Abstract
Lung transplantation is a proven treatment for selected patients with end-stage lung disease. However, the number of patients on the transplant waiting list far exceeds the number of available donor lungs, resulting in waiting list morbidity and mortality. The problem is further exacerbated by the low utilisation rate of available donor lungs, for fear of selecting a damaged lung and the resultant primary graft dysfunction. In the past decade, ex vivo lung perfusion (EVLP) has become part of standard lung transplant clinical practice in Canada and Europe, and it has been shown to improve the usage of available donor lungs by allowing physiological and radiologic evaluation of explanted donor lungs that are considered “marginal”. This allows clinicians a second opportunity to decide whether to proceed to transplantation, instead of declining an organ that appears questionable by standard clinical criteria. However there has been much research activity looking at EVLP as a platform for (I) molecular diagnosis, thereby further improving the diagnostic accuracy regarding quality of the donor lung; (II) organ repair, thereby allowing injured donor lungs to become clinically useable. This manuscript summarises some of the preclinical and clinical research from the Toronto group focusing on these promising aspects of EVLP which may further increase the number of useable donor lungs in lung transplantation.
Keywords: Ex vivo lung perfusion (EVLP), organ repair, molecular diagnosis
Introduction
Ex vivo lung perfusion (EVLP) has been shown to be an important advance in clinical lung transplantation (1). Prior to EVLP, many donor lungs would be declined by clinicians for fear of transplanting a damaged organ and the associated risks of primary graft dysfunction (PGD). This led to a utilization rate of only 15–20% of potentially available organs, the lowest amongst solid organ transplant (2). However it has been reported that that up to 40% of declined lungs are likely to be of adequate quality for transplantation (3).
The Toronto EVLP system involves explanting the injured donor lung in question with subsequent standard cold preservation with a low potassium dextran flush (Perfadex), followed by normothermic acellular ex vivo perfusion with Steen solution (4). This allows a further opportunity to evaluate questionable donor lungs over a period of 4 h where physiologic, radiologic, and bronchoscopic findings help clinicians to determine whether the lungs may be suitable for clinical transplantation. The Toronto group reported the result of the Human EVLP (HELP) study and showed that using EVLP in this fashion, over 80% of such donor lungs were in fact usable, and that the early and mid-term outcomes of lung transplant recipients of EVLP-treated lungs were non-inferior compared to patients who received donor lungs deemed useable according to conventional practice (1,5). EVLP has become an established part of the Toronto Lung Transplant Program, and donor lungs subjected to EVLP accounts for at least 20% of the total volume of our clinical lung transplantation activity.
Following the success of clinical EVLP, in recent years there has been active research into the possibility to further leverage the potential of EVLP beyond the current role of donor organ assessment using physiologic and radiologic parameters, with the ultimate goal of increasing the number of useable donor lungs, and improving the outcome of lung transplant. This article attempts to present some examples of preclinical and clinical studies that show the promise of EVLP as a platform for improved transplantation logistics, diagnostic and organ repair.
Extension of donor lung preservation
For practical implementation of EVLP, the explanted lungs are cooled down after EVLP leading to a second period of cold ischemia, called cold ischemia time (CIT)-2. The primary reason for this second cold preservation period is to provide hypothermic preservation of the organ during the surgical implantation period. In fact, the impact of this phase of preservation or its length has not been formally investigated since the inception of EVLP. Whereas intuitively, decreasing CIT-2 to a minimum may be expected to lead to improved graft function, a safe prolongation of post-EVLP preservation may facilitate transplant logistics, including recipient operation timing and a possibility of further transportation of the organ to other centers as previously reported (6) or organizing the transplant procedure in a more semi-elective fashion.
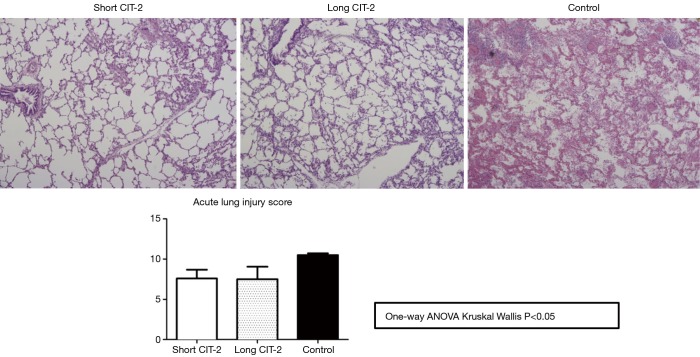
This question was addressed by the Toronto group in a study that investigated the effect of an extended CIT-2 on lung function after transplantation in a pig lung transplant model (7). Explanted pig lungs, preserved in Perfadex at 4 °C for 10 h, were subjected to 6 h of EVLP. Subsequently they were allocated to 2 groups: short CIT-2 (CIT-2 =2 h; n=5), and long CIT-2 (CIT-2 =10 h; n=5). In a control group, (n=6), explanted lungs were placed in cold static preservation for 24 h without EVLP. After the total preservation period, the left lung was transplanted in all groups.
After 4 h of reperfusion, oxygenation function, acute lung injury score, inflammatory markers, and cell death pathway markers, were similar between short and long CIT-2 groups. Interestingly, both EVLP groups fared significantly better than the control group in oxygenation function (P<0.05) (Figure 1).
Figure 1.
