Abstract
Background
Hydroxychloroquine (HCQ) is the standard of care medication for most SLE patients, however nonadherence is common. We investigated longitudinal patterns and predictors of nonadherence to HCQ in a U.S. SLE cohort of HCQ initiators.
Methods
We used Medicaid data from 28 states to identify adults 18–65 years with prevalent SLE. We included HCQ initiators following ≥6 months without use, and required ≥1 year of follow-up after first dispensing (index date). We used the proportion of days covered (PDC) to describe overall HCQ adherence (<80%=nonadherent) and novel group-based trajectory models (GBTM) to examine monthly patterns (<80% of days/month covered=nonadherent), during the first year of use. Multivariable multinomial logistic regression models were used to examine predictors of nonadherence.
Results
We identified 10,406 HCQ initiators with SLE. Mean age was 38 (±12) years, 94% were female, 42% black, 31% white; 85% had a mean PDC <80%. In our 4-group GBTM, 17% were persistent adherers, 36% persistent nonadherers, and 47% formed two dynamic patterns of partial adherence. Adherence declined for most patients over the first year. Compared to persistent adherers, the odds of nonadherence were increased for blacks and Hispanics vs. whites and for younger ages vs. older; increased SLE-related comorbidities were associated with reduced odds of nonadherence for persistent nonadherers (0.95, 95% CI 0.91–0.99).
Conclusions
Among HCQ initiators with SLE, we observed poor adherence which declined for most over the first year of use. HCQ adherence is a dynamic behavior and further studies of associated predictors, outcomes and interventions should reflect this.
Keywords: Adherence, hydroxychloroquine, systemic lupus erythematosus, pharmacoepidemiology, observational study, health services research
1.1 Introduction
Medication nonadherence is a serious problem among patients with systemic lupus erythematosus (SLE); less than half of patients adhere to their SLE-related medications as prescribed.(1) Clinical and epidemiologic factors unique to SLE may increase nonadherence including cognitive and psychological manifestations, high disease burden among lower socioeconomic status groups, the complexity and toxicity of the medication regimens, and SLE disease activity fluctuations. Hydroxychloroquine (HCQ) is considered the backbone of SLE therapy regardless of disease severity, and it is now standard of care for all SLE patients to take HCQ continuously beginning at the time of diagnosis.(2–4) HCQ use is disease stabilizing and associated with fewer disease flares, reduced disease activity overall and less organ damage accrual.(2, 4–7) Medically indicated discontinuation is uncommon with the exception of evidence of retinal toxicity, which results in most cases from cumulative exposure and occurs in 4–7.5% of patients taking HCQ for 10 years and in <1% during the first 5–7 years.(7, 8)
To date, most studies of HCQ adherence are small, cross-sectional, and based in academic cohorts. Moreover, they rely on one-time often self-reported measures of adherence failing to capture the dynamic nature of adherence behavior over time. Conflicting results regarding risk factors for nonadherence, and physicians’ inability to accurately predict who is likely to nonadhere, make it difficult to know who to target and how to intervene.(9, 10) In addition, most studies included prevalent users of HCQ and therefore conflate potentially different risk factors for nonadherence among patients initiating HCQ with those who have been taking it for years. We therefore aimed to use nationwide data on patients enrolled in Medicaid, the federal-state public health insurance for low-income individuals, to describe longitudinal patterns and predictors of HCQ adherence among SLE patients newly receiving this medication. To define distinct, dynamic HCQ adherence patterns, we used a well-described but novel method, group-based trajectory models (GBTM). GBTMs have been used in psychology and other social sciences, to model underlying longitudinal patterns where there are repeated measures available for individuals that may change over time.(11, 12) In the chronic disease literature, there are a few studies that use GBTM to describe patterns of adherence behavior and the method has been shown to better capture changes in adherence over time than standard composite measures such as the proportion of days covered (PDC).(13–16) To our knowledge, GBTMs have not been previously used to describe adherence among patients with SLE. We hypothesized that GBTMs would demonstrate distinct patterns of declining adherence over the first year of use and certain sociodemographic (e.g. young age, black race) and disease-related (e.g. absence of lupus nephritis) characteristics would predict patterns of sustained nonadherence.
1.2 Material and methods
1.2.1.Patient Cohort
We used the Medicaid Analytic eXtract (MAX) from the 29 most populated U.S. states from 2000–2010. HCQ dispensing data were unavailable in MAX for Medicaid beneficiaries living in Ohio and therefore this state was excluded, leaving 28 states in our analysis. MAX includes all billing claims, health care utilization, drug-dispensing data and demographic information for Medicaid beneficiaries. We identified patients aged 18–65 years with prevalent SLE based on >2 International Classification of Diseases, Ninth Revision (ICD-9) codes for SLE (710.0) from hospital discharge diagnoses or physician visit claims >30 days apart, and HCQ dispensing within 365 days of a SLE ICD-9 code. In prior studies using MAX, we required >3 ICD-9 codes for SLE however in this study we aimed to increase our ability to capture all patients with new onset SLE who initiated HCQ and therefore employed >2 codes for SLE plus one code for HCQ to accomplish this.(17) This algorithm with >2 ICD-9 codes and a related medication has been validated both in the rheumatoid arthritis literature, as well as in electronic health record based analyses among SLE patients with PPVs ranging from 77–89%.(18, 19) In addition, our interest here was HCQ adherence patterns and not SLE-associated outcomes, as it had been for the prior studies.(20, 21) We restricted our cohort to SLE patients with 183 days of continuous enrollment prior to first HCQ dispensing (index date) with no use of HCQ during this 183-day period. We restricted our cohort to incident users of HCQ to avoid potential bias from depletion of susceptibles.(22) Prevalent users of HCQ may be different from incident users because they remained on the drug (e.g. they did not have side effects or other reasons to discontinue or become nonadherent early on in use) and therefore we did not want to combine these groups in our analyses. We included patients who had >365 days of continuous follow-up after the index date. We excluded those with no dispensing data (N=233), and those who were hospitalized for the entire duration of follow-up (N=18). Additionally, we excluded patients who were missing zip code data as median household income was considered a potentially important covariate (N=253).
