Abstract
Quercetin, a common dietary flavone, is a competitive inhibitor of glucose uptake and is also thought to be transported into cells by GLUT1. In this study, we confirm that quercetin is a competitive inhibitor of GLUT1 and also demonstrate that newly synthesized compounds, WZB-117 and BAY-876 are robust inhibitors of GLUT1 in L929 cells. To measure quercetin interaction with L929 cells, we develop a new fluorescent assay using flow cytometry. The binding of quercetin and its inhibitory effects on 2-deoxyglucose (2DG) uptake showed nearly identical dose dependent effects, with both having maximum effects between 50 and 100 μM and similar half maximum effects at 8.9 and 8.5 μM respectively. The interaction of quercetin was rapid with t1/2 of 54 seconds and the onset and loss of its inhibitory effects on 2DG uptake were equally fast. This suggests that either quercetin is simply binding to surface GLUT1 or its transport in and out of the cell reaches equilibrium very quickly. If quercetin is transported, the co-incubation of quercetin with other glucose inhibitors should block quercetin uptake. However, we observed that WZB-117, an exofacial binding inhibitor of GLUT1 reduced quercetin interaction, while cytochalasin B, an endofacial binding inhibitor, enhanced quercetin interaction, and BAY-876 had no effect on quercetin interaction. Taken together, these data are more consistent with quercetin simply binding to GLUT1, but not actually being transported into L929 cells via the glucose channel in GLUT1.
1. Introduction
The flavone quercetin (3,3′,4′5,7-pentahydroxyflavone) is a natural polyphenol found in high concentrations in many fruits, vegetables, and wines [1, 2]. The average daily intake of flavonoids in a normal diet is approximately 23 mg of which quercetin makes up 60 to 70%[2]. Quercetin has a wide variety of physiological effects and has received significant research attention with over 15,000 studies reported in the literature including 650+ reviews and 230+ clinical trials. Much of the work, as illustrated by recent reviews, has focused on the potential of quercetin as a therapeutic in disease states such as diabetes, obesity and cancer. [1, 3-9]. Of potential relevance to its anticancer and antidiabetic activity is the documented direct interaction of quercetin with the GLUT family of proteins, particularly GLUT1 [10-16].
The facilitated glucose transporter, GLUT1 (SLC2A1), is expressed in a wide variety of cell types and, while generally responsible for basal uptake of glucose, it also responds to changing metabolic conditions [17]. Chronic exposure to cell stressors—such as hypoxia, hypoglycemia, and AMP kinase activation—increase GLUT1 protein expression [18-20]. In addition, this transporter appears to be overexpressed in a number of cancers, especially those driven by KRAS mutations, thereby accelerating glucose uptake in support of glycolytic metabolism [21-25]. Given the dependence of many cancers on GLUT1 transport activity, there is strong interest in small molecule inhibitors of GLUT1 as potential therapeutics. Potential inhibitors include a number of natural products such as quercetin [13, 14], curcumin [26, 27], and caffeine [28-30], as well as newly synthesized high affinity inhibitors such as WZB-117 [31, 32] and BAY-876 [33].
Quercetin is a competitive inhibitor of glucose entry via GLUT1 and a noncompetitive inhibitor of glucose exit. These studies suggest that quercetin binds to the exofacial surface of GLUT1[10, 14]. There is also evidence that suggests that quercetin itself is transported into cells via GLUT1 [11, 12] and GLUT4 [13]. The purposes of this study was: 1) to verify the inhibitory effects of quercetin on 2-deoxyglucose uptake in L929 cells, which exclusively express GLUT1 [34]; 2) to measure quercetin binding in L929 cells; and 3) to measure the effects of other GLUT1 inhibitors on quercetin binding to help map the quercetin binding site on GLUT1 and to determine if quercetin is transported by GLUT1 into cells. We hypothesized that if quercetin was taken up by GLUT1 in a fashion similar to glucose, inhibitors of glucose uptake should also inhibit quercetin uptake.
2. Materials and Methods
2.1 Chemicals
Quercetin, cytochalasin B, BAY-876, and caffeine were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO, USA), WZB-117 from Cayman Chemical Company (Ann Arbor, MI, USA), 2-deoxy-D-glucose- [1,2-3H] (2DG) from Moravek Biochemicals (Brea, CA, USA) and [4(n)-3H] cytochalasin B from Amersham Biosciences (Buckinghamshire, United Kingdom).
2.2 Cell culture
L929 mouse fibroblast cells were obtained from the American Type Culture Collection. The cells were grown under standard conditions at 37°C in an incubator supplied with humidified room air with 5% CO2, and maintained in low glucose (1 g/L) Dulbecco’s Modified Eagle Media (DMEM) (Gibco/Life Technologies, Grand Island, NY) supplemented with 5% fetal bovine serum (FBS). Cell detachment for passaging and seeding was performed with TrypLE Express dissociation enzyme (Life Technologies) after rinsing of attached cells with sterile phosphate buffered saline (PBS).
2.3 Glucose uptake assay
To initiate each experiment, 24-well plates were seeded with L929 cells either 1 or 2 days prior to experimentation. The cells were grown under standard conditions, and uptake experiments were done with cells near confluency, which is approximately 3.0 × 105 per well for L929 fibroblast cells. Glucose uptake was measured using the radiolabeled glucose analog 2-deoxyglucose (2DG) as previously described[28]. Briefly, the media was replaced with 0.2 mL of glucose-free HEPES buffer (140 mM NaCl, 5 mM KCl, 20 mM HEPES/Na pH=7.4, 2.5 mM MgSO4, 1 mM CaCl2, 2 mM NaPyruvate, 1 mM mannitol) supplemented with 1.0 mM (0.3 μCi/mL) 2DG (1,2-3H). 1.0 mM 2DG is below the Km of transport, 6-8 mM, and allows us to monitor linear uptake for longer times. After a 15-minute incubation, cells were washed twice with cold glucose-free HEPES. The cells were digested in 0.25 mL of 0.3 M NaOH and the 3H-2DG was measured using scintillation spectrometry.