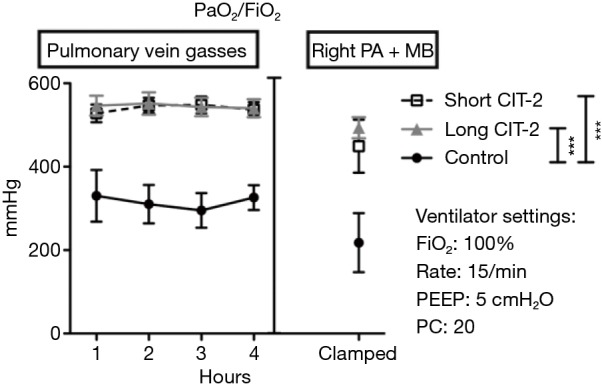
Pulmonary physiology after left single lung transplant and clamping right pulmonary artery. ***, P<0.05 by ANOVA with Tukey post-test. Data were presented as mean ± SEM. SEM, standard error of the mean; PEEP, positive end-expiratory pressure; PC, pressure control; CIT, cold ischemia time.
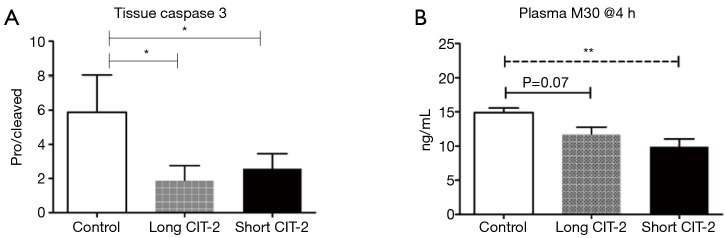
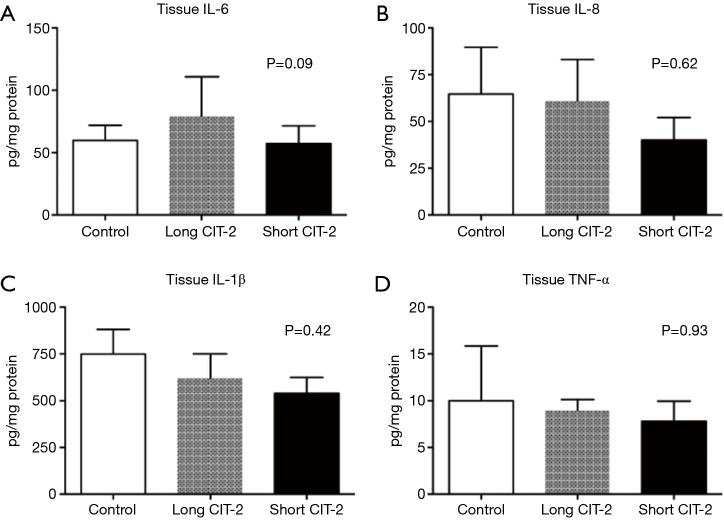
This study is limited by the fact that the explanted lungs were taken from healthy pigs, as opposed to brain dead or circulatory death subjects, which would more closely mimic the clinical scenario. Nevertheless, the study shows that in a pig model, post-transplant lung function in allografts that underwent a prolonged post EVLP CIT-2 (total preservation time: 26 h) was reliably and reproducibly excellent and equivalent to a short CIT-2 (total preservation time 18 h) (Figure 1). Histologic assessment by acute lung injury score (Figure 2), cell death markers (tissue caspase 3 and M30), and inflammatory markers (IL-6, IL-8, IL-1β, TNF-α) were not significantly different after extended CIT-2 (Figures 3,4). These findings support the combination of cold and warm preservation techniques to safely extend the preservation time of donor lungs. This may have important implications for clinical implementation of EVLP, organ allocation and transportation, and transformation of lung transplantation into a semi-elective procedure.
Figure 2.
Microscopic assessment of allografts after 4-h reperfusion (H&E staining at 200× magnification). CIT, cold ischemia time.
Figure 3.
Cell death markers not significantly different between EVLP groups. *, P<0.01 by one-way ANOVA with Tukey’s post-hoc test; **, P<0.01 by one-way ANOVA with Tukey’s post-hoc test. EVLP, ex vivo lung perfusion; CIT, cold ischemia time.
Figure 4.
Inflammatory markers are comparable between all groups at 4-h reperfusion. One-way ANOVA with Tukey’s post-hoc test was used for data analysis. CIT, cold ischemia time.
EVLP and molecular diagnosis
EVLP allows clinicians to gather objective physiologic parameters during perfusion and has significantly increased the utilization of marginal donor lungs. However, PGD still developed in 21% of donor lungs that were deemed satisfactory after EVLP assessment with physiologic and radiologic parameters and were used for clinical lung transplantation. EVLP provides the opportunity to identify potential biomarkers that may be predictive of post-transplant outcomes and allow further refinement of donor lung selection prior to implantation of the donor lung.
Metabolic profiling
During EVLP, the cold preserved donor lungs are rewarmed to normothermia and perfused for 4 h while being ventilated. These lungs inevitably undergo profound changes in metabolism and these changes may be reflected in the metabolites in the perfusion fluid in the EVLP circuit. The Toronto group postulated that by correlating the metabolic profile of the EVLP perfusion fluid with the development of PGD 3, the most severe form of PGD, potential biomarkers predictive of donor lung quality may be identified (8).
Between 2008 and 2011, EVLP was performed on 43 marginal donor lungs which were all transplanted. PGD 3 developed in 7 of these lungs, while the remaining 43 did not develop PGD 3. EVLP perfusate fluid was taken from the circuit at 1 h and four h of perfusion and underwent mass spectrometry non-targeted metabolic profiling. The metabolic profiles were correlated with the development of PGD 3 vs. non-PGD 3.
