Abstract
Background
Polymyxin B hemoperfusion is a strategy to remove circulating endotoxin in patients with sepsis. Previous systematic reviews derived from randomized and non-randomized studies suggested that use of polymyxin B hemoperfusion reduced mortality, based on the pooled data from various time points in the clinical course of sepsis. We conducted a meta-analysis of randomized controlled trials to assess the impact of polymyxin B hemoperfusion specifically on 28-day mortality in patients with sepsis and septic shock.
Methods
PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials were searched for eligible trials from inception through July 30, 2017. All randomized controlled trials were eligible if they examined the impact of polymyxin B hemoperfusion on 28-day mortality in patients with sepsis and septic shock. Risk of bias was evaluated with the Cochrane risk of bias assessment tool. Data were pooled using the DerSimonian and Laird random-effects model.
Results
Seven trials involving 586 participants were identified for the analysis. Use of polymyxin B hemoperfusion was not associated with a reduced risk of 28-day mortality [risk ratio (RR), 0.76; 95% CI, 0.54–1.07] compared with usual care. One unpublished trial also showed no significant 28-day survival benefit.
Conclusions
There is no evidence to support the use of polymyxin B hemoperfusion for patients with sepsis and septic shock with respect to 28-day mortality.
Keywords: Sepsis, septic shock, polymyxin B, hemoperfusion, meta-analysis
Introduction
Sepsis remains common, placing substantial burdens on patients and society. Although the mortality of severe sepsis has decreased over the last two decades, the reported mortality remains as high at 29.2% (1). Sepsis survivors are at a greater risk of recurrent infection, other morbidities, re-admission, and long-term mortality (2-6), and they tend to have impaired quality of life (6). Economic burdens are also substantial: the total hospital costs alone due to severe sepsis have increased to $24.3 billion annually in the United States (7). Given that the basic treatment of sepsis has largely remained unchanged, more effective treatment options for sepsis are needed (8).
Endotoxin, also called lipopolysaccharide, is present on the outer membrane of gram-negative bacteria, and it initiates and mediates the host response in sepsis. Polymyxin is a cyclic cationic polypeptide antibiotic with an ability to bind and neutralize endotoxin. Polymyxin B hemoperfusion is a strategy developed in Japan; polymyxin B is immobilized to polystyrene fibers in a hemoperfusion device and is thought to remove circulating endotoxin.
Previous systematic reviews have concluded that polymyxin B hemoperfusion was associated with a reduced risk of mortality (9-11). However, we suspect that two factors in these studies might have inflated the efficacy of polymyxin B hemoperfusion. First, some reviews pooled the data from both randomized, controlled studies and observational studies, thereby leaving some confounding factors uncontrolled. Second, one review pooled the mortality data from various time points in the clinical course of sepsis. It is known that short-term mortality increases with time, while later mortality is confounded by events unrelated to sepsis (12). Many of the original studies of polymyxin B hemoperfusion reported on mortality from 14 to 60 days, with the others from non-specified time points. However, a major and clinically important endpoint in sepsis research is 28-day mortality (12). We hypothesized that the efficacy of polymyxin B hemoperfusion on 28-day mortality is different from that available from previous systematic reviews.
Consequently, we conducted a meta-analysis of randomized, controlled trials to specifically assess the 28-day mortality benefit of polymyxin B hemoperfusion in patients with sepsis and septic shock, in comparison with usual care.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews (13). The protocol was registered at PROSPERO (CRD42016049581). We searched PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, CINAHL, and Igaku Chuo Zasshi (the largest Japanese database that includes literature and conference proceedings published in Japanese). Google Scholar was searched for studies that cited included trials, and the references of these trials were reviewed for potentially relevant articles. Toray Medical Co., Ltd that markets polymyxin B hemoperfusion was also contacted and asked for randomized controlled trials that tested the efficacy of polymyxin B hemoperfusion in patients with sepsis and septic shock. The search strategy is listed in Table 1. There were no restrictions on language or publication status, and the search was updated on July 30, 2017.
