Highlights
-
•
Oxidative Stress is the key mechanism involved in Lead, Mercury, Cadmium and Arsenic-induced kidney toxicity.
-
•
Proteinuria is common to all heavy metals as manifestation of kidney damage.
-
•
Chelation therapy is recommended based on the dose size of heavy metals, though fatal drawbacks limit their success.
-
•
Possible effectiveness of plants and plants derived compound against heavy metals is due to their antioxidant activity.
-
•
Other possible effectiveness of plant includes protecting integrity of mitochondria, Ca2+ homeostasis and apoptosis regulation.
Keywords: Heavy metals, Oxidative stress, Proteinuria, Kidney toxicity, Antioxidant
Abstract
Environmental pollution has become a concerning matter to human beings. Flint water crisis in the USA pointed out that pollution by heavy metal is getting worse day by day, predominantly by Lead, Cadmium, Mercury and Arsenic. Despite of not having any biological role in flora and fauna, they exhibit detrimental effect following exposure (acute or chronic). Even at low dose, they affect brain, kidney and heart. Oxidative stress has been termed as cause and effect in heavy metal-induced kidney toxicity. In treatment strategy, different chelating agent, vitamins and minerals are included, though chelating agents has been showed different fatal drawbacks. Interestingly, plants and plants derived compounds had shown possible effectiveness against heavy metals induced kidney toxicity. This review will provide detail information on toxicodynamics of Pb, Cd, Hg and As, treatment strategy along with the possible beneficiary role of plant derived compound to protect kidney.
1. Introduction
Heavy metals are ubiquitous in various forms in the environment. Primarily, human beings and animals are intoxicated by means of consumption of contaminated foods, contaminated water, mining, battery recycling and plastics. Due to the major route of excretion from the body, kidneys are more vulnerable to heavy metal toxicity, mostly to Lead (Pb), Cadmium (Cd), Mercury (Hg), Arsenic (As) [[1], [2], [3], [4]]. Depending on the duration and dose size (low to high), numerous fatal effects have been reported in blood composition, lung, and brain [5]. In general, removal of the patient from the site is the provision to treat toxicity. Depending on dose size and severity of the condition, different antidotes and chelating agents are prescribed. But, these agents intervene with essential metal ion like Ca2+, Cu2+, Zn2+, and results in aberrant physiologic function [6]. In addition, unbroken heavy metals are redistributed by these agents to potentiate toxicity at intracellular sites of liver and kidney [7]. To overcome these drawbacks, different nutrients and vitamins are suggested [8], whereas vitamins (antioxidants) at a same time could serves as chelating agent and reactive oxygen/nitrogen species (ROS/RNS) scavenger. Within few decades, numerous studies have been conducted on plants and plants derived compound against heavy metals, particularly on the kidney and reported the probable beneficial effect in in vitro & in vivo studies. Therefore, our current review was aimed to discuss the toxicodynamics and treatment of Pb, Cd, Hg and As toxicity on kidney and possible beneficiary role of plants & plant derived isolated compounds.
2. Lead (Pb) and kidney
Lead (Pb) is a toxic xenobiotic which causes different critical health conditions at the fatal stage [9,10]. Though it is toxic, it has been found or incorporated in different products including paints, water tape, cosmetics, fuel etc. for its different properties like low melting point, resistance to corrosion [11,12]. As a result, gastrointestinal, hematological, reproductive, immunomodulogical disorder have been recorded [[13], [14], [15], [16]]. Kidney is one of targeted site of Pb-toxicity for being major route of excretion from body and facilitates kidney damage via oxidative stress and lipid peroxidation [[17], [18], [19]].
2.1. Pb and proteinuria
Aminoaciduria, glycosuria, and phosphaturia were reported in acute Pb toxicity as marker for proximal tubule dysfunction [20].
2.2. Toxicodynamics of renal toxicity by Pb
Lead (Pb) can readily be absorbed by intestine, lung, less commonly through the skin and almost 90% of it binds to erythrocyte proteins (albumin) [21]. Through endocytosis and/or Erythrophagocytosis [22], it locates into different tissues and organs including liver, kidney where it exhibits oxidative damage on cells and tissue, and cellular organelles (e.g. by uncoupling the respiratory chain in mitochondria) [23]. Different hypothesis has been made to describe toxicodynamics of kidney toxicity by Pb. Hypothetically Pb2+ competes with Ca2+ and dysregulate the calcium homeostasis. In result, Ca2+ release from mitochondria is stimulated; initiate the opening of the mitochondrial transitional pore; in turn total mitochondrial damages, reactive species generation and oxidative stress including altered lipid metabolism [24,25]. Among the cells, proximal tubules are more susceptible to Pb-induced cellular damage followed by apoptosis [26]. Studies on primary cultures of rat proximal tubular (rPT) cell suggested that Pb2+ elevates cytosolic, mitochondrial calcium concentration, [Ca2+] and depletes endoplasmic reticulum’s (ER) [Ca2+] via acting on inositol 1,4,5-trisphosphate receptor (IP3Rs) [26]. Interestingly, study on HEK293 cells also ensured the involvement of Ca2+, where Canonical transient receptor potential TRPC1 actively participates in the cytotoxicity and entry of Pb2+ [27]. Besides, it also displaces essential metal ion like Zn2+ and Ca2+ in proteins and inhibits Cys2His2 zinc finger transcription factors [21].
From the different point of view, Pb also hinders the integrity of cell–cell junctions (tight junction) and modify cellular structure [28]. Altered polarity and vectorial transport of epithelial cells could also be a possible consequence of atypical cell–cell junction structure following reduced renal proximal tubule lumen and microvillae loss (Fig. 1).
Fig. 1.
Toxicodynamic of Pb–induced kidney toxicity.
2.3. Treatment strategy against Pb
The first step of treatment strategy is the removal of the patient from the site of exposure. If the patient is adult and blood Pb-level is >70 mg/dL, then chelation therapy with dimercaprol or succimer, meso-2,3-dimercaptosuccinic acid (DMSA) and CaNa2EDTA could be considered. Conversely, if Pb-level ranges in between 45 and 69 mg/dL for children, then oral chelation therapy with DMSA might be suggested [36]. But the repetitive dose of CaNa2EDTA could cause kidney damage particularly renal tubules, along with depleted essential metal ion like Zn, Cu, Ca concentration [6,37]. Profound role of vitamins and nutrients against Pb burden in the experimental animal were also documented. Antioxidants and nutrients such as vitamin C, E, B6, β carotene, Zinc, and Selenium are believed to be effective to reduce the lipid peroxidation (Vit C, E, and Selenium), oxidative stress with increase of endogenous antioxidant (Vit E, Vit B6, Zinc, and Selenium) and decrease the body Pb burden by increasing urinary Pb excretion (Vit C) [8]. Moreover, Zinc, Selenium, Iron and Calcium also reduce the Pb deposition by altering the either absorption or making chelates with Pb and improving the endogenous antioxidant defense system (e.g. SOD, GPx, GSH)[38].
Other than vitamins and minerals, researchers are also investigated the possible effectiveness of plants against Pb-induced kidney toxicity based on the ethnobotanical study. Different plants exhibited possible protective effect through reducing oxidative stress and protecting architecture of kidney reducing apoptosis. Collected evidences suggested that phenolic and flavonoid content, total antioxidant capacity (TAC), lignans, free radical scavenging activity of plants (Table 1), are believed to protect kidney against Pb by acting as monofunctional antioxidant mentioned by Antonio-García and Massó-Gonzalez, 2008 [39] or maintaining bifunctional role to upregulate the endogenous antioxidant protein via Keap1/Nrf2/ARE pathways and dealings with ROS [25,[30], [31], [32], [33],35].
Table 1.