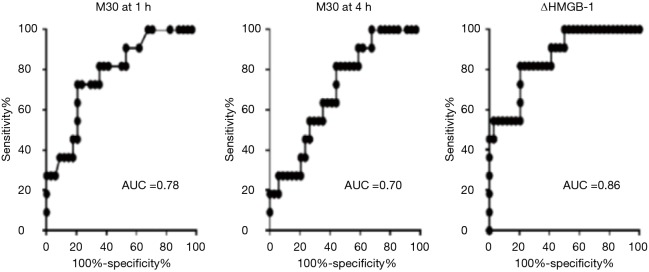
For EVLP perfusate at 1-h perfusion (EVLP-1 h), a logistic regression model based on the levels of palmitoyl-sphingomyelin, 5-aminovalerate, and decanoylcarnitine, yielded a receiver operator characteristics (ROC) curve with an area under the curve (AUC) of 0.987 in differentiating PGD 3 from non-PGD 3 outcomes (Figure 5). Permutation testing of the logistic regression model showed a high level of significance (P<0.0005). For the metabolic profile at EVLP perfusate at 4-h perfusion (EVLP-4 h), a logistic regression model based on the levels of N2-methylguanosine, 5-aminovalerate, oleamide, and decanoylcarnitine, yielded a ROC curve with AUC 0.985 in differentiating PGD 3 from non-PGD 3 outcomes (Figure 6). Permutation testing of the model showed a high level of significance (P<0.0005). Cross validation yielded an AUC (mean ± SD) of 87%±5% and 85%±6% for these models, respectively.
Figure 5.
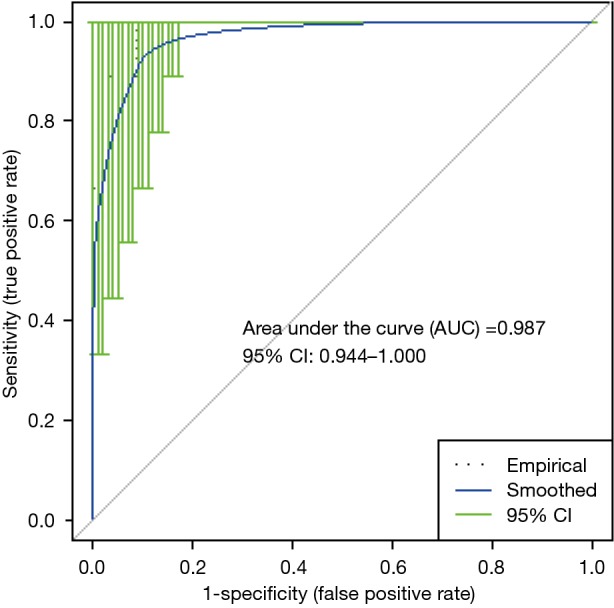
Receiver operator characteristics and area under the curve for EVLP-1 h PGD 3 (n=9) vs. non-PGD 3 (n=34). EVLP, ex vivo lung perfusion; PGD, primary graft dysfunction.
Figure 6.
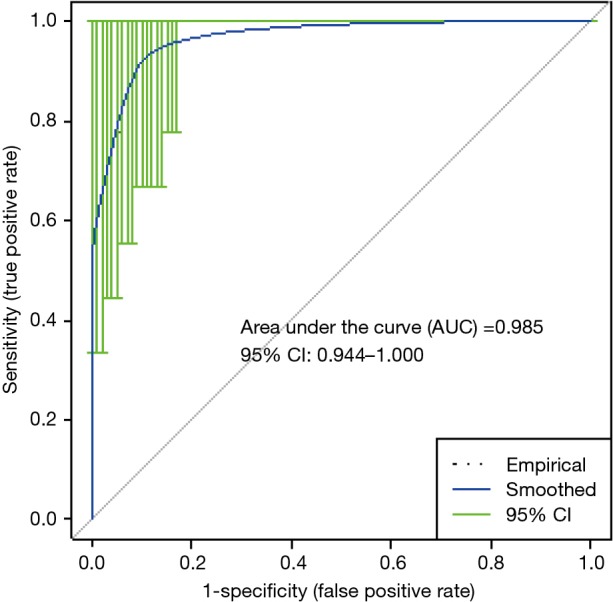
Receiver operator characteristics and area under the curve for EVLP-4 h PGD 3 (n=9) vs. non-PGD 3 (n=34). EVLP, ex vivo lung perfusion; PGD, primary graft dysfunction.
This preliminary exploratory study, which is the first report on the use of metabolic profiling in clinical lung transplantation and EVLP, yielded two panels of metabolites in EVLP perfusate that were highly correlated with the development of PGD 3, and these metabolites may be potential biomarkers that can be used during EVLP to improve donor lung selection and lung transplant outcomes. This will require further validation in a larger sample of EVLP perfusates. Based on the biochemical pathways where these metabolites are involved, further studies are needed to determine whether cell membrane remodeling, fatty acid oxidation and nucleotide degradation yield insight into the development of PGD 3.