Table 1. Search strategy.
| #1. Sepsis |
| #2. Septic shock |
| #3. Septicaemia OR septicemia |
| #4. Bacteremia OR bacteraemia |
| #5. Bacterial |
| #6. Endotoxic* |
| #7. Endotoxemia OR endotoxemia |
| #8. Endotoxin* |
| #9. Blood stream infection |
| #10. Toxic shock |
| #11. Severe sepsis |
| #12. Sepsis syndrome |
| #13. Shock septic |
| #14. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 |
| #15. Polymyxin B |
| #16. Polymyxin* |
| #17. Toraymyxin |
| #18. PMX |
| #19. PMX-20R |
| #20. Polymyxin B-hemoperfusion |
| #21. Polymyxin B-immobilized fiber column |
| #22. PMX-DHP |
| #23. PMX-HP |
| #24. Poly RX |
| #25. Aerosporin |
| #26. #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 |
| #27. Randomized controlled trial [pt] |
| #28. Controlled clinical trial [pt] |
| #29. Randomized [tiab] |
| #30. Placebo [tiab] |
| #31. Drug therapy [sh] |
| #32. Randomly [tiab] |
| #33. Trial [tiab] |
| #34. Groups [tiab] |
| #35. Humans [mh] |
| #36. #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 |
| #37. #14 AND #26 AND #35 |
PMX, polymyxin B hemoperfusion.
Parallel-group, randomized controlled trials that examined polymyxin B hemoperfusion in addition to usual care in patients with sepsis and septic shock were included. Trials that compared polymyxin B hemoperfusion and other hemofiltration therapy were excluded. The comparators were usual care of sepsis. A pair of the first author (AK) and another author (MK, SU, TT) independently reviewed the list of articles retrieved by the search strategy and selected potentially eligible articles.
The same two authors then extracted the data independently. The following information was extracted: (I) patient demographics (age and sex); (II) study characteristics (study sites); (III) information on interventions (number and duration of each polymyxin B hemoperfusion session); and (IV) outcomes of interest. The risk of bias was also assessed using the Cochrane risk of bias assessment tool (14). Any disagreements were resolved through consensus.
The primary outcomes were (I) 28-day mortality and (II) adverse events related to blood access and hemoperfusion. If the data on 28-day mortality were unavailable from a trial, the mortality data up to 30 days were pooled. The secondary outcomes included (I) ICU length of stay; (II) hospital length of stay; (III) duration of vasopressor requirement; (IV) any adverse events; and (V) costs. Dichotomous and continuous outcomes were combined using risk ratios (RRs) and weighted mean difference (WMD), respectively. Data were pooled using the DerSimonian and Laird random effects model, given the a priori known clinical heterogeneity among trials (15). Statistical heterogeneity was assessed visually with Galbraith plots (16) and statistically with the I2 and Q statistics (17). Prediction intervals provide a clear and appropriate treatment summary in future trials reflecting current estimates in meta-analyses (18). We also calculated a prediction interval for our meta-analysis of 28-day mortality. Publication bias or the small study effect was tested using Egger’s method (19).
Subgroup analyses were conducted by severity (severe sepsis and/or septic shock), source of infection, the number and duration of polymyxin B hemoperfusion sessions, and the study site, and the differences between subgroups were examined using the test of interaction. Sensitivity analysis was performed by excluding trials of high or unclear risk of bias in sequence generation, allocation concealment, and blinding of outcome assessors. Sensitivity analysis by calculating odds ratio (OR) or risk difference (RD) of mortality was also conducted. Since the risk of inaccurate estimate is anticipated in a meta-analysis with a small number of studies using DerSimonian and Laird random-effects model, we also conducted an analysis using Hartung-Knapp-Sidik-Jonkman random-effects model as a sensitivity analysis (20). Meta-regression analysis was also performed by publication year, sample size, and the mortality of control groups as potential covariates. The threshold of statistical significance was set at P<0.05. All analyses were conducted using Stata SE, version 15.0 (Stata Corp., College Station, TX, USA).
