-type (WT) B. burgdorferi strain B31-A3, or mutants of this strain lacking bba52 (Δbba52) or lp6.6 (Δlp6.6) and collected nymphs molted from these larvae. The spirochete loads per 100 ng of DNA extracted from nymphs after molting were determined by qPCR. We observed that the bba52-and lp6.6-deficient B. burgdorferi were present in flat nymphs at levels no different from each other or from the parental WT strain B31-A3 (Figure 3 left panel, Supplemental Table 3). These results are consistent with previous studies, indicating that neither BBA52 nor Lp6.6 is essential for spirochete survival in flat nymphs (Kumar et al., 2010; Promnares et al., 2009). These infected nymphs were then placed into the feeding chambers to feed on either human or quail blood for 5 days, and the bacterial loads and the number of blood cells ingested by ticks were obtained using qPCR. There were no statistical differences among the numbers of blood cells obtained by fed nymphs carrying each of the B. burgdorferi strains (Supplemental Figure 1). As shown in Figure 3 middle panel, we observed that WT B. burgdorferi strain B31-A3 survived in the ticks feeding on human blood (approximately 1034 spirochetes per 100 ng DNA). The bba52-deficient strain was present in human blood-fed ticks at levels indistinguishable from the parental WT strain B31-A3, indicating that BBA52 is dispensable for spirochete survival in ticks feeding on human blood (Figure 3, middle panel). In contrast, the lp6.6-deficient strain survived in the human blood-fed nymphs approximately 42-fold less well than the WT strain B31-A3 (p = 0.0083; Figure 3, middle panel and Supplemental Table 3). Additionally, when this mutant strain was complemented to express lp6.6 (lp6.6com), the burdens of the strain lp6.6com in either flat nymphs or the nymphs fed on human blood were no different from the burdens of the WT strain B31-A3 in flat nymphs (Figure 3, left panel) or human blood-fed nymphs (Fig. 3, middle panel). Further, this lp6.6com strain survived in the nymphs feeding on human blood at levels greater than the lp6.6-deficient strain (p=0.0066; Figure 3, middle panel and Supplemental Table 3). These findings suggest that Lp6.6, but not BBA52, confers spirochete survival in nymphs feeding on human blood.
Figure 3. Lp6.6 confers B. burgdorferi survival in nymphal ticks feeding on human but not quail blood.
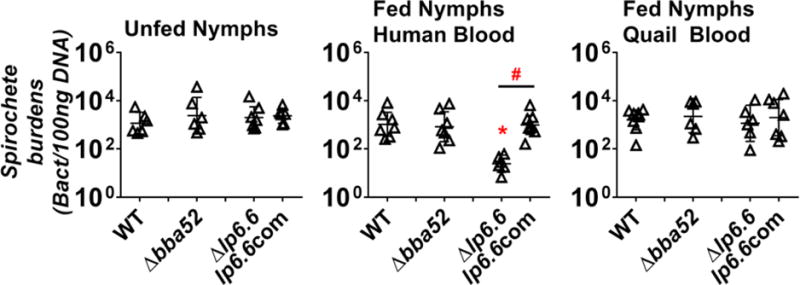
I. scapularis nymphal ticks infected with B. burgdorferi strain B31-A3 (WT), a bba52-deficient strain (Δbba52), an lp6.6-deficient strain (Δlp6.6), or this strain producing Lp6.6 (lp6.6com) were placed into artificial feeding chambers. The chambers were then placed into six-well cell culture plates with blood from humans or quail for 5 days. The blood feeding of nymphs carrying the spirochete strain lp6.6com was performed in a separate experiment. The bacterial loads in the nymphs prior to blood feeding (Unfed nymphs, Left panel) or after feeding on human blood (Fed nymphs human blood, Middle panel) or quail blood (Fed nymphs quail blood, Right panel) for 5 days were determined by qPCR. The results were then normalized to 100 ng total DNA. Each triangle indicates the bacterial burden derived from a single nymph. Shown are the geometric mean of bacterial loads ± 95% confidence interval of six nymphs per group. As all datasets were not in a normal distribution using a D’Agostino-Pearson Omnibus normality test, statistical significance was determined using a non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparisons test. The asterisk (*) indicates a significant difference (p < 0.05) in spirochete burdens relative to the strain B31-A3. The pound sign (#) indicates a significant difference (p < 0.05) in spirochete burdens between the Δlp6.6 and lp6.6com strains. The p-values between each group are shown in Supplemental Table 3.
