Abstract
Background
The standard first-line chemotherapy for patients with recurrent or metastatic nasopharyngeal carcinoma (R/M NPC) has not been well established. We conducted a pooled meta-analysis to evaluate the efficacy of commonly used first-line chemotherapy in this disease.
Methods
Electronic databases including PubMed, Embase, and Corchrane library were searched for eligible literatures. Objective response rate (ORR), disease control rate (DCR), progression free survival (PFS), and overall survival (OS) were pooled with the 95% confidence interval (CI) using R software.
Results
Totally 973 patients were available for analysis from 14 phase II single arm clinical trials and 2 phase III randomized clinical trials. Four regimens were identified including 5-fluorouracil plus platinum (FP), gemcitabine plus platinum (GP), taxanes plus platinum (TP), and triplet combination regimen. Of these four regimens, triplet combination regimen demonstrated best short-term efficacy with a highest ORR (0.74; 95% CI, 0.62–0.87), DCR (0.91; 95% CI, 0.87–0.95), and 6-month PFS rate (0.83; 95% CI, 0.75–0.91), while 1-year OS rate (0.74; 95% CI, 0.61–0.87) was a little lower than TP regimen. Meanwhile, TP regimen showed best prognosis with a highest 1-year OS rate of 0.79 (95% CI, 0.65–0.92) and pretty good short-term efficacy with an ORR of 0.60 (95% CI, 0.48–0.72) and a DCR of 0.92 (95% CI, 0.86–0.98) comparable with triplet combination therapy. FP regimen had the lowest ORR (0.52; 95% CI, 0.38–0.65) and 1-year OS rate (0.63; 95% CI, 0.57–0.69). Efficacy of GP regimen fell between FP and TP regimens with an ORR of 0.54 (95% CI, 0.38–0.65), a DCR of 0.85 (95% CI, 0.71–0.93), a 6-month PFS rate of 0.69 (95% CI, 0.60–0.78) and a 1-year OS rate of 0.71 (95% CI, 0.61–0.80).
Conclusions
Among four commonly used first-line chemotherapy regimens for R/M NPC, triplet combination regimen showed best short-term efficacy but failed to improve prognosis. TP regimen demonstrated fairly good short-term efficacy and best long-term efficacy, followed by GP regimen, while FP regimen was the lowest.
Keywords: Chemotherapy, first-line, metastatic, nasopharyngeal carcinoma, recurrent
Introduction
Nasopharyngeal carcinoma (NPC) is characterized by its unique geographic distribution (1). Southeast Asia has one of the highest incidence rates in the world with a prevalence of 20–30 incidence cases per 100,000 people (2). On the basis of high-level evidence, intense-modulated radiotherapy (IMRT) alone or with chemotherapy has become the primary treatment for early or locally advanced NPC, producing a 5-year survival rate of about 85% (3,4). Treatment failures are mainly systemic dissemination, which develop in approximately 20% of patients with locally advanced disease (5,6). Additionally, about 15% of patients present with distant metastases at primary diagnosis (7). The outcome for patients with recurrent or metastatic NPC (R/M NPC) is very poor, with a median overall survival (OS) of about 20 months (8).
NPC is a highly chemotherapy sensitive cancer. Platinum-containing doublet chemotherapy is generally regarded as the standard treatment for patients with R/M NPC. However, due to its unique geographic distribution and low overall incidence, just one phase III randomized clinical trials has conducted by Zhang et al. (9) to evaluate the efficacy and toxicity of gemcitabine plus cisplatin (GP) versus fluorouracil plus cisplatin (FP) in this disease. He demonstrated that the efficacy and tolerability of GP was superior to FP. This is the first and only randomized, phase III, head-to-head clinical trial of first-line chemotherapy in R/M NPC.
In addition to gemcitabine or fluorouracil in combination with platinum, taxanes (including paclitaxel and docetaxel) combined with platinum also has been widely used in practice, which mainly derived from experience of early or locally advanced NPC and several phase II sing arm clinical trials (10-13). Because of the scarcity of phase III clinical trials, whether a survival difference exists among patients receiving different regimens remains unknown. Therefore, we conduct this systematic review and pooled meta-analysis, to analyze the efficacy of commonly used first-line regimens for R/M NPC.
Methods
Search strategy and inclusion criteria
Literature search was conducted in PubMed, Embase, and Corchrane library from establishment date of the electronic database to 28th February, 2018. The following search terms, treated as free text combined with mesh terms, were used: recurrent or metastatic, NPC, clinical trials. The search was restricted to human studies published in English language. References lists of identified studies were hand-searched.
Studies met the following criteria were included: (I) study design: phase II single arm clinical trials or phase II/III randomized clinical trials; (II) patients: histological or cytological confirmed R/M NPC, unsuitable for local treatment, aged 18 years or older; (III) intervention: first-line chemotherapy; (IV) outcome: at least one outcome was available with regard to the treatment efficacy, which include objective response rate (ORR), disease control rate (DCR), progression free survival (PFS), and OS evaluated by Response Evaluation Criteria in Solid Tumors (RECIST). Exclusion criteria were: (I) phase I clinical trial; (II) chemotherapy combined with immunotherapy, targeted therapy or other treatment; (III) concurrent or sequential local treatment such as radiotherapy and surgery was conducted; (IV) patients with other head and neck cancer were included, meanwhile, outcome of patients with NPC was not reported independently; (V) unpublished studies.
Quality assessment and data extraction
Frist, all studies were imported to the literature management software Endnote X7 (http://endnote.com/) to eliminate duplicated records. Two authors independently conducted a preliminary screening of reports by reading titles and abstracts. Then the full texts of potentially relevant articles were downloaded for the second round of screening. When disagreement existed, two authors could discuss with each other or turned to a third reviewer to make the final decision.
The quality of included studies was assessed using the Down and Black checklist (D&B checklist), which is appropriate for both randomized and non-randomized clinical trials. This checklist consisted of 27 items distributed between five sub-scales. The total maximum score was 32. In general, a study scored 16 or more is ranked as high quality study (14).
Two reviewers independently extracted data from the identified studies. Any discrepancies were resolved by consensus. For each study, the following data were collected: year of publication, name of the first author, area of study; study design; baseline characteristics of including patients; intervention including regimens, dosages and cycles; outcomes including ORR, DCR, PFS and OS.