Results
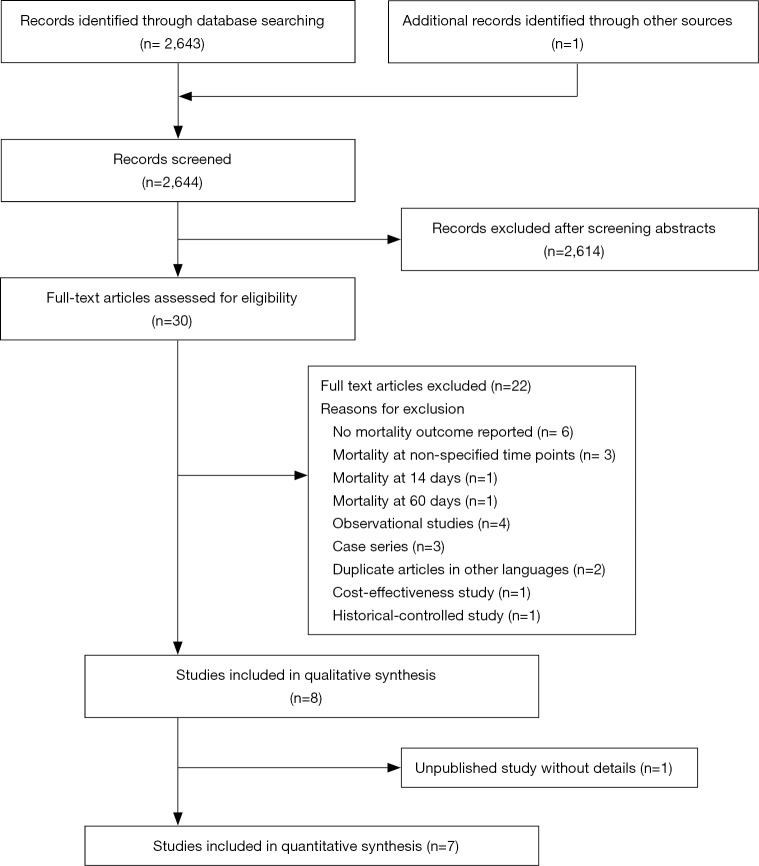
The search yielded 2,643 articles. After application of the inclusion and exclusion criteria, we excluded 2,614 studies mainly due to the study design or topics. One study that called itself randomized was excluded, because it was actually a historical control design (21). One study presented at an international conference was thereafter completed, and some latest data were obtained from the investigators (22). A total of 8 trials were thus eligible for our qualitative analysis. One large randomized trial that was completed but was excluded because the detailed information has not been published and therefore was not included in the quantitative analysis (23). We thus included 7 trials involving 586 patients in our quantitative analysis (22,24-29) (Figure 1).
Figure 1.
Study selection.
The sample size ranged from 35 to 232 (Table 2). The reported mean/median age of participants ranged from 53.8 to 72 years, and 36.1% to 42.2% were female. Three trials included patients with septic shock (25,27,28) and seven included severe sepsis/septic shock (22,24,26,29). The sources of infections were restricted to intra-abdominal infections in three trials (24,27,29), while the other three included miscellaneous sources of infection. Five trials presented the severity using the APACHE (Acute Physiology and Chronic Health Evaluation) score, ranging from 17.7 to 25.0 (24-26,28,29).
Table 2. Characteristics of included studies.
| Author/Year | Country | Population | Mean age (year) | Sample size (%, female) | Source of infection | APACHE | Timing of initiating PMX | Duration of each PMX session (h) | No. of PMX sessions |
|---|---|---|---|---|---|---|---|---|---|
| Nakamura/1999 | Japan | Septic shock | 53.8 | 50 [40] | Miscellaneous | 24.8 | 6th day | 2 | 2 |
| Nemoto/2001 | Japan | Severe sepsis/septic shock | 61.9 | 98 (38.8) | Miscellaneous | 22.4 | 1st day | 4 | 1 or 2 |
| Suzuki/2002 | Japan | Septic shock | 64.5 | 48 (36.1) | Miscellaneous | 25 | 1st day | 4 | 1 |
| Vincent/2005 | Belgium, UK, Germany, The Netherlands, Spain, Japan | Severe sepsis/septic shock | 57.6 | 35 (37.1) | Abdominal | 17.7 | 1st day | 2 | 1 |
| Cruz/2009 | Italy | Severe sepsis/septic shock | 63.8 | 64 (34.4) | Abdominal | 20.5 | 1st day | 2 | 2 |
| Payen/2015 | France | Septic shock | 72 | 232 (42.2) | Abdominal | NR | 1st day | 2 | 2 |
| Srisawat/2016 | Thailand | Severe sepsis/septic shock | 68 | 59 (NR) | Miscellaneous | NR | 1st day | 2 | 2 |
NR, not reported; PMX, polymyxin B hemoperfusion; APACHE, Acute Physiology and Chronic Health Evaluation.
Polymyxin B hemoperfusion was initiated within one day of the diagnosis of infection in six trials (22,24,26-29), with the remaining one starting within 6 days of the diagnosis of sepsis (25). Five trials performed polymyxin B hemoperfusion for 2 hours (22,24,25,27,29), and the remaining two for 4 hours. The number of polymyxin B hemoperfusion sessions varied across studies; two and four trials performed sessions once (28,29) and twice (22,24,25,27), respectively; and the remaining one conducted polymyxin B hemoperfusion once or twice according the patient’s condition (26).