Apoptosis markers
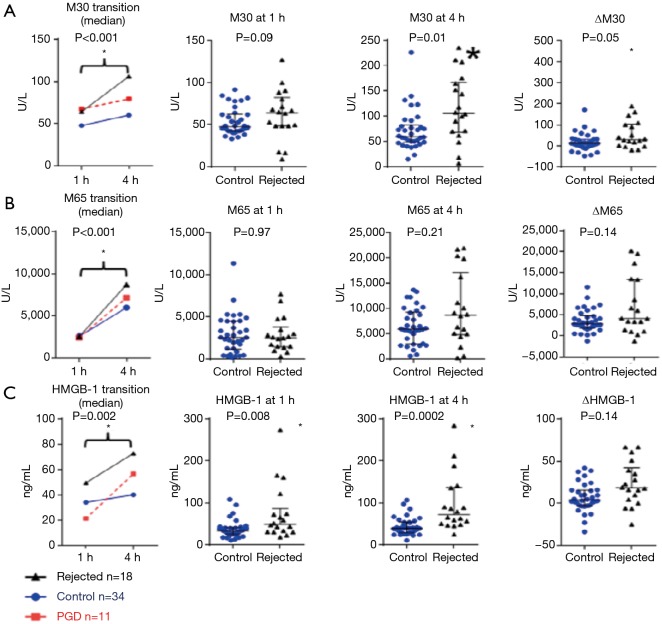
Another EVLP biomarker study from Toronto focused on the role of apoptosis during reperfusion of the transplanted lung (9). The degree of donor lung epithelial cell death is associated with inferior clinical outcomes after lung transplant. The levels of M30 (which reflects epithelial cell apoptosis), M65 (which reflects total epithelial cell death) and high mobility group box 1 (HMGB-1, which is related to cell death and inflammation) in EVLP perfusate were measured and correlated with the incidence of PGD after lung transplantation.
Out of 69 marginal lungs that underwent EVLP, 21 lungs were declined and 48 lungs were used for double lung transplant. Post transplantation, 34 recipients did not develop PGD 3. M30 was significantly elevated in the patients who developed PGD 3 compared to the non-PGD patients at one hour and 4 h of perfusion. The increase in HMGB-1 was significantly greater in the PGD 3 group than the non-PGD 3 group. In a Cox regression analysis, higher levels or greater increase in M30, and a greater increase in HMGB-1 were associated with higher mortality after lung transplant (Table 1, Figure 7). When compared to EVLP perfusate from donor lungs that were rejected for transplantation, the levels of death markers were higher in the rejected lungs than non-PGD lungs (Figure 8). This study shows that the levels of M30 and HMGB-1 in the EVLP perfusate correlate with the development of PGD 3 after lung transplant and may be useful as biomarkers to improve donor lung assessment during EVLP.
Table 1. Univariate Cox regression analysis with survival.
| Characteristicsa | Transplanted groupb (N=45) | Hazard ratio for death (95% CI)c | P value |
|---|---|---|---|
| Biomarkers | |||
| M30 level, U/L | |||
| At 1 h | 59.1±20.4 | 1.349 (1.039–1.752) | 0.025 |
| At 4 h | 88.2±82.9 | 1.093 (1.022–1.169) | 0.009 |
| ∆M30 | 29.1±74.9 | 1.094 (1.013–1.181) | 0.022 |
| M65 level, U/L | |||
| At 1 h | 3,199±2,602 | 1.000 (0.998–1.003) | 0.729 |
| At 4 h | 7,591±6,068 | 1.001 (1.000–1.002) | 0.200 |
| ∆M65 | 4,392±4,473 | 1.001 (1.000–1.002) | 0.130 |
| HMGB-1 level, ng/mL | |||
| At 1 h | 35.3±20.8 | 0.574 (0.313–1.050) | 0.072 |
| At 4 h | 49.8±26.1 | 1.087 (1.875–1.351) | 0.452 |
| ∆HMGB-1 | 14.5±22.9 | 1.383 (1.062–1.801) | 0.016 |
| Donor variables | |||
| Age, years | 43.1±14.6 | 0.996 (0.952–1.041) | 0.851 |
| Female | 23 (51.1) | 1.086 (0.562–2.098) | 0.806 |
| DCD | 18 (40.0) | 1.084 (0.181–6.503) | 0.930 |
| Smoking history | 26 (57.8) | 3.557 (0.725–17.710) | 0.121 |
| Cause of death: head trauma | 9 (20.0) | 0.550 (0.069–4.406) | 0.573 |
a, continuous variables are expressed as mean ± standard deviation and categoric variables as number (%); b, transplanted group = the control + primary graft dysfunction groups; c, hazard ratio is reported by increase of each 10 U/L for M30 and M65 and each 10 ng/mL for HMGB-1, respectively. CI, confidence interval; DCD, donation after cardiac death; HMGB-1, high mobility group box; ∆, refers to the difference between the value at 4 hours of ex vivo lung perfusion and the value at 1 hour of ex vivo lung perfusion.
Figure 7.
Receiving operator characteristics and area under the curve analyses for prediction of PGD by EVLP cell death markers. EVLP, ex vivo lung perfusion; PGD, primary graft dysfunction.
Figure 8.
Cell death markers in rejected lungs vs. transplanted lungs. Rejected lungs exhibit higher levels of perfusate cell death markers than control group lungs. (A) M30 levels; (B) M65 levels; (C) HMGB-1 levels. *, P<0.05. PGD, primary graft dysfunction.
EVLP as platform for organ repair
An area of active research is to investigate the potential for EVLP to allow individualized ex vivo treatment/repair of certain damaged donor lungs, and thereby further increase the number of useable donor lungs for clinical lung transplantation.