1.2.2. Adherence Measures
We assessed adherence in two ways using prescription refill data. Refill adherence has been shown to be a valid source of adherence information in population-based studies when directly observed methods are not possible.(23, 24) Prior studies demonstrate significant associations between refill adherence and other measures of adherence, as well as with serum drug levels and physiologic drug effects.(23, 25–28) Using prescription refill data, we calculated the overall proportion of days covered (PDC) for the 365-day follow-up period beginning at the index date. We calculated the PDC as the number of days covered divided by 365 days, multiplied by 100. We subtracted hospitalized days from the numerator and the denominator. In keeping with the chronic disease medication adherence literature (29), PDC >80% was considered adherent. In addition, we created a 12-month diary for each patient assigning a binary variable (0 (nonadherent) or 1 (adherent)) for each 30-day period indicating whether that period had >80% coverage (24 of the 30 days) with HCQ. We chose to use 30 days because 91% of our cohort received a 30-day supply of HCQ.
1.2.3. Covariates
We assessed covariates during the 183-day baseline period prior to and including the index date, unless otherwise specified. Demographic factors included age at the index date, sex, race/ethnicity (White, Black or African American, Hispanic or Latino, Asian (including Native Hawaiian and Pacific Islander), American Indian/Alaska Native, and other), region and state of residence at the index date. We included zip code median household income as a proxy for individual socioeconomic status using American Community Survey data (2006–2010). We assessed comorbidities including thromboembolism, pulmonary disease, chronic kidney disease, liver disease, cardiovascular disease, cerebrovascular disease, alcoholism, substance abuse, obesity or cancer/hematologic malignancy using >1 ICD-9 codes. For diabetes, we required an ICD-9 code for diabetes or the prescription of a diabetes-related medication. To determine smoking status, we used >1 ICD-9 code, CPT code for smoking cessation counseling, or dispensing of smoking cessation-related medications. We used the SLE risk adjustment index, which has been shown to be a better predictor of inpatient mortality among SLE patients than the Charlson comorbidity index, as a measure of SLE-related comorbidities.(30) We determined lupus nephritis using >2 ICD-9 hospital discharge diagnosis codes or physician billing claims for nephritis, proteinuria and/or renal failure occurring on or after one SLE diagnosis code.(31) We included antidepressant use as a marker of depression given the low positive predictive value of depression-related claims.(32)
As a marker of disease activity, we included number of laboratory tests for anti-dsDNA, BUN, creatinine, urinalysis and sediment, complement, ESR, and CRP. We included measures of health care utilization (number of emergency department (ED), outpatient visits and hospitalizations, and number hospitalized days), as well as preventive care using CPT codes for influenza and pneumococcal vaccinations. We assessed the number of other medications filled on the index date of new HCQ use, dispensing quantity, number of drugs, ever/never use of immunosuppressive medications (methotrexate, sulfasalazine, cyclophosphamide, azathioprine, mycophenolate mofetil, rituximab, tacrolimus or leflunomide), as well as corticosteroid use (mean daily prednisone-equivalent dose), anticoagulant use, nonsteroidal anti-inflammatory medications and selective and non-selective cyclooxygenase-2 inhibitors.
1.2.4 Statistical Methods
We calculated the overall PDC during the 365-day period following the index date. We used our binary indicators of adherence (0 (<80%), 1 (>80%)) for each 30-day period for the 365 days of follow-up to develop group-based trajectory models (GBTM) to classify patients by their HCQ adherence. GBTM has been shown to be the optimal technique for identifying latent patterns behind longitudinal data.(13, 33) First, a multinomial logistic regression model is used with no predictors and an intercept for each group to model the probability of each SLE patient’s probability of belonging to each adherence trajectory based on his/her own adherence pattern.(13) The GBTM accounts for repeated monthly measures of adherence for each individual and treats them as independent conditional on the trajectory group.(13) Each individual is then assigned to the group with the highest probability of membership. We modeled time in various forms (linear, quadratic and cubic) and found that the cubic terms best fit the adherence patterns uncovered in our data. We estimated trajectory models ranging from 2 to 6 groups. We did this because we aimed to find a GBTM that had good model fit but was not too complex and allowed for a more nuanced understanding of nonadherence patterns beyond a dichotomized comparison of overall “adherers” and “nonadherers.” We examined mean posterior probability values, or the average conditional probability of having been assigned to each trajectory group given a person’s adherence, with a probability of more than 80 percent for each group within each model considered to be acceptable. We compared model fit using Bayesian information criterion (BIC) with lower values considered preferable.(34) We looked for a reasonable distribution of subjects across the groups, and one clearly adherent trajectory (mean PDC >80%) for comparison. In choosing the most appropriate model, we aimed to balance what GBTM expert D.S. Nagin described as the “sometimes competing objectives of model parsimony and capturing the distinctive features of the data.”(34)
We then determined the association between baseline sociodemographic, clinical and health care utilization-related characteristics and the probability of trajectory group membership. We used multinomial logistic regression models to examine the odds of belonging to a nonadherent trajectory compared to the persistently adherent trajectory for the predictors including covariates during the 183 days prior to the index date, calendar year and state of residence at the index date.
We conducted additional analyses comparing the two trajectories that started off similar and then diverged 4–5 months after the index date. We used month 5 as our new index date and updated baseline covariates to include the period between the original index date and the fifth month of follow-up. We used multivariable logistic regression models to assess predictors of the two trajectories. We also compared monthly utilization between months 4 and 7, the time-period surrounding the point where the two curves diverged.
All analyses were conducted using SAS 9.4 (Cary, NC) and we used “Proc Traj,” an add-on package to base SAS to create our GBTMs. Data were obtained from the Centers for Medicare and Medicaid Services (CMS) through a Data Use Agreement and in accordance with CMS policies, all cell sizes <11 are suppressed. The Partners Healthcare Institutional Review Board approved this study.
1.3 Results
We identified 10,406 individuals with SLE who were new users of HCQ, had complete HCQ dispensing data, and 365-days of follow-up beginning at the date of HCQ initiation. The mean + SD age was 37.7 + 11.8 years, 94% were female, 42% were black, 31% white, 20% Hispanic (Table 1). During the baseline period, 10% had ICD-9 codes consistent with lupus nephritis, 27% with cardiovascular disease, 29% received an antidepressant medication and 59% received corticosteroids. During the 365-day follow-up period, the overall mean + SD PDC was 42% + 29; 15% of patients (N=1,575) had a composite PDC >80%.
Table 1.