Similarly, WT strain B31-A3 survived in nymphal ticks feeding on quail blood (1601 spirochetes per 100 ng DNA; Figure 3, right panel). In addition, the bba52- and lp6.6-deficient B. burgdorferi mutants, as well as the strain lp6.6com, survived in the quail blood-fed nymphs at levels indistinguishable from the parental WT strain B31-A3 (Figure 3, right panel). These results indicate that neither BBA52 nor Lp6.6 are essential for spirochete survival in nymphal ticks feeding on quail blood.
4. Discussion
Lyme borreliae have been isolated from ticks feeding on multiple vertebrate animals, including birds (e.g., quail) and mammals (e.g., humans and mice) (Kurtenbach et al., 2002), implying that these spirochetes produce proteins to support their survival in ticks feeding on these hosts. In fact, multiple B. burgdorferi proteins have been demonstrated to facilitate tick-to-mouse transmission of spirochetes (Kung et al., 2013; Radolf et al., 2012; Rosa et al., 2005). However, proteins critical for spirochete survival in ticks feeding on mice may not be important for spirochete survival in ticks feeding on other vertebrate hosts. To avoid the cumbersome work and costs of feeding ticks on live animals, different in vitro strategies, including microinjection, immersion, capillary feeding, and artificial feeding chambers, have been developed to introduce substances such as blood into ticks (Bonnet, 2012; Kuhnert, 1996). Not all of these methods are able to completely mimic the manner by which ticks feed on animals. For example, instead of passing by the mouthparts into the digestive tract, the microinjection methodology introduces substances into the tick’s gut through the cuticle, unlike feeding on animals (Bonnet, 2012). For immersion feeding, the prolonged time spent submerged can lead to an increase in nymph mortality, as the ticks may require days to obtain blood in this fashion (Gern and Rais, 1996; Policastro and Schwan, 2003; Randolph, 1998; Sojka et al., 2013). Although capillary feeding has been successfully used to inoculate ticks with blood (Antunes et al., 2014), the artificial feeding chamber methodology allows ticks to attach to a skin (or skin-like) membrane and ingest animal blood via hypostomes for as much time as necessary to feed. Thus, this technique is one of the in vitro feeding methodologies most fundamentally similar to how ticks take blood meals from animals. Some feeding chamber models use mouse or rabbit skin as membranes for in vitro feeding (Bonnet et al., 2007; Burkot et al., 2001; Cotte et al., 2008; Stone et al., 1983), while others use membranes made of abiotic materials, such as lens paper covered with silicone (Andrade et al., 2014; Kröber and Guerin, 2007a; Oliver et al., 2016). Though the silicone membrane-based model requires specific stimulants to promote tick feeding (e.g. animal hair and hair extract) (Andrade et al., 2014; Kröber and Guerin, 2007a; Oliver et al., 2016), this model avoids the laborious and time-consuming disadvantages of the skin-based model (Bonnet, 2012).
Using this silicone membrane-based artificial feeding chamber, we have shown that I. scapularis nymphal ticks not only obtain bovine blood as previously described (Andrade et al., 2014; Kröber and Guerin, 2007a; Oliver et al., 2016; Stone et al., 1983; Tajeri et al., 2016) but also acquire blood from humans and quail. We further tested the role that two B. burgdorferi proteins, BBA52 and Lp6.6, play in promoting spirochete survival in nymphs feeding on human or quail blood, as these proteins have been previously shown to promote spirochete survival in nymphs fed on mice (Kumar et al., 2010; Promnares et al., 2009). We observed that BBA52 is not required for B. burgdorferi to survive in nymphs acquiring blood from either humans or quail. Though Lp6.6 is not essential to promote spirochete survival in quail blood-fed nymphs, this protein is required for optimal B. burgdorferi survival in nymphs feeding on human blood. These observations suggest that the importance of Lp6.6 for spirochete survival in feeding ticks is dependent on the type of animal blood on which the ticks are feeding.