Ex vivo thrombolysis
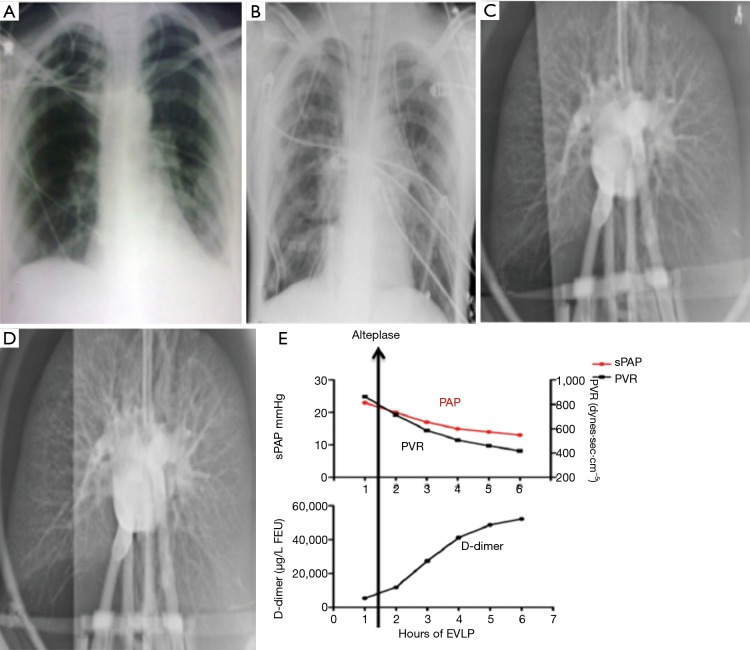
A case report by Machuca et al. describes a brain dead lung donor with a clinical picture of stroke and pulmonary embolism (10). The donor assessment revealed dilated right atrium and right ventricle, and a right ventricular systolic pressure (RVSP) 52 mmHg. The PaO2 FiO2 ratio was 266. At procurement the pulmonary artery pressure was 41/30 mmHg, and during donor lung explantation, large thrombi were extracted from the pulmonary artery during retrograde flush. The donor lung was placed on EVLP, during which time ex vivo thrombolysis was given. Following ex vivo thrombolysis, the pulmonary hemodynamics during EVLP improved with reduction of pulmonary artery pressure and pulmonary vascular resistance (PVR), and D-dimer levels in the EVLP perfusate was noted to have risen (Figure 9). Following EVLP, the donor lungs were deemed satisfactory, and double lung transplant was performed on a cystic fibrosis male patient, who had a satisfactory post-transplant outcome. CT chest done on POD 15 confirmed the pulmonary vasculature was clear. This case report is the first in the reported literature where injury specific treatment was administered ex vivo in a damaged donor lung, which was successfully salvaged for clinical lung transplantation.
Figure 9.
Ex vivo thrombolysis in donor lung. EVLP, ex vivo lung perfusion; sPAP, systolic pulmonary arterial pressure; PVR, pulmonary vascular resistance.
Ex vivo treatment of infected lung
A common reason for declining donor lungs in clinical transplantation is because of concerns of infection, and the risk increases with prolonged intubation and ventilation of intensive care patients, and affects up to 28% of intubated patients. It is known that 46–89% of donor lungs have positive bronchoalveolar lavage (BAL) bacterial cultures, and using such donor lungs significantly increase the risk of post-transplant infection and may lead to poor outcome. Conceptually, EVLP provides an ideal platform for the use of broad-spectrum antibiotics at adequate, or even supra-clinical doses, for the treatment of infected donor lungs, without collateral drug toxicity from the treatment to other organs, which might be a concern with lungs still inside the potential organ donor. This question was addressed by Nakajima et al. (11).
Human donor lungs which were rejected for clinical transplantation because of clinical concerns of infection, based on purulent secretions on bronchoscopy, infiltrates on chest X-ray, and intraoperative evaluation during planned organ retrieval, were randomly assigned to high-dose broad spectrum antibiotic treatment group (n=8), or control group (n=7). All lungs were placed on EVLP for 12 h. In the antibiotics group the lungs were treated with ciprofloxacin 400 mg or azithromycin 500 mg, and vancomycin 15 mg/kg and meropenem 2 g.
A quantitative decrease in bacterial counts in BAL was found in all antibiotics treated lungs, but only in two control cases. EVLP with antibiotics treatment significantly improved pulmonary oxygenation and compliance and reduced PVR. EVLP perfusate endotoxin levels at 12 h were significantly lower in antibiotics group. EVLP perfusate endotoxin levels at 12 h were strongly correlated with levels of TNF-α, IL-1β, MIP 1α and MIP 1β. Although this study is limited by the lack of post-transplant outcomes, this preclinical study using rejected human donor lungs with proven infection demonstrates that broad spectrum antibiotic therapy administered during EVLP is a promising avenue to pursue the goal of ex vivo organ repair. Further research is needed to fine tune the assessment, perhaps using molecular diagnostic approaches, and the treatment of infected donor lungs, to determine whether such lungs may be transplantable. This may eventually significantly expand the pool of useable donor lungs for clinical transplantation.
Ex vivo treatment of gastric acid aspiration
Nakajima et al. studied the problem of gastric acid aspiration which is common in potential organ donors with neurological deficits, and using donor lungs with this injury may lead to severe PGD (12). This results in a very high rate of decline of donor lungs with suspected or proven gastric acid aspiration.
In this preclinical study, the Toronto group investigated the role of EVLP in a pig model of gastric acid aspiration. They hypothesized that lung lavage can remove inflammatory components, and exogenous surfactant administration can supplement loss of endogenous surfactant caused by injury and lavage, maintain biophysical properties, and restore lung function damage caused by gastric acid aspiration.