The uptake media was supplemented with inhibitors from stock solutions as indicated in the figure legends. Stocks solutions of cytochalasin B, BAY-876, WZB-117, quercetin dissolved in ethanol (cytochalasin B) or DMSO (BAY-876, WZB-117, quercetin) were prepared at 200-400x. Quercetin was oxidized by mixing excess potassium iron III cyanide (K3Fe(CN)6) (2 mM) with 100 μM quercetin 15 minutes prior to exposure to cells (Fig 1B). For the kinetics experiments with or without 100 μM quercetin (Fig 1C), the concentration of 2DG in the uptake solution was varied as indicated in the figure legend.
Figure 1.
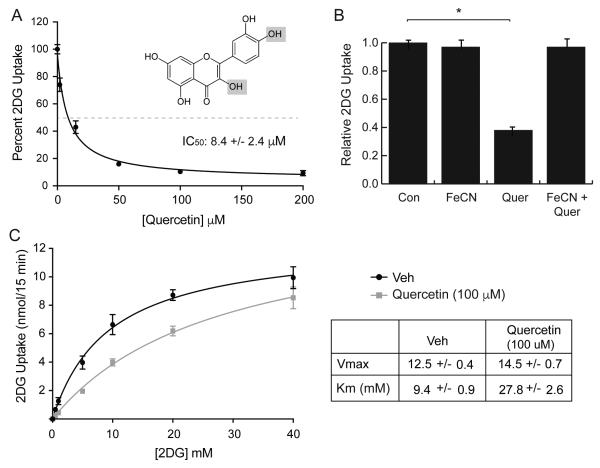
Effects of quercetin on 2DG uptake in L929 fibroblast cells. Panel A: Dose-dependent effects. 2DG uptake was measured at various concentrations of quercetin, ranging from 0-200 μM. Uptake data (n=8) were normalized to basal uptake (0 mM quercetin) at 100%, and displayed as means ± S.E. with a best-fit line to simple decay. All quercetin treatments were significantly lower at P < 0.01. Inset shows structure of quercetin with grey panels indicating the sites oxidized by ferric cyanide. Panel B: Oxidation of quercetin. 2DG uptake was measured in the presence of either no additions (Con), 2.0 mM potassium ferric cyanide (FeCN), 100 μM quercetin (Quer), or both (FeCn+Quer). Uptakes were normalized to control and reported as means ± S.E. Panel C: Kinetics of 2DG uptake. 2DG uptake was measured at 0.5, 1.0, 5.0, 10, 20, and 40 mM 2DG in the absence or presence of 100 μM quercetin and reported as nmol/15min/well. Data are means ± S.E. of quadruplicate samples from a representative experiment with a best-fit line to Michaelis-Menten kinetics. Vmax and Km are reported on the table. *Significant from control at P<0.01.
2.4 3H-Cytochalasin B binding
L929 cells were plated to 24-well plates and grown overnight as indicated above. Media was replaced with HEPES buffer supplemented with 1 μM 3H cytochalasin B at 0.3 μCi/mL and quercetin at 0, 10, 25, 50, or 100 μM. After a 15-minute incubation, cells were washed twice with cold glucose-free HEPES. The cells were digested in 0.25 mL of 0.3 M NaOH and the 3H-cytochalasin remaining with cells was measured using scintillation spectrometry. Background binding was measured as radioactivity remaining in the presence of 50 μM unlabeled cytochalasin B and subtracted from each experimental measurement.
2.5 Flow Cytometry
L929 cells were plated to 12-well plates at a density of 2.0 × 105 cells/well and grown overnight under standard conditions. The following day, cells were incubated for various times at 37°C in DMEM with varying concentrations of quercetin and other inhibitors as indicated in the figure legends and text. After treatment, the cells were washed with PBS, detached by trypsinization and then filtered into round-bottom polystyrene cytometry tubes to achieve single cell suspensions. Cells were pelleted by centrifugation, washed once with cold PBS and then maintained in the dark on ice until analysis. Flow cytometry was performed using a BD FACSCalibur instrument set to detect quercetin fluorescence using the 488 nm excitation laser and FL2 channel (585/40 nm filter) for emission detection. Background fluorescence and instrument settings were established with vehicle treated cells. For experiments involving fixed and permeabilized cells, a commercial kit that employs formalin fixation and permeabilization with saponin (Cytofix/Cytoperm kit, BD Biosciences, San Jose, CA) was used to prepare cells. Cells were incubated with the fixation reagent for 30 minutes on ice, washed once with PBS and then permeabilized at 4°C in buffered media containing saponin.
2.6 Statistical analysis
Each 2DG uptake experiment with quadruplicate samples was repeated a minimum of three times to ensure that results could be replicated. 2DG uptake data were measured as nmol/15 min/well ±standard error, normalized to control conditions and reported directly (kinetics) or as relative 2DG uptake. All flow cytometry experiments except those indicated in the text were performed a minimum of three times with duplicate samples for each condition. Statistical significance was determined by a two-tailed t-test and is reported at P< 0.05 or P<0.01. The software program, Prism v 6.0f, was used to fit the data and determine parameters such as Km, Vmax and IC50.
