Abstract
Consumption of diets high in fat and/or fructose content promotes tissue inflammation, oxidative stress, and insulin resistance, activating signals (e.g. NF-κB/JNK) that downregulate the insulin cascade. Current evidence supports the concept that select flavonoids can mitigate obesity and type 2 diabetes (T2D). This work investigated if supplementation with the anthocyanidins (AC) cyanidin and delphinidin could attenuate the adverse consequences of consuming a high fat diet (HFD) in mice. Consumption of an AC-rich blend mitigated HFD-induced obesity, dyslipidemia and insulin resistance (impaired responses to insulin and glucose). HFD-fed mice were characterized by increased liver lipid deposition and inflammation, which were also attenuated upon AC supplementation. HFD caused liver oxidative stress showing an increased expression of NADPH oxidases, generators of superoxide and H2O2, and high levels of oxidized lipid-protein adducts. This was associated with the activation of the redox sensitive signals IKK/NF-κB and JNK1/2, and increased expression of the NF-κB-regulated PTP1B phosphatase, all known inhibitors of the insulin pathway. In agreement with an improved insulin sensitivity, AC supplementation inhibited oxidative stress, NF-κB and JNK activation, and PTP1B overexpression. Thus, cyanidin and delphinidin consumption either through diet or by supplementation could be a positive strategy to control the adverse effects of Western style diets, including overweight, obesity, and T2D. Modulation of inflammation, oxidative stress, and NF-κB/JNK activation emerge as relevant targets of AC beneficial actions.
Abbreviations: AC, anthocyanidins; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide-1; GTT, glucose tolerance test; HFD, high fat diet; 4-HNE, 4-hydroxynonenal; IKK, IκB kinase; IRS1, insulin receptor substrate-1; ITT, insulin tolerance test; JNK, c-jun N-terminal kinase; MCP-1, monocyte chemoattractant protein-1, NAFLD, nonalcoholic fatty liver disease; NOS2, inducible nitric oxide synthase; NOX, NADPH oxidase; PTP1B, protein tyrosine phosphatase 1B; TNFα, tumor necrosis factor alpha; T2D, type 2 diabetes
Graphical abstract
Highlights
-
•
Anthocyanidins (AC) mitigate high fat diet (HFD)-induced obesity and insulin resistance in mice.
-
•
AC inhibits HFD-induced liver lipid deposition and inflammation.
-
•
AC inhibit HFD-induced liver NADPH oxidase upregulation and oxidative stress.
-
•
AC inhibit the activation of redox sensitive signals that cause insulin resistance.
-
•
AC could be beneficial against the adverse effects of Western style diets.
1. Introduction
Overweight and obesity put individuals at risk of major health problems including type 2 diabetes (T2D), nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Consumption of Western style diets can be a major contributing factor to the increased rates of overweight and obesity in human populations, while consumption of select fruits and vegetables could attenuate these conditions. Evidence for the latter is conflicting when considering overall intakes, types of fruits and vegetables consumed, and other variables associated with population studies [1], [2], [3]. On the other hand, a large body of evidence in experimental animals suggests a benefit of select phytochemicals present in fruits and vegetables in the development of obesity and associated pathologies triggered by consumption of high fructose and/or high fat diets.
Among phytochemicals, anthocyanidins (AC) are flavonoids being actively investigated for their potential to mitigate unhealthy conditions, particularly metabolic disorders. In this regard, mounting evidence supports a potential beneficial action of AC consumption on T2D [4] and cardiovascular health [5]. Furthermore, AC-rich food consumption is inversely correlated with overall mortality [6]. AC are flavonoids that exist in nature as anthocyanins, the glycosylated forms of AC. They provide color to grapes, berries, blueberries, black currants, bilberries, purple corn, and black rice, among other fruits and vegetables. With the basic three-ring structure of flavonoids, AC are characterized by double bonds in the three rings and a positive charge in the B ring on the oxygen atom [7]. Different hydroxyl substitutions in number and position define different AC, e.g. delphinidins, malvidins, and peonidins. These differences in substitutions can have a major impact on AC biological actions in animals. In this regard, we recently observed that 3-O-glucosides of cyanidin and delphinidin were more efficient than malvidin, petunidin and peonidin 3-O-glucosides at inhibiting tumor necrosis factor alpha (TNFα)-induced activation of transcription factor NF-κB in Caco-2 cells [8].
Dietary energy overload can cause tissue inflammation, oxidative stress, and insulin resistance. Excess fat consumption leads to the activation of inflammatory and redox-regulated events including: i) the IκB kinase (IKK), and downstream the transcription factor NF-κB; and ii) the mitogen activated kinase c-jun N-terminal kinase (JNK). Activation of both JNK [9], [10] and IKK [11] and the increased expression of the NF-κB-regulated protein tyrosine phosphatase 1B phosphatase (PTP1B) [12] downregulate the insulin signaling pathway leading to insulin resistance. Inflammation, oxidative stress, and chronic NF-κB activation also contribute to other major adverse consequences of obesity, e.g. NAFLD and cardiovascular disease [13], [14], [15].
Identifying fruits and vegetables and their active components that can provide protection against the adverse effects of consuming Western style diets has the potential to have a major impact on human health. Moreover, understanding the mechanisms by which these components act modifying cell functions is crucial to define public recommendations in terms of diets and potential supplementation. This work investigated the capacity of a diet enriched in the AC cyanidin and delphinidin to mitigate in mice the development of obesity, dyslipidemia, steatosis, and insulin resistance promoted by the chronic consumption of a HFD. The beneficial effects of AC were mainly associated with the attenuation of liver inflammation, oxidative stress, and downregulation of the redox sensitive JNK and IKK/NF-κB. These findings stress the concept that cyanidins and delphinidins can provide benefits against excess fat consumption and its adverse health consequences.
2. Materials and methods
2.1. Materials
Cholesterol and triglyceride concentrations were determined using kits purchased from Wiener Lab Group (Rosario, Argentina). Glucose levels were measured using a kit purchased from Sigma-Aldrich Co (St. Louis, MO). Concentrations of insulin, glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP), leptin and adiponectin were determined using kits purchased from Crystal Chem Inc. (Downers Grove, IL). Antibodies for monocyte chemoattractant protein-1 (MCP-1) (#2029), TNFα (#11948), phospho (Ser176/180) - IKKα/β (#2697), IKKα (#2682), JNK2 (#9258) and β-actin (#12620) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies for F4/80 (sc-25830), nitric oxide synthase 2 (NOS2) (sc-649), phospho (Thr183/Tyr185) - JNK (sc-6254) and NOX3 (sc-67005) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for 4-hydroxynonenal (4-HNE) (ab46545), NOX4 (ab133303) and gp91phox (ab129068) were from Abcam, Inc. (Cambridge, MA). The antibody for PTP1B (ABS40) was from EMD Millipore (Hayward, CA). PVDF membranes were obtained from BIO-RAD (Hercules, CA). The Enhanced chemiluminescence (ECL) Western blotting system was from Thermo Fisher Scientific Inc. (Piscataway, NJ). All other chemicals were purchased from Sigma-Aldrich Co (St. Louis, MO). The AC-rich blend was provided by NSE Products, Inc. (Provo, UT) and its composition is shown in Supplemental Table 1.