Baseline characteristics of new users of HCQ with SLE enrolled in Medicaid
| Baseline characteristics* | HCQ New Users (N=10,406) |
|---|---|
| Age – mean + SD | 37.7 + 11.8 |
| Age group – N (%) | |
| 18–34 years | 4614 (44) |
| 35–50 years | 3951 (38) |
| 51–65 years | 1841 (18) |
| Female – N (%) | 9800 (94) |
| Race/ethnicity | |
| Black | 4365 (42) |
| White | 3239 (31) |
| Hispanic | 2047 (20) |
| Asian | 400 (4) |
| American Indian/Alaska Native | 121 (1) |
| Other | 234 (2) |
| Region | |
| Northeast | 2507 (24) |
| Midwest | 1607 (15) |
| South | 3789 (36) |
| West | 2503 (24) |
| Median household income + – mean + SD | 4.5 + 1.7 |
| SLE risk adjustment index – mean + SD | 1.0 + 1.9 |
| Comorbidities- N (%) | |
| Substance abuse | 154 (1) |
| Alcoholism | 57 (1) |
| Malignancy | 235 (2) |
| Cardiovascular disease | 2818 (27) |
| Cerebrovascular disease | 311 (3) |
| Chronic kidney disease | 73 (1) |
| Diabetes mellitus | 972 (9) |
| Chronic liver disease | 342 (3) |
| Chronic lung disease | 1167 (11) |
| Lupus nephritis | 1059 (10) |
| Obesity | 240 (2) |
| Thromboembolic disease | 359 (3) |
| Smoking | 627 (6) |
| Antidepressant use | 3019 (29) |
| Preventive care – N (%) | |
| Influenza vaccine | 184 (2) |
| Pneumococcal vaccine | 63 (1) |
| Immunosuppressive medication use- N (%) | |
| Azathioprine | 540 (5) |
| Cyclophosphamide | 38 (0.4) |
| Leflunomide | 66 (1) |
| Methotrexate | 601 (6) |
| Mycophenolate mofetil | 378 (4) |
| Sulfasalazine | 88 (1) |
| Tacrolimus | 55 (1) |
| Corticosteroids | |
| Ever use – N (%) | 6160 (59) |
| Mean daily prednisone-equivalent dose + SD | 2.9 mg + 17 Median: 0 (0, 2.8) |
| Mean number of medications – mean + SD | 4.1 + 3.4 |
| Healthcare utilization | |
| ED Visits – median (25, 75) | 0 (0, 1) |
| Hospitalizations – Median (25, 75) | 0 (0, 1) |
| Outpatient visits – median (25, 75) | 2 (0, 6) |
| Hospitalized days – mean + SD | 3.9 + 11 |
Determined from the 183 days prior to and including the index date (the date of first HCQ dispensing)
Determined at the zip code level; mean + SD divided by 10,000
1.3.1. Group-based Trajectory Model (GBTM)
We found that a four-group trajectory model allowed for the most nuanced exploration of nonadherence while also providing a good fit for our data (Figure 1). The five- and six-group models had larger BICs, group posterior probabilities <0.8, and unbalanced sample size distributions. The BICs for the two- and three- group models were smaller. However, the two-group model did not have an adherent trajectory with a mean PDC >80%. To both uphold model parsimony and understand unique features of nonadherent patterns in depth, we chose the four-group model over the three-group model. In keeping with Nagin’s principles, the BICs were similar, sample sizes were balanced, and both had all group posterior probabilities >0.8, however the four-group model uncovered distinctive patterns that enabled further exploration (34) (Supplemental Figure 1).
Figure 1.
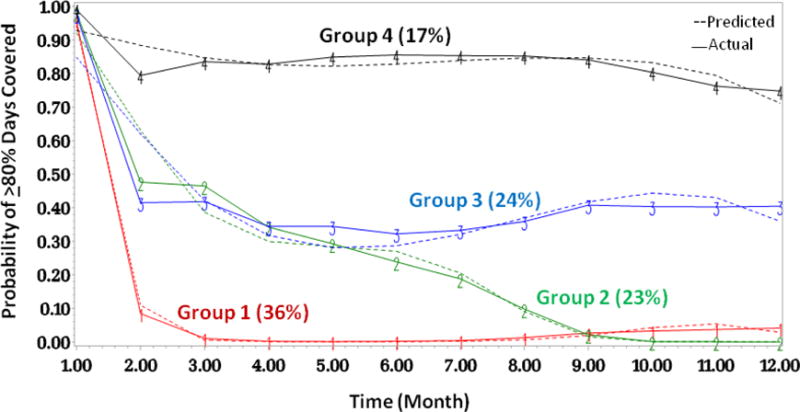
Four-group trajectory model of adherence patterns for new HCQ users with SLE; Group 1 are persistent nonadherers, Group 4 are persistent adherers
The four-group model revealed four distinct patterns (Figure 1). Group 1, which includes 36% of the cohort, are persistent nonadherers with few if any refills of HCQ after the initial dispensing. Group 4, which includes 17% of the cohort, are persistent adherers, with, on average, >80% of days covered for nearly all months over the course of the year of follow-up with a slightly declining trend from months 10–12. Groups 2 and 3 are intermediate nonadherers with more dynamic patterns than groups 1 and 4. The trajectories for Groups 2 and 3 are similar until month 5 when they diverge; Group 3 improves slightly and then reaches a plateau whereas Group 2 becomes nearly completely nonadherent for the remainder of follow-up. Except for Group 3, adherence declined by the end of the year of follow-up compared with the first 90-days. For Group 1, the overall mean + SD PDC was 15% + 10, for Group 2, 37% + 15, for Group 3, 57% + 15, and for Group 4, 88% + 8. There were no individuals in Group 1 with mean PDCs >80%, 3 (<1%) in Group 2, 133 (5%) in Group 3, and 1439 (84%) in Group 4.
Baseline characteristics for the four trajectories are presented in Table 2. The mean age was highest among the persistent adherers (Group 4) and lowest among persistent nonadherers (Group 1) (p<0.001). The highest percentage of individuals in Groups 1, 2 and 3 were black compared to Group 4 where the highest percentage were white. The median household income was similar across groups with slightly higher income among individuals in Group 4 compared to Group 1 (p=0.01). The SLE risk adjustment index was highest in Group 4 suggesting a higher burden of SLE-related comorbidities (p<0.001). Similarly, the mean number of medications dispensed (p<0.001) and the mean daily prednisone-equivalent dose were both higher in Group 4 compared to Group 1 suggesting a more ill population (p<0.001).
Table 2.