Risk of bias assessment
Three of seven trials (43%) each had adequate sequence generation and concealed allocation (Table 3) (24,27,29). Outcome assessors were considered to be adequately blinded in two trials (29%) (24,27). Four studies (57%) were at low risk of incomplete outcome data (22,24,27,29). One trial was deemed at high risk of bias, because of the imbalance in the characteristics of participants; fungi from surgical samples were more frequently detected in the polymyxin B hemoperfusion-treated group than in the control group (24% vs. 12%), thus rendering the polymyxin B hemoperfusion-treated group more susceptible to the risk of morbidity and mortality (27). Two trials clearly disclosed industry sponsorship (24,27).
Table 3. Risk of bias in included studies.
| Trial ID | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessors | Incomplete outcome data | Selective outcome reporting | Other source of bias | Industry sponsorship |
|---|---|---|---|---|---|---|---|---|
| Nakamura/1999 | Unclear | Unclear | High | Unclear | Unclear | Unclear | Low | Unclear |
| Nemoto/2001 | Unclear | Unclear | High | Unclear | Unclear | Unclear | Low | Unclear |
| Suzuki/2002 | Unclear | Unclear | High | Unclear | Unclear | Low | Low | Unclear |
| Vincent/2005 | Low | Low | High | Unclear | Low | Unclear | Low | Unclear |
| Cruz/2009 | Low | Low | High | Low | Low | Low | Low | Yes |
| Payen/2015 | Low | Low | High | Low | Low | Low | High | Yes |
| Srisawat/2016 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Unclear |
Primary outcomes
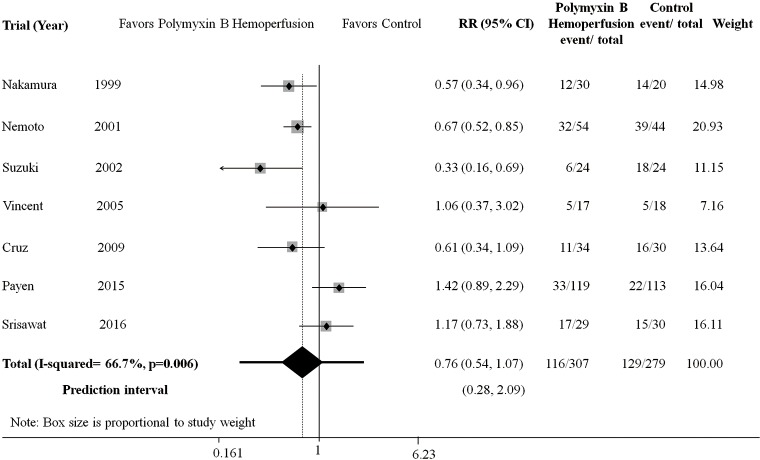
A total of 7 trials involving 527 patients were included for analysis (22,24-29). Use of polymyxin B hemoperfusion was not significantly associated with improved mortality (RR 0.76; 95% CI, 0.54–1.07; P=0.112; df =6; I2=66.7%), with a prediction interval of (95% CI, 0.28–2.09) (Figure 2). There was no evidence of publication bias (P=0.79).
Figure 2.
Relative risk of 28-day mortality between polymyxin B hemoperfusion and control.
A total of 5 trials reported adverse events related to use of polymyxin B hemoperfusion. Nemoto et al. and Suzuki et al. both reported no serious adverse events related to use of polymyxin B hemoperfusion (26,28). Vincent et al. reported one episode of fever related to the device (29). Cruz et al. reported cartridge clotting in 4 patients (6%), followed by hypotension in 1 patient (1.5%) and tachycardia in 2 (3%) (24). Payen et al. reported severe adverse events in the polymyxin B hemoperfusion-treated group, including hemorrhagic episodes in 4 patients (3.4%) (27).
Secondary outcomes
Three trials involving 331 patients were included for analysis of ICU length of stay (24,27,29). Use of polymyxin B hemoperfusion was not associated with shorter length of ICU stay (WMD 2.52 days; 95% CI, −6.29 to 11.34; P=0.58; df =2; I2=82.2%).
Only one trial involving 64 patients was available for analysis of length of hospital stay (24). Use of polymyxin B hemoperfusion was not associated with shorter length of hospital stay.
Two trials involving 267 patients reported on patients experiencing at least one adverse event in each group (27,29). Use of polymyxin B hemoperfusion was not associated with the occurrence of at least one adverse event (RR 1.07; 95% CI, 0.92–1.24; P=0.53).