2.2. Determination of AC blend composition
The AC-rich blend was analyzed using a liquid chromatography method [16], [17] as previously described [8]. Briefly, the blend was dissolved in water, filtered through PTFE (0.22 µm) membranes and separations performed using an Agilent series 1200 instrument (Agilent Technologies, Santa Clara, CA) with a Kinetex F5 pentafluorophenyl HPLC column (2.6 µm, 100 × 4.6 mm) and SecurityGuard® cartridge (PFP, 4.0 × 2.0 mm) (Phenomenex, Torrance, CA). A flow rate of 0.70 ml/min and a column temperature of 37 °C was set. The injector temperature was 4 °C with an injection volume of 7 l. Detection was done by UV–Vis DAD (wavelength monitored at 280 and 520 nm) and ESI-MS-MS for mass spectral results. Quantification was based on DAD peak area absorbance at 520 nm. A binary gradient was employed consisting of 1.0% formic acid (v/v) in water (mobile phase A) and 1.0% formic acid (v/v) in acetonitrile (mobile phase B). Gradient was as follows: 1% B at 0 min, 7.5% B at 7 min, 7.6% B at 14 min, 10% B at 17 min, 12% B at 18.5 min, 30% B at 24 min, 90% B at 25 min, 1% B at 26–30 min. Mass spectral data were acquired using an Agilent 6430 triple-quadrupole mass spectrometer with electrospray injection (Agilent Technologies, Santa Clara, CA, USA) set to scan mode with the following optimal MS/MS source parameters: nebulizer at 40 psi, capillary voltage + 4000 V (or − 3500 V), gas temperature 325 °C, and flow of 5 l/min. Sheath gas was 250 °C and sheath flow of 11 l/min. AC were identified after ionization in their molecular cation form under MS positive ion mode. Parent ions (m/z) were previously described [8].
2.3. Animals and animal care
All procedures were in agreement with standards for care of laboratory animals as outlined in the NIH Guide for the Care and Use of Laboratory Animals; experimental protocols were approved before implementation by the University of California, Davis Animal Use and Care Administrative Advisory Committee. Procedures were administered under the auspices of the Animal Resource Services of the University of California, Davis.
Healthy male C57BL/6J mice (20–25 g) (10 mice/group) were fed for 14 w either: i) a diet containing approximately 10% total calories from fat (Control, C group); ii) the control diet plus 40 mg AC/kg body weight (CA group); iii) a diet containing approximately 60% total calories from fat (lard) (HF group); or iv) the high fat diet supplemented with 2 (HFA2 group), 20 (HFA20 group) or 40 (HFA40 group) mg AC/kg body weight.
Body and food intake were measured weekly throughout the study. After 14 w on the dietary treatments, mice were euthanized by cervical dislocation, blood was collected from the sub-mandibular vein into heparinized tubes, and plasma was obtained after centrifugation at 1000×g for 15 min at 4 °C. Different adipose tissue pads, and liver were collected and weighed. Tissues were flash frozen in liquid nitrogen and then stored at − 80 °C for further analysis.
2.4. Metabolic measurements
For insulin tolerance tests (ITT), mice were fasted for 4 h and injected i.p. with 1 U human insulin/kg body weight. Blood glucose values were measured before and at 15, 30, 45, 60, 90 and 120 min post-injection. For glucose tolerance tests (GTT), overnight fasted mice were injected with D-glucose (2 g/kg body weight), and blood glucose was measured before and at 15, 30, 60, and 120 min post-injection. For both tests, glucose levels were measured using a glucometer (Easy Plus II, Home Aid Diagnostics Inc, Deerfield Beach, FL). At the end of the study, plasma total cholesterol, triglycerides, glucose, insulin, adiponectin, leptin, GLP-1 and GIP concentrations were determined following manufacturer's guidelines.
2.5. Determination of fecal and liver triglyceride content
Fecal triglyceride content was measured using a modified method to that proposed by Folch et al. [18]. Fecal samples were collected over 24 h from single cages (3–4 mice) and dried at 37 °C for 24 h. Dried feces (0.5 g) were ground to a fine powder using a mortar and pestle. The lipid extraction was performed by homogenizing the fecal powder with 500 ml of chloroform-methanol (2:1, v/v) solution. Samples were mixed for 5 min and centrifuged at 1000×g for 10 min at room temperature and the lower liquid phase containing the extracted lipids in chloroform-methanol was collected and evaporated overnight. Analysis of triglyceride content was performed by saponification using a method described by Weber et al. [19] with minor modifications. Briefly, the lipid residue was digested by incubation with 500 µl of a KOH (30% w/v):ethanol (1:2 v:v) solution for 30 min at 60 °C. An aliquot (200 µl) was combined with 215 µl of 1 M MgCl2. After centrifugation for 15 min at 2000×g at room temperature, 2 µl of the supernatant were collected and analyzed for glycerol content using the enzymatic triglyceride kit TG Color GPO/PAP AA (Wiener Lab, Rosario, Argentina).
Analysis of liver triglyceride content was performed after extraction and saponification, basically as previously described for feces. Briefly, a 100 µl aliquot of 10% (w/v) liver homogenate was mixed with 300 µl of a KOH (30% w/v):ethanol (1:2, v:v) solution and evaporated overnight at 55 °C. The following day, 1 ml of 50% (v/v) ethanol was added and samples centrifuged for 5 min at 10,000×g at room temperature. Of the resulting supernatant, 200 µl were added with 215 µl of 1 M MgCl2 and placed on ice for 10 min. After centrifugation at 10,000×g for 5 min at room temperature, 10 µl of the supernatant were analyzed for triglyceride content as described above.
2.6. Histological analyses
The liver was removed and samples fixed overnight in 4% (w/v) neutralized paraformaldehyde solution. Samples were subsequently washed twice in phosphate buffer saline solution, dehydrated, and then embedded in paraffin for histological analysis. Sections (5 µm thickness) were obtained from paraffin blocks and placed on glass slides. Hematoxylin and eosin staining was performed following standard procedures. Sections were examined using an Olympus BX51 microscope (Olympus America Inc., Center Valley, PA). Hepatic histological examination was performed using the NAFLD activity score (NAS) described by Kleiner et al. [20]. Three randomly selected fields per animal were assessed and analyzed using Pro Plus 5.1 software (Media Cybernetics, Rockville, MD).
2.7. Western blot analysis
Livers were homogenized as previously described [21]. Aliquots of total homogenates containing 25–40 μg protein were denatured with Laemmli buffer, separated by reducing 7.5–12.5% polyacrylamide gel electrophoresis, and electroblotted to PVDF membranes. Membranes were blocked for 2 h in 5% (w/v) bovine serum albumin and subsequently incubated in the presence of the corresponding primary antibodies (1:1000 dilution) overnight at 4 °C. After incubation for 90 min at room temperature in the presence of secondary antibodies (HRP conjugated) (1:10,000 dilution), the conjugates were visualized using enhanced chemiluminescence.
2.8. Electrophoretic mobility shift assay (EMSA)
NF-κB-DNA binding was assessed in the nuclear fractions obtained from liver as previously described [22], [23]. The EMSA was performed by end labeling the oligonucleotide containing the consensus sequences for NF-κB with [γ-32P] ATP. The oligonucleotide was end-labeled using T4 polynucleotide kinase and purified using Chroma Spin-10 columns. Samples were incubated with the labeled oligonucleotide (20,000–30,000 cpm) for 20 min at room temperature in 1X binding buffer [5X binding buffer: 50 mM Tris-HCl buffer, pH 7.5, containing 20% (v/v) glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, and 0.25 mg/ml poly(dI-dC)]. The products were separated by electrophoresis in a 6% (w/v) non-denaturing polyacrylamide gel using 0.5 X TBE (45 mM Tris/borate, 1 mM EDTA) as the running buffer. The gels were dried and the radioactivity quantified in a Phosphoimager 840 (Amersham Pharmacia Biotech. Inc., Piscataway, NJ).