Statistical analysis
Statistical analyses of ORR, DCR, 6-month PFS rate and 1-year OS rate were pooled with the corresponding 95% confidence interval (CI) using the software R version 3.2.2 (http://cran.r-project.org/). When OS and PFS could not be extracted from the original study, the data were deciphered from the K-M survival curves using Engauge software (version 4.1, http://digitizer.sourceforge.net). The heterogeneity between trials was estimated by inconsistency statistic (I2). Heterogeneity was considered non-significant when P>0.05. Because studies included in our study was mostly single arm phase II clinical trials, heterogeneity could be more obvious than randomized clinical trials, even if we had only included high quality studies with a D&B checklist score of 16 or more. So random-effect model was used to compute the pooled prevalence whether heterogeneity existed or not.
Results
Characteristics of identified studies
As shown in Figure 1, 27 studies that met the inclusion criteria were identified from 1,601 studies (15-25). Sixteen out of the 27 included studies were considered high quality with D&B checklist scores equal or above 16, and were included in the meta-analysis. Therefore, there were totally 973 patients were available for analysis from 14 phase II single arm clinical trials and 2 phase III randomized clinical trials. Four regimens were identified including 5-fluorouracil plus platinum (FP, comprised of 3 studies) (9,26,27), gemcitabine plus platinum (GP, comprised of 5 studies) (9,28-31), taxanes plus platinum (TP, comprised of 5 studies) (10-13,32), and triplet combination regimen (comprised of 4 studies) (33-36). Details of the identified studies are shown in Table 1.
Figure 1.
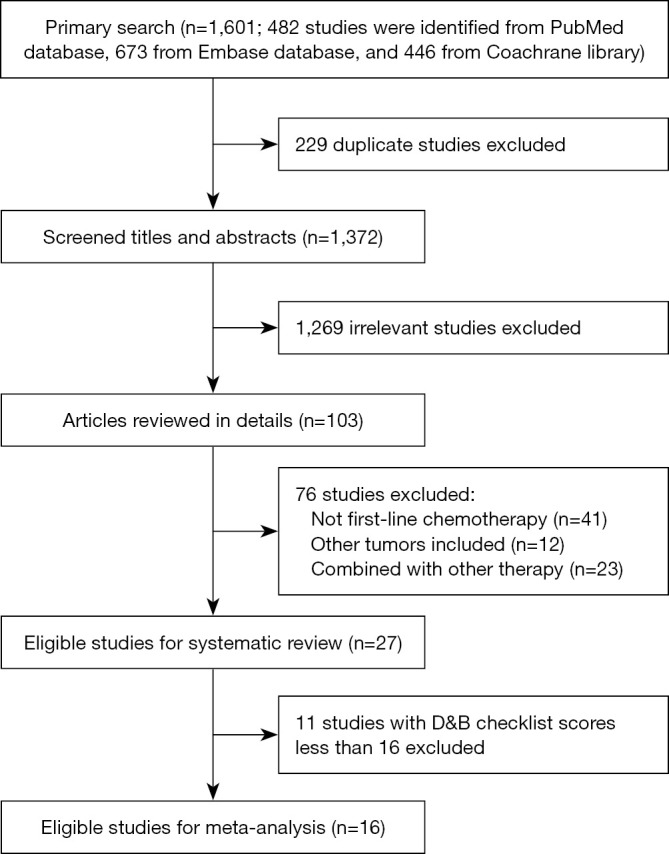
Flow chart demonstrating the process of study selection.
Table 1. Baseline characteristics of included studies.
| Study | Area | Study design | N | Chemotherapy regimen | ORR (%) | DCR (%) | mPFS (months) | mOS (months) | Score |
|---|---|---|---|---|---|---|---|---|---|
| 2017, Zhang (9) | China | Phase 3 RCT | 181 | Arm A: gemcitabine 1 g/m2 d1, 8 + DDP 80 mg/m2 d1 Q3w | 64 | 90 | 7 | 29.1 | 27 |
| 181 | Arm B: 5-FU 4 g/m2 civ96h + DDP 80 mg/m2 d1 Q3w. Maximum of 6 cycles | 42 | 86 | 5.6 | 20.9 | ||||
| 2016, Zhang (32) | China | Phase 2 single arm | 37 | Docetaxel 75 mg/m2 d1 + lobaplatin 30 mg/m2 d1 Q3w | 67.6 | 81.8 | 9.4 | 18.3 | 16 |
| 2015, Hsieh (28) | Taiwan | Phase 2 single arm | 52 | Gemcitabine 1.25 g/m2 d1, 8 + DDP 75 mg/m2 d1 Q3w | 51.9 | 84.6 | 9.8 | 14.6 | 18 |
| 2015, Peng (10) | China | Phase 2 single arm | 73 | Docetaxel 75 mg/m2 d1 + nedaplatin 80 mg/m2 d1 Q3w. 2 cycles at least | 65.8 | 95.9 | 7.9 | 15.7 | 17 |
| 2013, Chen (33) | China | Phase 2 single arm | 95 | Paclitaxel 135 mg/m2 d1 + 5-Fu 0.6–1 g/m2/d civ over 120 h + DDP 25mg/m2/d d1-3 Q3w | 78.9 | 93.6 | 8.6 | 22.7 | 18 |
| 2013, Hsieh (15) | Asia | Phase 2 single arm | 22 | DDP 50 mg/m2 d1, 22 + mitomycin C 6 mg/m2 d1 + oral tegafur uracil 300 mg/m2/d d1–14 + oral leucovorin 60 mg/d d22–35, Q6w | 59 | 63.7 | 10 | 16 | 13 |
| 2012, Chua (26) | Asia | Phase 2 single arm | 39 | Capecitabine 1 g/m2 twice daily for 14 d+ DDP 100 mg/m2 d1 Q3w | 53.8 | 92.3 | 7.3 | 28 | 20 |
| 2012, Ji (11) | Korea | Phase 2 single arm | 46 | Docetaxel 35 mg/m2 d1, 8 + DDP 70 mg/m2 d1, Q3w | 70.2 | 93.6 | 9.6 | 28.5 | 25 |
| 2012, Li (29) | China | Phase 2 RCT | 30 | Arm A: CIK + gemcitabine 1 g/m2 d1, 8 + DDP 20 mg/m2/d d1–5 Q4w, maximum 4 cycles | 70 | 76.7 | 26 | NA | 16 |
| 30 | Arm B: gemcitabine 1 g/m2 d1, 8 + DDP 20 mg/m2/d d1–5 Q4w, maximum 4 cycles | 46.7 | 56.7 | 19 | 23 | ||||