No trials reported on the duration that catecholamine was required and cost for the polymyxin B hemoperfusion-treated and control groups. Payen et al. reported that the number of days without catecholamine was similar between the polymyxin B hemoperfusion-treated and control groups during the first 7 days (27).
Subgroup analyses
There were no significant differences in mortality between the subgroups by severity (P=0.70), source of infection (P=0.33), number of polymyxin B hemoperfusion sessions (P=0.13), or duration of each polymyxin B hemoperfusion session (P=0.21) (Table 4). Subgroup analysis by region found that, although barely non-significant, the studies from Japan showed a larger efficacy of polymyxin B hemoperfusion in comparison with that from other regions.
Table 4. Summary of subgroup analyses.
| Subgroup | No. of trials | Total sample size | Risk ratio (95% CI) | Heterogeneity | Test of interaction (P value) | |||
|---|---|---|---|---|---|---|---|---|
| Q | df | I2, % | P value for I2 | |||||
| Severity | 0.70 | |||||||
| Septic shock | 3 | 330 | 0.67 (0.29–1.54) | 12.76 | 2 | 84.3 | 0.002 | |
| Severe sepsis/septic shock | 4 | 256 | 0.79 (0.57–1.09) | 5.21 | 3 | 42.4 | 0.157 | |
| Source of infection | 0.33 | |||||||
| Miscellaneous | 4 | 255 | 0.66 (0.44–0.99) | 9.24 | 3 | 67.5 | 0.026 | |
| Abdominal infection | 3 | 331 | 0.98 (0.54–1.78) | 4.89 | 2 | 59.6 | 0.087 | |
| Duration of each polymyxin B hemoperfusion session | 0.21 | |||||||
| 2 hours | 5 | 440 | 0.91 (0.61–1.35) | 9.43 | 4 | 57.6 | 0.051 | |
| 4 hours | 2 | 146 | 0.52 (0.27–1.00) | 3.13 | 1 | 68.1 | 0.077 | |
| Number of polymyxin B hemoperfusion sessions | 0.13 | |||||||
| Once | 2 | 83 | 0.56 (0.18–1.72) | 3.15 | 1 | 68.2 | 0.076 | |
| Once or twice | 1 | 98 | 0.67 (0.52–0.85) | 0.00 | 0 | NA | NA | |
| Twice | 4 | 405 | 0.89 (0.57–1.40) | 9.37 | 3 | 68.0 | 0.025 | |
| Regions where a trial was conducted | 0.06 | |||||||
| Japan | 3 | 196 | 0.57 (0.41–0.87) | 3.22 | 2 | 44 | 0.20 | |
| Non-Japanese nations | 4 | 390 | 1.05 (0.71–1.53) | 5.11 | 3 | 58 | 0.165 | |
NA, not applicable.
Sensitivity analysis
Mortality was reported in a sufficient number of trials to conduct sensitivity analyses. Sensitivity analyses with trials at low risk of bias in sequence generation, allocation concealment, blinding of outcome assessors, and incomplete outcome reporting showed results consistent with the primary analysis (Table 5). Sensitivity analysis with two trials that had disclosed industry sponsorship also provided a consistent finding (Table 5). Sensitivity analyses also suggested that use of polymyxin B hemoperfusion was not associated with a reduced risk of mortality when summarized in OR (OR 0.50; 95% CI, 0.22–1.14; P=0.099; df =6; I2=76.4%) or RD (RD −0.16; −0.34 to 0.02; P=0.088; df =6; I2=81.4%). The analysis using the Hartung-Knapp-Sidik-Jonkman random-effects model also yielded a result similar to the primary analysis (RR 0.76; 95% CI, 0.52–1.10; P=0.15).
Table 5. Sensitivity analysis of mortality by risk of bias and industry sponsorship.
| Risk of bias | No. of trials | Total sample size | Risk ratio (95% CI) | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| Q | df | I2, % | P value for I2 | ||||
| Low risk in sequence generation | 3 | 331 | 0.98 (0.54–1.77) | 4.89 | 2 | 59.1 | 0.087 |
| Low risk in allocation concealment | 3 | 331 | 0.98 (0.54–1.77) | 4.89 | 2 | 59.1 | 0.087 |
| Low risk in blinding of outcome assessors | 2 | 296 | 0.95 (0.41–2.19) | 4.88 | 1 | 79.5 | 0.027 |
| Low risk in incomplete outcome reporting | 4 | 390 | 1.05 (0.71–1.53) | 5.10 | 3 | 41.1 | 0.165 |
| Presence of industry sponsorship | 2 | 296 | 0.95 (0.41–2.19) | 4.88 | 1 | 79.5 | 0.027 |
Meta-regression analysis
Meta-regression analysis of mortality was conducted to examine the association between effect size and some covariates (Table 6). Mortality tended to decrease in recent trials (regression coefficient 0.05; 95% CI, 0.06–0.09; P=0.033). Although not significant, mortality tended to decrease with an increased risk of mortality in the control group (P=0.066).