Possible beneficial effect of plants against Pb-induced kidney toxicity.
| Plant Name | Effect on kidney by Pb | Outcome | References |
|---|---|---|---|
| Spirulina maxima | Decreases antioxidant activity and increases LPO | Attenuates about all alteration | [25] |
| Smilax glabra (SG) | Increases Pb concentration, increases LPO and decreases endogenous antioxidant activity, tubular dilatation, vacuolar, hemorrhage, cellular debris and glomerulus hyper cellularity | Attenuates about all alteration | [29] |
| Flaxseed oil | Increases NO, MDA both in serum and kidney tissue, increases ROS, increases uric acid, blood urea and creatinine, increases renal accumulation and decreases antioxidant activity, increases apoptosis in renal tubules along with tubular dilatation, vacuolated tubules, pockets of hemorrhages and shrunken glomeruli. | Attenuates about all alteration | [30] |
| Coriandrum sativum (coriander) | Increases LPO and decreases antioxidant activity, dilates tubule, vacuolated and swollen proximal convoluted tubules, Shrink glomeruli with widen urinary space and presence of inflammatory cells in intertubular spaces | Attenuates histopathological condition along with significant increase of antioxidant activity | [31] |
| Hesperetin (5,7,3’-trihydroxy-4-methoxyl flavanone) | Increases MDA, decreases antioxidant activity and kidney function | Normalizes all parameter | [32] |
| Salvia miltiorrhiza (SM) | Decreases antioxidant activity and kidney function, increases deposition in kidney and oxidative stress, histological changes in the cortex and remarkable swelling in the proximal tubular epithelial cells, diminishing glomeruli of the kidney along with renal apoptosis | Attenuates about to normal | [33] |
| Ginger (Zingiber officinale) | Decreases antioxidant level and activity, architecture changes including necrosis in tubular epithelial cell, in between glomeruli and intratubular region and inflammatory cell in intratubular region | Attenuates about to normal | [34] |
| Curcumin from Curcuma longa | Increases renal deposition and oxidative stress, decreases kidney function, glomeruli atrophy, large urinary space, necrotic, and vacuolated proximal tubules. blood congestion in the interstitial tissue and hypervascularization. Renal corpuscle with fragmented glomeruli and wide urinary space, necrotic and swollen proximal tubules, distal tubules with dilated lumen and damaged epithelium. |
Reduces Pb deposition as well as oxidative stress and protects architectural integrity | [35] |
3. Cadmium (Cd) and kidney
The Cd pollution was first recognized by its complication named “Itai-itai", following the second world war in Japan [40]. A few decades later, the experimental study revealed the harmful consequences of Cd depicting severe damages and histological changes in kidney along with renal dysfunction [41,42].
3.1. Cd and proteinuria
Proteinuria had been reported in workers belongs to Zn and Cd industry [43,44] and confirmed by different experimental study whereas Cd secretion was increased to 50-fold with proteinuria [45].
3.2. Toxicodynamic of renal toxicity by Cd
Following exposure, Cd is transported either from GI or lungs to blood plasma where it binds to albumin and a little of it secretes into the bile from liver. In addition to albumin, large amount of Cd make a complex with metallothionein (MT) that can easily be filtered by glomerulus, reabsorbed at proximal tubule and distal tubule by adsorptive endocytosis with the help of ZIP8 transporter situated on the apical surface of renal tubular cells [46,47]. Following entry into tubular cell, lysosome breaks the complex to free Cd; initiates the damage to kidneys through perturbing [Ca2+] homeostasis, electrochemical gradient [41], inducing oxidative stress, inflammatory cell infiltration and downregulating mitochondrial coenzymes Q (e.g. Q9 and Q10) [[48], [49], [50], [51], [52], [53],54]. Regarding Cd-induced programed cell death of kidney, free radicals and ER-stress attribute separately depending on the types of cell. For example, increasing intracellular Ca2+ overload and depolarizing the mitochondrial membrane potential ER stress activates caspases (9 and 3) to initiate apoptosis via ERK pathway, while excessive ROS activate glycogen synthase kinase (GSK-3β) to carry out either phagocytosis or apoptosis of mesangial cell [55]. Moreover, entrance of Cd into proximal tubules reduces cadherin-dependent (Ca2+ dependent) cell-cell junctions [56]. According to Prozialeck and Edwards, 2007, Cd also targets on cell-adhesion molecule possibly through regulating protein kinase C activation and MAPK signaling pathway prior to mitochondrial injury (Fig. 2) [56].
Fig. 2.
Toxicodynamic of Cd-induced kidney toxicity.
3.3. Treatment strategy against Cd
According to Agency for Toxic Substances and Disease Registry (ATSDR), for acute high dose toxicity of Cd, fluid replacement, mechanical ventilation, and oxygen supply are recommended [57]. Likewise to Pb toxicity for the chronic exposure, removal of the patient is the first step; proper clothing and eating habit are also considered. But no chelation therapy is recommended. Regarding long time chelation therapy, Nordberg, 1984 doubted about effectiveness [58].
Various studies on plants dictated the possible effective role against Cd-induced kidney toxicity (Table 2). This effectiveness might be due to the free radical quenching activity of phytoconsituents of plants, together with possible capabilities to reduce Cd absorption and accumulation, oxidative stress, and to increase the endogenous antioxidant activity, kidney function protecting nephron [[48], [49], [50], [51], [52], [53]].
Table 2.
Possible beneficial effect of plants against Cd-induced kidney toxicity.
| Plant Name | Effect of Cd on kidney | Outcome | References |
|---|---|---|---|
| Quercetin | Decreases enzymatic antioxidants activity and non-enzymatic status, Decreases kidney function, increases LPO, tubular necrosis, degeneration, dilation, desquamation, thickening of basement membrane and luminal cast formation |
Attenuates alteration considering all premises | [48] |
|
Lactobacillus plantarum CCFM8610 |
Perturbation of Ca, Fe, Mn, Zn concentration Increases MDA, decreases CAT, SOD, GPx acivity and GSH level. Cloudy swelling and necrosis of tubules and dilation of glomeruli. |
Normalizes the pathological condition through oral route rather IP administration | [49] |
| Flax seed oil (Linum usitatissimum) | Decreases antioxidant activity, increases NO level, MDA | Normalizes the pathological condition | [51] |
| Curcumin Curcuma longa L | No observation | Decreases hepatic LPO, increases CAT, GPX activity, GSH level | [52] |
| Resveratrol (3,5,4-trihydroxystilbene) | |||
| melatonin | |||
| Naringenin | Increases serum urea, uric acid, creatinine level, increases LPO, carbonyl compound, hydroperoxides, Decreases non-enzymatic and enzymatic antioxidant status. necrosis and cloudy swelling of tubules, inflammatory cell infiltration, tubular necrosis, desquamation and degeneration of glomeruli and renal tubules |
Normalizes the pathological condition | [53] |
4. Mercury (Hg) and kidney
Mercury is ubiquitous in nature and available in three forms: elemental mercury, organic mercury like methyl mercury and ethyl mercury, and finally inorganic mercury i.e. mercuric mercury. All form have notorious effects on organs including kidney [78] where males are more susceptible over female [59,60]. Human being can be exposed to Hg through many ways like contaminated food, battery industry or dental amalgam [61] and mercury-containing product handling including mining [78]. “Minamata disease” in Japan and disaster happened in Iraq from 1955 to 1972 pointed out the detrimental effect on human [62].
4.1. Hg and proteinuria
Proteinuria was also reported in Hg toxicity. An experimental study revealed female are more vulnerable to kidney toxicity exposed to Hg and effect on pars recta tubule cells are assumed to contribute in proteinuria [63]. Besides, damages of the proximal tubular cell and freely accessible through the glomerulus filter, sensitivity of lysosome could also attribute to proteinuria [64].
4.2. Toxicodynamics of renal toxicity by Hg
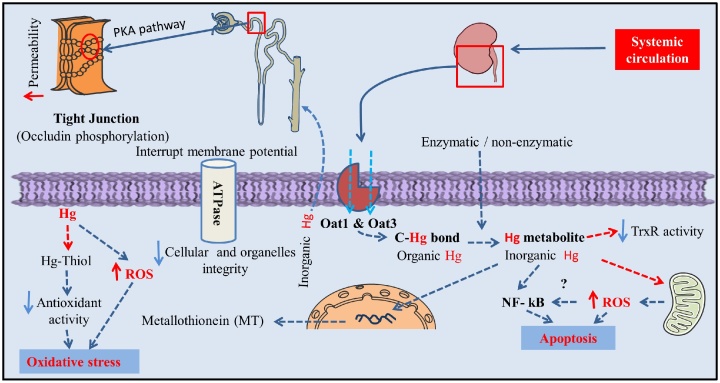
Long-term exposure to both organic and inorganic mercury is deleterious for health including kidney [65]. From systemic circulation, Hg is up taken by organic anion transport 1 (Oat1) and organic anion transport 3 (Oat3) into kidney specially into proximal tubules [60,66]. Here, the cleavage of carbon-mercury bond converts organic to inorganic mercury as metabolites either by the enzymatic or non-enzymatic process [67]. At the same time, Hg deposition is closely related to ROS generation, mRNA expression of MT, apoptosis and proximal tubule damage [[68], [69], [70]].