3. Results
3.1 Quercetin is a competitive inhibitor of 2DG uptake in L929 fibroblast cells
Quercetin has been shown to be an inhibitor of GLUT1 transport activity in a variety of cells including U937, HL-60, Jurkat, transformed CHO, and human erythrocytes as well as an inhibitor of GLUT4 in adipocytes [10, 12-16, 35]. Our initial experiments were designed to confirm this inhibitory action of quercetin in mouse L929 fibroblast cells, which express only GLUT1 [34]. We measured the effects of increasing concentrations of quercetin on 2DG uptake in L929 cells. The results reported in Fig 1A are expressed as a percentage of control and indicate that quercetin inhibits uptake in a dose dependent manner with maximum inhibition of about 90% achieved between 50 and 100 μM and an IC50 of 8.5 μM. These results are virtually identical to results observed in HL-60 cells [14]. When 100 μM quercetin is incubated with 2.0 mM potassium ferric cyanide the inhibitory action of quercetin is lost (Fig 1B), confirming previous work that oxidized quercetin does not inhibit 2DG uptake [12]. The results of a kinetics experiment shown in Fig 1C reveal that quercetin is a competitive inhibitor of 2DG uptake in L929 cells with no statistical change in the Vmax of uptake, but an increase in the Km from 9.5 to 27.9 mM. This matches previous results [14].
3.2 Inhibition of Quercetin is immediate and quickly reversible
The inhibition of glucose uptake by quercetin appears to be the result of a direct binding to the exofacial side of GLUT1 [10, 14]. However, it has also been reported that quercetin is transported by GLUT1 [12] and that it can compete for cytochalasin B binding [14], which is known to bind to the endofacial side of GLUT1 [36-38]. These data taken together suggest that quercetin can inhibit 2DG uptake by either binding directly to an exofacial site, or by being transported and then binding to an endofacial site. We addressed this possibility of dual inhibition by investigating the effects of time on quercetin inhibition of 2DG uptake. We hypothesized that if quercetin binds to an exofacial site, we should see immediate inhibition; however, if it is also transported into cells and binds to an endofacial site, we might observe an increase in inhibition over time. Also, if quercetin is transported into cells, we would predict a slow recovery of the inhibitory effects reflecting the time require for quercetin to be lost from the cells. This slow recovery is precisely what was observed in the recovery of caffeine inhibition which binds to an endofacial site [28,30]. However, we did not observe either on these results with quercetin.
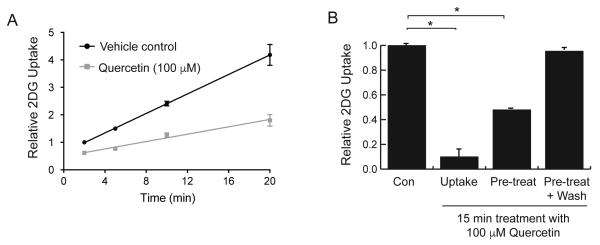
2DG uptake was measured in the absence and presence of quercetin over time (Fig 2A) and resulted in an immediate inhibition at the earliest measurement of 2 minutes. We observed that this inhibition was linear over a 20-minute time-course. When cells were incubated with 100 μM quercetin only during the 15 minutes of uptake, we observed about 90% inhibition. However, when cells were exposed to the same dose of quercetin for 15 minutes prior to measuring 2DG uptake, during which time quercetin was absent, we observed an immediate partial recovery to 50% inhibition. Furthermore, if cells were washed once with PBS after initial exposure to quercetin, they displayed a full recovery of transport activity (Fig 2B). This rapid reversibility of quercetin’s inhibitory effect seems inconsistent with a transport model, and more likely reflects a reversible binding of the flavone to GLUT1 molecules on the cell surface.
Figure 2.
Effects of quercetin treatment times on 2DG uptake in L929 cells. Panel A: Inhibition is immediate and constant. 2DG uptake was measured in the presence or absence of 100 μM quercetin for 2, 5, 10, or 20 minutes, normalized to control uptake at 2 minutes and expressed as means of relative uptake ± S.E. All quercetin effects were significantly lower than control at P<0.01. Panel B: Recovery from quercetin effects. Cells were exposed to 100 μM quercetin for 15 minutes either during the measurement of uptake only, or just prior to measurement of uptake with or without a wash before measurement of uptake without quercetin. 2DG uptakes were measured, normalized to control (no exposure to quercetin) and expressed as means of relative uptake ± S.E. *Significant from control at P<0.01.
3.3. Measurement of fluorescent quercetin uptake by flow cytometry
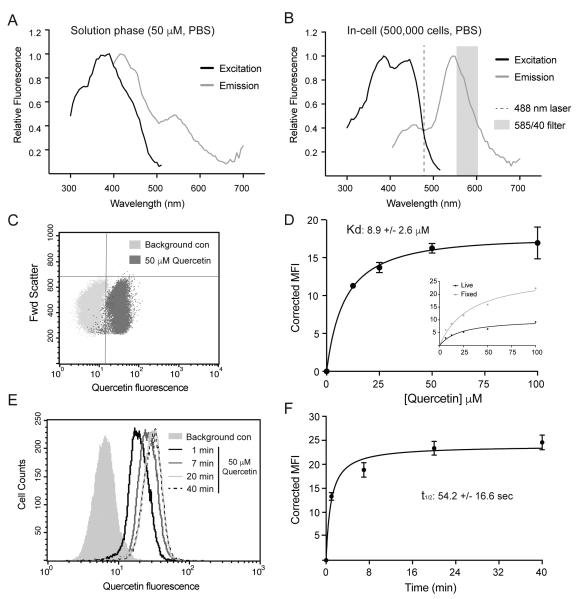
To better understand the mode of uptake for quercetin via GLUT1 (either simple binding or transport), we needed an assay to measure quercetin uptake. Quercetin is a naturally fluorescent molecule whose fluorescent characteristics change when it binds proteins [39]. We therefore investigated whether we could take advantage of this property to measure and quantify the association of quercetin with live cells. Analysis of quercetin fluorescence in PBS solution yielded excitation and emission spectra with a large degree of overlap (Fig 3A). These spectra were significantly shifted in the presence of L929 cells, however, especially with regard to the emission spectrum (Fig 3B). This shift in emission spectrum allowed us to employ flow cytometry, set at 488 nm excitation and a 585 nm emission detection, to measure the binding of 50 μM quercetin to L929 cells (Fig 3C). To measure the dose-dependent effect of quercetin on cellular fluorescence, cells were incubated with varying concentrations of quercetin for 15 minutes prior to analysis by flow cytometry. The mean fluorescent signal obtained from measuring cells without exposure to quercetin was subtracted from the mean fluorescent signal obtained after exposure to quercetin to obtain a corrected mean fluorescent intensity (MFI). The results, shown on Fig 3D, indicate maximum uptake of quercetin between 50-100 μM and a Kd of 8.9 μM in L929 cells. This is virtually identical to quercetin’s inhibitory effects on 2DG uptake (Fig 1) and is consistent with a model in which binding of quercetin to GLUT1 directly inhibits its glucose transport activity.