Though the function of Lp6.6 remains unknown, the roles of other spirochete proteins that promote similar phenotypes of spirochete survival in fed nymphs have been previously identified. Some proteins promote global regulation of the B. burgdorferi signaling pathways in response to the host-specific environments, such as the blood meals in nymphs (Caimano et al., 2007; Caimano et al., 2011; Fisher et al., 2005; He et al., 2011; Hubner et al., 2001). Other proteins serve as transporters or enzymes to contribute to nutrient acquisition and/or metabolism in nymphs’ blood meals (Eggers et al., 2011; Jewett et al., 2009; Nogueira et al., 2012; Ouyang et al., 2009; Purser et al., 2003; Strother and de Silva, 2005). These previous findings may provide insight into research investigating the potential role that Lp6.6 plays during Lyme infection. In addition, our finding that Lp6.6 confers host blood-specific spirochete survival in nymphs implies that the proteins required for B. burgdorferi to survive in the blood meals of ticks varies by the source of the blood. In fact, the nutrients and metabolites in host blood differ among different animals (Hamdy, 1977; Sojka et al., 2013; Wickramasekara et al., 2008), which raises the possibility that the spirochete proteins required for B. burgdorferi to take up or metabolize the nutrients in blood meals from different hosts may vary. This could be one of the reasons why B. burgdorferi needs different proteins to promote spirochete survival in ticks feeding on blood from different hosts. In addition, blood from vertebrate animals contains numerous innate immune defense mechanisms (e.g., the complement system and antimicrobial peptides), which are harmful for spirochetes (Akira et al., 2006; Ganz, 2003; Zipfel and Skerka, 2009). The ability of these mechanisms to eliminate Lyme borreliae varies among vertebrate hosts (Kraiczy, 2016b). This is likely due to the polymorphisms of the components of these innate immune mechanisms. For example, the complement components or complement regulatory proteins share approximately 60% identity among vertebrate hosts (Horstmann et al., 1985; Kristensen and Tack, 1986). Similarly, less than 40% of sequence identity has been found in the antimicrobial peptide cathelicidin LL-37 among different vertebrate animals (Tomasinsig and Zanetti, 2005). B. burgdorferi has been shown to produce multiple proteins to evade these innate immune mechanisms (Carrasco et al., 2015; Kraiczy, 2016a). Thus, the fact that these mechanisms from different hosts differ in their ability to eliminate spirochetes leads to an intriguing possibility that the spirochete proteins essential for B. burgdorferi evasion of these innate immune mechanisms in the blood meals from different hosts vary. This could potentially be another reason to explain why B. burgdorferi requires different proteins to promote its survival in ticks feeding on the blood from different hosts.
Using a similar artificial feeding chamber model, Koci et al. recently observed that a B. burgdorferi strain devoid of a protein Lmp1 displays reduced levels of spirochete survival in ticks feeding on bovine blood (Koci et al., 2018). However, our findings strongly suggest that a survival defect of spirochetes in ticks feeding on one blood source does not necessarily reflect a similar defect in ticks feeding on another. Thus, our study demonstrates how the feeding chamber model can be used to delineate the roles that Lyme borreliae proteins play in promoting host blood-specific spirochete survival in feeding ticks. This information may facilitate the further study of host tropisms exhibited by Lyme borreliae in the enzootic cycle.
5. Conclusions
In this study, we utilized a previously reported artificial feeding chamber system to demonstrate that Lp6.6 differs in its importance for B. burgdorferi survival in nymphs feeding on blood from humans or quail. This is the first time that such feeding chambers have been used to identify B. burgdorferi proteins that promote spirochete survival in ticks feeding on the blood from various vertebrate hosts. The results derived from the use of this technique will illuminate the potential roles of B. burgdorferi proteins in feeding ticks, and lead to the development of further studies to identify the mechanisms by which these proteins contribute to tick-to-host transmission. Such information will further promote the development of therapeutics targeting these proteins for the benefit of human health.