Organic anion transport 1 (Oat1) and organic anion transport 3 (Oat3) localize mainly in the lysosome of in the proximal tubule [66], uptake Hg into the kidney. As Hg2+ has a greater affinity to bind with thiol-containing enzymes [67], it inactivates the enzymes with thiol group through irreversible oxidation [71]; results in depletion of total thiol content and oxidative stress [72]. Inactivation of sulfhydryl protein (e.g. Na+/K+-ATPase) also affects the cellular integrity interrupting membrane potential and volume of cells as well as cellular organelles [71]. Therefore, its consequences were observed with free radical generation (myeloperoxidase activity and MDA), and protein urea (activities of N-acetyl-beta-D-glucosaminidase (NAG)) [59,68]. Absence of detoxifying protein [63] or reduced selenolthiol containing antioxidant activity [i.e thioredoxin reductase (TrxR)] [73] also facilitate the proximal tubules damage. Interesting to note that NF- kB also play a crucial role in apoptosis linked renal damage increasing sensitivity to apoptosis which have been found both in vitro and in vivo studies [74]. But no immunological defect or glomerular damage at low dose of inorganic Hg were evidenced in the early study of Langworth et al., 1992 [75].
Mercury also reduces the function of tight junction protein in kidney and perturbs cellular permeability. According to Kawedia et al., 2008, Hg decreases transepithelial electrical resistance (TER) and facilitates the phosphorylation of tight junction protein, occludin via a protein kinase A (PKA) dependent mechanism [76] (Fig. 3).
Fig. 3.
Toxicodynamic of Hg-induced kidney toxicity.
4.3. Treatment strategy against Hg
According to Agency for Toxic Substances and Disease Registry (ATSDR), removal of the patient from the site is essential as prehospital management. Sometime chelation therapy might be considered on the basis of dose [77]. Different chelating agent like DMSA, 2,3-dimercapto-1-propanesulfonic acid (DMPS), Dpenicillamine (DPCN), or dimercaprol (BAL) are recommended for acute inorganic Hg poisoning, whereas for children FDA approved DMSA. The physician also recommends Glutathione and N-acetylcysteine (NAC) but facilitates the deposition of Hg at brain and kidney [36].
For decades, numerous approaches have been taken to find out possible effectiveness of plants against Hg-induced kidney toxicity (Table 3). A number of plants/ plant derived compound demonstrated possible kidney protective effect by reducing Hg accumulation, free radicals, oxidative stress; by increasing total thiol content & endogenous antioxidants, due to in part of antioxidant activity of phytoconstituents.
Table 3.
Possible beneficial effect of plants against Hg-induced kidney toxicity.
| Plant Name | Effect of Hg on kidney | Outcome | References |
|---|---|---|---|
| Ginkgo biloba extract | Increases ROS generation, tissue collagen, Myeloperoxidase (MPO) activity, Malondialdehyde (MDA), decreases endogenous antioxidant activity | Restores all biochemical alteration by Hg intoxication | [59] |
| Tea Polyphenols and Schisandrin B | Increases activity of NAG, ALP. LDH, decreases activity of endogenous antioxidant, increases ROS generation, LPO, and apoptosis, histological alteration | Restores histological alteration, decreases oxidative stress, decreases activity of NAG, ALP. LDH. | [68] |
| Curcumin | Increases LPO, decreases CAT, SOD, GPx activity and glutathione level, histological alteration, mRNA expression of MT | Reverses the histological alteration, mRNA expression of MT, decreases oxidative stress | [69] |
| Vitamin E | Increases activity of ALP. LDH, increases LPO, decreases CAT, SOD, GPx activity and glutathione level, increases creatinine, BUN, increases Hg concentration, histological alteration | Restores histological alteration, decreases oxidative stress, decreases activity of ALP. LDH, decreases BUN, creatinine, decreases Hg concentration | [70] |
| Pomegranate seed oil | Increases MDA level, decreases total thiol content, increases blood urea and creatinine, protein conc. in urine, histological alteration | Decreases oxidative stress (MDA), decreases blood urea, creatinine and protein in urine, restores thiol content, presence of mild lesion in kidney | [79] |
| Tribulus terrestris fruit extract | Increases LPO, decreases CAT, SOD, GSH level. | Decreases LPO, increases CAT, SOD, GSH level. | [80] |
| Eruca sativa seed extract | Increases BUN and creatinine, decreases activity of CAT, SOD, GPx, GR, decreases SH group, increases thiobarbituric acid reactive substances, presences of focal necrosis, and congested glomeruli and stroma in kidney histology | Scavenges DPPH, hydrogen peroxide (H2O2), hydroxyl radical (.OH) and nitric oxide (NO), superoxide. | [81] |
| Arabic gum | Increases blood creatinine, blood urea nitrogen, thiobarbituric acid reactive substances, Reduces activity of glutathione peroxidase (GSH-Px) and catalase, increases NO level Glomerular and tubular necrosis, interstitial nephritis, and desquamation of the tubular epithelial cells in the renal cortex in kidney histology |
Normalizes the pathological conditions | [81] |
| Juglans sinensis Dode extract | Decreases GFR, increases plasma creatinine, increases LPO, decreases renal p-aminohippurate uptake | Normalizes the pathological conditions | [82] |
5. Arsenic (As) and kidney
Arsenic (As) is a naturally occurring toxic metalloid prevalent in the earth’s crust. Drinking water is the major source of naturally occurring inorganic As. Around the world, 200 million of people are intoxicated by drinking water [83]. Developing countries like Bangladesh are most vulnerable to As poisoning and 57 million of people are at risk. According to Peters et al., 2014, 72% of wells water does not comply with the limits of world health organization at an area named Araihazar in Bangladesh during 2000, and more than 50% of the 6–11 million tube-wells containing As concentrations above the WHO guideline value of 10 mL/L [85].
Arsenic is eliminated by urinary excretion; therefore it can accumulate in the kidneys. Chronic kidney disease (CKD) is closely associated with exposure to As, which can be characterized with decrease estimated glomerulus filtration rate (eGFR) and inflammation. Besides cardiovascular disease, respiratory illness & cancer in the urinary tract (e.g. Bladder and kidney), skin and lung have been mentioned in various studies [84,86,87].
5.1. As and proteinuria
Intoxication with As also causes proteinuria including beta(2)-microglobulin urea [88] and higher in MT-null mice than the wild-type which depicts As detoxification by MT [89].
5.2. Toxicodynamics of renal toxicity by As
A co-relation with As accumulation and depletion of endogenous antioxidant activity is established and it is believed to accelerate tissue damage introducing oxidative stress condition [[90], [91], [92], [93]]. Thereafter, the deposited inorganic As in kidney could be converted into methylated metabolite by the enzyme arsenic (+3 oxidation state) methyltransferase (As3mt), in consequence hydronephrosis of kidney [94].
Briefly, binding of trivalent As to thiol-containing antioxidant, leads to series of events: decrease antioxidant activity [95], changes fatty acid composition [96], renal DNA damage and cell death modulating [Ca2+] concentration [97] and downregulates Nef-2 expression [91]. Arsenic also interrupt the mitochondrial electron transport chain by inhibiting function or activity of Isocitrate dehydrogenase, α -Ketoglutarate dehydrogenase, Succinate dehydrogenase, NADH-dehydrogenase, Cytochrome c oxidase, increasing LPO and decreasing antioxidant activity in mitochondria [98].