Baseline characteristics by trajectory group (N=10,406)
| Baseline characteristics* | Group 1 (persistent nonadherers) |
Group 2 (intermediate nonadherers) |
Group 3 (intermediate nonadherers) |
Group 4 (persistent adherers) |
|---|---|---|---|---|
| N (%) | 3772 (36) | 2431 (23) | 2481 (24) | 1722 (17) |
| Age – mean + SD | 36.7 + 11.6 | 37.1 + 11.7 | 37.8 + 11.8 | 40.4 + 12.2 |
| Age group – N (%) | ||||
| 18–34 years | 1808 (48) | 1120 (46) | 1067 (43) | 619 (36) |
| 35–50 years | 1388 (37) | 923 (38) | 975 (39) | 665 (39) |
| 51–65 years | 576 (15) | 388 (16) | 439 (18) | 438 (25) |
| Female – N (%) Race/ethnicity |
3568 (95) | 2301 (95) | 2333 (94) | 1598 (93) |
| Black | 1694 (45) | 1069 (44) | 1062 (43) | 540 (31) |
| White | 1166 (31) | 675 (28) | 717 (29) | 681 (40) |
| Hispanic | 692 (18) | 518 (21) | 513 (21) | 324 (19) |
| Asian | 99 (3) | 90 (4) | 100 (4) | 111 (6) |
| AI/AN | 46 (1) | 27 (1) | 28 (1) | 20 (1) |
| Other | 75 (2) | 52 (2) | 61 (2) | 46 (3) |
| Region | ||||
| Northeast | 843 (22) | 579 (24) | 626 (25) | 459 (27) |
| Midwest | 627 (17) | 357 (15) | 357 (14) | 266 (15) |
| South | 1455 (39) | 922 (38) | 867 (35) | 545 (32) |
| West | 847 (22) | 573 (24) | 631 (25) | 452 (26) |
| Median household income + – mean + SD | 4.4 + 1.7 | 4.5 + 1.6 | 4.5 + 1.7 | 4.6 + 1.7 |
| SLE risk adjustment index – mean + SD | 0.9 + 1.8 | 1.0 + 1.8 | 1.1 + 2.0 | 1.3 + 2.2 |
| Comorbidities- N (%) | ||||
| Substance abuse | 71 (2) | 33 (1) | 27 (1) | 23 (1) |
| Alcoholism | 23 (1) | 11 (0.5) | 17 (1) | NR |
| Malignancy | 78 (2) | 48 (2) | 63 (3) | 46 (3) |
| Cardiovascular disease | 942 (25) | 658 (27) | 709 (29) | 509 (30) |
| Cerebrovascular disease | 99 (3) | 72 (3) | 84 (3) | 56 (3) |
| Chronic kidney disease | 27 (1) | 15 (1) | 20 (8) | 11 (1) |
| Diabetes mellitus | 314 (8) | 221 (9) | 242 (10) | 195 (11) |
| Chronic liver disease | 112 (3) | 81 (3) | 85 (3) | 64 (4) |
| Chronic lung disease | 402 (11) | 299 (12) | 269 (11) | 197 (11) |
| Lupus nephritis | 366 (10) | 244 (10) | 252 (10) | 197 (11) |
| Obesity | 90 (2) | 45 (2) | 62 (2) | 43 (2) |
| Thromboembolic disease | 109 (3) | 84 (3) | 84 (3) | 82 (5) |
| Smoking | 215 (6) | 149 (6) | 148 (6) | 115 (7) |
| Antidepressant use Preventive care – N (%) | 1045 (28) | 739 (30) | 685 (28) | 550 (32) |
| Influenza vaccine | 66 (2) | 32 (1) | 48 (2) | 38 (2) |
| Pneumococcal vaccine | 21 (1) | 11 (0.5) | 17 (1) | 14 (1) |
| Immunosuppressive medication use- N (%) | ||||
| Azathioprine | 160 (4) | 107 (4) | 151 (6) | 122 (7) |
| Cyclophosphamide | 11 (0.3) | NR | NR | NR |
| Leflunomide | 20 (0.5) | 15 (1) | 11 (0.4) | 20 (1) |
| Methotrexate | 204 (5) | 138 (6) | 156 (6) | 103 (6) |
| Mycophenolate mofetil | 122 (3) | 87 (4) | 99 (4) | 70 (4) |
| Sulfasalazine | 34 (1) | 19 (8) | 20 (8) | 15 (1) |
| Tacrolimus | 18 (0.5) | 13 (1) | NR | 15 (1) |
| Corticosteroids | ||||
| Ever use – N (%) | 2108 (56) | 1475 (61) | 1521 (61) | 1056 (61) |
| Mean daily prednisone-equivalent dose + SD | 2.5mg + 5.8 Median 0 (0, 2.4) |
2.9mg + 6.5 Median 0 (0, 2.9) |
2.8mg + 6.0 Median 0 (0, 3) |
4mg + 40 Median 0 (0, 3.3) |
| HCQ Prescription <30 days- N (%) | 3557 (94) | 2131 (88) | 2270 (91) | 1490 (87) |
|
Mean number of medications – mean + SD Healthcare utilization |
3.7 + 3.2 | 4.2 + 3.4 | 4.2 + 3.3 | 5.3 + 3.9 |
| ED Visits – median (25, 75) | 0 (0, 1) Mean 0.95 + 2.4 |
0 (0, 1) Mean 0.91 + 2.0 |
0 (0, 1) Mean 0.82 + 1.9 |
0 (0, 1) Mean 0.76 + 1.9 |
| Inpatient – median (25, 75) | 0 (0, 1) Mean 0.57 + 1.1 |
0 (0, 1) Mean 0.60 + 1.2 |
0 (0, 1) Mean 0.60 + 1.1 |
0 (0, 1) Mean 0.61 + 1.2 |
| Outpatient– median (25, 75) | 2 (0, 6) Mean 3.9 + 4.8 |
2 (0, 6) Mean 3.9 + 4.9 |
2 (0, 6) Mean 3.7+ 4.8 |
2 (0,7) Mean 4.0 + 5.0 |
| Hospitalized days – mean + SD | 3.5 + 9.5 | 4.2 + 12.7 | 3.9 + 10.0 | 4.3 + 11.0 |
Determined from the 183 days prior to and including the index date (the date of first HCQ dispensing)
Determined at the zip code level; mean + SD divided by 10,000
1.3.2. Trajectory Predictors
We estimated multinomial logistic regression models to examine predictors of the different trajectories with Group 4 (persistent adherers) as the reference. We found increased odds of belonging to all three nonadherent trajectories for individuals aged 18–34 years and 35–50 years, compared to those 51–65 years (Table 3). We similarly found increased odds of belonging to Groups 1, 2 or 3 compared to Group 4 associated with black race and Hispanic ethnicity, compared to white. We found reduced odds (OR 0.64, 95% CI 0.47–0.87) of belonging to Group 1 vs. Group 4 (persistent adherers) among Asians compared to whites.
Table 3.