Supplementary Material
Acknowledgments
We thank Aurelie Kern, Linden Hu, Magdia de Jesus, and Heather Gallagher for valuable technical advice, Emily Mallick, Ashley Marcinkiewicz, Linda Gebhardt, and Paul Masters for critical reading of the manuscript, Kevin Rooney for technical support of tick feeding assay, Tim Abrams, Dean Abrams, and Ashley Marcinkiewicz for providing deer hairs, John Leong for providing B. burgdorferi strain B31-A3, and Sudha Chaturvedi for allowing us to use her Bead Homogenizer.
Funding sources
This work was supported by New York State Department of Health Wadsworth Center Start-Up Grant (to Y.L. and T.H.) and National Institutes of Health R01AI080615 and R01AI116620 (to U.P. and X.Y.).
Abbreviations
- B31-A3
Borrelia burgdorferi strain B31-A3
- Δbba52
a bba52-deficient B. burgdorferi in the strain B31-A3 background
- Δlp6.6
an lp6.6-deficient B. burgdorferi in the strain B31-A3 background
- lp6.6com
an lp6.6-complemented B. burgdorferi in a Δlp6.6 strain background
- dpf
days post-feeding
- IACUC
Institutional Animal Care and Use Committee
- Ct
threshold cycle
- WT
wild-type
- qPCR
quantitative PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics statement
All experiments involving in the use of animal products were performed in strict accordance with all provisions of the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the PHS policy on Humane Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Wadsworth Center, New York State Department of Health (Protocol docket number 16-451). All efforts were made to minimize animal suffering.
Declarations of interest
none
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Andrade JJ, Xu G, Rich SM. A silicone membrane for in vitro feeding of Ixodes scapularis (Ixodida: Ixodidae) Journal of medical entomology. 2014;51:878–879. doi: 10.1603/me13180. [DOI] [PubMed] [Google Scholar]
- Antunes S, Merino O, Mosqueda J, Moreno-Cid JA, Bell-Sakyi L, Fragkoudis R, Weisheit S, Perez de la Lastra JM, Alberdi P, Domingos A, de la Fuente J. Tick capillary feeding for the study of proteins involved in tick-pathogen interactions as potential antigens for the control of tick infestation and pathogen infection. Parasites & vectors. 2014;7:42. doi: 10.1186/1756-3305-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Burgdorfer W, Grunwaldt E, Steere AC. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. The Journal of clinical investigation. 1983;72:504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, Jouglin M, Malandrin L, Becker C, Agoulon A, L’Hostis M, Chauvin A. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134:197–207. doi: 10.1017/S0031182006001545. [DOI] [PubMed] [Google Scholar]
- Bonnet SLX. Laboratory artificial infection of hard ticks: A tool for the analysis of tick-borne pathogen transmission. Acarologia. 2012;52:453–464. [Google Scholar]
- Bullard R, Allen P, Chao CC, Douglas J, Das P, Morgan SE, Ching WM, Karim S. Structural characterization of tick cement cones collected from in vivo and artificial membrane blood-fed Lone Star ticks (Amblyomma americanum) Ticks and tick-borne diseases. 2016;7:880–892. doi: 10.1016/j.ttbdis.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess EC, Gendron-Fitzpatrick A, Wright WO. Arthritis and systemic disease caused by Borrelia burgdorferi infection in a cow. Journal of the American Veterinary Medical Association. 1987;191:1468–1470. [PubMed] [Google Scholar]
- Burgess EC, Wachal MD, Cleven TD. Borrelia burgdorferi infection in dairy cows, rodents, and birds from four Wisconsin dairy farms. Veterinary microbiology. 1993;35:61–77. doi: 10.1016/0378-1135(93)90116-o. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Happ CM, Dolan MC, Maupin GO. Infection of Ixodes scapularis (Acari: Ixodidae) with Borrelia burgdorferi using a new artificial feeding technique. Journal of medical entomology. 2001;38:167–171. doi: 10.1603/0022-2585-38.2.167. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Molecular microbiology. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, Akins DR, Pal U, Radolf JD. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infection and immunity. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco SE, Troxell B, Yang Y, Brandt SL, Li H, Sandusky GE, Condon KW, Serezani CH, Yang XF. Outer surface protein OspC is an antiphagocytic factor that protects Borrelia burgdorferi from phagocytosis by macrophages. Infection and immunity. 2015;83:4848–4860. doi: 10.1128/IAI.01215-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotte V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis HJ, Lecuelle B, Lilin T, Vayssier-Taussat M. Transmission of Bartonella henselae by Ixodes ricinus. Emerging infectious diseases. 2008;14:1074–1080. doi: 10.3201/eid1407.071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura ST, da Fonseca AH, Fernandes CG, Butler JF. Artificial feeding of Amblyomma cajennense (Fabricius, 1787) (Acari:Ixodidae) through silicone membrane. Mem Inst Oswaldo Cruz. 1997;92:545–548. doi: 10.1590/s0074-02761997000400019. [DOI] [PubMed] [Google Scholar]
- Drummond MGBBSAF, Dalsecco LS, Brasil RSAF, Teixeira LV, Oliveira DAA. A versatile real-time PCR method to quantify bovine contamination in buffalo products. Food Control. 2013;29:131–137. [Google Scholar]
- Eggers CH, Caimano MJ, Malizia RA, Kariu T, Cusack B, Desrosiers DC, Hazlett KR, Claiborne A, Pal U, Radolf JD. The coenzyme A disulphide reductase of Borrelia burgdorferi is important for rapid growth throughout the enzootic cycle and essential for infection of the mammalian host. Molecular microbiology. 2011;82:679–697. doi: 10.1111/j.1365-2958.2011.07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Wiederanders RE. Blood copper variation among species. The American journal of physiology. 1967;213:1183–1185. doi: 10.1152/ajplegacy.1967.213.5.1183. [DOI] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature reviews Immunology. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Gern L, Rais O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae) Journal of medical entomology. 1996;33:189–192. doi: 10.1093/jmedent/33.1.189. [DOI] [PubMed] [Google Scholar]
- Hamdy BH. Biochemical and physiological studies of certain ticks (Ixodoidea). Excretion during ixodid feeding. Journal of medical entomology. 1977;14:15–18. doi: 10.1093/jmedent/14.1.15. [DOI] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, Yang XF. Cyclic di-GMP is essential for the survival of the lyme disease spirochete in ticks. PLoS pathogens. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann RD, Pangburn MK, Muller-Eberhard HJ. Species specificity of recognition by the alternative pathway of complement. Journal of immunology. 1985;134:1101–1104. [PubMed] [Google Scholar]
- Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai E, Tanaka S, Braga IS, 3rd, Itakura C, Isogai H, Kimura K, Fujii N. Experimental Borrelia garinii infection of Japanese quail. Infection and immunity. 1994;62:3580–3582. doi: 10.1128/iai.62.8.3580-3582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai H, Isogai E, Masuzawa T, Yanagihara Y, Matsubara M, Shimanuki M, Seta T, Fukai K, Kurosawa N, Enokidani M, et al. Seroepidemiological survey for antibody to Borrelia burgdorferi in cows. Microbiol Immunol. 1992;36:1029–1039. doi: 10.1111/j.1348-0421.1992.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Jewett MW, Lawrence KA, Bestor A, Byram R, Gherardini F, Rosa PA. GuaA and GuaB are essential for Borrelia burgdorferi survival in the tick-mouse infection cycle. Journal of bacteriology. 2009;191:6231–6241. doi: 10.1128/JB.00450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A, Zhou CW, Jia F, Xu Q, Hu LT. Live-vaccinia virus encapsulation in pH-sensitive polymer increases safety of a reservoir-targeted Lyme disease vaccine by targeting gastrointestinal release. Vaccine. 2016;34:4507–4513. doi: 10.1016/j.vaccine.2016.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Molecular microbiology. 2011;81:219–231. doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koci J, Bernard Q, Yang X, Pal U. Borrelia burgdroferi surface protein Lmp1 facilitates pathoegen dissemination through ticks as studied by an artificial membrane feeding system. Scientific Report. 