Meanwhile, As decreases the reabsorption and increases kidney dysfunction decreasing BBM enzyme activity [99] and membrane-bound ATPase-like Na+/K + ATPase, Ca2+ATPase, Mg2+ ATPase [91]. Kidney dysfunction (i.e. increased estimate glomerulus filtration rate) is also closely associated with ROS-mediated inflammation, observed in vivo study [87]; possibly triggering TNF-α mediated apoptosis [100]. Successively, it also modulates CpG site at -168 bases upstream of transcription start site in the IL-8 promoter and its demethylation along with CBP/P300 recruitment; increased H3 histone acetylation leads to IL-8 mediated kidney pathology [101]. Apart from above, As also possess immunosuppressive effect, whereas it suppresses Th1 (IF-γ, IL-12), Th2 (IL-4, IL-10) and complementary protein C3a in the kidney; makes it susceptible to pathogenic attack [102]. Interestingly, acute As toxicity causes decrease of mitochondrial function and up-regulates the expression of multidrug resistance-associated protein 2 (MRP2) which helps to excrete into urine and generate tolerance of kidney against As [103]
The metabolomic study suggested that altered energy metabolism (e.g. glycolysis, Krebs cycle), amino acid metabolism, choline metabolism, methionine cycle (transmethylation), purine metabolism and degradation of membrane phospholipids contributes to cell apoptosis in As toxicity [104]. In this context, attribution of profound damages to brush border membrane (BBM), mitochondria of renal proximal tubule could be, due to, perturbed carbohydrate metabolism, decreased TCA cycle, gluconeogenesis and or HMP-shunt pathway alteration [105]. Moreover, As also regulates the expression of various phase I and phase II aryl hydrocarbon receptors (AhR)-regulated metabolizing enzymes [phase I enzymes {cytochrome P450 1A1 (CYP1A1),CYP1A2} and phase II enzymes {NAD(P)H: quinone oxidoreductase-1 (NQO1), glutathione-S-transferase A1 (GSTA1)}] [106] (Fig. 4).
Fig. 4.
Toxicodynamic of As-induced kidney toxicity.
5.3. Treatment strategy against Arsenic
Agency for Toxic Substances and Disease Registry (ATSDR) recommended aggressive intravenous fluid replacement therapy, gastric lavage, activated charcoal with a cathartic (e.g. sorbitol) for acute high dose As poisoning. Hemodialysis and chelation therapy with (2-3-dimercapto-1-propanesulfonate (DMPS), meso 2, 3-dimer-captosuccinic acid (DMSA) are also suggested. Nutrient and vitamins like Se, retinol could also be effective for promoting excretion of As from kidney [107].
List of plants and plant derived compounds which could be beneficial against As-mediated kidney toxicity is presented in Table 4. These plants and plant derived compounds exhibited their possible beneficial effects by increasing antioxidant activity, reducing oxidative stress, specially free radical quenching activity [[108], [109], [110]], increasing antioxidant enzyme activity regulating Nrf-2 [91] and chelating activity against As [111]. Moreover, few of them might be effective to protect kidney in different ways: decreasing As accumulation [112], protecting mitochondria [98] & altered carbohydrate metabolism [113], increasing enzyme activity of BBM & membrane protein decreasing free fatty acid [114], DNA damage [93,115] and NF-kB, TNF-mediated apoptosis [91,100].
Table 4.
Possible beneficial effect of plants against As-induced kidney toxicity.
| Plant / agent/extract name | Effect on kidney by arsenic |
Outcome | References |
|---|---|---|---|
| Silibinin | Increases apoptosis, NADPH oxidase, iNOS and NF-kB overexpression, decreases the activity of antioxidants and ATPase | Decreases caspase-3 mediated tubular cell apoptosis and decreases the NADPH oxidase, iNOS and NF-kB expression, upregulates the Nrf2 expression in the renal tissue, restores the activity of antioxidants and membrane bound ATPase. | [91] |
| Astaxanthin | Kidney dysfunction, arsenic deposition, decreases levels of Na+-K + ATPase and sulfydryl. Decreases endogenous antioxidant level, increases LPO, increases ROS generation, DNA damage, renal interstitial hemorrhage, focal necrosis, expansion and hyperemia in renal capsule, plasmolysis of epithelium, and cellular swelling of the renal tubular cell |
Normalizes the pathological conditions | [93] |
| Ascorbic acid and/or α-tocopherol | Increases LPO, decreases antioxidants and enzymes present in mitochondria. | Normalizes the pathological conditions | [98] |
| Ascorbic acid and/or α-tocopherol | Increases level of TNF- α, increases apoptosis | Decreases TNF- α level and apoptosis | [100] |
| Naringenin | Increases urea, creatinine in blood, deceases glutathione level, CAT, SOD, GST, GPx activity, dilation of tubules, necrosis and swelling of the epithelial cells with slight loss in brush border integrity | Increases antioxidant potential and radical quenching action, decreases oxidative stress and restores renal function | [108] |
| Quercetin and monoisoamyl 2, 3- dimercaptosuccinic acid | Increases TBARS, arsenic deposition and depletion of GPx, SOD, CAT activity | Increseases antioxidant and thiol content | [109] |
| Acetyl-L-Carnitine | Increases MDA, and decreases SH content, GST, SOD, CAT, tubulointerstitial inflammation and cytoplasmic vacuolization with necrosis of tubules |
Increases antioxidant activity | [110] |
| Selenium lentil diets | Increases arsenic concentration with lower urinary excretion. | Decreases arsenic conc., increases blood GSH level and urinary excretion | [111] |
| Resveratrol (pretreatment) | Increases oxidative stress, decreases endogenous antioxidant, thiol content, selenium content and increases kidney arsenic accumulation, BUN, creatinine, cortex edema, tubular cell swelling, interstitial edema, glomeruli dilation and hyperemia, pyknotic nuclei and severe necrosis, and denudation of the tubular cells | Normalizes the pathological conditions | [112] |
| Flaxseed oil | Perturbation of carbohydrate metabolism, decreases enzyme activity of BBM, kidney dysfunction, decreases activity of endogenous antioxidant with oxidative stress and histopatholical condition | Restores carbohydrate metabolism, activities of BBM, kidney function and antioxidant along with architecture | [113] |
| Biochanin A | Increases BUN, creatinine and urea, increases MDA, phospholipid, free fatty acid, decreases SOD, CAT, GSH level, degenerated renal tubules, elongation of urinary space and mild infiltration of inflammatory cells | Decreases BUN, creatinine and urea, decreases free fatty acid, increases antioxidant level, presence of mild degeneration of renal tubules. | [114] |
| Trichosanthes dioica root | Increases LPO, decreases GSH, and increases GSSG, decreases activity of GPx and GR, CAT, SOD and increases % of DNA fragmentation. | Normalizes the pathological conditions | [115] |
| Folate and/or Vitamin B12 | Increases urea and creatinine in blood, shrinkage of glomerular capsule, tubular lumen and tissue degeneration | Decreases urea and creatinine in blood, increases the integrity of cellular structure | [116] |
| Hesperidin and Lipoic Acid | Tubular epithelium vacuolation, Interstitial blood. |
Restores the histopathology about to normal | [117] |
6. Conclusion
This comprehensive review on heavy metals and kidney toxicity dictates that we can easily be exposed to heavy metals from surroundings until proper management has been implied throughout the world to reduce environmental pollution by heavy metals. Following toxicity, they exhibits their notorious effect especially on kidney by facilitating oxidative stress, competing with essential metals like Selenium (Se), Zn and Ca, mitochondrial dysfunction, Ca2+ and ROS-mediated apoptosis. In the kidney, Cell-cell junction serves as the permeability and absorption of epithelial cells. Alteration by heavy metal was also evidenced except arsenic (no available data), which results in proteinuria and kidney dysfunction. Due to the fatal side effect of chelation therapy, concurrent treatment of combined therapy is more preferred than monotherapy, even at lower doses. Besides, vitamins and minerals together with potent antioxidant derived from plants might be used to treat acute exposure after thorough investigation and clinical trial. To sum up, antioxidants from plants and different supplements might play a crucial role in settling new approach to treat heavy metal poisoning.
Conflict of interest
Authors have declared no conflict of interest.
Source of funding
This work was financially supported by Walailak University (WU59122), Thailand.
Acknowledgment
Thankful to Walailak University for financial assistance.
Contributor Information
Mohammad Nasiruddin Rana, Email: nasiriiuc09@yahoo.com.
Jitbanjong Tangpong, Email: rjitbanj@wu.ac.th.
Md. Masudur Rahman, Email: masud@pharm.iiuc.ac.bd.
References
- 1.WHO, WHO | Arsenic, WHO (2016). http://www.who.int/ipcs/assessment/public_health/arsenic/en/ (Accessed 11 April 2017).
- 2.United Nations Environment Programme., International Labour Organisation., World Health Organization., International Program on Chemical Safety., Inorganic lead., World Health Organization, 1995.