| 2012, You (30) | North America | Phase 2 single arm | 19 | Gemcitabine 1,000 mg/m2 d1, 8 + DDP 70 mg/m2 or carboplatin AUC 5 d1, Q3w. Then switch to erlotinib 150 mg/d Q28d after 6 cycles, or prior if PD | 37 | 95 | 6.3 | NA | 20 |
| 2009, Ma (31) | Hong Kong | Phase 2 single arm | 40 | Gemcitabine 1,000 mg/m2 d1 + oxaliplatin 100 mg/m2 d2 Q2W. Maximum of 12 cycles | 56.1 | 90.20 | 8.9 | 19.6 | 18 |
| 2008, Leong (34) | Singapore | Phase 2 single arm | 28 | Gemcitabine 1,000 mg/m2 + paclitaxel 70 mg/m2 + carboplatin AUC 2.5, d1, 8 Q3w, maximum total of 6 cycles. If PR/CR then continue with weekly 5-FU 450 mg/m2 + leucovorin 30 mg/m2, until PD or maximum treatment duration of 48 weeks | 86 | 89.3 | 8 | 22 | 21 |
| 2008, Li (27) | China | Phase 2 single arm | 48 | Capecitabine 1,000 mg/m2 d1–14 + DDP 80 mg/m2 d1 Q3w. Maximum of 6 cycles | 62.5 | 81.3 | 7.7 | 13.5 | 16 |
| 2005, Chua (12) | Asia | Phase 2 single arm | 19 | Docetaxel 75 mg/m2 d1 + DDP 75 mg/m2 d1 Q3w. Protocol was later modified to 60 mg/m2 for both agents | 62.5 | 100 | 5.6 | 12.4 | 23 |
| 2005, Leong (35) | Singapore | Phase 2 single arm | 32 | Paclitaxel 70 mg/m2 d1, 8 + carboplatin AUC =5 d1 + gemcitabine 1,000 mg/m2 d1, 8 Q3w, maximum total of 8 cycles | 78 | 84.4 | 8.1 | 18.6 | 18 |
| 2004, Ciuleanu (16) | Europe | Phase 2 single arm | 40 | Paclitaxel 175 mg/m2 + carboplatin AUC =6, Q3w | 28 | NA | 3.5 | 11.5 | 15 |
| 2002, McCarthy (13) | North America | Phase 2 single arm | 9 | Docetaxel 75 mg/m2 d1 + DDP 75 mg /m2 d1, Q3w | 22 | NA | 8.4 | NA | 19 |
| 1999, Hasbini (36) | Europe | Phase 2 single arm | 44 | 5FU 800 mg/m2 d1–4 + epirubicin 70 mg/m2 d1 + DDP 100 mg/m2 d1 Q4w. Mitomycin C 10 mg/m2 cycle 1 d1, cycle 3 d1, and cycle 5 d1. Maximum of 6 cycles | 52 | 86.4 | 9 | 14 | 16 |
| 1999, Tan (17) | Asia | Phase 2 single arm | 32 | Paclitaxel 175 mg/m2 + carboplatin AUC =6, Q3w | 75 | NA | 7 | 12 | 13 |
| 1998, Au (18) | Asia | Phase 2 single arm | 24 | Paclitaxel 175 mg/m2 d1 Q3w | 21.7 | 56.4 | 2.5 | 12 | 14 |
| 1998, Siu (19) | North America | Phase 2 single arm | 90 | Schedule 1A: cyclophosphamide 250 mg/m2 + doxorubicin 25 mg/m2 + DDP 50 mg/m2 + methotrexate 50 mg/m2 + bleomycin 15 mg/m2, Q4w | All 73; ALD 86; VMLD 41; MMD 80 | NA | NA | All patients 16; VALD 47; MLD16; MMD 14. | 9 |
| Schedule 1B: cyclophosphamide 200 mg + doxorubicin 20 mg/m2 + DDP 50 mg/m2 + methotrexate 50 mg/m2 + bleomycin 10 mg/m2 + folinic acid 10 mg every 6 h for 4 doses, Q4w | |||||||||
| Schedule 2A: cyclophosphamide 350 mg/m2 + doxorubicin 35 mg/m2 + DDP 70 g/m2 + methotrexate 50 mg/m2 + bleomycin 15 mg/m2, Q4w | |||||||||
| Schedule 2B: cyclophosphamide 350 mg/m2 + doxorubicin 35 mg/m2 + DDP 70 mg/m2 + methotrexate 50 mg/m2 + bleomycin 10 mg/m2 + folinic acid 10 mg every 6 h for 4 doses, Q3w | |||||||||
| 1997, Fountzilas (20) | Europe | Phase 2 single arm | 14 | Paclitaxel 200 mg/m2, carboplatin AUC 7, Q4w, with G-CSF | 57 | NA | 16.5 | NA | 13 |
| 1997, Jelic (21) | Europe | Phase 2 single arm | 80 | Arm A: zorubicin 325 mg/m2/24 h d1 | 20 | NA | NA | NA | 15 |
| Arm B: zorubicin 250 mg/m2/24 h d1 + DDP 30 mg/m2/24 h d2–5 Q4w | 67.5 | NA | |||||||
| 1996, Stein (22) | Africa | Phase 2 single arm | 18 | DDP 50 mg/m2 + ifosfamide 3g/m2 d1–2, Q3–4w | 59 | NA | 6.5 | 13.6 | 10 |
| 1994, Au (23) | Asia | Phase 2 single arm | 24 | 5-FU 1g/m2 d1–5+DDP 33.3 mg/m2/d d1–3 Q3w | 66 | NA | 8 | 11 | 8 |
| 1990, Villalon (24) | Asia | Phase 2 single arm | 24 | Mitoxantrone 12–14 mg/m2, Q3w | 38 | NA | 4.4 | 5.3 | 10 |
| 1987, de Graeff (25) | Europe | Phase 2 single arm | 4 | Doxorubicin 50 mg/m2 Q3w, CCNU 120 mg/m2 Q6w | 80 | NA | NA | NA | 9 |
ORR, objective response rate; DCR, disease control rate; PFS, progression free survival; OS, overall survival; 5-Fu, 5-fluorouracil, DDP, cisplatin; NA, not applicable.