Multinomial logistic regression model of the odds of being in the Group 1 (persistent nonadherers), Group 2 or 3 (intermediate nonadherers) trajectories compared with being in the Group 4 trajectory (persistent adherers, reference)
| Baseline characteristics N=10,406 |
Group 1 (persistent nonadherers) OR (95% CI) |
Group 2 (intermediate nonadherers) OR (95% CI) |
Group 3 (intermediate nonadherers) OR (95% CI) |
|---|---|---|---|
| Age group | |||
| 18–34 years | 1.66 (1.39–1.98) | 1.66 (1.38–2.02) | 1.42 (1.17–1.71) |
| 35–50 years | 1.33 (1.13–1.57) | 1.38 (1.16–1.66) | 1.32 (1.11–1.58) |
| 51–65 years | Ref. | Ref. | Ref. |
| Male (Female = ref) | 0.87 (0.68–1.10) | 0.85 (0.66–1.11) | 0.88 (0.68–1.14) |
| Race/ethnicity | |||
| White | Ref. | Ref. | Ref. |
| Black | 1.74 (1.49–2.04) | 1.95 (1.65–2.31) | 1.81 (1.53–2.14) |
| Hispanic | 1.40 (1.16–1.68) | 1.66 (1.36–2.02) | 1.51 (1.24–1.83) |
| Asian | 0.64 (0.47–0.87) | 0.92 (0.67–1.26) | 0.86 (0.63–1.17) |
| AI/AN | 1.05 (0.60–1.84) | 1.15 (0.62–2.12) | 1.11 (0.61–2.03) |
| Median household income | 0.99 (0.96–1.03) | 1.01 (0.96–1.05) | 1.00 (0.96–1.04) |
| SLE risk adjustment index | 0.95 (0.91–0.99) | 0.96 (0.92–1.00) | 0.99 (0.95–1.04) |
| Diabetes mellitus | 1.25 (1.01–1.56) | 1.17 (0.93–1.49) | 1.18 (0.94–1.48) |
| Lupus nephritis | 1.06 (0.80–1.41) | 0.90 (0.66–1.22) | 0.71 (0.53–0.96) |
| Antidepressant use (Never=ref) | 1.18 (1.02–1.37) | 1.30 (1.12–1.52) | 1.11 (0.95–1.29) |
| Corticosteroids use (Never=ref) | 0.87 (0.76–1.00) | 0.99 (0.86–1.14) | 1.07 (0.93–1.23) |
| Number of medications | 0.90 (0.88–0.92) | 0.94 (0.92–0.96) | 0.92 (0.90–0.94) |
| Healthcare utilization | |||
| ED Visits | 1.05 (1.01–1.08) | 1.03 (0.99–1.07) | 1.02 (0.98–1.06) |
| Hospitalizations | 1.12 (1.03–1.22) | 1.05 (0.96–1.14) | 1.06 (0.98–1.16) |
| Outpatient visits | 1.00 (0.98–1.01) | 0.99 (0.97–1.01) | 1.00 (0.98–1.01) |
Model additionally adjusted for U.S. state, geographic region, calendar year at index date, index date HCQ dispensing amount, laboratory tests, pain medications, preventive care (influenza vaccine, pneumococcal vaccine), immunosuppressive medication use, comorbidities (substance abuse, alcoholism, malignancy, cardiovascular disease, cerebrovascular disease, chronic kidney disease, obesity, thromboembolic disease, chronic lung disease, smoking), and mean daily corticosteroid dose. All variables were determined during the 183 days prior to and including the index date. Group 4 (persistent adherers) is there reference.
In terms of comorbidities, we found increased odds of belonging to Group 1 (persistent nonadherers) vs. 4 (persistent adherers) associated with diabetes (OR 1.25, 95% CI 1.01–1.56) and decreased odds for each unit increase in the SLE risk adjustment index (0.95, 95% CI 0.91–0.99). There were reduced odds of belonging to Group 3 vs. 4 associated with lupus nephritis (OR 0.71, 95%CI 0.53–0.96). The odds of belonging to nonadherent Groups 1,2 and 3 vs. Group 4 were inversely related to the total number of medications filled. There were increased odds of belonging to nonadherent Groups 1 and 2 vs. 4 associated with antidepressant use.
We used multivariable logistic regression to examine predictors of belonging to Group 2 vs. Group 3 beginning at month 5, the point at which the curves diverged and Group 2 became nearly completely nonadherent while Group 3 plateaued (Table 4). We found increased odds of belonging to Group 2 associated with younger age (OR 1.20, 95% CI 1.01–1.44) and the use of antidepressants (OR 1.25, 95% CI 1.10–1.43). We examined health care utilization separately by month for months 4 through 7 (Table 5) and found that beginning at month 4, patients in Group 3 had more hospitalizations, and beginning at month 5, longer hospitalizations compared to those in Group 2. We observed a trend towards more outpatient visits for Group 3 compared to Group 2 during months 6 and 7.
Table 4.
Multivariable logistic regression model comparing Group 2 (N=2431, declining adherence) to Group 3 (N=2481), plateaued adherence, reference) at the point of divergence*
| Predictors* | Group 2** |
|---|---|
| Odds Ratio (95% CI) | |
| Age group | |
| 18–34 years | 1.20 (1.01–1.44) |
| 35–50 years | 1.07 (0.0.90–1.27) |
| 51–65 years | Ref |
| Male (Female = ref) | 0.97 (0.75–1.25) |
| Race/ethnicity | |
| White | Ref |
| Black | 1.09 (0.93–1.27) |
| Hispanic | 1.13 (0.94–1.36) |
| Asian | 1.09 (0.79–1.51) |
| AI/AN | 1.07 (0.61–1.88) |
| Median household income | 1.01 (0.97–1.05) |
| SLE risk adjustment index | 0.96 (0.92–1.01) |
| Diabetes mellitus | 1.01 (0.0.81–1.26) |
| Lupus nephritis | 1.32 (0.99–1.74) |
| Antidepressant use (Never=ref) | 1.16 (1.01–1.34) |
| Corticosteroids use (Never=ref) | 0.93 (0.82–1.06) |
| Number of medications | 1.02 (1.00–1.04) |
| Healthcare utilization | |
| ED Visits | 1.01 (0.99–1.05) |
| Hospitalizations | 0.99 (0.92–1.07) |
| Outpatient visits | 0.99 (0.98–1.01) |
| Number of Laboratory tests | |
| ESR | 1.09 (0.79–1.23) |
| BUN | 1.24 (1.04–1.49) |
| Creatinine | 0.96 (0.84–1.09) |
| Complement (C3 or C4) | 0.94 (0.82–1.06) |
Predictors from 6 months prior to first HCQ dispensing and updated through month 4 of follow-up; nonadherence patterns assessed from months 5 through 12.