2.9. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) using Statview 5.0 (SAS Institute Inc., Cary, NC). Fisher least significance difference test was used to examine differences between group means. A repeated measure ANOVA with Tukey-Kramer multiple comparison test was used to analyze changes in body weight and food intake. A P value < 0.05 was considered statistically significant. Data are shown as means ± SEM.
3. Results
3.1. Diets
To prepare the diets containing AC, in particular cyanidin and delphinidin, control and high fat diets were added with a blend of berry and black rice extracts. The polyphenol composition of the AC-rich blend was determined as described in Methods and is shown in Table 1. It can be estimated that the AC containing diets provided AC glycosides in the following percentages: 66% cyanidin, 28% delphinidin and 5.6% peonidin of the total AC content.
Table 1.
Polyphenol composition of the AC-rich blend.
| Compounds | mg/g dry weight extract | |
|---|---|---|
| Anthocyanidins | Cyanidin | 95 |
| Delphinidin | 40 | |
| Peonidin | 8 | |
| Malvidin | ND | |
| Petunidin | ND | |
| Pelargonidin | ND | |
| Benzoic acids | 3-methylgallate | ND |
| 4-hydroxybenzoic acid | ND | |
| Ferulic acid | 0.1 | |
| Fumaric acid | ND | |
| Gallic acid | 0.8 | |
| Protocatechuic acid | 14.9 | |
| Quinic acid | < 0.02 | |
| Shikimic acid | ND | |
| Syringic acid | < 0.02 | |
| Vanillic acid | 0.4 | |
| Hydroxycinnamates | Caffeic acid | 1.0 |
| Chlorogenic acid | 7.5 | |
| p-Coumaric | 3.4 | |
| Sinapinic acid | 0.7 | |
| Flavonols | Kaempferol-3-galactose | ND |
| Kaempferol-3-glucose | 3.4 | |
| Kaempferol | < 0.05 | |
| Naringenin | < 0.05 | |
| Quercetin | 0.1 | |
| Quercetin-3-galactose | 1.2 | |
| Quercetin-3-Rutin | 0.1 | |
| catechins | Catechin | ND |
| Epicatechin | < 0.001 | |
| Gallocatechins | ND |
ND: Not detected.
3.2. Animal outcomes
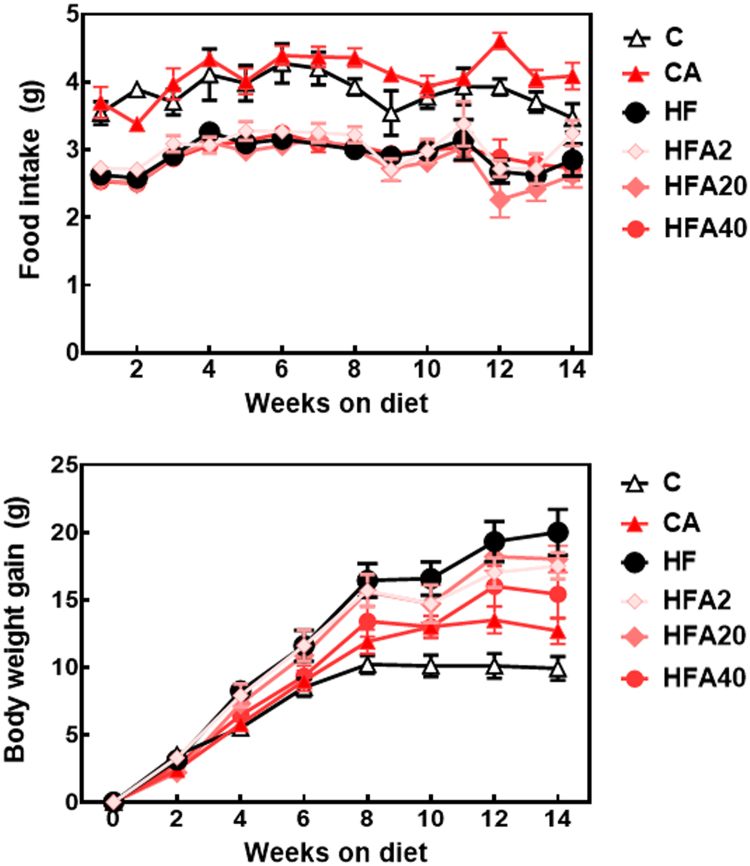
Daily food intake in the groups fed the HFD was significantly lower than in those fed the control and CA diets (Table 2, Fig. 1A). However, the calorie intake was similar within groups (14.4 and 14.8 kcal/d for control- and HFD-fed mice, respectively). Weekly food intake did not significantly vary within groups (Fig. 1A).
Table 2.
Metabolic parameters from mice fed for 14 w a control (Control) or a high fat diet (HF), without or with supplementation with 2, 20, or 40 mg AC/kg body weight (HFA2, HFA20, HFA40, CA40). Cholesterol, triglycerides, glucose, and insulin were measured in plasma. Values are shown as means ± SE (n = 10). Values having different superscripts are significantly different (p < 0.05, one way ANOVA).
| Parameter | Control | CA40 | HF | HFA2 | HFA20 | HFA40 |
|---|---|---|---|---|---|---|
| Daily food intake (g/d) | 3.7 ± 0.2a | 4.1 ± 0.1c | 3.0 ± 0.1b | 3.0 ± 0.1b | 2.8 ± 0.1b | 2.9 ± 0.03b |
| Body weight (g) | 33.7 ± 0.8a | 35.8 ± 1.0a | 44.1 ± 1.8b | 41.6 ± 1.2bc | 41.0 ± 1.2bc | 38.1 ± 2.2c |
| Brown fat (mg) | 149 ± 12a | 213 ± 24b | 259 ± 20b | 204 ± 32 cd | 201 ± 20 cd | 178 ± 20a |
| Epididymal fat (g) | 1.23 ± 0.10a | 1.45 ± 0.15a | 2.36 ± 0.10b | 2.28 ± 0.21b | 2.49 ± 0.09b | 2.22 ± 0.29b |
| Visceral fat (g) | 0.53 ± 0.10a | 0.55 ± 0.07ac | 1.28 ± 0.12b | 1.02 ± 0.12b | 1.16 ± 0.18b | 0.89 ± 0.12c |
| Subcutaneous fat (g) | 0.98 ± 0.06a | 1.59 ± 0.19a | 3.54 ± 0.23b | 2.78 ± 0.36b | 3.43 ± 0.27b | 2.71 ± 0.49b |
| Retroperitoneal fat (g) | 0.40 ± 0.27a | 0.54 ± 0.07a | 1.26 ± 0.09b | 0.95 ± 0.07cb | 1.25 ± 0.15b | 0.84 ± 0.13c |
| Total cholesterol (mg/dl) | 126 ± 3a | 134 ± 5a | 160 ± 3b | 147 ± 7b | 156 ± 6b | 138 ± 5a |
| Triglycerides (mg/dl) | 84 ± 2a | 84 ± 2a | 92 ± 1b | 86 ± 2a | 86 ± 3a | 86 ± 2a |
| Fasted glucose (mg/dl) | 164 ± 8a | 200 ± 12a | 230 ± 11b | 206 ± 17b | 225 ± 11b | 161 ± 13a |
| Fasted insulin (ng/ml) | 0.53 ± 0.04ac | 0.47 ± 0.04c | 1.22 ± 0.10b | 0.88 ± 0.20a | 1.08 ± 0.24b | 0.61 ± 0.10ac |
Fig. 1.