Table 6. Meta-regression analysis of mortality.
| Variables | Regression coefficient (95% CI) | P value |
|---|---|---|
| Sample size of a trial | 0.004 (−0.002 to 0.010) | 0.149 |
| Publication year | 0.05 (0.06 to 0.09) | 0.033 |
| Mortality rate of control groups | −1.29 (−2.70 to 0.13) | 0.066 |
Discussion
The present analysis suggests that there was a non-significant tendency to favor polymyxin B hemoperfusion in terms of 28-day mortality in patients with severe sepsis and septic shock. However, the wide confidential interval for the efficacy of polymyxin B hemoperfusion on 28-day mortality suggests that polymyxin B hemoperfusion can have not only a non-significant beneficial effect but also a harmful effect on 28-day mortality. Our analysis using the prediction interval suggests that polymyxin B hemoperfusion can either be beneficial or harmful with respect to 28-day mortality in future trials. Further, few of the included trials are considered at low risk of bias. Our sensitivity analyses based on few such trials or other analyses were consistent with the primary analysis, thereby confirming the robustness of our primary finding. With all such evidence, there is no argument to support the use of polymyxin B hemoperfusion in patients with severe sepsis and septic shock, unlike previous reviews that favor the use of polymyxin B hemoperfusion.
Three systematic reviews and a meta-analysis have concluded that use of polymyxin B hemoperfusion was associated with reduced risk of mortality (9-11). However, there are substantial differences in the study design between those studies and the present analysis. Cruz et al. conducted a meta-analysis of randomized, controlled trials as subgroup analysis in 2007 (10). They pooled the data from different time points (14, 28, 30, and 60 days), resulting in substantial clinical heterogeneity. Mitaka et al. and Chang et al. performed a systematic review of studies that examined the benefits of polymyxin B hemoperfusion in 2011 and 2017, respectively (9,11). They pooled the data of 28-day mortality from both randomized, controlled trials and observational studies. The Cochrane Collaboration, however, does not recommend pooling randomized and non-randomized studies, because unpredictable effects of uncontrolled confounders in non-randomized studies and both methodological and clinical heterogeneity are anticipated (14). In contrast, in the present study, data on 28-day mortality were pooled only from randomized, controlled trials to minimize the impact of uncontrolled bias and to strictly examine the efficacy of polymyxin B hemoperfusion on 28-day mortality. As stated previously, an inflated summary effect of polymyxin B hemoperfusion in previous studies might have derived from pooling data from different time points in the clinical course of sepsis or from uncontrolled confounding factors.
Our finding on 28-mortality had a substantial statistical heterogeneity (I2=66.7%). We thus conducted subgroup and meta-regression analyses to investigate the cause of this heterogeneity. Our subgroup found that, while severity of sepsis, source of infection, or duration and number of polymyxin B hemoperfusion session might not be the cause of the heterogeneity, studies from Japan showed a larger efficacy of polymyxin B hemoperfusion. Our meta-regression analyses found that the efficacy of polymyxin B hemoperfusion became smaller in recent trials.
We included only seven studies and the findings of our meta-regression may have been susceptible to outlier studies. However, our meta-regression analyses were conducted based to on three hypotheses. First, it has been reported that most large treatment effects emerge from small-sized studies (30,31). Our analysis did not show this tendency. Second, it is known that mortality has declined in patients with sepsis enrolled in usual care arms of multicenter, randomized trials over the last two decades due to improved processes of sepsis care (1). Our meta-regression showed a significant tendency for a smaller effect size of polymyxin B hemoperfusion associated with recent trials (P=0.033). We suspected that the merit of adding polymyxin B hemoperfusion to usual care might be smaller in recent years, because the sepsis care might have improved with time, leading to the decreased mortality. Third, the severity of patients in original studies might have differed across studies. There was no uniform reporting of severity across studies, and mortality in the control groups was used as a covariate. Our meta-regression showed an insignificant tendency for a greater effect size of polymyxin B hemoperfusion to be associated with greater mortality in the control groups (P=0.066). The merit of adding polymyxin B hemoperfusion to usual care in severe patients is not demonstrated.