2018;8:1910–1918. doi: 10.1038/s41598-018-20208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P. Hide and Seek: How Lyme Disease Spirochetes Overcome Complement Attack. Frontiers in immunology. 2016a;7:385. doi: 10.3389/fimmu.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P. Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Vet Sci. 2016b;3:12–26. doi: 10.3390/vetsci3020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T, Tack BF. Murine protein H is comprised of 20 repeating units, 61 amino acids in length. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:3963–3967. doi: 10.1073/pnas.83.11.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröber T, Guerin PM. An in vitro feeding assay to test acaricides for control of hard ticks. Pest management science. 2007a;63:17–22. doi: 10.1002/ps.1293. [DOI] [PubMed] [Google Scholar]
- Kröber T, Guerin PM. In vitro feeding assays for hard ticks. Trends in parasitology. 2007b;23:445–449. doi: 10.1016/j.pt.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kuhnert F. Feeding of Hard Ticks In Vitro: New Perspectives for Rearing and for the Identification of Systemic Acaricides. Altex. 1996;13:76–87. [PubMed] [Google Scholar]
- Kumar M, Kaur S, Kariu T, Yang X, Bossis I, Anderson JF, Pal U. Borrelia burgdorferi BBA52 is a potential target for transmission blocking Lyme disease vaccine. Vaccine. 2011;29:9012–9019. doi: 10.1016/j.vaccine.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Yang X, Coleman AS, Pal U. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. The Journal of infectious diseases. 2010;201:1084–1095. doi: 10.1086/651172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung F, Anguita J, Pal U. Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector. Future Microbiol. 2013;8:41–56. doi: 10.2217/fmb.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Kraiczy P. Host association of Borrelia burgdorferi sensu lato–the key role of host complement. Trends in microbiology. 2002;10:74–79. doi: 10.1016/s0966-842x(01)02298-3. [DOI] [PubMed] [Google Scholar]
- Lahdenne P, Porcella SF, Hagman KE, Akins DR, Popova TG, Cox DL, Katona LI, Radolf JD, Norgard MV. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infection and immunity. 1997;65:412–421. doi: 10.1128/iai.65.2.412-421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Benoit V, Yang X, Martinez-Herranz R, Pal U, Leong JM. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the lyme disease spirochete. PLoS pathogens. 2014;10:e1004238. doi: 10.1371/journal.ppat.1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay ARBJL. A simple and improved PCR-based technique for white-tailed deer (Odocoileus virginianus) sex identification. Conservation Genetics. 2008;9:443–447. [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of immunology. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Nogueira SV, Smith AA, Qin JH, Pal U. A surface enolase participates in Borrelia burgdorferi-plasminogen interaction and contributes to pathogen survival within feeding ticks. Infection and immunity. 2012;80:82–90. doi: 10.1128/IAI.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD, Lynn GE, Burkhardt NY, Price LD, Nelson CM, Kurtti TJ, Munderloh UG. Infection of Immature Ixodes scapularis (Acari: Ixodidae) by Membrane Feeding. Journal of medical entomology. 2016;53:409–415. doi: 10.1093/jme/tjv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, Norgard MV. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Molecular microbiology. 2009;74:1331–1343. doi: 10.1111/j.1365-2958.2009.06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Dai J, Li X, Neelakanta G, Luo P, Kumar M, Wang P, Yang X, Anderson JF, Fikrig E. A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. The Journal of infectious diseases. 2008;197:148–155. doi: 10.1086/523764. [DOI] [PubMed] [Google Scholar]