After cold preservation for 10 h, pig lungs damaged by gastric acid were placed on EVLP for 6 h, during which time these lungs underwent: (I) no treatment, (II) lung lavage, (III) exogenous surfactant administration, and (IV) lung lavage followed by surfactant. These lungs were then transplanted (single left lung transplant) and evaluated after 4 h of perfusion.
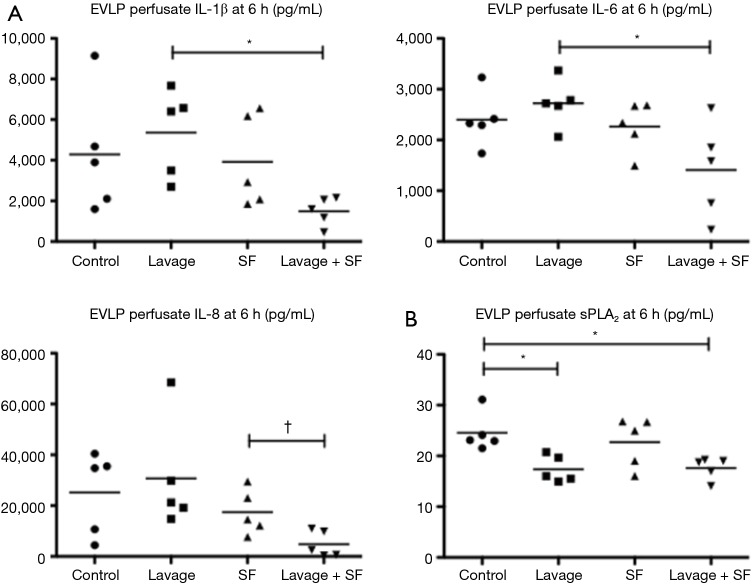
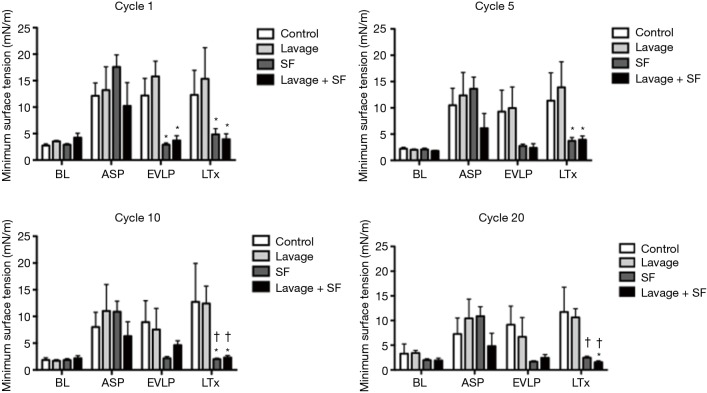
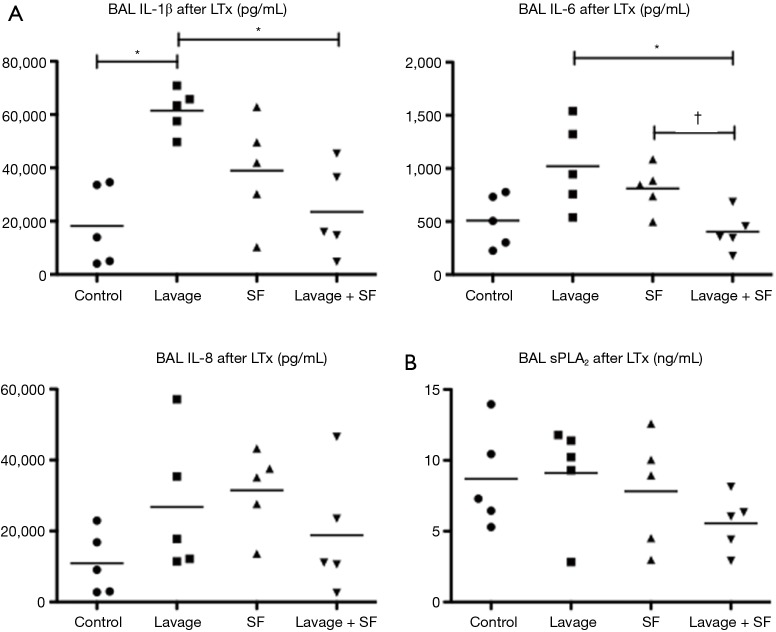
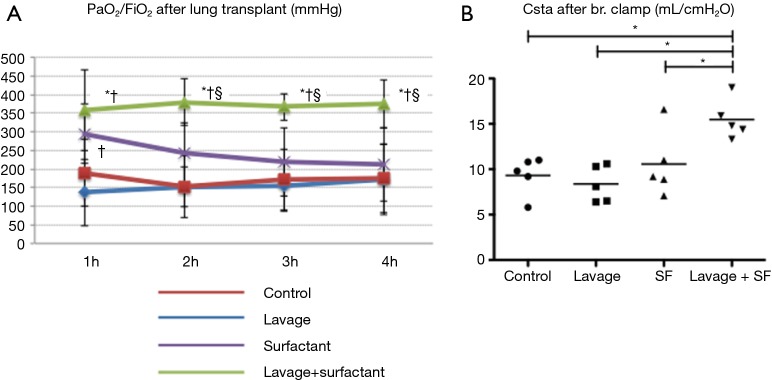
They found that physiologic function significantly improved after adding surfactant during EVLP (Figure 10). The levels of IL-1B, Il-6, IL-8 (all closely related to 30-day mortality in clinical lung transplant) and secretory phospholipase A2 in the EVLP perfusate were significantly lower in the lavage + surfactant group (Figure 11). Total phosphatidylcholine was increased, and minimum surface tension recovered to normal levels in the bronchoalveolar fluid after adding surfactant (Figure 12). Surfactant dysfunction is thought to be caused by the conversion of the active surfactant phosphatidylcholine to the inactive form lysophosphatidylcholine by phospholipase A2. Lysophosphatidylcholine in the bronchoalveolar fluid was found to be significantly lower in the lavage + surfactant group (Table 2). The levels of inflammatory mediators IL-1β and IL-6 in the BAL taken 4 h after transplantation were significantly lower in the lavage + surfactant group (Figure 13). Post-transplant lung function was significantly better in the lavage + surfactant group, compared to all other groups (Figure 14).