Recently, one randomized trial has assessed the efficacy of polymyxin B hemoperfusion in septic patients. The large trial termed “Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock (EUPHRATES)” has been completed (32). This study was conducted in 50 ICUs in the United States and Canada. Included patients were required to have persistent septic shock despite adequate fluid resuscitation who were on vasopressors for more than 2 and less than 30 h, and to have an endotoxin activity assay (EAA) ≥0.60. The patients were then randomized to receive treatment with two sessions of polymyxin B hemoperfusion or sham hemoperfusion. Their inclusion criteria were revised, and some patients were excluded after the interim analysis found no benefit of polymyxin B hemoperfusion according to the initial protocol. This trial finally included 460 patients. Although the detailed information has not been officially published, the press release on May 30, 2017 announced that the trial showed a non-statistically significant reduction in 28-day mortality of less than 5% in the per protocol population (23). The sample size enrolled in the EUPHRATES study (n=460) is comparable to 78% of that included in the present analysis (n=587), suggesting that the impact of the EUPHRATES study is comparable to the present study finding. Thus, there might be no significant 28-day mortality benefit with the use of polymyxin B hemoperfusion, which is consistent with the present study finding.
The present study has some strengths. First, an extensive search for relevant studies was conducted by including the Japanese database. Although only one relevant new trial since the publication of the two previous reviews was included, the relevant company was contacted to ensure that the search was exhaustive. This might have made the search comprehensive. Second, subgroup and meta-regression analyses were adequately performed. Specifically, subgroup and meta-regression analyses could partly explain the source of substantial heterogeneity in mortality and provide clinically important explanations. Third, the focus was on patient-oriented outcomes in the present study, as recommended by the Cochrane Collaboration. Previous systematic reviews focused on transient physiological parameters, such as mean arterial pressure and the Sequential Organ Failure Assessment score, and laboratory outcomes including endotoxin levels, which are surrogate markers of sepsis. In the present study, the outcomes examined were ICU and hospital lengths of stay, as well as mortality. These outcomes should be clinically more important to patients and healthcare professionals.
The present study also has some limitations. First, the included trials differed in terms of sample size, disease severity, source of infection, and durations and sessions of polymyxin B hemoperfusion. Primary outcome analysis of mortality showed high levels of statistical heterogeneity, but meta-regression analysis suggested that this heterogeneity was due to the publication years. Second, adverse effects, which are of crucial relevance to critically ill patients, were not examined in detail. The Consolidated Standards of Reporting Trials (CONSORT) statement requires that trial investigators report “harms” associated with interventions (33). All included trials were published after enactment of the CONSORT statement, but five trials focused only on ‘severe’ adverse events, with two reporting at least one adverse event. As seen in research of other areas (34-37), harms associated with polymyxin B hemoperfusion are under-reported. This would further limit the applicability of polymyxin B hemoperfusion in patients with severe sepsis and septic shock. Third, the number of included trials was smaller than that in previous systematic reviews, despite the comprehensive search. However, it was possible to strictly examine 28-day mortality, which made the analysis more directly and clinically relevant to clinical practice.
Conclusions
There is no evidence to support the use of polymyxin B hemoperfusion in patients with sepsis and septic shock with respect to 28-day mortality.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med 2014;42:625-31. 10.1097/CCM.0000000000000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T, Derhovanessian A, De Cruz S, et al. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med 2014;29:87-95. 10.1177/0885066612467162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortego A, Gaieski DF, Fuchs BD, et al. Hospital-based acute care use in survivors of septic shock. Crit Care Med 2015;43:729-37. 10.1097/CCM.0000000000000693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin AJ, Rice DA, Simpson KN, et al. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med 2015;43:738-46. 10.1097/CCM.0000000000000859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013;309:355-63. 10.1001/jama.2012.216476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winters BD, Eberlein M, Leung J, et al. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 2010;38:1276-83. 10.1097/CCM.0b013e3181d8cc1d [DOI] [PubMed] [Google Scholar]