Efficacy
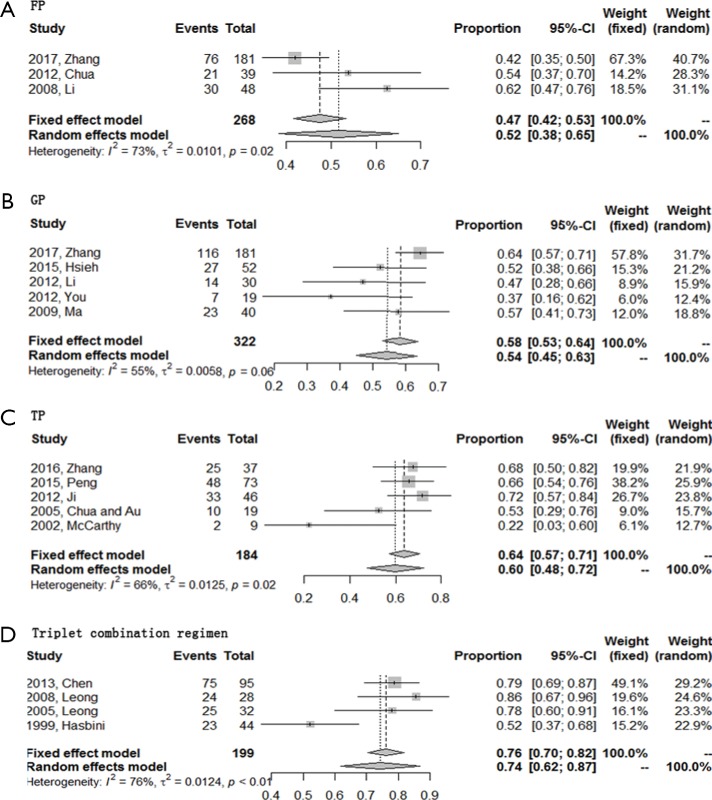
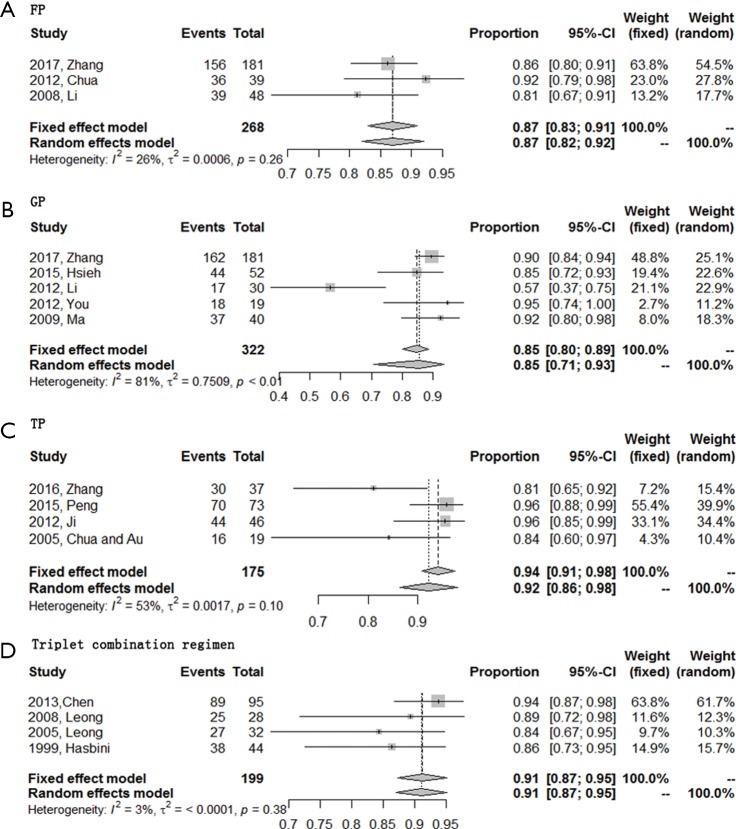
ORR was evaluable for all 16 included studies while DCR could not be extracted from one study about TP regimen. Of these four regimens, triplet combination regimen demonstrated best short-term efficacy with a highest ORR (0.74; 95% CI, 0.62–0.87), followed by TP regimen with an ORR of 0.60 (95% CI, 0.48–0.72). DCR of these two regimens were comparable [0.91 (95% CI, 0.87–0.95) for triplet combination regimen and 0.92 (95% CI, 0.86–0.98) for TP regimen, respectively]. GP and FP regimen together ranked the last with an ORR of 0.54 (95% CI, 0.45–0.63), a DCR of 0.85 (95% CI, 0.71–0.93) for GP regimen, and an ORR of 0.52 (95% CI, 0.38–0.65), a DCR of 0.87 (95% CI, 0.82–0.92) for FP regimen (Figures 2,3).
Figure 2.
Forest plots of the ORR of four first-line chemotherapy regimens in R/M NPC. (A) Triplet combination regimen; (B) TP regimen; (C) GP regimen; (D) FP regimen. R/M NPC, recurrent or metastatic nasopharyngeal carcinoma; TP, taxanes plus platinum; GP, gemcitabine plus platinum; FP, 5-fluorouracil plus platinum; ORR, objective response rate.
Figure 3.
Forest plots of the DCR of four first-line chemotherapy regimens in R/M NPC. (A) Triplet combination regimen; (B) TP regimen; (C) GP regimen; (D) FP regimen. R/M NPC, recurrent or metastatic nasopharyngeal carcinoma; TP, taxanes plus platinum; GP, gemcitabine plus platinum; FP, 5-fluorouracil plus platinum; DCR, disease control rate.