Figure 3.
Measurement of quercetin uptake via fluorescence detection. Excitation and emission spectra of quercetin fluorescence in PBS (Panel A) or in the presence of 500,000 cells in PBS (Panel B). Dashed line shows excitation laser and grey band shows detection filter of the flow cytometer. Panel C: dot plot showing quercetin’s fluorescence intensity of individual cells after exposure to 0 or 50 μM quercetin for 15 minutes. Panel D: dose response of fluorescence intensity after a 15-minute exposure to 12.5, 25, 50, or 100 μM quercetin. Data for triplicate measurements are reported as mean intensity fluorescence ± S.E. Inset shows results of a single dose dependent experiment with 15-minute exposures to either live cells or fixed and permeablized cells. Panel E: results of a single experiment where L929 cells were exposed to 50 μM quercetin for 1, 7, 20, or 40 minutes. Data is reported as a histogram of cells at increasing fluorescence. Panel F: composite results from duplicate samples with results reported as mean intensity fluorescence ± S.E.
Because the total pool of GLUT1 transporters in cells is divided between the plasma membrane and intracellular recycling vesicles, we also performed a single experiment in which the dose dependent uptake of quercetin was compared in live versus fixed and permeablized cells. As expected, the fluorescent signal is enhanced when quercetin has access to internal GLUT1 in the fixed and permeablized cells (inset, Fig 3D). If quercetin is transported into the cytosol by GLUT1, we would expect a steady, linear increase in quercetin fluorescence as a function of time. As seen in Fig 3E and Fig 3F, however, a time-course of quercetin exposure results in a rapid increase in fluorescence with a t1/2 of 54 seconds.
3.4 Effects of GLUT1 inhibitors, BAY-876 and WZB-117, on quercetin uptake
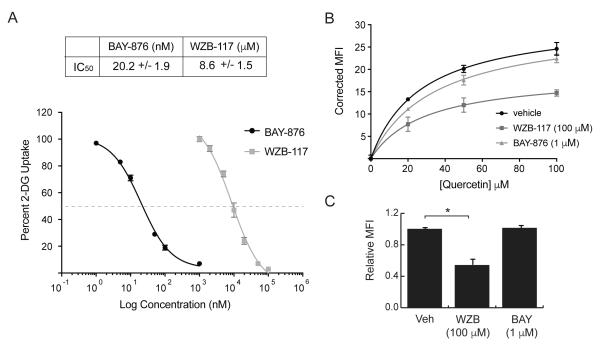
If quercetin is transported by GLUT1, it should be possible to block its uptake into the cytoplasm using other GLUT1 inhibitors. To address this issue experimentally, we chose to use BAY-876 and WZB-117, two relatively new and potent inhibitors of GLUT1 [32, 33]. Prior studies suggest that WZB-117 binds to the exofacial side of GLUT1 [32], while the binding site of BAY-876 has not been determined. As shown in Fig 4A, both of these compounds inhibit 2DG uptake by over 90%, with BAY-876 having significantly higher potency (IC50 = 20 nM) than WZB-117 (IC50 = 8.5 μM).
Figure 4.
Effects of GLUT1 inhibitors on 2DG uptake and quercetin binding. Panel A: 2DG uptake was measured in L929 cells in the presence of either BAY-876(0-1 μM) or WZB-117 (0-100 μM). Uptakes for each inhibitor were normalized to control (no inhibitor) and reported as percent uptake ± S.E with best fit line. Panel B: quercetin fluorescence was measured after a 15-minute exposure to 20, 50, or 100 μM quercetin in the presence of no inhibitor (vehicle) or maximum effective concentrations of either WZB-117 (100 μM) or BAY-876 (1.0 μM). Corrected mean fluorescence intensity ± S.E. from duplicate samples is shown. Panel C: quercetin fluorescence was measured after a 15-minute exposure to 100 μM quercetin in the presence of no inhibitor (vehicle) either 100 μM WZB-117 or 1.0 μM BAY-876. Results from three separate experiments were normalize to vehicle and reported as relative mean fluorescence intensity ± S.E. *Significant from control at P<0.01.
Having validated the inhibitory function of these two compounds in L929 fibroblasts, we next asked whether they would similarly decrease quercetin uptake by these cells. Quercetin fluorescence was measured by flow cytometry after a 15-minute incubation of 20, 50, and 100 μM concentrations in the presence of a maximally effective concentration of either BAY-876 (1 μM) or WZB-117 (100 μM). The results shown in Fig 4B indicate that quercetin uptake was inhibited by WZB-117, but not by BAY-876. The effects of 100 μM WZB-117 or 1 μM BAY-876 on the uptake of quercetin at its highest concentration (100 μM) are shown in Fig 4C.