Effects of supplementation with an AC-rich blend on metabolic parameters in HFD-fed rats. A- Food intake and B- body weight gain. Mice were fed a control diet (empty triangles), the control diet supplemented with 40 mg AC/kg body weight (full triangles), a HFD (black circles), or the HFD supplemented with 2 (light pink diamonds), 20 (dark pink diamonds), or 40 mg AC/kg body weight (red circles). Results are shown as means ± SE and are the average of 9–10 animals/group. Differences between the HF and HFA40 body weight gain values are significant between weeks 4 and 14 on the diets (p < 0.05, repeated measures ANOVA).
Starting at week 4 and through the following weeks, the body weight gain for C, CA, and HFA40 groups was significantly lower than for the HF group (Fig. 1B). At week 14, there was a dose-dependent decrease in body weight depending on the amount of AC in the diet (Table 2). At the end of the study, consumption of the HFD caused a 31% higher body weight compared to controls, while the body weight of HFA40 mice was 14% lower than in HF mice.
HFD-induced obesity was associated with an increased weight of the different fat pads (brown, epididymal, visceral, subcutaneous and retroperitoneal) (Table 2). HFA40 mice showed a brown fat content similar to C mice, and 72% and 49% lower visceral and retroperitoneal fat accumulation, respectively, compared to HF mice. Brown fat weight in CA mice was 43% higher than in the C group.
Consumption of the HFD also caused dyslipidemia. Plasma cholesterol and triglyceride levels were 26% and 9% higher in HF than in C mice, respectively (Table 2). Supplementation of HFD-fed mice with AC prevented the increase of plasma triglyceride concentrations at all the AC concentrations tested, while plasma cholesterol increase was only prevented at the highest AC supplementation level, i.e. HFA40 group.
While there were no significant differences in the amount of triglycerides excreted with the feces among the control, HF and CA groups (10.2 ± 0.8, 12.0 ± 0.3 and 12.0 ± 0.1 mg/g feces, respectively), fecal triglyceride amount in the HFA40 group (15.6 ± 3.6 mg/g feces) was significantly higher than in the control group (p < 0.04). Fecal cholesterol content was significantly higher (p < 0.05) in the HF and HFA40 groups (8.0 ± 0.3 and 6 ± 0.8 mg/g feces, respectively) than in control and CA groups (2.7 ± 0.1 and 3.2 ± 0.3, respectively).
3.3. Supplementation with AC improved glucose homeostasis in mice fed a high fat diet
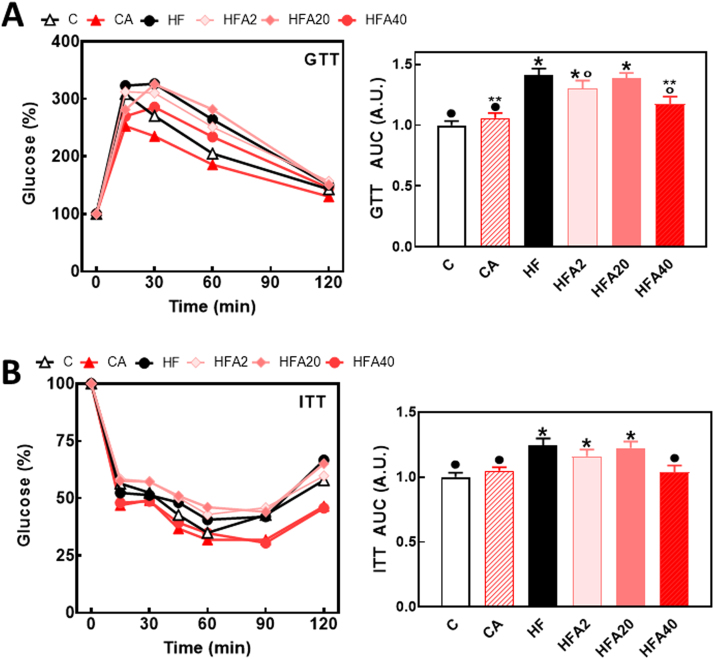
Fasting plasma glucose and insulin were 40% and 130% higher, respectively, in the HF compared to the C group. In the HFA40 group, both parameters showed values similar to those observed in the C and CA groups, while in the HFA2 group only plasma insulin was similar to control values (Table 2). Consumption of the HFD diet altered the response of mice to insulin (ITT) and glucose (GTT) tolerance tests (Fig. 2A, B). The area under the curve for the GTT and ITT was 41% and 24% higher, respectively, in HF than in C and CA mice. Supplementation with AC at the highest dose tested caused a 59% reduction of the increase in GTT area under the curve, and total prevention of the increase in ITT area under the curve. Given that supplementation with 40 mg AC/kg body weight was the AC level that provided the strongest level of protection against HFD-induced insulin resistance, subsequent experiments were focused only on the HFA40 group.
Fig. 2.
Effects of supplementation with an AC-rich extract on GTT and ITT responses in HFD-fed rats. GTT (A) and ITT (B) were performed on weeks 9 and 11 on the diets, respectively. Mice were fed a control diet (empty triangles, empty bars), the control diet supplemented with 40 mg AC/kg body weight (full triangles, dashed bars), a HFD (black circles, black bars), or the HFD supplemented with 2 (light pink diamonds, light pink bars), 20 (dark pink diamonds, dark pink bars), or 40 mg AC/kg body weight (red circles, red bars). The area under the curve for GTT and ITT tests were calculated, values referred to the control group, and shown as bars in the right panels. Results are shown as means ± SE and are the average of 9–10 animals/group. Values having different superscripts are significantly different (p < 0.05, one-way ANOVA).
3.4. Supplementation with AC improved plasma hormone profiles
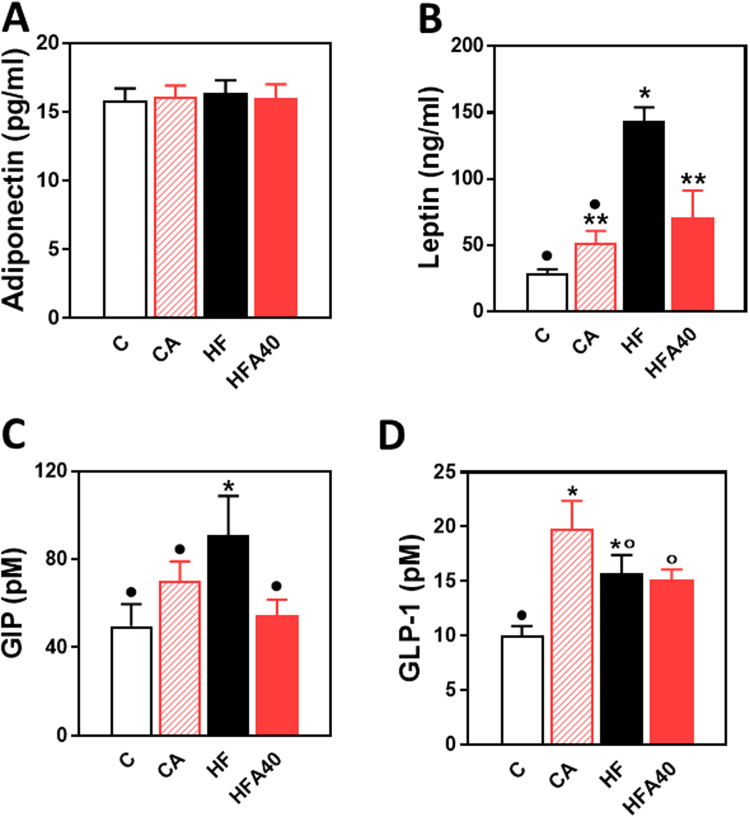
We next investigated the levels of select hormones, which are relevant to the regulation of glucose homeostasis, and that are produced by the adipose tissue, i.e. adiponectin and leptin, and by gastrointestinal enteroendocrine cells, i.e. GIP and GLP-1. While plasma adiponectin levels were similar among groups, plasma leptin was 4 times higher in the HF compared to the C group (Fig. 3 A, B). The increase in leptin observed in HF mice was partially (63%) prevented in HFA40 mice. HFD consumption caused a significant increase (83%) in plasma GIP that was not observed in the HFA40 group (Fig. 3 C). Plasma GLP-1 concentration was significantly higher in HF, HFA40 and CA groups (57%, 52%, and 98%, respectively) compared to the C group (Fig. 3 D).