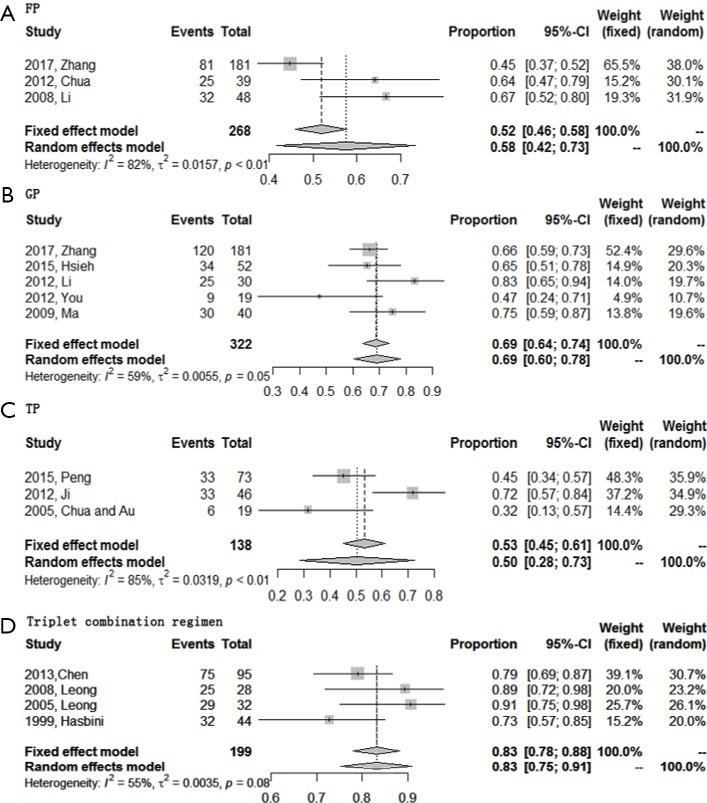
A total of 15 studies reported or could deciphered 6-month PFS rate from K-M survival curve except two studies about TP regimen. Pooled meta-analysis indicated that the 6-month PFS rate of triplet combination regimen ranked top again (0.83; 95% CI, 0.75–0.91), followed by GP regimen (0.69; 95% CI, 0.60–0.78), and then was FP regimen (0.58; 95% CI, 0.42–0.73). Surprisingly, the 6-month PFS rate of TP regimen was the lowest (0.50; 95% CI, 0.28–0.73), which might contribute from the least sample size of this group (Figure 4).
Figure 4.
Forest plots of the 6-month PFS rate of four first-line chemotherapy regimens in R/M NPC. (A) Triplet combination regimen; (B) TP regimen; (C) GP regimen; (D) FP regimen. R/M NPC, recurrent or metastatic nasopharyngeal carcinoma; TP, taxanes plus platinum; GP, gemcitabine plus platinum; FP, 5-fluorouracil plus platinum; PFS, progression free survival.
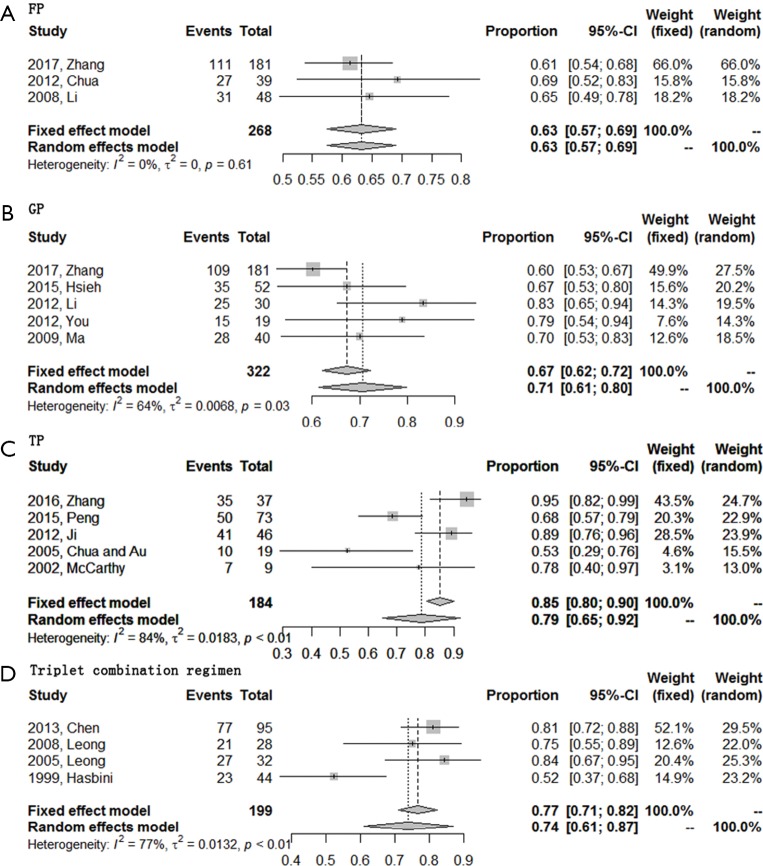
All 16 included studies were available for 1-year OS rate analysis. TP regimen showed highest 1-year OS rate of 0.79 (95% CI, 0.65–0.92). Secondly, GP regimen and triplet combination regimen showed similar 1-year OS rates, with a rate of 0.71 (95% CI, 0.61–0.80) for GP regimen and 0.74 (95% CI, 0.61–0.87) for triplet combination regimen. FP regimen always ranked the last with a 1-year OS rate of 0.63 (95% CI, 0.57–0.69) (Figure 5). The total efficacy of four first-line chemotherapy regimens in R/M NPC was list at length in Table 2.
Figure 5.
Forest plots of the 1y-OS rate of four first-line chemotherapy regimens in R/M NPC. (A) Triplet combination regimen; (B) TP regimen; (C) GP regimen; (D) FP regimen. R/M NPC, recurrent or metastatic nasopharyngeal carcinoma; TP, taxanes plus platinum; GP, gemcitabine plus platinum; FP, 5-fluorouracil plus platinum; OS, overall survival.
Table 2. Efficacy of first-line chemotherapy in RM/NPC.
| Regimen | ORR (95% CI) | DCR (95% CI) | 6-month PFS rate (95% CI) | 1-year OS rate (95% CI) |
|---|---|---|---|---|
| FP | 0.52 (0.38–0.65) | 0.87 (0.82–0.92) | 0.58 (0.42–0.73) | 0.63 (0.57–0.69) |
| GP | 0.54 (0.45–0.63) | 0.85 (0.71–0.93) | 0.69 (0.60–0.78) | 0.71 (0.61–0.80) |
| TP | 0.60 (0.48–0.72) | 0.92 (0.86–0.98) | 0.50 (0.28–0.73) | 0.79 (0.65–0.92) |
| Triplet combination regimen | 0.74 (0.62–0.87)† | 0.91 (0.87–0.95)† | 0.83 (0.75–0.91)† | 0.74 (0.61–0.87) |
†, these pooled data derived from studies using WHO criteria as efficacy evaluation tool. R/M NPC, recurrent or metastatic nasopharyngeal carcinoma; FP, 5-fluorouracil plus platinum; GP, Gemcitabine plus platinum; TP, taxanes plus platinum; ORR, objective response rate; DCR, disease control rate; PFS, progression free survival; OS, overall survival.