3.5 Combined effects of cytochalasin B and quercetin
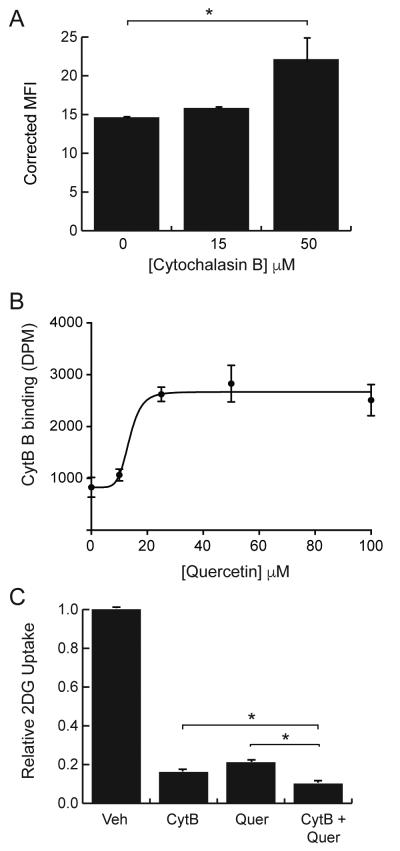
The classic inhibitor of GLUT proteins is cytochalasin B, which binds to the endofacial site of GLUT1 and is a competitive inhibitor of glucose exit [37, 38]. The effects of cytochalasin B on quercetin uptake were measured by incubating cells for 15 minutes with 100 μM quercetin plus, 0, 15, or 50 μM cytochalasin B. The results, reported on Fig 5A, indicate that cytochalasin B does not inhibit, but actually enhances quercetin uptake. In addition, quercetin also enhances cytochalasin B binding in a dose dependent manner (Fig 5B). One interpretation of these data is that the exofacial binding of quercetin enhances or stabilizes the endofacial binding of cytochalasin B and vice versa. Since both inhibitors appear to simultaneously bind to GLUT1, we would predict that the combined inhibitory effects should be greater than the effects of either inhibitor alone. To test this, we measured the 2DG uptake in the presence of either 50 μM cytochalasin B, 100 μM quercetin, or both. The uptake results were normalized to uptake in cells treated with the vehicle, DMSO, and are reported on Fig 5C. As predicted, the combined inhibitory effect of cytochalasin B and quercetin was greater than the individual effect of either inhibitor.
Figure 5.
Combined effects of quercetin and cytochalasin B on inhibitor binding and 2DG uptake. Panel A: fluorescence was measured by flow cytometry of L929 cells exposed for 15-minutes to 100 μM quercetin plus 0, 15 or 50 μM cytochalasin B. Quercetin fluorescence is reported as corrected mean fluorescence intensity. Panel B: binding of radioactive cytochalasin B in the presence of 10, 25, 50, or 100 μM quercetin was measured for triplicate samples and reported as DPM ± S.E. Panel C: 2DG uptake was measured in the presence of either no inhibitor or 50 μM cytochalasin B, or 100 μM querctin or both. Uptakes were normalized to vehicle treatment (DMSO) and reported as relative 2DG uptake ± S.E. *Significant from control at P<0.01.
4. Discussion
Quercetin is a relatively abundant bioactive flavonoid with a wide variety of documented physiological effects, which has gained popularity as a nutritional supplement [40]. There is increasing interest in quercetin’s anticancer properties (for recent reviews see [5, 6, 40-43]). It is important to note that many cancers are highly glycolytic and have increased lactate production in spite of the presence of adequate oxygen and the maintenance of normal oxidative metabolism. Known as aerobic fermentation, or the Warburg effect, this increased metabolism of glucose is often achieved by an increased expression of GLUT1 [21, 44, 45]. Thus, GLUT1 has emerged as a potential target for the development of new anticancer therapies in spite of the potential negative effects of GLUT1 inhibition on neural glucose transport [32, 46].
The initial goal of this study was to investigate the effects quercetin on glucose uptake in L929 mouse fibroblast cells, which rely exclusively on GLUT1 for glucose uptake [34]. Quercetin inhibits 2DG uptake in L929 cells in a dose dependent manner with a maximum inhibition of about 90% inhibition achieved between 50 and 100 μM and a IC50 of 8.5 μM. We also showed quercetin increased the Km of 2DG uptake without a change in the Vmax of transport, which is consistent with competitive inhibition. These data are virtually identical to data from HL-60 cells where the IC50 was reported as 8 μM [14]. We also confirmed earlier studies that the oxidized form of quercetin does not inhibit 2DG uptake [12].
In our hands, the inhibitory activity of quercetin is immediate (concurrent with 2DG uptake), and is rapidly reversible upon simple washing of cells. This finding was different from what we had anticipated, since inhibitors that are transported into the cytoplasm typically take time to diffuse back out of cells, and therefore exert prolonged inhibitory effects. The rapid recovery of 2DG uptake after a single washing step was our first hint that quercetin may be simply binding to the GLUT 1 and not transported by the GLUT as previously reported [11-13].
To approach the question of whether quercetin simply binds to GLUT1 or is also transported, we developed a novel assay for measuring quercetin in cells. We took advantage of the natural fluorescence of quercetin and the dramatic red shift of the emission spectra that occurs as quercetin binds to cells [39]. This shift allowed us to use flow cytometry to measure fluorescence of quercetin in individual cells. The dose dependent uptake of quercetin was maximized between 50 and 100 μM, with a Kd of 8.9 μM–values that are essentially identical to the inhibitory effects of quercetin on 2DG uptake. The time-course of quercetin uptake was rapid, with half max uptake time of 54 seconds that again matches the rapid onset and loss of its inhibition on 2DG uptake.