Fig. 3.
Effects of supplementation with an AC-rich blend on hormones relevant to glucose homeostasis in HFD-fed rats. A-Adiponectin, B- leptin, C- GIP and D- GLP-1 were measured in plasma of mice fed for 14 w- a control diet (empty bars), the control diet supplemented with 40 mg AC/kg body weight (dashed bars), a HFD (black bars), or the HFD supplemented with 40 mg AC/kg body weight (red bars). Results are shown as means ± SE and are the average of 9–10 animals/group. Values having different superscripts are significantly different (p < 0.05, one-way ANOVA).
3.5. Supplementation with AC attenuated steatosis and inflammation in the liver of mice fed a high fat diet
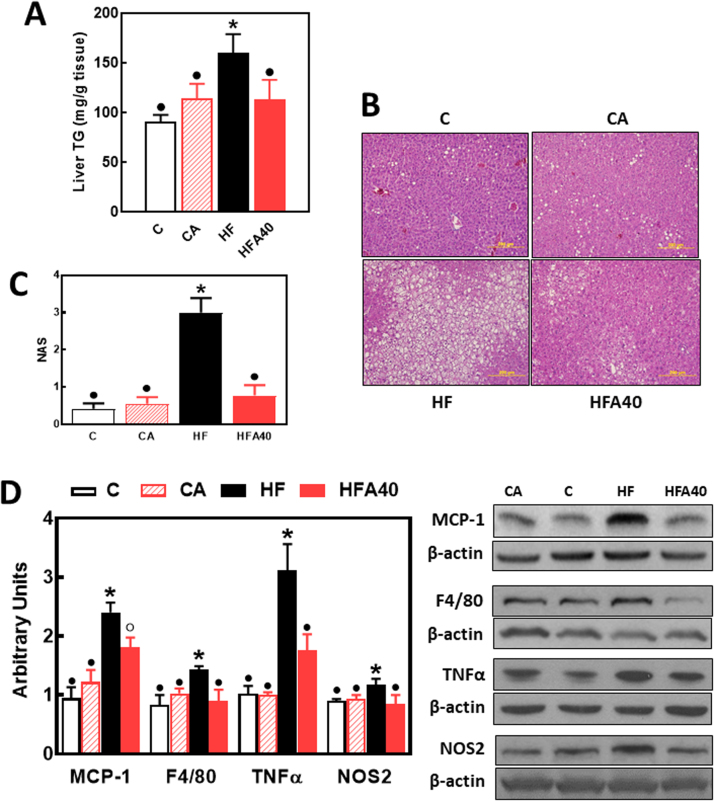
Consumption of the HFD caused steatosis and liver inflammation. Liver triglyceride levels were 76% higher in the HFD-fed compared to the C group. This increase in liver triglycerides was not observed in the HFA40 group. (Fig. 4A). Lipid deposition was also assessed by histological analysis after hematoxylin-eosin staining (Fig. 4B). Consumption of the HFD caused an increased lipid deposition in the liver that was not observed in HFA40 mouse liver. The NAFLD activity score (NAS) was significantly higher (6.4 folds) in the liver from HF compared to C, CA, and HFA40 mice (Fig. 4C).
Fig. 4.
Effects of supplementation with an AC-rich blend on steatosis and hepatic inflammation in HFD-fed rats. Mice were fed a control diet (empty bars), the control diet supplemented with 40 mg AC/kg body weight (dashed bars), a HFD (black bars), or the HFD supplemented with 40 mg AC/kg body weight (red bars). At week 14 on the corresponding diets the following parameters were measured: A- liver triglyceride content, B- fat liver deposition as evaluated by hematoxylin/eosin tissue staining, C- NAFLD activity score (NAS), and D- proteins involved in inflammation: MCP-1, F4/80, TNFα, and NOS2. Western blot bands were quantified and values referred to β-actin levels (loading control). Results for CA, HF and HFA40 were referred to control group values (C). Results are shown as mean ± SE of 6 animals/group. Values having different superscripts are significantly different (p < 0.05, one-way ANOVA test).
Several proteins involved in the inflammatory response were measured in liver by Western blot (Fig. 4D). The chemokine MCP-1, the cytokine TNFα, the macrophage marker F4/80, and the enzyme NOS2 were all upregulated in the liver of HF mice. Supplementation with AC either partially (MCP-1) or fully (F4/80, TNFα, and NOS2) prevented these increases. No significant differences were observed between C and CA in markers of steatosis and liver inflammation.
3.6. Supplementation with AC improved parameters of oxidative stress and redox signaling involved in insulin resistance in the liver of mice fed a high fat diet
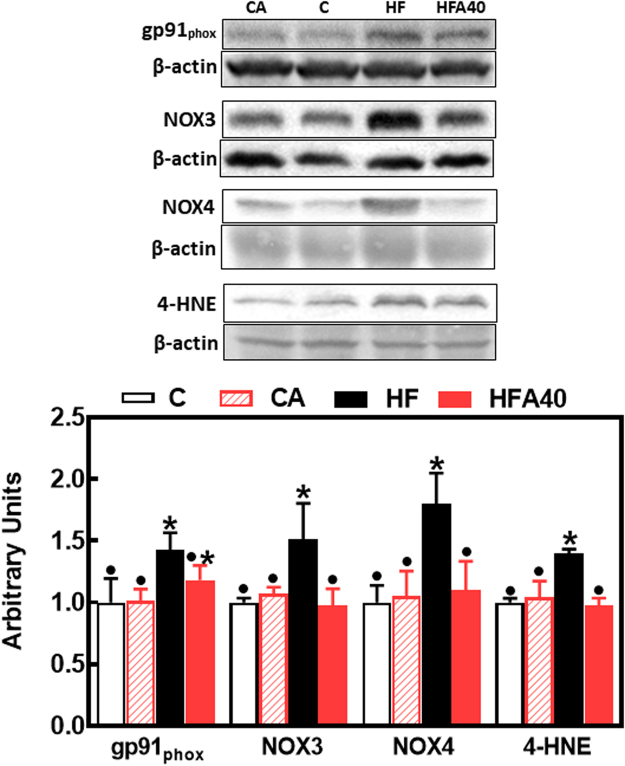
HFD-associated alterations in glucose homeostasis can be due to an upregulation of NADPH oxidase (NOX) isoforms leading to hepatic oxidative stress, and the activation of redox-sensitive signals that promote insulin resistance. Consumption of the HFD caused the upregulation of liver NOX2 (gp91phox), NOX3 and NOX4 (Fig. 5). Thus, gp91phox, NOX3 and NOX4 levels were 43%, 51% and 80% higher, respectively, in HF compared to C mice. AC supplementation prevented HFD-mediated upregulation of NOX3 and NOX4, but not that of gp91phox. The increased NOXs expression was paralleled by a 40% increase in the levels of 4-hydroxynonenal-protein adducts in HFD-fed mice, which was prevented by AC supplementation (Fig. 5).