Group 3 is the reference.
Model is additionally adjusted for U.S. state, geographic region, calendar year at index date, index date HCQ dispensing amount, additional laboratory tests, pain medications, preventive care (influenza vaccine, pneumococcal vaccine), immunosuppressive medication use, comorbidities (substance abuse, alcoholism, malignancy, cardiovascular disease, cerebrovascular disease, chronic kidney disease, obesity, thromboembolic disease, chronic lung disease, smoking), and mean daily corticosteroid dose.
Table 5.
Healthcare utilization for Group 2 (N=2431, declining adherence) and Group 3 (N= 2481, plateaued adherence) in months 4–7
| Healthcare utilization | Month 4 | Month 5 | Month 6 | Month 7 | ||||
|---|---|---|---|---|---|---|---|---|
| Group 2 |
Group 3 |
Group 2 |
Group 3 |
Group 2 |
Group 3 |
Group 2 |
Group 3 |
|
| Outpatient visits- mean ± SD | 8.4 ± 7.0 | 8.4 ± 6.8 | 9.3 ± 7.7 | 9.3 ± 7.5 | 10.1 ±8.2 | 10.2 ± 8.1 | 10.9 ± 8.7 | 11.1 ± 8.7 |
| ED visits- mean ± SD | 1.9 ± 3.5 | 1.8 ± 3.5 | 2.1 ± 3.7 | 2.0 ± 3.8 | 2.3 ± 4.0 | 2.2 ± 4.0 | 2.4 ± 4.3 | 2.4 ± 4.4 |
| Inpatient visits- mean ± SD | 0.25 ±1.0 | 0.29 ± 1.0 | 0.26 ± 1.0 | 0.32 ± 1.1 | 0.28 ± 1.1 | 0.34 ± 1.2 | 0.31 ± 1.1 | 0.37 ± 1.3 |
| Hospitalized days- mean ± SD | 1.6 ± 8.0 | 1.9 ± 7.8 | 1.7 ± 8.3 | 2.1 ± 8.7 | 1.9 ± 8.6 | 2.3 ± 9.9 | 2.1 ± 9.5 | 2.4 ±10.5 |
Bolded values indicate statistically significant difference (p<0.05) comparing groups 2 and 3 within the month
1.4. Discussion
In this longitudinal study of Medicaid beneficiaries with SLE, adherence among HCQ initiators was poor starting one month after the first dispensing, and for most patients, adherence declined over the first year of use. While prior studies demonstrated that nonadherence among SLE patients is common, nonadherence among SLE patients enrolled in Medicaid is even more pronounced.(10, 35, 36) Our model revealed a group of persistent nonadherers, which comprised 36% of our cohort, who had very few HCQ refills after the initial dispensing. We identified a small group of persistent adherers (Group 4, 17%), although even this group also experienced a decline in adherence beginning at 9 months.
In contrast to prior studies, which either measure adherence cross-sectionally or using a composite measure, we could explore the nuances of adherence patterns over the first year of use, which was especially relevant for the intermediate nonadherers (Groups 2 and 3). We found that using the PDC, a commonly used composite measure, we would have misclassified 136 patients with nonadherent patterns as adherent and 283 patients with persistently adherent patterns as nonadherent. We found that the 5-month mark represented a critical juncture that may represent a clinical opportunity to intervene before adherence worsens among these “undecided” groups. We found that patients who plateaued had more frequent and longer hospitalizations suggesting both that they were more seriously ill, and that they likely had more interactions with the healthcare system to have their medications renewed. We also observed a trend towards more outpatient visits in this group suggesting that sustained access to outpatient care may increase the likelihood a patient continues the medication he/she is prescribed. Five months might also be the point at which patients feel that they have adequately trialed the medication and if there is no symptomatic improvement, they discontinue. With the growing body of literature suggesting long-term preventive effects from HCQ, increased provider and patient education at this juncture may be beneficial. (2–4, 6, 7, 37)
Interestingly, we did not find many strong predictors within our available set of covariates that were significantly associated with declining adherence (Group 2) versus plateaued adherence (Group 3) at 5 months. We did not find an association with demographic factors which suggests that while age and race/ethnicity might contribute initially to who is likely to be a persistent adherer or nonadherent, these factors may not play an important role in determining who continues to be modestly adherent versus who discontinues after an initial period. We did find an association with increased antidepressant use among those with declining adherence. Prior studies similarly show depression as a risk factor for poorer adherence and it is possible that among patients with depression the threshold to discontinue a medication that they may not see a tangible benefit after a period of time may be lower.(38)
In order to investigate whether patterns of adherence are distinct by drug, we have separately used group-based trajectory models to examine adherence to azathioprine and mycophenolate mofetil also within the Medicaid SLE population.(39) Interestingly, while we similarly noted poor nonadherence to both drugs with fewer than 20% of individuals characterized as adherent to either drug, we found that each drug had a pattern of adherence that differed from that of HCQ. While both drugs had subsets of the population who were persistent adherers and persistent nonadherers, the paths of the intermediate nonadherers were distinct. This suggests that while there may be certain patients who will adhere or will not adhere consistently, for those in a more “undecided” category, characteristics of the drug itself, such as side effect profile, may play a role in adherence behavior. In addition, the patients receiving azathioprine and mycophenolate mofetil are likely sicker than those receiving HCQ, and this, as well as ongoing disease activity, may also contribute to adherence behavior. Overall, our finding of high rates of nonadherence among SLE patients is in line with prior studies that repeatedly show that more than half of SLE patients are nonadherent regardless of the adherence assessment method or the population under investigation.(40) Our findings are also consistent with adherence estimates for other chronic disease medications both among Medicaid beneficiaries and among the commercially insured.(41, 42)
Previous studies have highlighted several potential predictors for nonadherence including black race, increased comorbidities, depression and polypharmacy.(38, 43, 44) We found that younger age, Black race, Hispanic ethnicity, and antidepressant medication use increased the odds of nonadherence. However, in our cohort, corticosteroid use, polypharmacy and higher SLE risk adjustment scores, all associated with increased SLE severity, reduced the odds of persistent nonadherence. Our findings suggest that patients who may have more active and severe SLE may be more likely to adhere to their HCQ. It is possible that among patients with milder SLE, while it is the standard of care to continue HCQ in all SLE patients unless contraindicated, the patients or physicians may have felt the medication was unnecessary. Overall, it is important to note that the magnitude of all of the statistically significant odds ratios was small. This reaffirms findings from prior studies that note that adherence behavior is complex and not readily predictable by a defined set of measurable variables.