The flow cytometry assay we developed, as well as previously employed quercetin uptake assays [13, 14], are not able to distinguish between simple quercetin binding to the cell surface and its incorporation into the cell. The 2DG uptake results with quercetin, therefore, could be consistent with a transport process that rapidly comes to equilibrium, similar to what is observe in 3-O-methylglucose uptake assays. However, the observation that the total quercetin binding capacity of permeabilized cells is more than double that of live cells suggests that transported quercetin should be retained in the cell by binding to internalized GLUT1. This increased binding capacity of permeabilized cells is also consistent with an alternate interpretation of the time-dependent quercetin binding data. The binding of quercetin was modeled as a simple equilibrium binding process with a t1/2 = 54 sec. However, these data are also consistent with a rapid initial binding of quercetin to GLUT1 followed by slow doubling of the binding over 40 minutes as GLUT1 cycles from and to the cell surface gradually ‘labeling’ the vesicular GLUT1 pool. It has been reported that the recycling time for GLUT1 after endocytosis is 45 minutes [47]. This model of GLUT1 regulation differs from what would be expected in erythrocytes, which do not traffic surface nutrient transporters in the same way as other cell types such as fibroblasts and epithelial cells.
To help resolve the question of simple binding versus transport, we reasoned that if quercetin is transported by GLUT1, we should be able to block its uptake by other GLUT1 inhibitors. We initially investigated the effects of two newly identified GLUT1 inhibitors, BAY-876 and WZB-117. WZB-117 was first identified as a small molecule inhibitor of GLUT1 that had anticancer activity in a nude mouse model [31]. Subsequently, the inhibitory and binding properties of WZB-117 were systematically investigated in erythrocytes and transformed HEK292 cells where it was shown to be an exofacial inhibitor binding preferentially to the central glucose channel in the outwardly open GLUT1 conformation [32]. More recently, BAY-876 was identified as the first highly selective inhibitor of GLUT1 with a reported IC50 of 2 nM for GLUT1, which was 150-5000 times lower than its IC50 for GLUT2, GLUT3, or GLUT4 [33]. There are no additional studies utilizing this inhibitor which delineate its mechanism of action. Our data reveal that both of these compounds are robust inhibitors of GLUT1-mediated 2DG uptake in L929 cells. The IC50 for WZB-117 inhibition of 2DG uptake in L929 cells is close to the values reported for its inhibition of 3-O-methylglucose uptake in erythrocytes (IC50 = 6.2 μM) [32] and its inhibition of cancer growth (IC50 = 10 μM) [31]. In contrast, the IC50 for BAY-876 is about 10-fold higher than previously reported [33]. This discrepancy can likely be attributed to the utilization of different assays for measuring glucose uptake and different cell types. In this study we utilized a 2DG uptake assay in L929 fibroblast cells, which is a more direct measure of GLUT1 transport activity while the previous study that utilized a luciferase assay for cellular ATP content in rotenone-poisoned CHO cells as a proxy for GLUT transport activity.
Somewhat unexpectedly, WZB-117 and BAY-876 differed in their effect on quercetin uptake. WZB-117 reduced quercetin uptake, consistent with inhibiting transport, but the more robust glucose uptake inhibitor, BAY-876, had no effect on quercetin uptake. Our findings with cytochalasin B are also inconsistent with quercetin transport. This classic potent inhibitor of GLUT1 activity actually increases the binding of quercetin to L929 cells. Unlike WZB-117, cytochalasin B is an endofacial binding inhibitor of the GLUT1 [37, 38], which provides some information about the mode by which quercetin engages transporters. We also found that quercetin enhances cytochalasin B binding, which suggests that both inhibitors can simultaneously bind to GLUT1. A dual binding of quercetin and cytochalasin B is consistent with the observation that the combined inhibitory effects of these two compounds are greater than the inhibition of either alone.
The most straightforward interpretation of these data is that cyochalasin B binds at an endofacial site on GLUT1, which stabilizes the external binding of quercetin, and conversely, the external binding of quercetin on GLUT1 stabilizes the endofacial binding of cytochalasin B. This model conflicts with a previous study that demonstrated that quercetin inhibited cytochalasin B binding to GLUT1 [14]. That study, however, measured binding in unsealed erythrocyte ghosts where quercetin has access to both the internal and external surfaces of GLUT1. We propose that quercetin does not inhibit cytochalasin B binding in live L929 cells because it is not transported into the cells, and therefore does not have access to the endofacial cytochalasin B binding site. Our observation of the dual binding of quercetin and cytochalasin B is similar to the effects of WZB-117 on cytochalasin B binding. WZB-117 did not inhibit cytochalasin B binding in intact human erythrocytes, but did inhibit cytochalasin B binding in protein depleted membranes where WZB-117 has access to both surfaces of the erythrocyte membrane [32]. The authors concluded from that observation that WZB-117 was not transported by GLUT1 into erythrocytes.
The simple carrier model for GLUT1 transport activity proposes that the transporter alternates between an outward facing glucose binding site and an inward, cytoplasmic, facing glucose binding site. Cytochalasin B is an endofacial inhibitor and appears to preferentially bind to the open, inward facing conformation [32, 48, 49]. The mutual enhancement of quercetin and cytochalasin B binding suggests that both compounds preferentially bind to GLUT1 in the inward facing glucose binding site conformation. Thus, WZB-117, which binds to outward facing conformation, would be expected to reduce the preferential binding of quercetin to the inward facing conformation, which is exactly what we observe. This is in precise agreement with the model for quercetin inhibition of GLUT1 proposed from kinetic and binding studies in erythrocytes [10]. The observation that BAY-876 does not affect quercetin binding indicates that further studies are needed to map its binding or interactions with GLUT1.
5. Conclusions
In conclusion, this study confirmed that quercetin is a competitive inhibitor of 2DG uptake and also demonstrated that WZB-117 and BAY-876 are robust inhibitors of GLUT1 in L929 cells. We measured quercetin binding utilizing a newly developed flow cytometry assay and show that quercetin’s binding directly mirrors its inhibitory activity on 2DG uptake. Based on the immediate onset and loss of both quercetin’s uptake and its inhibitory activity, as well as the failure of robust glucose inhibitors to block quercetin’s interaction, we suggest that in L929 cells, quercetin simply binds, but is not transported into cells via the glucose transport channel in GLUT1.