- 3.Programme international sur la sécurité des substances chimiques., Organisation mondiale de la santé . World Health Organization; Cadmium: 1992. Programme des Nations Unies pour l’environnement., Organisation internationale du travail. [Google Scholar]
- 4.Friberg L. World Health Organization; 1991. United Nations Environment Programme., International Labour Organisation., World Health Organization., International Program on Chemical Safety., Inorganic mercury. [Google Scholar]
- 5.Monnet-Tschudi F., Zurich M.-G., Boschat C., Corbaz A., Honegger P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev. Environ. Health. 2006;21:105–117. doi: 10.1515/reveh.2006.21.2.105. http://www.ncbi.nlm.nih.gov/pubmed/16898674 (Accessed 16 April 2017) [DOI] [PubMed] [Google Scholar]
- 6.Aposhian H.V., Maiorino R.M., Gonzalez-Ramirez D., Zuniga-Charles M., Xu Z., Hurlbut K.M., Junco-Munoz P., Dart R.C., Aposhian M.M. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology. 1995;97:23–38. doi: 10.1016/0300-483x(95)02965-b. http://www.ncbi.nlm.nih.gov/pubmed/7716789 (Accessed 3 April 2017) [DOI] [PubMed] [Google Scholar]
- 7.Flora S.J.S., Pachauri V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health. 2010;7:2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu P.-C., Guo Y.L. Antioxidant nutrients and lead toxicity. Toxicology. 2002;180:33–44. doi: 10.1016/s0300-483x(02)00380-3. http://www.ncbi.nlm.nih.gov/pubmed/12324198 (Accessed 3 April 2017) [DOI] [PubMed] [Google Scholar]
- 9.Annabi Berrahal A., Nehdi A., Hajjaji N., Gharbi N., El-Fazâa S. Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. C. R. Biol. 2007;330:581–588. doi: 10.1016/j.crvi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Reglero M.M., Taggart M.A., Monsalve-González L., Mateo R. Heavy metal exposure in large game from a lead mining area: effects on oxidative stress and fatty acid composition in liver. Environ. Pollut. 2009;157:1388–1395. doi: 10.1016/j.envpol.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Soliman M.M., Baiomy A.A., Yassin M.H. Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in Wistar rats. Biol. Trace Elem. Res. 2015;167:91–102. doi: 10.1007/s12011-015-0280-0. [DOI] [PubMed] [Google Scholar]
- 12.Shotyk W., Le Roux G. Biogeochemistry and cycling of lead. Met. Ions Biol. Syst. 2005;43:239–275. doi: 10.1201/9780824751999.ch10. http://www.ncbi.nlm.nih.gov/pubmed/16370121 (Accessed 25 April 2017) [DOI] [PubMed] [Google Scholar]
- 13.Ademuyiwa O., Ugbaja R.N., Rotimi S.O., Abam E., Okediran B.S., Dosumu O.A., Onunkwor B.O. Erythrocyte acetylcholinesterase activity as a surrogate indicator of lead-induced neurotoxicity in occupational lead exposure in Abeokuta, Nigeria. Environ. Toxicol. Pharmacol. 2007;24:183–188. doi: 10.1016/j.etap.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Vega-Dienstmaier J.M., Salinas-Piélago J.E., del M., Gutiérrez-Campos R., Mandamiento-Ayquipa R.D., del M., Yara-Hokama C., Ponce-Canchihuamán J., Castro-Morales J. Lead levels and cognitive abilities in Peruvian children. Rev. Bras. Psiquiatr. 2006;28:33–39. doi: 10.1590/s1516-44462006000100008. [DOI] [PubMed] [Google Scholar]
- 15.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. 2006;11:114–127. http://www.ncbi.nlm.nih.gov/pubmed/16813461 (Accessed 3 April 2017) [PubMed] [Google Scholar]
- 16.Rana M.N., Tangpong J. In vitro free radical scavenging and anti-genotoxic activities of Thunbergia laurifolia aqueous leaf extract. J. Heal. Res. 2017;31:127–133. [Google Scholar]
- 17.Garçon G., Leleu B., Marez T., Zerimech F., Haguenoer J.-M., Furon D., Shirali P. Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: usefulness of alpha-glutathione S-transferase. Sci. Total Environ. 2007;377:165–172. doi: 10.1016/j.scitotenv.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.El-Nekeety A.A., El-Kady A.A., Soliman M.S., Hassan N.S., Abdel-Wahhab M.A. Protective effect of Aquilegia vulgaris (L.) Against lead acetate-induced oxidative stress in rats. Food Chem. Toxicol. 2009;47:2209–2215. doi: 10.1016/j.fct.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Farrag A.-R.H., Mahdy K.A., Abdel Rahman G.H., Osfor M.M. Protective effect of Nigella sativa seeds against lead-induced hepatorenal damage in male rats. Pak. J. Biol. Sci. 2007;10:2809–2816. doi: 10.3923/pjbs.2007.2809.2816. http://www.ncbi.nlm.nih.gov/pubmed/19090181 (Accessed 3 April 2017) [DOI] [PubMed] [Google Scholar]
- 20.Goyer R.A. Lead toxicity: a problem in environmental pathology. Am. J. Pathol. 1971;64:167–182. http://www.ncbi.nlm.nih.gov/pubmed/4326632 (Accessed 3 April 2017) [PMC free article] [PubMed] [Google Scholar]
- 21.Gonick H.C. Lead-binding proteins: a review. J. Toxicol. 2011;2011:686050. doi: 10.1155/2011/686050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon S.-Y., Bae O.-N., Noh J.-Y., Kim K., Kang S., Shin Y.-J., Lim K.-M., Chung J.-H. Erythrophagocytosis of lead-exposed rrythrocytes by renal tubular cells: possible role in lead-induced nephrotoxicity. Environ. Heal. Perspect. 2015;123:120–127. doi: 10.1289/ehp.1408094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes J.L., Molina-Jijón E., Rodríguez-Muñoz R., Bautista-García P., Debray-García Y., Namorado M.D.C. Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed Res. Int. 2013;2013:730789. doi: 10.1155/2013/730789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahamed M., Siddiqui M.K.J. Environmental lead toxicity and nutritional factors. Clin. Nutr. 2007;26:400–408. doi: 10.1016/j.clnu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Ponce-Canchihuamán J.C., Pérez-Méndez O., Hernández-Muñoz R., Torres-Durán P.V., Juárez-Oropeza M.A. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis. 2010;9(35) doi: 10.1186/1476-511X-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Wang Z.-K., Jiao P., Zhou X.-P., Yang D.-B., Wang Z.-Y., Wang L. Redistribution of subcellular calcium and its effect on apoptosis in primary cultures of rat proximal tubular cells exposed to lead. Toxicology. 2015;333:137–146. doi: 10.1016/j.tox.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H., Li W., Xue Y., Zou F. TRPC1 is involved in Ca2+ influx and cytotoxicity following Pb2+ exposure in human embryonic kidney cells. Toxicol. Lett. 2014;229:52–58. doi: 10.1016/j.toxlet.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Moreno L.G., Quintanar-Escorza M.A., González S., Mondragón R., Cerbón-Solorzáno J., Valdés J., Calderón-Salinas J.V. Effects of lead intoxication on intercellular junctions and biochemical alterations of the renal proximal tubule cells. Toxicol. Vitr. 2009;23:1298–1304. doi: 10.1016/j.tiv.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Xia D., Yu X., Liao S., Shao Q., Mou H., Ma W. Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J. Ethnopharmacol. 2010;130:414–420. doi: 10.1016/j.jep.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Abdel Moneim A.E., Dkhil M.A., Al-Quraishy S. The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J. Hazard. Mater. 2011;194:250–255. doi: 10.1016/j.jhazmat.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 31.L. Kansal, L. Kansal, V. Sharma, A. Sharma, S. Lodi, S.H. Sharma, PROTECTIVE ROLE OF CORIANDRUM SATIVUM (CORIANDER) EXTRACTS AGA INST LEAD NITRATE INDUCED OXIDATIVE STRESS AND TISSUE DAMAGE IN THE LIVER AND KIDNEY IN MALE MICE. a, (n.d.) http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.401.8966 (Accessed 3 April 2017).