Figure 10.
Gastric acid aspiration injured donor lung function during EVLP. EVLP, ex vivo lung perfusion; Csta, static compliance; PVR, pulmonary vascular resistance. *, P<0.05 vs. control group; †, P<0.05 vs. lavage group.
Figure 11.
Perfusate inflammatory mediators and secretary phospholipase A2 (sPLA2) levels. EVLP perfusate (A) inflammatory mediators interleukins and (B) secretary phospholipase A2 levels at 6 hours were reduced by lung lavage and surfactant replacement. EVLP, ex vivo lung perfusion; PVR, pulmonary vascular resistance; SF, surfactant. *, P<0.05; †, P=0.0245.
Figure 12.
Minimum surface tension during dynamic compression. BL, baseline; ASP, after aspiration injury; EVLP, ex vivo lung perfusion; LTx, lung transplantation; SF, surfactant. *, P<0.05 vs. lavage group; †, P<0.05 vs. control group.
Table 2. Lysophosphatidylcholine in bronchoalveolar fluid.
| Variables | Control (mean ± SD) | Lavage (mean ± SD) | Surfactant (mean ± SD) | Lavage+ surfactant (mean ± SD) | P value |
|---|---|---|---|---|---|
| Total PC (ng/kg body weight) | 5,714±4,122 | 4,640±1,929 | 11,772±9,260 | 7,497±4,521 | 0.2800 |
| PC 30/0 | 375.5±258.6 | 257.5±108.0 | 1,460.3±1,373.6 | 798.6±521.7 | 0.0400 |
| PC 32/0 | 2,723.8±1,973.2 | 2,309.8±953.4 | 4,817.6±3,258.1 | 3,090.9±1,682.2 | 0.3900 |
| PC 34/0 | 283.7±228.6 | 217.4±110.8 | 546.1±420.4 | 416.6±263.2 | 0.2800 |
| PC 34/1 | 1,002.1±740.2 | 752.0±266.4 | 2,752.6±2,488.0 | 1,808.0±1,179.9 | 0.1100 |
| PC 34/2 | 652.2±479.3 | 544.5±261.4 | 1,237.9±987.9 | 699.0±466.8 | 0.5400 |
| PC 36/1 | 113.5±67.6 | 96.1±56.7 | 284.4±275.5 | 227.7±157.0 | 0.2600 |
| PC 36/2 | 271.9±184.9 | 241.2±141.6 | 415.2±314.7 | 279.3±149.8 | 0.7900 |
| PC 36/4 | 126.2±125.9 | 85.8±44.5 | 141.3±110.9 | 78.5±56.8 | 0.6600 |
| PC 38/4 | 116.1±84.5 | 102.5±57.4 | 103.0±69.8 | 71.6±48.4 | 0.7000 |
| PC 38/6 | 37.8±38.0 | 23.8±11.5 | 32.0±22.8 | 20.8±14.8 | 0.6300 |
| PC 40/6 | 11.4±7.2 | 9.1±3.6 | 8.0±4.1 | 6.1±3.7 | 0.3400 |
| Total LPC (ng/kg body weight) | 12.07±7.70 | 11.88±7.11 | 18.73±11.87 | 7.49±4.85* | 0.1500 |
| LPC 16:0 | 7.57±4.66 | 8.04±5.07 | 12.31±7.90 | 4.80±3.20 | 0.1700 |
| LPC 18:0 | 2.26±1.36 | 1.84±0.89 | 3.67±2.85 | 1.58±1.10 | 0.3700 |
| LPC 18:1 | 2.24±1.75 | 2.00±1.30 | 2.76±1.73 | 1.11±0.57 | 0.3900 |
| Total PC/LPC | 585±287 | 505±274 | 675±369 | 1009±143 | 0.0438 |
*, P=0.0317 vs. surfactant group by Mann-Whitney test. LPC, lysophosphatidylcholine; PC, phosphatidylcholine; SD, standard deviation; PC 30/0, PC 16:0/14:0 and PC 14:0/16:0; PC 32/0, PC 16:0/16:0, PC 14:0/18:0 and PC 18:0/14:0; PC 34/0, PC 16:0/18:0 and PC 18:0/16:0; PC 34/1, PC 16:0/18:1; PC 34/2, PC 16:0/18:2; PC 36/1, PC 18:0/18:1 and PC 18:1/18:0; PC 36/2, PC 18:0/18:2; PC 36/4: PC 16:0/20:4; PC 38/4, PC 18:0/20:4; PC 38/6, PC 16:0/22:6; PC 40/6, PC 18:0/20:6.