There were limitations to this work. First, we used dispensing data to infer adherence however, filling a medication does not guarantee that a medication was taken. While 91% of our cohort received a 30 day or less supply of HCQ, the small subset receiving 60 or 90-day supplies may appear adherent for longer than they were. We conducted a sensitivity analyses looking specifically at this group and found similar trajectory patterns but the declines in adherence, as would be expected, started 2–3 month later than in our primary model. The mean + SD PDC for the 91% with 30-day or less HCQ prescriptions was 41% + 29%, very much the same as our full cohort. We also used a monthly cutoff of >80% (24 of 30 days covered) to classify a patient as adherent in keeping with prior chronic disease studies. However, it is unclear if there is a difference in clinical outcomes associated with this cutoff. In addition, while we feel that medically indicated discontinuation within the first year of use of HCQ is uncommon, it is certainly possible that some patients may have stopped their medication because they were told to do so by their physician and we cannot distinguish this from nonadherence using claims data. Similarly, the claims-based definition we used to identify SLE patients may have identified individuals with “probable SLE” as well, as these early undifferentiated patients are often prescribed HCQ. However, as HCQ is most often the initial medication prescribed for SLE, and because retinal toxicity is rare early on, we aimed to understand adherence in the first year of use. Side effects such as gastrointestinal upset, allergic reaction, or hyperpigmentation (45), may occasionally preclude its continued use. While HCQ has been shown to be safe during pregnancy and breastfeeding, it is possible that some women may have chosen to discontinue after consultation with their physicians. This may be reflected by the increased odds of belonging to a nonadherent trajectory among younger age groups. In addition, the use of claims data limits our ability to understand SLE disease activity, which may parallel adherence patterns. We did account for predictors that are markers of SLE disease activity and severity such as the SLE risk adjustment index, medication use (corticosteroid dose and immunosuppressive medications), and comorbidities such as lupus nephritis. We also do not have information regarding disease duration since this is not an inception cohort of SLE patients. In addition, while we were able to examine healthcare utilization (number of hospitalizations, outpatient and emergency department visits), we do not have data available regarding continuity of outpatient care, or access to subspecialty care, which may also influence adherence behavior.
The Medicaid population is a high-risk, vulnerable population with a high burden of comorbidities and adverse outcomes. Therefore, while it is an important population to study, findings might not be broadly generalizable as nonadherence is likely higher in this population. It is possible that there may be misclassification, particularly of comorbidities, since primarily ICD-9 codes alone were used for identification. It is also possible that there are important predictors, such as individual-level socioeconomic status, which are not available in Medicaid claims data but may play a significant role in adherence behavior. We also restricted our population to adults because there may be different factors associated with adherence among children and adolescents with SLE. Further studies are needed to examine adherence behavior among adolescents, particularly during the vulnerable transition from pediatric to adult care.
This work also has a number of strengths. We included a large non-academic cohort of HCQ initiators with SLE and used a well-established method not previously applied to SLE medication use that enabled us to understand patterns of adherence over the first year of use. Our patient population was racially and ethnically diverse and 28 U.S. states were represented and we adjusted our analyses by state to account for potential differences in Medicaid enrollment and drug policies. Adherence was measured longitudinally rather than cross-sectionally as it has been done in most prior SLE-related studies, and therefore we captured changes in adherence over time. We restricted our cohort to HCQ initiators and therefore did not conflate patterns and risk factors among patients receiving this medication for years with those for whom it was newly prescribed. In addition, rather than using a long-term average measure alone, such as the PDC, as most prior claims-based studies do, we used month-by-month measures which have been shown to better represent the nuances of adherence behaviors.(13) While we found a similar percentage formed the persistently adherent trajectory (17%) as were classified as adherent using the PDC composite measure (15%), we were able to delve into predictors of different patterns of nonadherence. Notably, we were able to understand certain factors that contributed to individuals trending from intermediate to complete nonadherence, which has the potential to inform strategies physicians take to counsel patients and identify the most vulnerable groups.
Overall, our study demonstrated that HCQ adherence is a dynamic behavior that declines over the first year of use. While claims data do not allow us to understand the reasons for nonadherence, it is clear from our findings that the majority of patients prescribed HCQ are not taking the medication as prescribed often beginning the month after first dispensing. Potentially modifiable factors, such as improving sustained access to healthcare not only for those who are more severely ill, might prevent intermediate nonadherers, or “undecided” patients from moving towards increasingly nonadherent pathways. In addition, with the knowledge of the extremely poor adherence among this especially vulnerable patient population, increased counseling and support programs both at the time of first HCQ prescription and throughout the first year of use, are needed to promote more sustained patterns of adherence for all patients.
Supplementary Material
Acknowledgments
Funding: This study was funded by the Rheumatology Research Foundation Investigator Award (CH Feldman), NIH 1K23 AR071500-01 (CH Feldman), NIH NIAMS R01 057327 (KH Costenbader), K24 AR066109 (KH Costenbader) and K24 AR055989 (DH Solomon)
Abbreviations
- SLE
Systemic lupus erythematosus
- HCQ
hydroxychloroquine
- PDC
proportion of days covered
- GBTM
group-based trajectory models
- MAX
Medicaid Analytic eXtract
- ED
emergency department
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have relevant financial disclosures.