Research Highlights.
Quercetin is a competitive inhibitor of 2DG uptake in GLUT1 expressing cells
BAY-876 and WZB-117 are also potent inhibitors of GLUT1 activity
Cytochalasin B, an endofacial-binding inhibitor of GLUT1, enhances quercetin binding
WZB-117 inhibits and BAY-876 has no effect on quercetin binding
Quercetin binds to an exofacial site, but is not likely transported by GLUT1.
Acknowledgements
This research was supported by a NIH R15 grant (DK08193-1A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- [2].Lopez-Lopez G, Moreno L, Cogolludo A, Galisteo M, Ibarra M, Duarte J, Lodi F, Tamargo J, Perez-Vizcaino F. Nitric oxide (NO) scavenging and NO protecting effects of quercetin and their biological significance in vascular smooth muscle. Mol Pharmacol. 2004;65:851–859. doi: 10.1124/mol.65.4.851. [DOI] [PubMed] [Google Scholar]
- [3].Eid HM, Haddad PS. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr Med Chem. 2017;24:355–364. doi: 10.2174/0929867323666160909153707. [DOI] [PubMed] [Google Scholar]
- [4].Gelen V, Sengul E, Gedikli S, Gur C, Ozkanlar S. Therapeutic effect of quercetin on renal function and tissue damage in the obesity induced rats. Biomed Pharmacother. 2017;89:524–528. doi: 10.1016/j.biopha.2017.02.057. [DOI] [PubMed] [Google Scholar]
- [5].Haghi A, Azimi H, Rahimi R. A Comprehensive Review on Pharmacotherapeutics of Three Phytochemicals, Curcumin, Quercetin, and Allicin, in the Treatment of Gastric Cancer. J Gastrointest Cancer. 2017;48:314–320. doi: 10.1007/s12029-017-9997-7. [DOI] [PubMed] [Google Scholar]
- [6].Haque I, Subramanian A, Huang CH, Godwin AK, Van Veldhuizen PJ, Banerjee S, Banerjee SK. The Role of Compounds Derived from Natural Supplement as Anticancer Agents in Renal Cell Carcinoma: A Review. Int J Mol Sci. 2017;19 doi: 10.3390/ijms19010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Massi A, Bortolini O, Ragno D, Bernardi T, Sacchetti G, Tacchini M, De Risi C. Research Progress in the Modification of Quercetin Leading to Anticancer Agents. Molecules. 2017;22 doi: 10.3390/molecules22081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Williamson G. The role of polyphenols in modern nutrition. Nutr Bull. 2017;42:226–235. doi: 10.1111/nbu.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhao Y, Chen B, Shen J, Wan L, Zhu Y, Yi T, Xiao Z. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxid Med Cell Longev. 2017;2017:1459497. doi: 10.1155/2017/1459497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perez A, Ojeda P, Ojeda L, Salas M, Rivas CI, Vera JC, Reyes AM. Hexose transporter GLUT1 harbors several distinct regulatory binding sites for flavones and tyrphostins. Biochemistry. 2011;50:8834–8845. doi: 10.1021/bi200748b. [DOI] [PubMed] [Google Scholar]
- [11].Vlachodimitropoulou E, Sharp PA, Naftalin RJ. Quercetin-iron chelates are transported via glucose transporters. Free Radic Biol Med. 2011;50:934–944. doi: 10.1016/j.freeradbiomed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- [12].Cunningham P, Afzal-Ahmed I, Naftalin RJ. Docking studies show that D-glucose and quercetin slide through the transporter GLUT1. The Journal of biological chemistry. 2006;281:5797–5803. doi: 10.1074/jbc.M509422200. [DOI] [PubMed] [Google Scholar]
- [13].Strobel P, Allard C, Perez-Acle T, Calderon R, Aldunate R, Leighton F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem J. 2005;386:471–478. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vera JC, Reyes AM, Velasquez FV, Rivas CI, Zhang RH, Strobel P, Slebe JC, Nunez-Alarcon J, Golde DW. Direct inhibition of the hexose transporter GLUT1 by tyrosine kinase inhibitors. Biochemistry. 2001;40:777–790. doi: 10.1021/bi001660j. [DOI] [PubMed] [Google Scholar]
- [15].Martin HJ, Kornmann F, Fuhrmann GF. The inhibitory effects of flavonoids and antiestrogens on the Glut1 glucose transporter in human erythrocytes. Chem Biol Interact. 2003;146:225–235. doi: 10.1016/j.cbi.2003.06.001. [DOI] [PubMed] [Google Scholar]
- [16].Park JB. Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochem Biophys Res Commun. 1999;260:568–574. doi: 10.1006/bbrc.1999.0890. [DOI] [PubMed] [Google Scholar]
- [17].Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! American journal of physiology. Endocrinology and metabolism. 2009;297:E836–848. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boado RJ, Pardridge WM. Glucose deprivation and hypoxia increase the expression of the GLUT1 glucose transporter via a specific mRNA cis-acting regulatory element. Journal of neurochemistry. 2002;80:552–554. doi: 10.1046/j.0022-3042.2001.00756.x. [DOI] [PubMed] [Google Scholar]
- [19].Wertheimer E, Sasson S, Cerasi E, Ben-Neriah Y. The ubiquitous glucose transporter GLUT-1 belongs to the glucose-regulated protein family of stress-inducible proteins. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2525–2529. doi: 10.1073/pnas.88.6.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kitzman HH, Jr., McMahon RJ, Williams MG, Frost SC. Effect of glucose deprivation of GLUT 1 expression in 3T3-L1 adipocytes. The Journal of biological chemistry. 1993;268:1320–1325. [PubMed] [Google Scholar]