- 32.Yang Z., Liu Z., Wang J., Zhu H. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J. Pharmacol. 2013;45:395. doi: 10.4103/0253-7613.115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L., Zhang Y., Ma J., Dong W., Song Q., Zhang J., Chu L. Salvia miltiorrhiza injection ameliorates renal damage induced by lead exposure in mice. Sci. World J. 2014;2014:1–9. doi: 10.1155/2014/572697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy Y.A., Chalamaiah M., Ramesh B., Balaji G., Indira P. Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J. Food Sci. Technol. 2014;51:908–914. doi: 10.1007/s13197-011-0568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdel-Moneim A.M., El-Toweissy M.Y., Ali A.M., Awad Allah A.A.M., Darwish H.S., Sadek I.A. Curcumin ameliorates lead (Pb2++)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol. Trace Elem. Res. 2015;168:206–220. doi: 10.1007/s12011-015-0360-1. [DOI] [PubMed] [Google Scholar]
- 36.Sharma B., Singh S., Siddiqi N.J. Biomedical implications of heavy metals induced imbalances in redox systems. Biomed. Res. Int. 2014;2014:640754. doi: 10.1155/2014/640754. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Porru S., Alessio L. The use of chelating agents in occupational lead poisoning. Occup. Med. (Lond.) 1996;46:41–48. doi: 10.1093/occmed/46.1.41. http://www.ncbi.nlm.nih.gov/pubmed/8672793 (Accessed 3 April 2017) [DOI] [PubMed] [Google Scholar]
- 38.Ahamed M., Siddiqui M.K.J. Low level lead exposure and oxidative stress: current opinions. Clin. Chim. Acta. 2007;383:57–64. doi: 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Antonio-García M.T., Massó-Gonzalez E.L. Toxic effects of perinatal lead exposure on the brain of rats: involvement of oxidative stress and the beneficial role of antioxidants. Food Chem. Toxicol. 2008;46:2089–2095. doi: 10.1016/j.fct.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 40.Programme international sur la sécurité des substances chimiques., Organisation mondiale de la santé., Programme des Nations Unies pour l’environnement., Organisation internationale du travail., Cadmium., World Health Organization, 1992. http://www.inchem.org/documents/ehc/ehc/ehc134.htm (Accessed 3 April 2017).
- 41.Nordberg G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009;238:192–200. doi: 10.1016/j.taap.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Prodan L. Cadmium poisoning: II. Experimental cadmium poisoning. J. Ind. Hyg. 1932;14:174–196. https://www.cabdirect.org/cabdirect/abstract/19322701232 (Accessed 3 April 2017) [Google Scholar]
- 43.Lauwerys R.R., Buchet J.P., Roels H.A., Brouwers J., Stanescu D. Epidemiological survey of workers exposed to cadmium. Arch. Environ. Health. 1974;28:145–148. doi: 10.1080/00039896.1974.10666455. http://www.ncbi.nlm.nih.gov/pubmed/4810886 (Accessed 5 April 2017) [DOI] [PubMed] [Google Scholar]
- 44.Bonnell J.A. Emphysema and proteinuria in men casting copper-cadmium alloys. Br. J. Ind. Med. 1955;12:181–195. doi: 10.1136/oem.12.3.181. http://www.ncbi.nlm.nih.gov/pubmed/13240021 (Accessed 5 April 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friberg L. Further investigations on chronic cadmium poisoning; A study on rabbits with radioactive cadmium. AMA Arch. Ind. Hyg. Occup. Med. 1952;5:30–36. http://www.ncbi.nlm.nih.gov/pubmed/14884779 (Accessed 5 April 2017) [PubMed] [Google Scholar]
- 46.Wang B., Schneider S.N., Dragin N., Girijashanker K., Dalton T.P., He L., Miller M.L., Stringer K.F., Soleimani M., Richardson D.D., Nebert D.W. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. AJP Cell Physiol. 2006;292:C1523–C1535. doi: 10.1152/ajpcell.00409.2006. [DOI] [PubMed] [Google Scholar]
- 47.Barbier O., Jacquillet G., Tauc M., Poujeol P., Cougnon M. Acute study of interaction among cadmium, calcium, and zinc transport along the rat nephron in vivo. AJP Ren. Physiol. 2004;287:F1067–F1075. doi: 10.1152/ajprenal.00120.2004. [DOI] [PubMed] [Google Scholar]
- 48.Renugadevi J., Milton Prabu S. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp. Toxicol. Pathol. 2010;62:471–481. doi: 10.1016/j.etp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Zhai Q., Wang G., Zhao J., Liu X., Narbad A., Chen Y.Q., Zhang H., Tian F., Chen W. Protective effects of lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl. Environ. Microbiol. 2014;80:4063–4071. doi: 10.1128/AEM.00762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amamou F., Nemmiche S., kaouthar Meziane R., Didi A., Yazit S.M., Chabane-Sari D. Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem. Toxicol. 2015;78:177–184. doi: 10.1016/j.fct.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Karaca S., Eraslan G. The effects of flaxseed oil on cadmium-induced oxidative stress in rats. Biol. Trace Elem. Res. 2013;155:423–430. doi: 10.1007/s12011-013-9804-7. [DOI] [PubMed] [Google Scholar]
- 52.Eybl V., Kotyzova D., Koutensky J. Comparative study of natural antioxidants – curcumin, resveratrol and melatonin – in cadmium-induced oxidative damage in mice. Toxicology. 2006;225:150–156. doi: 10.1016/j.tox.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Renugadevi J., Prabu S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256:128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Dua T.K., Dewanjee S., Khanra R., Bhattacharya N., Bhaskar B., Zia-Ul-Haq M., De Feo V. The effects of two common edible herbs, Ipomoea aquatica and Enhydra fluctuans, on cadmium-induced pathophysiology: a focus on oxidative defence and anti-apoptotic mechanism. J. Transl. Med. 2015;13:245. doi: 10.1186/s12967-015-0598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L.-Y., Wu K.-H., Chiu W.-T., Wang S.-H., Shih C.-M. The cadmium-induced death of mesangial cells results in nephrotoxicity. Autophagy. 2009;5:571–572. doi: 10.4161/auto.5.4.8311. http://www.ncbi.nlm.nih.gov/pubmed/19333000 (Accessed 5 April 2017) [DOI] [PubMed] [Google Scholar]
- 56.Prozialeck W.C., Edwards J.R. Cell adhesion molecules in chemically-induced renal injury. Pharmacol. Ther. 2007;114:74–93. doi: 10.1016/j.pharmthera.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agency for Toxic Substances and Disease Registry (ATSDR), Cadmium (Cd) Toxicity: How Should Patients Exposed to Cadmium Be Treated and Managed? | ATSDR - Environmental Medicine & Environmental Health Education - CSEM, (n.d.). https://www.atsdr.cdc.gov/csem/csem.asp?csem=6&po=16 (accessed April 5, 2017).