References
- 1.Marengo MF, Waimann CA, de Achaval S, Zhang H, Garcia-Gonzalez A, Richardson MN, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus. 2012;21(11):1158–65. doi: 10.1177/0961203312447868. [DOI] [PubMed] [Google Scholar]
- 2.A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. N Engl J Med. 1991;324(3):150–4. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 3.Akhavan PS, Su J, Lou W, Gladman DD, Urowitz MB, Fortin PR. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J Rheumatol. 2013;40(6):831–41. doi: 10.3899/jrheum.120572. [DOI] [PubMed] [Google Scholar]
- 4.Pons-Estel GJ, Alarcon GS, Gonzalez LA, Zhang J, Vila LM, Reveille JD, et al. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res (Hoboken) 2010;62(3):393–400. doi: 10.1002/acr.20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durcan L, Clarke WA, Magder LS, Petri M. Hydroxychloroquine Blood Levels in Systemic Lupus Erythematosus: Clarifying Dosing Controversies and Improving Adherence. J Rheumatol. 2015;42(11):2092–7. doi: 10.3899/jrheum.150379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fessler BJ, Alarcon GS, McGwin G, Jr, Roseman J, Bastian HM, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52(5):1473–80. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–8. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 8.Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453–60. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- 9.Mushlin AI, Appel FA. Diagnosing potential noncompliance. Physicians’ ability in a behavioral dimension of medical care. Archives of internal medicine. 1977;137(3):318–21. doi: 10.1001/archinte.137.3.318. [DOI] [PubMed] [Google Scholar]
- 10.Koneru S, Shishov M, Ware A, Farhey Y, Mongey AB, Graham TB, et al. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis and rheumatism. 2007;57(6):1000–6. doi: 10.1002/art.22898. [DOI] [PubMed] [Google Scholar]
- 11.Nagin DS, Odgers CL. Group-Based Trajectory Modeling (Nearly) Two Decades Later. J Quant Criminol. 2010;26(4):445–53. doi: 10.1007/s10940-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 13.Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Medical care. 2013;51(9):789–96. doi: 10.1097/MLR.0b013e3182984c1f. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhou H, Cai B, Kahler KH, Tian H, Gabriel S, et al. Group-based trajectory modeling to assess adherence to biologics among patients with psoriasis. Clinicoecon Outcomes Res. 2014;6:197–208. doi: 10.2147/CEOR.S59339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juarez DT, Williams AE, Chen C, Daida YG, Tanaka SK, Trinacty CM, et al. Factors affecting medication adherence trajectories for patients with heart failure. Am J Manag Care. 2015;21(3):e197–205. [PMC free article] [PubMed] [Google Scholar]
- 16.MacEwan JP, Forma FM, Shafrin J, Hatch A, Lakdawalla DN, Lindenmayer JP. Patterns of Adherence to Oral Atypical Antipsychotics Among Patients Diagnosed with Schizophrenia. J Manag Care Spec Pharm. 2016;22(11):1349–61. doi: 10.18553/jmcp.2016.22.11.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnado A, Casey C, Carroll RJ, Wheless L, Denny JC, Crofford LJ. Developing Electronic Health Record Algorithms that Accurately Identify Patients with Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farhangian ME, Huang WW, Feldman SR. Adherence to Oral and Topical Medications in Cutaneous Lupus Erythematosus is not Well Characterized. Dermatol Ther (Heidelb) 2015;5(2):91–105. doi: 10.1007/s13555-015-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcon GS, Costenbader KH. Racial/Ethnic variation in all-cause mortality among United States medicaid recipients with systemic lupus erythematosus: a Hispanic and asian paradox. Arthritis Rheumatol. 2015;67(3):752–60. doi: 10.1002/art.38981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson M, Suissa S. Avoiding common pitfalls in the analysis of observational studies of new treatments for rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(6):805–10. doi: 10.1002/acr.20124. [DOI] [PubMed] [Google Scholar]
- 23.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. Journal of clinical epidemiology. 1997;50(1):105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 24.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Medical care. 1988;26(8):814–23. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Medical care. 2006;44(5):471–7. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]
- 26.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43(1):78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 27.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–5. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner JF, Fihn SD, Blair B, Inut TS. Appropriate reductions in compliance among well-controlled hypertensive patients. Journal of clinical epidemiology. 1991;44(12):1361–71. doi: 10.1016/0895-4356(91)90097-s. [DOI] [PubMed] [Google Scholar]
- 29.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–40. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for in-hospital mortality. J Rheumatol. 2000;27(6):1408–13. [PubMed] [Google Scholar]
- 31.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19(6):741–3. doi: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noyes K, Liu H, Lyness JM, Friedman B. Medicare beneficiaries with depression: comparing diagnoses in claims data with the results of screening. Psychiatr Serv. 2011;62(10):1159–66. doi: 10.1176/ps.62.10.pss6210_1159. [DOI] [PubMed] [Google Scholar]
- 33.Twisk J, Hoekstra T. Classifying developmental trajectories over time should be done with great caution: a comparison between methods. Journal of clinical epidemiology. 2012;65(10):1078–87. doi: 10.1016/j.jclinepi.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Nagin DS. Group-Based Modeling of Development. Boston, MA: Harvard University Press; 2005. [Google Scholar]
- 35.Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH. Medication Nonadherence Is Associated With Increased Subsequent Acute Care Utilization Among Medicaid Beneficiaries With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2015;67(12):1712–21. doi: 10.1002/acr.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira-Santos M, Verani JF, Klumb EM, Albuquerque EM. Evaluation of adherence to drug treatment in patients with systemic lupus erythematosus in Brazil. Lupus. 2011;20(3):320–9. doi: 10.1177/0961203310383736. [DOI] [PubMed] [Google Scholar]
- 37.Jung H, Bobba R, Su J, Shariati-Sarabi Z, Gladman DD, Urowitz M, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis and rheumatism. 2010;62(3):863–8. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 38.Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 2009;61(2):240–6. doi: 10.1002/art.24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman CH, Collins JE, Zhang Z, Kawachi I, Solomon DH, Costenbader KH. Azathioprine and mycophenolate mofetil adherence in a nationwide Medicaid cohort with systemic lupus erythematosus [abstract] Arthritis Rheumatol. 2016;2016(68) doi: 10.1002/acr.23792. Suppl 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehat P, Atiquzzaman M, Esdaile JM, AviNa-Zubieta A, De Vera MA. Medication Nonadherence in Systemic Lupus Erythematosus: A Systematic Review. Arthritis Care Res (Hoboken) 2017;69(11):1706–13. doi: 10.1002/acr.23191. [DOI] [PubMed] [Google Scholar]
- 41.Choudhry NK, Krumme AA, Ercole PM, Girdish C, Tong AY, Khan NF, et al. Effect of Reminder Devices on Medication Adherence: The REMIND Randomized Clinical Trial. JAMA Intern Med. 2017;177(5):624–31. doi: 10.1001/jamainternmed.2016.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey JE, Hajjar M, Shoib B, Tang J, Ray MM, Wan JY. Risk factors associated with antihypertensive medication nonadherence in a statewide Medicaid population. Am J Med Sci. 2014;348(5):410–5. doi: 10.1097/MAJ.0b013e31825ce50f. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Gonzalez A, Richardson M, Garcia Popa-Lisseanu M, Cox V, Kallen MA, Janssen N, et al. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2008;27(7):883–9. doi: 10.1007/s10067-007-0816-6. [DOI] [PubMed] [Google Scholar]
- 44.Mosley-Williams A, Lumley MA, Gillis M, Leisen J, Guice D. Barriers to treatment adherence among African American and white women with systemic lupus erythematosus. Arthritis Rheum. 2002;47(6):630–8. doi: 10.1002/art.10790. [DOI] [PubMed] [Google Scholar]
- 45.Jallouli M, Frances C, Piette JC, Huong du LT, Moguelet P, Factor C, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149(8):935–40. doi: 10.1001/jamadermatol.2013.709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