Efficacy by different regions
Epstein-Barr virus (EBV) infection is proposed to be one of the main contributing factors in endemic regions, while human papilloma virus (HPV) infection is thought to account more for cases in non-endemic areas. So we conduct sub-group analysis to find out if the etiology cause may affect sensitivity to chemotherapy. Among Asian patients, GP regimen showed better 6-month PFS rate (P=0.04), TP regimen showed better ORR (P=0.011), and triplet combination regimens showed better ORR (P<0.001) and 1-year OS rate (P<0.001) compared with non-Asian patients. Thus, sensitivity to first-line chemotherapy among Asian patients was consistent with our conclusions. However, in non-Asian patients, triplet combination regimen showed best short-term efficacy with highest ORR, 6-month PFS rate, GP regimen showed best long-term efficacy with highest DCR, 1-year OS rate, and TP regimen ranked the last. No clinical trials using FP regimen were conducted in non-Asia areas in our analysis (Table S1, more data can be found in the article. The interested reader can read a supplementary appendix online).
Table S1. Efficacy of first-line chemotherapy in RM/NPC in Asia versus non-Asia regions.
| Regimen | ORR | DCR | 6-month PFS rate | 1-year OS rate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asia | Non-Asia | P | Asia | Non-Asia | P | Asia | Non-Asia | P | Asia | Non-Asia | P | ||||
| FP | 0.44 | – | – | 0.88 | – | – | 0.53 | – | – | 0.63 | – | – | |||
| GP | 0.57 | 0.37 | 0.098 | 0.84 | 0.95 | 0.328 | 0.71 | 0.47 | 0.040 | 0.69 | 0.79 | 0.448 | |||
| TP | 0.67 | 0.22 | 0.011 | 0.92 | – | – | 0.50 | – | – | 0.79 | 0.78 | 1.000 | |||
| Triplet combination regimen | 0.80 | 0.52 | <0.001 | 0.92 | 0.86 | 0.239 | 0.86 | 0.73 | 0.067 | 0.81 | 0.52 | <0.001 | |||
†, these pooled data derived from studies using WHO criteria as efficacy evaluation tool. R/M NPC, recurrent or metastatic nasopharyngeal carcinoma; FP, 5-fluorouracil plus platinum; GP, Gemcitabine plus platinum; TP, taxanes plus platinum; ORR, objective response rate; DCR, disease control rate; PFS, progression free survival; OS, overall survival.
Sensitivity analysis
RECIST has been widely used for efficacy evaluation in solid tumor. The first version of RECIST was released in 1999 (37) and the second in 2009 (38). Several identified studies were conducted before 2000 and adopted WHO criteria (39) to assess efficacy, which may contributed to heterogeneity in our analysis. Therefore, to reduce heterogeneity between studies, sensitivity analyses were conducted by excluding those studies using WHO evaluation criteria.
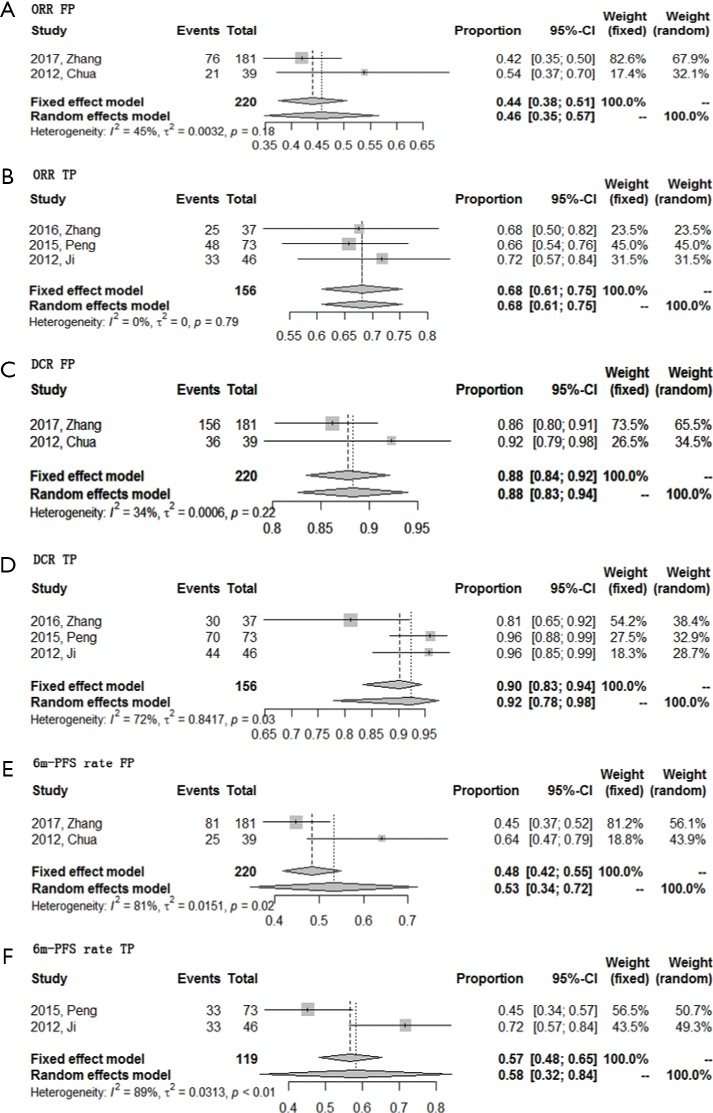
There were totally 6 included studies adopting WHO criteria to evaluate efficacy, 1 in the FP group, 2 in the TP group, and 3 in the triplet combination regimen group. After removing 3 studies, there was only 1 study left in the triplet combination regimen, making it inapplicable for pooled meta-analysis and sensitivity analysis. So sensitivity analyses were conducted in the FP and TP group. The results of the sensitivity analyses were similar compared to the pooled result using all studies (Figure S1, more data can be found in the article. The interested reader can read a supplementary appendix online).
Figure S1.
Sensitivity analyses of first-line triplet combination regimen in R/M NPC. (A) ORR for FP regimen; (B) ORR for TP regimen; (C) DCR for FP regimen; (D) DCR for TP regimen; (E) 6-month PFS rate for FP regimen; (F) 6-month PFS rate for TP regimen. R/M NPC, recurrent or metastatic nasopharyngeal carcinoma; ORR, objective response rate; FP, 5-fluorouracil plus platinum; TP, taxanes plus platinum; DCR, disease control rate; PFS, progression free survival.