- 58.Nordberg G.F. Chelating agents and cadmium toxicity: problems and prospects. Environ. Health Perspect. 1984;54:213–218. doi: 10.1289/ehp.8454213. http://www.ncbi.nlm.nih.gov/pubmed/6428872 (Accessed 5 April 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Şener G., Sehirli Ö., Tozan A., Velioğlu-Övunç A., Gedik N., Omurtag G.Z. Ginkgo biloba extract protects against mercury(II)-induced oxidative tissue damage in rats. Food Chem. Toxicol. 2007;45:543–550. doi: 10.1016/j.fct.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Hazelhoff M.H., Bulacio R.P., Torres A.M. Gender related differences in kidney injury induced by mercury. Int. J. Mol. Sci. 2012;13:10523–10536. doi: 10.3390/ijms130810523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barregard L., Fabricius-Lagging E., Lundh T., Mölne J., Wallin M., Olausson M., Modigh C., Sallsten G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: impact of different exposure sources. Environ. Res. 2010;110:47–54. doi: 10.1016/j.envres.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Rafati-Rahimzadeh M., Rafati-Rahimzadeh M., Kazemi S., Moghadamnia A. Current approaches of the management of mercury poisoning: need of the hour. DARU J. Pharm. Sci. 2014;22:46. doi: 10.1186/2008-2231-22-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fowler B.A. The morphologic effects of dieldrin and methyl mercuric chloride on pars recta segments of rat kidney proximal tubules. Am. J. Pathol. 1972;69:163–178. http://www.ncbi.nlm.nih.gov/pubmed/5080703 (Accessed 5 April 2017) [PMC free article] [PubMed] [Google Scholar]
- 64.Reyes J.L., Molina-Jijón E., Rodríguez-Muñoz R., Bautista-García P., Debray-García Y., Namorado M.D.C. Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed. Res. Int. 2013;2013:730789. doi: 10.1155/2013/730789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka-Kagawa T., Suzuki M., Naganuma A., Yamanaka N., Imura N. Strain difference in sensitivity of mice to renal toxicity of inorganic mercury. J. Pharmacol. Exp. Ther. 1998;285:335–341. http://www.ncbi.nlm.nih.gov/pubmed/9536029 (Accessed 5 April 2017) [PubMed] [Google Scholar]
- 66.Hultman P., Eneström S. Localization of mercury in the kidney during experimental acute tubular necrosis studied by the cytochemical Silver Amplification method. Br. J. Exp. Pathol. 1986;67:493–503. http://www.ncbi.nlm.nih.gov/pubmed/3741775 (Accessed 5 April 2017) [PMC free article] [PubMed] [Google Scholar]
- 67.Zalups R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000;52:113–143. http://www.ncbi.nlm.nih.gov/pubmed/10699157 (Accessed 5 April 2017) [PubMed] [Google Scholar]
- 68.Liu W., Xu Z., Yang H., Deng Y., Xu B., Wei Y. The protective effects of tea polyphenols and schisandrin B on nephrotoxicity of mercury. Biol. Trace Elem. Res. 2011;143:1651–1665. doi: 10.1007/s12011-011-8996-y. [DOI] [PubMed] [Google Scholar]
- 69.Agarwal R., Goel S.K., Behari J.R. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J. Appl. Toxicol. 2010;30 doi: 10.1002/jat.1517. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal R., Goel S.K., Chandra R., Behari J.R. Role of vitamin E in preventing acute mercury toxicity in rat. Environ. Toxicol. Pharmacol. 2010;29:70–78. doi: 10.1016/j.etap.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Kade I.J., Joseph I. Mercury toxicity on sodium pump and organoseleniums intervention: a paradox. J. Biomed. Biotechnol. 2012;2012:1–12. doi: 10.1155/2012/924549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boroushaki M.T., Mollazadeh H., Rajabian A., Dolati K., Hoseini A., Paseban M., Farzadnia M. Protective effect of pomegranate seed oil against mercuric chloride-induced nephrotoxicity in rat. Ren. Fail. 2014;36:1581–1586. doi: 10.3109/0886022X.2014.949770. [DOI] [PubMed] [Google Scholar]
- 73.Branco V., Ramos P., Canário J., Lu J., Holmgren A., Carvalho C., Holmgren A., Carvalho C. Biomarkers of adverse response to mercury: histopathology versus thioredoxin reductase activity. J. Biomed. Biotechnol. 2012;2012:1–9. doi: 10.1155/2012/359879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dieguez-Acuna F.J., Polk W.W., Ellis M.E., Simmonds P.L., Kushleika J.V., Woods J.S. Nuclear factor B activity determines the sensitivity of kidney epithelial cells to apoptosis: implications for mercury-induced renal failure. Toxicol. Sci. 2004;82:114–123. doi: 10.1093/toxsci/kfh236. [DOI] [PubMed] [Google Scholar]
- 75.Langworth S., Elinder C.G., Sundquist K.G., Vesterberg O. Renal and immunological effects of occupational exposure to inorganic mercury. Br. J. Ind. Med. 1992;49:394–401. doi: 10.1136/oem.49.6.394. http://www.ncbi.nlm.nih.gov/pubmed/1606025 (Accessed 5 April 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawedia J.D., Jiang M., Kulkarni A., Waechter H.E., Matlin K.S., Pauletti G.M., Menon A.G. The protein kinase A pathway contributes to Hg2++-induced alterations in phosphorylation and subcellular distribution of occludin associated with increased tight junction permeability of salivary epithelial cell monolayers. J. Pharmacol. Exp. Ther. 2008;326:829–837. doi: 10.1124/jpet.107.135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agency for Toxic Substances and Disease Registry (ATSDR), ATSDR - Medical Management Guidelines (MMGs): Mercury, (n.d.). https://www.atsdr.cdc.gov/MMG/MMG.asp?id=106&tid=24 (Accessed 5 April 2017).
- 78.Agency for Toxic Substances and Disease Registry (ATSDR), ATSDR - Public Health Statement: Mercury, (n.d.). https://www.atsdr.cdc.gov/phs/phs.asp?id=112&tid=24 (Accessed 5 April 2017).
- 79.Boroushaki M.T., Hosseini A., Dolati K., Mollazadeh H., Rajabian A. Protective effect of pomegranate seed oil against mercuric chloride-induced hepatotoxicity in rat. Acta Pol. Pharm. - Drug Res. 2016;73:991–997. [PubMed] [Google Scholar]
- 80.Kavitha A.V., Jagadeesan G. Role of Tribulus terrestris (Linn.) (Zygophyllacea) against mercuric chloride induced nephrotoxicity in mice, Mus Musculus (Linn.) J. Environ. Biol. 2006;27:397–400. http://www.ncbi.nlm.nih.gov/pubmed/17436531 (Accessed 5 April 2017) [PubMed] [Google Scholar]
- 81.Sarwar Alam M., Kaur G., Jabbar Z., Javed K., Athar M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem. Toxicol. 2007;45:910–920. doi: 10.1016/j.fct.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 82.Ahn C.B., Song C.H., Kim W.H., Kim Y.K. Effects of Juglans sinensis Dode extract and antioxidant on mercury chloride-induced acute renal failure in rabbits. J. Ethnopharmacol. 2002;82:45–49. doi: 10.1016/s0378-8741(02)00124-1. [DOI] [PubMed] [Google Scholar]
- 83.World Health Organization, Guidelines for Drinking-water Quality THIRD EDITION INCORPORATING THE FIRST AND SECOND ADDENDA Volume 1 Recommendations | Karla Paola Acosta Herrera - Academia.edu, (n.d.). http://www.academia.edu/6051932/Guidelines_for_Drinking-water_Quality_THIRD_EDITION_INCORPORATING_THE_FIRST_AND_SECOND_ADDENDA_Volume_1_Recommendations (Accessed 5 April 2017).
- 84.Peters B.A., Hall M.N., Liu X., Neugut Y.D., Pilsner J.R., Levy D., Ilievski V., Slavkovich V., Islam T., Factor-Litvak P., Graziano J.H., Gamble M.V. Creatinine, arsenic metabolism, and renal function in an arsenic-exposed population in Bangladesh. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hawkesworth S., Wagatsuma Y., Kippler M., Fulford A.J., Arifeen S.E., Persson L.-A., Moore S.E., Vahter M. Early exposure to toxic metals has a limited effect on blood pressure or kidney function in later childhood, rural Bangladesh. Int. J. Epidemiol. 2013;42:176–185. doi: 10.1093/ije/dys215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saint-Jacques N., Parker L., Brown P., Dummer T.J., Williamson G., Rennie D., Moher D., Becker B., Sipe T., Thacker S., Lotan Y., Fletcher T., Guallar E., Alberg A., Chakraborti D. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ. Heal. 2014;13:44. doi: 10.1186/1476-069X-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peters B.A., Liu X., Hall M.N., Ilievski V., Slavkovich V., Siddique A.B., Alam S., Islam T., Graziano J.H., Gamble M.V. Arsenic exposure, inflammation, and renal function in Bangladeshi adults: effect modification by plasma glutathione redox potential. Free Radic. Biol. Med. 2015;85:174–182. doi: 10.1016/j.freeradbiomed.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robles-Osorio M.L., Pérez-Maldonado I.N., Martín D., Campo D., Montero-Perea D., Avilés-Romo I., Sabath-Silva E., Sabath E. Urinary arsenic levels and risk of renal injury in a cross-sectional study in open population. Rev. Investig. Clín. 2012;64:609–614. http://www.medigraphic.com/pdfs/revinvcli/nn-2012/nn126IIe.pdf (Accessed 6 April 2017) [PubMed] [Google Scholar]