- Perner J, Sobotka R, Sima R, Konvickova J, Sojka D, Oliveira PL, Hajdusek O, Kopacek P. Acquisition of exogenous haem is essential for tick reproduction. Elife. 2016;5:e12318. doi: 10.7554/eLife.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policastro PF, Schwan TG. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. Journal of medical entomology. 2003;40:364–370. doi: 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- Promnares K, Kumar M, Shroder DY, Zhang X, Anderson JF, Pal U. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Molecular microbiology. 2009;74:112–125. doi: 10.1111/j.1365-2958.2009.06853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Molecular microbiology. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nature reviews Microbiology. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE. Ticks are not Insects: Consequences of Contrasting Vector Biology for Transmission Potential. Parasitology today. 1998;14:186–192. doi: 10.1016/s0169-4758(98)01224-1. [DOI] [PubMed] [Google Scholar]
- Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nature reviews Microbiology. 2005;3:129–143. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- Sojka D, Franta Z, Horn M, Caffrey CR, Mares M, Kopacek P. New insights into the machinery of blood digestion by ticks. Trends in parasitology. 2013;29:276–285. doi: 10.1016/j.pt.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. Lyme borreliosis. Nature reviews Disease primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BF, Commins MA, Kemp DH. Artificial feeding of the Australian paralysis tick, Ixodes holocyclus and collection of paralysing toxin. International journal for parasitology. 1983;13:447–454. doi: 10.1016/s0020-7519(83)80007-1. [DOI] [PubMed] [Google Scholar]
- Strother KO, de Silva A. Role of Borrelia burgdorferi linear plasmid 25 in infection of Ixodes scapularis ticks. Journal of bacteriology. 2005;187:5776–5781. doi: 10.1128/JB.187.16.5776-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Shijuuku T, Fukamachi T, Zaunders J, Guillemin G, Cooper D, Kelleher A. Prolonged transcriptional silencing and CpG methylation induced by siRNAs targeted to the HIV-1 promoter region. Journal of RNAi and gene silencing: an international journal of RNA and gene targeting research. 2005;1:66–78. [PMC free article] [PubMed] [Google Scholar]
- Tajeri S, Razmi G, Haghparast A. Establishment of an Artificial Tick Feeding System to Study Theileria lestoquardi Infection. PloS one. 2016;11:e0169053. doi: 10.1371/journal.pone.0169053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasinsig L, Zanetti M. The cathelicidins–structure, function and evolution. Curr Protein Pept Sci. 2005;6:23–34. doi: 10.2174/1389203053027520. [DOI] [PubMed] [Google Scholar]
- Turner JC, Anderson HM, Gandal CP. Species differences in red blood cell phosphatides separated by column and paper chromatography. Biochimica et biophysica acta. 1958;30:130–134. doi: 10.1016/0006-3002(58)90249-x. [DOI] [PubMed] [Google Scholar]
- Uno Y, Usui T, Fujimoto Y, Ito T, Yamaguchi T. Quantification of interferon, interleukin, and Toll-like receptor 7 mRNA in quail splenocytes using real-time PCR. Poultry science. 2012;91:2496–2501. doi: 10.3382/ps.2012-02283. [DOI] [PubMed] [Google Scholar]
- van Dam AP. Diversity of Ixodes-borne Borrelia species–clinical, pathogenetic, and diagnostic implications and impact on vaccine development. Vector borne and zoonotic diseases. 2002;2:249–254. doi: 10.1089/153036602321653833. [DOI] [PubMed] [Google Scholar]
- Wickramasekara S, Bunikis J, Wysocki V, Barbour AG. Identification of residual blood proteins in ticks by mass spectrometry proteomics. Emerging infectious diseases. 2008;14:1273–1275. doi: 10.3201/eid1408.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, He M, He JJ, Yang XF. Role of the surface lipoprotein BBA07 in the enzootic cycle of Borrelia burgdorferi. Infection and immunity. 2010;78:2910–2918. doi: 10.1128/IAI.00372-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang X, Kumar M, Pal U. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. The Journal of infectious diseases. 2009;200:1318–1330. doi: 10.1086/605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nature reviews Immunology. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