- [21].Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. Journal of cellular physiology. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- [22].Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochimica et biophysica acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Szablewski L. Expression of glucose transporters in cancers. Biochimica et biophysica acta. 2013;1835:164–169. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [24].Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S, Diaz LA, Jr, Velculescu VE, Lengauer C, Kinzler KW, Vogelstein B, Papadopoulos N. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Green A, Krause J, Rumberger JM. Curcumin is a direct inhibitor of glucose transport in adipocytes. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2014;21:118–122. doi: 10.1016/j.phymed.2013.08.014. [DOI] [PubMed] [Google Scholar]
- [27].Gunnink LK, Alabi OD, Kuiper BD, Gunnink SM, Schuiteman SJ, Strohbehn LE, Hamilton KE, Wrobel KE, Louters LL. Curcumin directly inhibits the transport activity of GLUT1. Biochimie. 2016;125:179–185. doi: 10.1016/j.biochi.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gunnink LK, Busscher BM, Wodarek JA, Rosette KA, Strohbehn LE, Looyenga BD, Louters LL. Caffeine inhibition of GLUT1 is dependent on the activation state of the transporter. Biochimie. 2017;137:99–105. doi: 10.1016/j.biochi.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ojeda PG, Perez AA, Ojeda L, Vargas-Uribe M, Rivas CI, Salas M, Vera JC, Reyes AM. Non-Competitive Blocking of Human Glut1 Hexose Transporter by Methylxanthines Reveals an Exofacial Regulatory Binding Site. American journal of physiology. Cell physiology. 2012 doi: 10.1152/ajpcell.00145.2012. [DOI] [PubMed] [Google Scholar]
- [30].Sage JM, Cura AJ, Lloyd KP, Carruthers A. Caffeine inhibits glucose transport by binding at the GLUT1 nucleotide-binding site. American journal of physiology. Cell physiology. 2015;308:C827–834. doi: 10.1152/ajpcell.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- [32].Ojelabi OA, Lloyd KP, Simon AH, De Zutter JK, Carruthers A. [Google Scholar]
- [33].Siebeneicher H, Cleve A, Rehwinkel H, Neuhaus R, Heisler I, Muller T, Bauser M, Buchmann B. Identification and Optimization of the First Highly Selective GLUT1 Inhibitor BAY-876. ChemMedChem. 2016;11:2261–2271. doi: 10.1002/cmdc.201600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liong E, Kong SK, Au KK, Li JY, Xu GY, Lee YL, Kwok TT, Choy YM, Lee CY, Fung KP. Inhibition of glucose uptake and suppression of glucose transporter 1 mRNA expression in L929 cells by tumour necrosis factor-alpha. Life sciences. 1999;65:PL215–220. doi: 10.1016/s0024-3205(99)00408-7. [DOI] [PubMed] [Google Scholar]
- [35].Park JB, Levine M. Intracellular accumulation of ascorbic acid is inhibited by flavonoids via blocking of dehydroascorbic acid and ascorbic acid uptakes in HL-60, U937 and Jurkat cells. The Journal of nutrition. 2000;130:1297–1302. doi: 10.1093/jn/130.5.1297. [DOI] [PubMed] [Google Scholar]
- [36].Barnett JE, Holman GD, Chalkley RA, Munday KA. Evidence for two asymmetric conformational states in the human erythrocyte sugar-transport system. Biochem J. 1975;145:417–429. doi: 10.1042/bj1450417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Basketter DA, Widdas WF. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol. 1978;278:389–401. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Deves R, Krupka RM. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochimica et biophysica acta. 1978;510:339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- [39].IG. Baran C, Ursu I, Baran V, Calinescu O, Iftime A, Ungureanu R, Tofolean IT. Fluorecent Properties of Quercetin in Human Leukemia Jurkat T-cells. Rom. Journ. Phys. 2011;56:388–398. [Google Scholar]
- [40].Anand David AV, Arulmoli R, Parasuraman S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Parvaresh A, Razavi R, Rafie N, Ghiasvand R, Pourmasoumi M, Miraghajani M. Quercetin and ovarian cancer: An evaluation based on a systematic review. J Res Med Sci. 2016;21:34. doi: 10.4103/1735-1995.181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- [43].Griffiths K, Aggarwal BB, Singh RB, Buttar HS, Wilson D, De Meester F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases. 2016;4 doi: 10.3390/diseases4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang J, Ye C, Chen C, Xiong H, Xie B, Zhou J, Chen Y, Zheng S, Wang L. Glucose transporter GLUT1 expression and clinical outcome in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:16875–16886. doi: 10.18632/oncotarget.15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- [46].Keating E, Martel F. Antimetabolic Effects of Polyphenols in Breast Cancer Cells: Focus on Glucose Uptake and Metabolism. Front Nutr. 2018;5:25. doi: 10.3389/fnut.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nakayama T, Kamiguchi H, Akagawa K. Syntaxin 1C, a soluble form of syntaxin, attenuates membrane recycling by destabilizing microtubules. J Cell Sci. 2012;125:817–830. doi: 10.1242/jcs.081943. [DOI] [PubMed] [Google Scholar]
- [48].Carruthers A, Helgerson AL. Inhibitions of sugar transport produced by ligands binding at opposite sides of the membrane. Evidence for simultaneous occupation of the carrier by maltose and cytochalasin B. Biochemistry. 1991;30:3907–3915. doi: 10.1021/bi00230a015. [DOI] [PubMed] [Google Scholar]
- [49].Baker GF, Basketter DA, Widdas WF. Asymmetry of the hexose transfer system in human erythrocytes. Experiments with non-transportable inhibitors. J Physiol. 1978;278:377–388. doi: 10.1113/jphysiol.1978.sp012310. [DOI] [PMC free article] [PubMed] [Google Scholar]