Fig. 5.
Effects of supplementation with an AC-rich blend on parameters of liver oxidative stress in HFD mice. Mice were fed a control diet (empty bars), the control diet supplemented with 40 mg AC/kg body weight (dashed bars), a HFD (black bars), or the HFD supplemented with 40 mg AC/kg body weight (red bars). At week 14 on the corresponding diets the following parameters were measured in liver by Western blot: gp91phox, NOX3, NOX4 and HNE-protein adducts, Bands were quantified and values referred to β-actin levels (loading control. Results for CA, HF and HFA40 were referred to control group values (C). Results are shown as mean ± SE of 6 animals/group. Values having different superscripts are significantly different (p < 0.05, one-way ANOVA test).
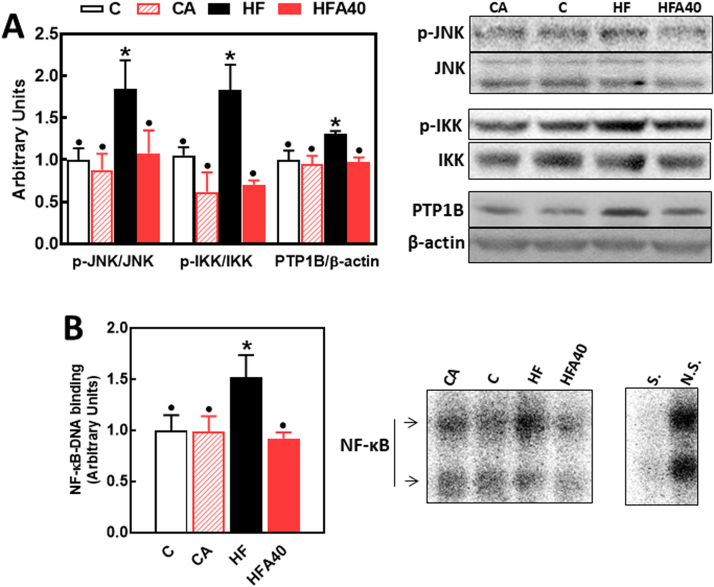
The phosphorylation of the redox-sensitive signals JNK (Thr183/Tyr185) and IKK (Ser176/180) was 84% and 83% higher, respectively, in the liver of HF compared to C mice (Fig. 6A). The increased phosphorylation of both JNK and IKK was not observed in HFA40 mice. In agreement with the activation of IKK, an upstream event in the pathway, NF-κB-DNA binding, was 52% higher in liver nuclear fractions from HF than in C and HFA40 mice (Fig. 6B). Activation of the NF-κB signaling pathway was associated with an increased expression of PTP1B, which in the liver of HF mice was 31% higher than in C and HFA40 mice (Fig. 6A).
Fig. 6.
Effects of supplementation with an AC-rich blend on redox-sensitive signals that modulate insulin sensitivity in HFD mice. Mice were fed a control diet (empty bars), the control diet supplemented with 40 mg AC/kg body weight (dashed bars), a HFD (black bars), or the HFD supplemented with 40 mg AC/kg body weight (red bars). At week 14 on the corresponding diets the following parameters were measured in liver: (A) by Western blot: phosphorylation of JNK and IKK and PTP1B levels (bands were quantified and values referred to total protein levels (JNK, IKK) or β-actin levels (PTP1B); B- NF-κB-DNA binding in liver nuclear fractions measured by EMSA. Representative EMSA images are shown on the right panel. To assess the specific bands, a control nuclear fraction was incubated in the presence of a 100-fold molar excess of unlabeled oligonucleotide containing the consensus sequence for a nonspecific (N.S.) (FOXO-1) or specific (S.) (NF-κB) transcription factor before the binding assay. For both A and B, results for CA, HF and HFA40 were referred to control group values (C). Results are shown as mean ± SE of 6 animals/group. Values having different superscripts are significantly different (p < 0.05, one-way ANOVA test).
4. Discussion
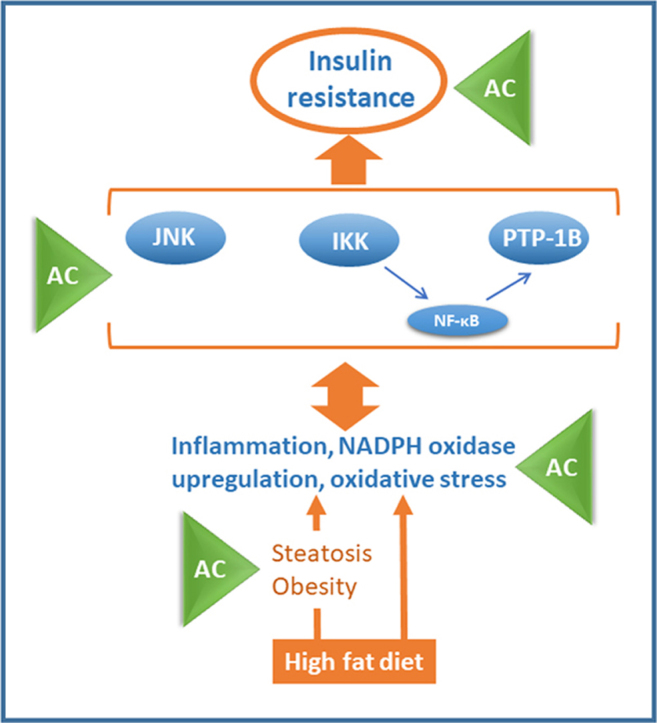
Chronic consumption of a HFD by mice led to the development of obesity, adiposity, dyslipidemia, steatosis, liver inflammation and insulin resistance. Simultaneous consumption of a diet rich in AC, i.e. cyanidins and delphinidins, attenuated all these adverse effects. In addition, the AC-associated improvement of inflammation, oxidative stress, and insulin sensitivity was in part associated with their capacity to modulate NF-κB and JNK.