A minimum of 10 studies is needed to assess potential publication bias so it was not applicable in our study.
Conclusions
As we all know, outcome of R/M NPC was very poor with a median OS of about 20 months (8). Unique geographic distribution and low overall incidence of this disease makes it difficult for development of phase III randomized clinical trials. As a result, the standard first-line chemotherapy for patients with R/M NPC has not been well established so far. 5-fluorouracil (10,30,35), gemcitabine (9,16,31-33), and taxanes (including paclitaxel and docetaxel) (10-13) combine with platinum have been widely used in practice. However, the evidence mainly derived from experience in early or locally advanced NPC or from phase II single arm clinical trials in R/M NPC. Besides, efficacy of above commonly used regimen for R/M NPC was reported inconsistently. To resolve the problem, we conduct this systematic review and pooled meta-analysis evaluating the efficacy of commonly used regimens for R/M NPC in first-line setting.
Our study showed that although triplet combination regimen demonstrated best short-term efficacy with highest ORR and 6-month PFS rate, it failed to improve prognosis of these patients compared with TP and GP regimen. This might due to intolerable high incidence adverse events of triplet combination regimen. On one hand, the serious toxicity may result in dosage and cycle reduction. On the other hand, excessive adverse events may reduce patients’ confidence and choice in follow-up treatment. TP regimen seems to be more effective compared with other three regimens regarding to the fairly perfect ORR, DCR, and highest 1-year OS rate. So far, no randomized phase III clinical trials has been developed to evaluate the efficacy of first-line TP regimen in R/M NPC, more evidence is needed to verify the conclusion.
In 2016, Zhang et al. (9) reported a phase III randomized clinical trial comparing the efficacy and toxicity of GP versus FP as first-line chemotherapy in R/M NPC, which was the first head-to-head randomized study in this disease. It is reported that ORR was higher in the GP group [0.64 vs. 0.42, relative risk 1.5 (95% CI, 1.2–1.9), P<0.0001] while DCR was similar for both groups (0.9 vs. 0.86). GP prolonged PFS [7.0 vs. 5.6 months; hazard ratio 0.55 (95% CI, 0.44–0.68), P<0.0001]. These results are consistent with our study. Meta-analysis showed ORR and DCR of GP and FP regimens are similar, while 1-year OS rate of GP regimen is a little lower than TP regimen but higher than FP regimen.
Our study is the first pooled meta-analysis to evaluate the efficacy of commonly used first-line chemotherapy in R/M NPC. However, there exist some limitations. First of all, the sample size of our study was not big because limited clinical trials in R/M NPC. For example, in the FP subgroup only three studies were identified. Secondly, all of the outcome data were obtained from literature review instead of individual patient data, which caused incomplete data for some outcomes. Thirdly, due to the long time span, different adverse event evaluation criteria and incomplete report of adverse events of the included studies, tolerability was not included in our meta-analysis. Finally, studies included in our study were mostly single arm phase II clinical trials, making heterogeneity more obvious than randomized clinical trials. We took some measures to reduce the heterogeneity, such as included high-quality studies. However, heterogeneity still exists. Due to the limited size of identified studies in each regimen group, further subgroup analysis could not be conducted to evaluate source of heterogeneity. So random-effect model was used to compute the pooled rate whether heterogeneity existed or not.
In conclusion, among four commonly used chemotherapy regimen for R/M NPC in the first-line setting, TP regimen showed the highest efficacy, followed by GP regimen, while FP regimen was the lowest. Besides, compared with TP and GP, triplet combination regimen has higher short-term efficacy but failed to improve prognosis of these patients. Further phase III randomized clinical trials are needed to verify our conclusions.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal cancer. Cancer Treat Res 2003;114:275-93. 10.1007/0-306-48060-3_11 [DOI] [PubMed] [Google Scholar]
- 2.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 2011;30:114-9. 10.5732/cjc.010.10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. 10.1016/S1470-2045(15)70126-9 [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Hong S, Wang Y, et al. Development and External Validation of Nomograms for Predicting Survival in Nasopharyngeal Carcinoma Patients after Definitive Radiotherapy. Sci Rep 2015;5:15638. 10.1038/srep15638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 1992;23:261-70. 10.1016/0360-3016(92)90740-9 [DOI] [PubMed] [Google Scholar]
- 6.Li AC, Xiao WW, Shen GZ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget 2015;6:24511-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013;31:2861-9. 10.1200/JCO.2012.46.0816 [DOI] [PubMed] [Google Scholar]
- 8.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005;365:2041-54. 10.1016/S0140-6736(05)66698-6 [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016;388:1883-92. 10.1016/S0140-6736(16)31388-5 [DOI] [PubMed] [Google Scholar]
- 10.Peng PJ, Lv BJ, Tang C, et al. Phase II trial of docetaxel combined with nedaplatin for patients with recurrent and metastatic nasopharyngeal carcinoma. Drug Des Devel Ther 2015;9:6401-5. 10.2147/DDDT.S95946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji JH, Yun T, Kim SB, et al. A prospective multicentre phase II study of cisplatin and weekly docetaxel as first-line treatment for recurrent or metastatic nasopharyngeal cancer (KCSG HN07-01). Eur J Cancer 2012;48:3198-204. 10.1016/j.ejca.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 12.Chua DT, Sham JS, Au GK. A phase II study of docetaxel and cisplatin as first-line chemotherapy in patients with metastatic nasopharyngeal carcinoma. Oral Oncol 2005;41:589-95. 10.1016/j.oraloncology.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 13.McCarthy JS, Tannock IF, Degendorfer P, et al. A Phase II trial of docetaxel and cisplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. Oral Oncol 2002;38:686-90. 10.1016/S1368-8375(01)00134-8 [DOI] [PubMed] [Google Scholar]