- 89.Liu J., Liu Y., Habeebu S.M., Waalkes M.P., Klaassen C.D. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology. 2000;147:157–166. doi: 10.1016/s0300-483x(00)00194-3. [DOI] [PubMed] [Google Scholar]
- 90.Kim J.-H., Kang J.-C. The arsenic accumulation and its effect on oxidative stress responses in juvenile rockfish, Sebastes schlegelii, exposed to waterborne arsenic (As3++) Environ. Toxicol. Pharmacol. 2015;39:668–676. doi: 10.1016/j.etap.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Prabu S.M., Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol. Biol. Rep. 2012;39:11201–11216. doi: 10.1007/s11033-012-2029-6. [DOI] [PubMed] [Google Scholar]
- 92.Sasaki A., Oshima Y., Fujimura A. An approach to elucidate potential mechanism of renal toxicity of arsenic trioxide. Exp. Hematol. 2007;35:252–262. doi: 10.1016/j.exphem.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Wang X., Zhao H., Shao Y., Wang P., Wei Y., Zhang W., Jiang J., Chen Y., Zhang Z. Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats. Nutr. Res. Pract. 2014;8:46–53. doi: 10.4162/nrp.2014.8.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yokohira M., Arnold L.L., Pennington K.L., Suzuki S., Kakiuchi-Kiyota S., Herbin-Davis K., Thomas D.J., Cohen S.M. Effect of sodium arsenite dose administered in the drinking Water on the urinary bladder epithelium of female arsenic (+3 oxidation State) methyltransferase knockout mice. Toxicol. Sci. 2011;121:257–266. doi: 10.1093/toxsci/kfr051. [DOI] [PubMed] [Google Scholar]
- 95.Kotyzová D., Bludovská M., Eybl V. Differential influences of various arsenic compounds on antioxidant defense system in liver and kidney of rats. Environ. Toxicol. Pharmacol. 2013;36:1015–1021. doi: 10.1016/j.etap.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Kharroubi W., Dhibi M., Mekni M., Haouas Z., Chreif I., Neffati F., Hammami M., Sakly R. Sodium arsenate induce changes in fatty acids profiles and oxidative damage in kidney of rats. Environ. Sci. Pollut. Res. 2014;21:12040–12049. doi: 10.1007/s11356-014-3142-y. [DOI] [PubMed] [Google Scholar]
- 97.Florea A.-M., Splettstoesser F., Büsselberg D. Arsenic trioxide (As2O3) induced calcium signals and cytotoxicity in two human cell lines: SY-5Y neuroblastoma and 293 embryonic kidney (HEK) Toxicol. Appl. Pharmacol. 2007;220:292–301. doi: 10.1016/j.taap.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 98.Ramanathan K., Shila S., Kumaran S., Panneerselvam C. Ascorbic acid and alpha-tocopherol as potent modulators on arsenic induced toxicity in mitochondria. J. Nutr. Biochem. 2003;14:416–420. doi: 10.1016/s0955-2863(03)00076-7. http://www.ncbi.nlm.nih.gov/pubmed/12915223 (Accessed 6 April 2017) [DOI] [PubMed] [Google Scholar]
- 99.Rizwan S., Naqshbandi A., Farooqui Z., Khan A.A., Khan F. Protective effect of dietary flaxseed oil on arsenic-induced nephrotoxicity and oxidative damage in rat kidney. Food Chem. Toxicol. 2014;68:99–107. doi: 10.1016/j.fct.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 100.Ramanathan K., Anusuyadevi M., Shila S., Panneerselvam C. Ascorbic acid and α-tocopherol as potent modulators of apoptosis on arsenic induced toxicity in rats. Toxicol. Lett. 2005;156:297–306. doi: 10.1016/j.toxlet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Singh R.D., Tiwari R., Khan H., Kumar A., Srivastava V. Arsenic exposure causes epigenetic dysregulation of IL-8 expression leading to proneoplastic changes in kidney cells. Toxicol. Lett. 2015;237:1–10. doi: 10.1016/j.toxlet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 102.Banerjee S., Mitra T., Purohit G.K., Mohanty S., Mohanty B.P. Immunomodulatory effect of arsenic on cytokine and HSP gene expression in Labeo rohita fingerlings. Fish Shellfish Immunol. 2015;44:43–49. doi: 10.1016/j.fsi.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 103.Miller D.S., Shaw J.R., Stanton C.R., Barnaby R., Karlson K.H., Hamilton J.W., Stanton B.A. MRP2 and acquired tolerance to inorganic arsenic in the kidney of killifish (Fundulus heteroclitus) Toxicol. Sci. 2007;97:103–110. doi: 10.1093/toxsci/kfm030. [DOI] [PubMed] [Google Scholar]
- 104.García-Sevillano M.A., Contreras-Acuña M., García-Barrera T., Navarro F., Gómez-Ariza J.L. Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry. Anal. Bioanal. Chem. 2014;406:1455–1469. doi: 10.1007/s00216-013-7564-z. [DOI] [PubMed] [Google Scholar]
- 105.Shahid F., Rizwan S., Khan M.W., Khan S.A., Naqshbandi A., Yusufi A.N.K. Studies on the effect of sodium arsenate on the enzymes of carbohydrate metabolism, brush border membrane, and oxidative stress in the rat kidney. Environ. Toxicol. Pharmacol. 2014;37:592–599. doi: 10.1016/j.etap.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 106.Anwar-Mohamed A., Abdelhamid G., Amara I.E.A., El-Kadi A.O.S. Differential modulation of aryl hydrocarbon receptor regulated enzymes by arsenite in the kidney, lung, and heart of C57BL/6 mice. Arch. Toxicol. 2012;86:897–910. doi: 10.1007/s00204-012-0855-x. [DOI] [PubMed] [Google Scholar]
- 107.Agency for Toxic Substances and Disease Registry (ATSDR), Arsenic Toxicity Case Study: How Should Patients Exposed to Arsenic Be Treated and Managed? | ATSDR - Environmental Medicine & Environmental Health Education - CSEM, (n.d.). https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=13 (Accessed 6 April 2017).
- 108.Mershiba S.D., Dassprakash M.V., Saraswathy S.D. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol. Biol. Rep. 2013;40:3681–3691. doi: 10.1007/s11033-012-2444-8. [DOI] [PubMed] [Google Scholar]
- 109.Mishra D., Flora S.J.S. Quercetin administration during chelation therapy protects arsenic-induced oxidative stress in mice. Biol. Trace Elem. Res. 2008;122:137–147. doi: 10.1007/s12011-007-8064-9. [DOI] [PubMed] [Google Scholar]
- 110.Sepand M.R., Razavi-Azarkhiavi K., Omidi A., Zirak M.R., Sabzevari S., Kazemi A.R., Sabzevari O. Effect of acetyl-l-carnitine on antioxidant status, lipid peroxidation, and oxidative damage of arsenic in rat. Biol. Trace Elem. Res. 2016;171:107–115. doi: 10.1007/s12011-015-0436-y. [DOI] [PubMed] [Google Scholar]
- 111.Sah S., Vandenberg A., Smits J. Treating chronic arsenic toxicity with high selenium lentil diets. Toxicol. Appl. Pharmacol. 2013;272:256–262. doi: 10.1016/j.taap.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W., Liu Y., Ge M., Jing J., Chen Y., Jiang H., Yu H., Li N., Zhang Z. Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats. Nutr. Res. Pract. 2014;8:220. doi: 10.4162/nrp.2014.8.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rizwan S., Naqshbandi A., Khan F. Dietary flaxseed oil supplementation mitigates the effect of lead on the enzymes of carbohydrate metabolism, brush border membrane, and oxidative stress in rat kidney tissues. Biol. Trace Elem. Res. 2013;153:279–290. doi: 10.1007/s12011-013-9669-9. [DOI] [PubMed] [Google Scholar]
- 114.Jalaludeen A.M., Lee W.Y., Kim J.H., Jeong H.Y., Ki K.S., Kwon E.G., Song H. Therapeutic efficacy of biochanin a against arsenic-induced renal and cardiac damage in rats. Environ. Toxicol. Pharmacol. 2015;39:1221–1231. doi: 10.1016/j.etap.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 115.Bhattacharya S., Haldar P.K. Ameliorative effect Trichosanthes dioica root against experimentally induced arsenic toxicity in male albino rats. Environ. Toxicol. Pharmacol. 2012;33:394–402. doi: 10.1016/j.etap.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 116.Acharyya N., Deb B., Chattopadhyay S., Maiti S. Arsenic-induced antioxidant depletion, oxidative DNA breakage, and tissue damages are prevented by the combined action of folate and vitamin B12. Biol. Trace Elem. Res. 2015;168:122–132. doi: 10.1007/s12011-015-0324-5. [DOI] [PubMed] [Google Scholar]
- 117.Pires das Neves R., Fernandes E., Soares E., Bastos M., Pereira M., Carvalho F., Carvalho M. Protective activity of hesperidin and lipoic acid against sodium arsenite acute toxicity in mice. Toxicol. Pathol. 2004;32:527–535. doi: 10.1080/01926230490502566. [DOI] [PubMed] [Google Scholar]