Diets rich in fat and carbohydrates are in part responsible for the increasing global burden of overweight and obesity. The dietary consumption of a HFD by mice mimics the consequences of Western style diets in humans. The amount of ACs provided are comparable in quality and amount to that achievable through food consumption and/or rational amounts of dietary supplements in humans. We observed that mice eating the HFD and AC gained less weight than those fed the HFD alone, despite consuming similar amounts of calories. In line with these results, AC at the highest amount provided, i.e. 40 mg/kg body weight, led to lower weight of brown, visceral, and retroperitoneal fat pads, but not epididymal or subcutaneous fat, compared to the non-supplemented HFD-fed mice. AC-mediated decrease in visceral fat is particularly relevant given the role of this fat pad in the development of systemic adverse effects through the release of adipokines, growth factors and inflammatory molecules. Thus, visceral fat accumulation is associated with the development of metabolic syndrome [24] and associated diseases, e.g., NAFLD [25] and cardiovascular disease [26]. AC supplementation also attenuated the hyperlipidemia and steatosis associated with HFD consumption. These results disagree with previous reports that pure AC but not AC in berry extracts have the capacity to improve dyslipidemia [27]. These differences may be related to differences in experimental design, but stress the relevance of the overall food matrix in AC absorption and metabolism, and subsequently on their biological actions. Part of the mentioned effects on obesity and steatosis could be due to the actions of AC at the gastrointestinal tract [28]. In this regard, factors that can contribute to the capacity of AC to mitigate body weight gain and excess tissue lipid deposition may include the modulation of GLP-1, a hormone known to reduce adiposity [29], and/or a decreased fat absorption associated to the inhibition of pancreatic lipase, which is essential for dietary triglyceride absorption in the intestine [30]. With regard to the latter, both cyanidin and cyanidin-3,5-diglucoside were shown to inhibit the enzyme in vitro [31]. Pancreatic lipase inhibition would also be consistent with the current finding of high fecal triglyceride levels in the AC-supplemented and HFD-fed mice.
The increasing incidence of T2D worldwide has paralleled that of overweight and obesity. As previously reported [32], [33]. HFD consumption by C57BL/6J mice led to insulin resistance as evidenced by high fasted plasma glucose and insulin levels, and impaired ITT and GTT tests. At the highest concentration tested, the AC blend improved all these parameters. Accordingly, an AC-rich blueberry extract was found to improve parameters of insulin sensitivity in HFD-fed mice, although under the tested conditions the ITT and GTT were not affected by fat consumption [34]. The above beneficial effects could be in part related to the capacity of AC to modulate hormones that regulate different aspects of glucose homeostasis. Adipokines (adiponectin, leptin) and incretins (GLP-1, GIP) contribute to the regulation of satiety and/or glucose homeostasis. While plasma adiponectin levels were not affected, plasma leptin was increased because of HFD consumption, as it is observed in diet-induced obesity [35], The attenuation of hyperleptinemia by AC supplementation in HFD-fed mice may in part reflect a decreased fat pad mass and an improved capacity to modulate food intake and energy balance. In terms of the incretins, GIP and GLP-1 increase insulin secretion after food consumption influencing glucose control. Furthermore, GLP-1 promotes satiety [36] and improves basal and postprandial lipidemia [37]. The prevention by AC of HFD-mediated GIP increase may reflect AC-mediated improved capacity to regulate glucose homeostasis. While AC supplementation did not affect HFD-mediated increase in plasma GLP-1, it increased GLP-1 plasma levels in mice fed the control diet. Consistently with the latter, delphinidin was found to increase GLP-1 secretion in GLUTag cells [38]. The capacity of cyanidin and/or delphinidin to increase plasma GLP-1 may be an important mechanism underlying, in part, their anti-obesity and anti-T2D actions. In fact, GLP-1 is a relevant therapeutic target for the control of T2D [29].
The liver is one of the central organs in the maintenance of glucose and lipid homeostasis. HFD consumption disrupted this homeostasis, and in parallel caused liver fat deposition and inflammation. The coordinated AC-mediated improvement of HFD-induced liver steatosis, inflammation, and systemic insulin resistance can be explained through the interplay among these adverse conditions. In terms of biochemical mechanisms, inflammation, oxidative stress and the activation of NF-κB are events that establish a self-feeding cycle that can be initiated by excess nutrient consumption [39], [40]. The capacity of AC to mitigate HFD-triggered hepatic inflammation and NF-κB/JNK activation can be in part due to AC capacity to modulate liver oxidative stress. AC cannot exert direct antioxidant actions except at the gastrointestinal tract where they can reach high enough concentrations that allow to scavenge oxidants at a significant extent [41]. On the other hand, in the liver and other organs, the regulation of oxidative stress is mainly due to indirect antioxidant actions exerted by AC, mainly their metabolites, through the modulation of the production of superoxide anion and nitric oxide [42]. In fact, we observed that AC supplementation prevented HFD-mediated upregulation of NOX3 and NOX4, and of the pro-inflammatory inducible nitric oxide synthase, NOS2. AC supplementation also prevented the high levels of 4-HNE-protein adducts in HFD-mouse liver. Consistently, nutrient overload can cause increased formation of 4-HNE which irreversibly form adducts with macromolecules (e.g. proteins), which can modify cell function, and contribute to T2D and NAFLD development [43].
Inactivation of the redox sensitive transcription NF-κB can be central to AC beneficial actions. We observed that AC supplementation inhibited in the liver HFD-induced NF-κB activation, as evidenced by both decreased IKK phosphorylation, and NF-κB-DNA binding. Accordingly, we previously observed that cyanidin and delphinidin 3-O-glucosides inhibited TNFα-induced NF-κB activation and downstream loss of monolayer integrity in Caco-2 intestinal cells [8]. Cyanidin inhibited the inflammatory response and redox imbalance (glutathione decrease) triggered by TNFα in Caco-2 cells, which was attributed in part to NF-κB inhibition and Nrf2 upregulation [44]. NF-κB and JNK pathways are key players in the development of insulin resistance. Activation of the NF-κB upstream kinase IKK [45] and of JNK [9], [10] leads to the phosphorylation in serine residues of the insulin receptor substrate-1 (IRS1) causing a downregulation of the insulin cascade. In addition, NF-κB activation also induces the transcription of PTP1B [46], a tyrosine phosphatase which dephosphorylates and inactivates the insulin receptor and IRS1. Thus, disruption of the high fat-diet induced cycle of inflammation, oxidative stress and NF-κB/JNK activation can be central in the capacity of AC to mitigate HFD-induced insulin resistance. Furthermore, it can be argued that the effects of AC on insulin resistance are in part derived from an upstream regulation of oxidant levels and/or inflammation.
In summary, supplementation with a cyanidin and delphinidin-rich blend, mitigated the adverse consequences of HFD consumption, i.e. obesity, dyslipidemia, steatosis, and insulin resistance. Inhibition of inflammation, oxidative stress and NF-κB/JNK activation emerge as mechanisms underlying those AC-mediated benefits. In addition, AC could also act in part by inhibiting pancreatic lipase and subsequently dietary lipid absorption, and by modulating incretins involved in glucose and lipid homeostasis. Increased AC cyanidin/delphinidin consumption either through diet or by supplementation could be a plausible strategy to control the adverse effects of Western style diets.
Acknowledgements
Funding was provided by an unrestricted research grant from Pharmanex Research, NSE Products Inc., Provo, UT, USA. PIO is correspondent researcher from CONICET, Argentina.
Acknowledgments
Conflict of interest
AM, SMW, and SNH are employed by Pharmanex Research, NSE Products Inc., Provo, UT, USA, a company that provided the test blend. ALW, CGF and PIO have received unrestricted research grants from NSE Products Inc. as well as from other food companies and government agencies with an interest in health and nutrition.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.05.012.