- 14.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CH, Hsu CL, Wang CH, et al. Cisplatin, tegafur-uracil and leucovorin plus mitomycin C: an acceptably effective and toxic regimen for patients with recurrent or metastatic nasopharyngeal carcinoma. Biomed J 2013;36:229-36. 10.4103/2319-4170.113375 [DOI] [PubMed] [Google Scholar]
- 16.Ciuleanu TE, Fountzilas G, Ciuleanu E, et al. Paclitaxel and carboplatin in relapsed or metastatic nasopharyngeal carcinoma: a multicenter phase II study. J BUON 2004;9:161-5. [PubMed] [Google Scholar]
- 17.Tan EH, Khoo KS, Wee J, et al. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol 1999;10:235-7. 10.1023/A:1008390929826 [DOI] [PubMed] [Google Scholar]
- 18.Au E, Tan EH, Ang PT. Activity of paclitaxel by three-hour infusion in Asian patients with metastatic undifferentiated nasopharyngeal cancer. Ann Oncol 1998;9:327-9. 10.1023/A:1008255220284 [DOI] [PubMed] [Google Scholar]
- 19.Siu LL, Czaykowski PM, Tannock IF. Phase I/II study of the CAPABLE regimen for patients with poorly differentiated carcinoma of the nasopharynx. J Clin Oncol 1998;16:2514-21. 10.1200/JCO.1998.16.7.2514 [DOI] [PubMed] [Google Scholar]
- 20.Fountzilas G, Skarlos D, Athanassiades A, et al. Paclitaxel by three-hour infusion and carboplatin in advanced carcinoma of nasopharynx and other sites of the head and neck. A phase II study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 1997;8:451-5. 10.1023/A:1008279503428 [DOI] [PubMed] [Google Scholar]
- 21.Jelic S, Kovcin V, Milanovic N, et al. Randomized study of zorubicin versus zorubicin-cisplatin in undifferentiated carcinoma of the nasopharynx (UCNT). Ann Oncol 1997;8:739-44. 10.1023/A:1008210527637 [DOI] [PubMed] [Google Scholar]
- 22.Stein ME, Ruff P, Weaving A, et al. A phase II study of cisplatin/ifosfamide in recurrent/metastatic undifferentiated nasopharyngeal carcinoma among young blacks in southern Africa. Am J Clin Oncol 1996;19:386-8. 10.1097/00000421-199608000-00014 [DOI] [PubMed] [Google Scholar]
- 23.Au E, Ang PT. A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol 1994;5:87-9. 10.1093/oxfordjournals.annonc.a058703 [DOI] [PubMed] [Google Scholar]
- 24.Villalon DM, Go Machica ME. Phase II evaluation of mitoxantrone in patients with advanced nasopharyngeal carcinoma. Philippine J Internal Med 1990;28:169-76. [Google Scholar]
- 25.de Graeff A, de Groot JA, Schornagel JH. Chemotherapy with doxorubicin and CCNU in advanced undifferentiated carcinoma of the nasopharynx. A retrospective report on five patients. Neth J Med 1987;31:111-6. [PubMed] [Google Scholar]
- 26.Chua DT, Yiu HH, Seetalarom K, et al. Phase II trial of capecitabine plus cisplatin as first-line therapy in patients with metastatic nasopharyngeal cancer. Head Neck 2012;34:1225-30. 10.1002/hed.21884 [DOI] [PubMed] [Google Scholar]
- 27.Li YH, Wang FH, Jiang WQ, et al. Phase II study of capecitabine and cisplatin combination as first-line chemotherapy in Chinese patients with metastatic nasopharyngeal carcinoma. Cancer Chemother Pharmacol 2008;62:539-44. 10.1007/s00280-007-0641-2 [DOI] [PubMed] [Google Scholar]
- 28.Hsieh JC, Hsu CL, Ng SH, et al. Gemcitabine plus cisplatin for patients with recurrent or metastatic nasopharyngeal carcinoma in Taiwan: a multicenter prospective Phase II trial. Jpn J Clin Oncol 2015;45:819-27. 10.1093/jjco/hyv083 [DOI] [PubMed] [Google Scholar]
- 29.Li JJ, Gu MF, Pan K, et al. Autologous cytokine-induced killer cell transfusion in combination with gemcitabine plus cisplatin regimen chemotherapy for metastatic nasopharyngeal carcinoma. J Immunother 2012;35:189-95. 10.1097/CJI.0b013e318241d9de [DOI] [PubMed] [Google Scholar]
- 30.You B, Le Tourneau C, Chen EX, et al. A Phase II trial of erlotinib as maintenance treatment after gemcitabine plus platinum-based chemotherapy in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Am J Clin Oncol 2012;35:255-60. 10.1097/COC.0b013e31820dbdcc [DOI] [PubMed] [Google Scholar]
- 31.Ma BB, Hui EP, Wong SC, et al. Multicenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma--correlation with excision repair cross-complementing-1 polymorphisms. Ann Oncol 2009;20:1854-9. 10.1093/annonc/mdp065 [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Chen J, Yang S, et al. An open-label, single-arm phase II clinical study of docetaxel plus lobaplatin for Chinese patients with pulmonary and hepatic metastasis of nasopharyngeal carcinoma. Anticancer Drugs 2016;27:685-8. 10.1097/CAD.0000000000000370 [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Wang FH, An X, et al. Triplet combination with paclitaxel, cisplatin and 5-FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemother Pharmacol 2013;71:371-8. 10.1007/s00280-012-2020-x [DOI] [PubMed] [Google Scholar]
- 34.Leong SS, Wee J, Rajan S, et al. Triplet combination of gemcitabine, paclitaxel, and carboplatin followed by maintenance 5-fluorouracil and folinic acid in patients with metastatic nasopharyngeal carcinoma. Cancer 2008;113:1332-7. 10.1002/cncr.23687 [DOI] [PubMed] [Google Scholar]
- 35.Leong SS, Wee J, Tay MH, et al. Paclitaxel, carboplatin, and gemcitabine in metastatic nasopharyngeal carcinoma: a Phase II trial using a triplet combination. Cancer 2005;103:569-75. 10.1002/cncr.20804 [DOI] [PubMed] [Google Scholar]
- 36.Hasbini A, Mahjoubi R, Fandi A, et al. Phase II trial combining mitomycin with 5-fluorouracil, epirubicin, and cisplatin in recurrent and metastatic undifferentiated carcinoma of nasopharyngeal type. Ann Oncol 1999;10:421-5. 10.1023/A:1008342828496 [DOI] [PubMed] [Google Scholar]
- 37.James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst 1999;91:523-8. 10.1093/jnci/91.6.523 [DOI] [PubMed] [Google Scholar]
- 38.Nishino M, Jackman DM, Hatabu H, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 2010;195:W221-8. 10.2214/AJR.09.3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AB, Hoogstraten B, Staquet M, et al. Reporting Results of Cancer Treatment. Cancer 1981;47:207-14. [DOI] [PubMed] [Google Scholar]