Figure 13.
Inflammatory mediators in BAL post-LTx. BAL, bronchoalveolar lavage; LTx, lung transplantation; SF, surfactant; sPLA2, secretory phospholipase A2. *, P<0.05; †, P=0.0159.
Figure 14.
Post lung transplant lung function. SF, surfactant. *, P<0.05 vs. control group; †, P<0.05 vs. lavage group; §, P<0.05 vs. surfactant group.
Although the use of lung lavage and surfactant administration have been previously described for gastric aspiration, in the clinical setting where a potential donor shows deteriorating lung function, this has practical limitations. In this acute pig lung transplant model, the use of lung lavage and surfactant during EVLP is associated with reduction of key inflammatory mediators, and is shown to result in superior post-transplant lung physiologic function. This preclinical large animal study demonstrates the promise of EVLP as a platform for repair as well as organ evaluation, and further studies are needed to identify the best strategy to recondition donor lungs injured by gastric acid aspiration for clinical lung transplantation in the future.
Conclusions
In summary, this review attempts to illustrate with some examples of preclinical and clinical studies from the Toronto lung transplant program, that the lung transplant community is only just starting to utilize EVLP in the expansion of the pool of useable donor lungs for clinical transplantation. EVLP can potentially transform the logistics of clinical lung transplantation by allowing extended cold storage without affecting the post-transplant outcomes. The identification and validation of reliable molecular biomarkers that can enable clinicians to make a decision regarding the quality of a marginal donor lung will greatly improve the accuracy in donor lung selection and therefore the outcome of lung transplantation. Marginal donor lungs deemed to be unsuitable for clinical transplantation during initial clinical and EVLP evaluation may undergo injury-specific ex vivo organ repair, which may then render them suitable for transplantation after detailed reevaluation. Moreover, researchers are looking at other molecular diagnosis platforms, such as gene expression profiling in the context of EVLP (13), and other treatment modalities, including the use of adenoviral gene therapy in animal models of EVLP lung transplant (14). Clearly these findings need to be validated to confirm whether they can be translated into clinical lung transplant practice. Nevertheless, it seems promising that EVLP may have an important role beyond the current application of physiologic evaluation of marginal donor lungs.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cypel M, Yeung JC, Liu M, et al. Normothermic Ex vivo Lung Perfusion in Clinical Lung Transplantation. New Eng J Med 2011;364:1431-40. 10.1056/NEJMoa1014597 [DOI] [PubMed] [Google Scholar]
- 2.Tuttle-Newhall JE, Krishnan SM, Levy MF, et al. Organ donation and utilization in the United States: 1998-2007. Am J Transplant 2009;9:879-93. 10.1111/j.1600-6143.2009.02565.x [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Wang Y, Fang X, et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet 2002;360:619-20. 10.1016/S0140-6736(02)09774-X [DOI] [PubMed] [Google Scholar]
- 4.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319-25. 10.1016/j.healun.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 5.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. The Journal of thoracic and cardiovascular surgery 2012;144:1200-6. 10.1016/j.jtcvs.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 6.Wigfield CH, Cypel M, Yeung J, et al. Successful emergent lung transplantation after remote ex vivo perfusion optimization and transportation of donor lungs. Am J Transplant 2012;12:2838-44. 10.1111/j.1600-6143.2012.04175.x [DOI] [PubMed] [Google Scholar]
- 7.Hsin MK, Iskender I, Nakajima D, et al. Extension of donor lung preservation with hypothermic storage after normothermic ex vivo lung perfusion. J Heart Lung Transplant 2016;35:130-6. 10.1016/j.healun.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 8.Hsin MK, Zamel R, Cypel M, et al. Metabolic Profile of Ex Vivo Lung Perfusate Yields Biomarkers for Lung Transplant Outcomes. Ann Surg 2018;267:196-7. 10.1097/SLA.0000000000002016 [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K, Cypel M, Juvet S, et al. Higher M30 and high mobility group box 1 protein levels in ex vivo lung perfusate are associated with primary graft dysfunction after human lung transplantation. J Heart Lung Transplant 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Machuca TN, Hsin MK, Ott HC, et al. Injury-specific ex vivo treatment of the donor lung: pulmonary thrombolysis followed by successful lung transplantation. Am J Respir Crit Care Med 2013;188:878-80. 10.1164/rccm.201302-0368LE [DOI] [PubMed] [Google Scholar]
- 11.Nakajima D, Cypel M, Bonato R, et al. Ex Vivo Perfusion Treatment of Infection in Human Donor Lungs. Am J Transplant 2016;16:1229-37. 10.1111/ajt.13562 [DOI] [PubMed] [Google Scholar]
- 12.Nakajima D, Liu M, Ohsumi A, et al. Lung Lavage and Surfactant Replacement During Ex Vivo Lung Perfusion for Treatment of Gastric Acid Aspiration-Induced Donor Lung Injury. J Heart Lung Transplant 2017;36:577-85. 10.1016/j.healun.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 13.Yeung JC, Zamel R, Klement W, et al. Towards Donor Lung Recovery - Gene Expression Changes During Ex Vivo Lung Perfusion of Human Lungs. Am J Transplant 2018. [Epub ahead of print] 10.1111/ajt.14700 [DOI] [PubMed] [Google Scholar]
- 14.Cypel M, Liu M, Rubacha M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med 2009;1:4ra9. 10.1126/scitranslmed.3000266 [DOI] [PubMed] [Google Scholar]