Appendix A. Supplementary material
Supplementary material
References
- 1.Mytton O.T., Nnoaham K., Eyles H., Scarborough P., Ni Mhurchu C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health. 2014;14:886. doi: 10.1186/1471-2458-14-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser K.A., Brown A.W., Bohan Brown M.M., Shikany J.M., Mattes R.D., Allison D.B. Increased fruit and vegetable intake has no discernible effect on weight loss: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2014;100:567–576. doi: 10.3945/ajcn.114.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwingshackl L., Hoffmann G., Kalle-Uhlmann T., Arregui M., Buijsse B., Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PLoS One. 2015;10:e0140846. doi: 10.1371/journal.pone.0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo X., Yang B., Tan J., Jiang J., Li D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016;70:1360–1367. doi: 10.1038/ejcn.2016.142. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2017 doi: 10.1016/j.mam.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Ivey K.L., Jensen M.K., Hodgson J.M., Eliassen A.H., Cassidy A., Rimm E.B. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br. J. Nutr. 2017;117:1470–1477. doi: 10.1017/S0007114517001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crozier A., Del Rio D., Clifford M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010;31:446–467. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Cremonini E., Mastaloudis A., Hester S.N., Verstraeten S.V., Anderson M., Wood S.M., Waterhouse A.L., Fraga C.G., Oteiza P.I. Anthocyanins inhibit tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Food Funct. 2017;8:2915–2923. doi: 10.1039/c7fo00625j. [DOI] [PubMed] [Google Scholar]
- 9.Hirosumi J., Tuncman G., Chang L., Gorgun C.Z., Uysal K.T., Maeda K., Karin M., Hotamisligil G.S. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre V., Uchida T., Yenush L., Davis R., White M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J. Biol. Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 11.Gao Z., Hwang D., Bataille F., Lefevre M., York D., Quon M.J., Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J. Biol. Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 12.Haj F.G., Zabolotny J.M., Kim Y.B., Kahn B.B., Neel B.G. Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B-/-mice. J. Biol. Chem. 2005;280:15038–15046. doi: 10.1074/jbc.M413240200. [DOI] [PubMed] [Google Scholar]
- 13.Tornatore L., Thotakura A.K., Bennett J., Moretti M., Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557–566. doi: 10.1016/j.tcb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Tarantino G., Savastano S., Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J. Gastroenterol. 2010;16:4773–4783. doi: 10.3748/wjg.v16.i38.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborn O., Olefsky J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 16.de Ferrars R.M., Czank C., Saha S., Needs P.W., Zhang Q., Raheem K.S., Botting N.P., Kroon P.A., Kay C.D. Methods for isolating, identifying, and quantifying anthocyanin metabolites in clinical samples. Anal. Chem. 2014;86:10052–10058. doi: 10.1021/ac500565a. [DOI] [PubMed] [Google Scholar]
- 17.Kay C.D., Kroon P.A., Cassidy A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009;53:S92–S101. doi: 10.1002/mnfr.200800461. [DOI] [PubMed] [Google Scholar]
- 18.Folch J., Lees M., Stanley G.H. Sloane. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 19.Weber T.E., Trabue S.L., Ziemer C.J., Kerr B.J. Evaluation of elevated dietary corn fiber from corn germ meal in growing female pigs. J. Anim. Sci. 2010;88:192–201. doi: 10.2527/jas.2009-1896. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., Yeh M., McCullough A.J., Sanyal A.J., Nonalcoholic Steatohepatitis Clinical Research N. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Bettaieb A., Vazquez-Prieto M.A., Lanzi C.R., Miatello R.M., Haj F.G., Fraga C.G., Oteiza P.I. (-)-Epicatechin mitigates high fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic. Biol. Med. 2014;72:247–256. doi: 10.1016/j.freeradbiomed.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie G.G., Carrasquedo F., Delfino J.M., Keen C.L., Fraga C.G., Oteiza P.I. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18:167–169. doi: 10.1096/fj.03-0402fje. [DOI] [PubMed] [Google Scholar]
- 23.Contreras T.C., Ricciardi E., Cremonini E., Oteiza P.I. (-)-Epicatechin in the prevention of tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Arch. Biochem. Biophys. 2015;573:84–91. doi: 10.1016/j.abb.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 25.van der Poorten D., Milner K.L., Hui J., Hodge A., Trenell M.I., Kench J.G., London R., Peduto T., Chisholm D.J., George J. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–457. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 26.Britton K.A., Fox C.S. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 27.Prior R.L., Wu X., Gu L., Hager T., Hager A., Wilkes S., Howard L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol. Nutr. Food Res. 2009;53:1406–1418. doi: 10.1002/mnfr.200900026. [DOI] [PubMed] [Google Scholar]
- 28.Oteiza P.I., Fraga C.G., Mills D.A., Taft D.H. Flavonoids and the gastrointestinal tract: local and systemic effects. Mol. Asp. Med. 2018 doi: 10.1016/j.mam.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Rajeev S.P., Wilding J. GLP-1 as a target for therapeutic intervention. Curr. Opin. Pharmacol. 2016;31:44–49. doi: 10.1016/j.coph.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Buchholz T., Melzig M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015;81:771–783. doi: 10.1055/s-0035-1546173. [DOI] [PubMed] [Google Scholar]
- 31.You Q., Chen F., Wang X., Luo P.G., Jiang Y. Inhibitory effects of muscadine anthocyanins on alpha-glucosidase and pancreatic lipase activities. J. Agric. Food Chem. 2011;59:9506–9511. doi: 10.1021/jf201452v. [DOI] [PubMed] [Google Scholar]
- 32.Cremonini E., Bettaieb A., Haj F.G., Fraga C.G., Oteiza P.I. (-)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch. Biochem. Biophys. 2016;599:13–21. doi: 10.1016/j.abb.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cremonini E., Wang Z., Bettaieb A., Adamo A.M., Daveri E., Mills D.A., Kalanetra K.M., Haj F.G., Karakas S., Oteiza P.I. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: implications for steatosis and insulin resistance. Redox Biol. 2018;14:588–599. doi: 10.1016/j.redox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S., Keirsey K.I., Kirkland R., Grunewald Z.I., Fischer J.G., de La Serre C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J. Nutr. 2018;148:209–219. doi: 10.1093/jn/nxx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Git K.C., Adan R.A. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes. Rev. 2015;16:207–224. doi: 10.1111/obr.12243. [DOI] [PubMed] [Google Scholar]
- 36.Dailey M.J., Moran T.H. Glucagon-like peptide 1 and appetite. Trends Endocrinol. Metab. 2013;24:85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutz T.A., Osto E. Glucagon-like peptide-1, glucagon-like peptide-2, and lipid metabolism. Curr. Opin. Lipidol. 2016;27:257–263. doi: 10.1097/MOL.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 38.Kato M., Tani T., Terahara N., Tsuda T. The anthocyanin delphinidin 3-rutinoside stimulates glucagon-like peptide-1 secretion in murine GLUTag cell line via the Ca2+/calmodulin-dependent kinase II pathway. PLoS One. 2015;10:e0126157. doi: 10.1371/journal.pone.0126157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 40.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 41.Galleano M., Verstraeten S.V., Oteiza P.I., Fraga C.G. Antioxidant actions of flavonoids: thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010;501:23–30. doi: 10.1016/j.abb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Fraga C.G., Oteiza P.I., Galleano M. Plant bioactives and redox signaling: (-)-epicatechin as a paradigm. Mol. Asp. Med. 2018 doi: 10.1016/j.mam.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Sasson S. Nutrient overload, lipid peroxidation and pancreatic beta cell function. Free Radic. Biol. Med. 2017;111:102–109. doi: 10.1016/j.freeradbiomed.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Ferrari D., Speciale A., Cristani M., Fratantonio D., Molonia M.S., Ranaldi G., Saija A., Cimino F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-alpha and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016;264:51–58. doi: 10.1016/j.toxlet.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J., Shoelson S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zabolotny J.M., Kim Y.B., Welsh L.A., Kershaw E.E., Neel B.G., Kahn B.B. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J. Biol. Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material