- 7.Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754-61. 10.1097/CCM.0b013e318232db65 [DOI] [PubMed] [Google Scholar]
- 8.Suffredini AF, Munford RS. Novel therapies for septic shock over the past 4 decades. JAMA 2011;306:194-9. 10.1001/jama.2011.909 [DOI] [PubMed] [Google Scholar]
- 9.Chang T, Tu YK, Lee CT, et al. Effects of Polymyxin B Hemoperfusion on Mortality in Patients With Severe Sepsis and Septic Shock: A Systemic Review, Meta-Analysis Update, and Disease Severity Subgroup Meta-Analysis. Crit Care Med 2017;45:e858-64. 10.1097/CCM.0000000000002362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz DN, Perazella MA, Bellomo R, et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care 2007;11:R47. 10.1186/cc5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitaka C, Tomita M. Polymyxin B-immobilized fiber column hemoperfusion therapy for septic shock. Shock 2011;36:332-8. 10.1097/SHK.0b013e318225f839 [DOI] [PubMed] [Google Scholar]
- 12.Marshall JC, Vincent JL, Guyatt G, et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med 2005;33:1708-16. 10.1097/01.CCM.0000174478.70338.03 [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 14.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 1st ed. Wiley Cochrane Series. Chichester, England: John Wiley & Sons, Ltd, 2008. [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 16.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988;7:889-94. 10.1002/sim.4780070807 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IntHout J , Ioannidis JP, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247. 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guolo A, Varin C. Random-effects meta-analysis: the number of studies matters. Stat Methods Med Res 2017;26:1500-18. 10.1177/0962280215583568 [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Matsuda T, Suzuki Y, et al. Polymyxin B-Immobilized Fiber Hemoperfusion in Patients with Sepsis. Dial Transplant 2003;32:602-9. [Google Scholar]
- 22.Srisawat N, Peerapornratana S, Laoveeravat P, et al. The immunomodulation effect of Polymyxin-B Hemoperfusion in severe sepsis/septic shock: a randomized controlled trial. Intensive Care Med Exp 2016;4:A377. [Google Scholar]
- 23.SPECTRAL Medical Inc. Spectral files final PMA module for Toraymyxin™ with FDA. (Last access as of October 17, 2017). Available online: http://www.spectraldx.com/assets/spectral-rls-05.30.17.pdf
- 24.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA 2009;301:2445-52. 10.1001/jama.2009.856 [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Ebihara I, Shoji H, et al. Treatment with polymyxin B-immobilized fiber reduces platelet activation in septic shock patients: decrease in plasma levels of soluble P-selectin, platelet factor 4 and beta-thromboglobulin. Inflamm Res 1999;48:171-5. 10.1007/s000110050442 [DOI] [PubMed] [Google Scholar]
- 26.Nemoto H, Nakamoto H, Okada H, et al. Newly developed immobilized polymyxin B fibers improve the survival of patients with sepsis. Blood Purif 2001;19:361-8; discussion 368-9. 10.1159/000046966 [DOI] [PubMed] [Google Scholar]
- 27.Payen DM, Guilhot J, Launey Y, et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med 2015;41:975-84. 10.1007/s00134-015-3751-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki H, Nemoto H, Nakamoto H, et al. Continuous hemodiafiltration with polymyxin-B immobilized fiber is effective in patients with sepsis syndrome and acute renal failure. Ther Apher 2002;6:234-40. 10.1046/j.1526-0968.2002.00416.x [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, Laterre PF, Cohen J, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005;23:400-5. 10.1097/01.shk.0000159930.87737.8a [DOI] [PubMed] [Google Scholar]
- 30.Pereira TV, Horwitz RI, Ioannidis JP. Empirical evaluation of very large treatment effects of medical interventions. JAMA 2012;308:1676-84. 10.1001/jama.2012.13444 [DOI] [PubMed] [Google Scholar]
- 31.Dechartres A, Trinquart L, Boutron I, et al. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. 10.1136/bmj.f2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein DJ, Foster D, Schorr CA, et al. The EUPHRATES trial (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock): study protocol for a randomized controlled trial. Trials 2014;15:218. 10.1186/1745-6215-15-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherer RW, Crawley B. Reporting of randomized clinical trial descriptors and use of structured abstracts. JAMA 1998;280:269-72. 10.1001/jama.280.3.269 [DOI] [PubMed] [Google Scholar]
- 34.Kuriyama A, Maeda H, Sun R, et al. Topical application of corticosteroids to tracheal tubes to prevent postoperative sore throat in adults undergoing tracheal intubation: a systematic review and meta-analysis. Anaesthesia 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Kuriyama A, Umakoshi N, Sun R. Prophylactic Corticosteroids for Prevention of Postextubation Stridor and Reintubation in Adults: A Systematic Review and Meta-analysis. Chest 2017;151:1002-10. 10.1016/j.chest.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 36.Kuriyama A, Aga M, Maeda H. Topical benzydamine hydrochloride for prevention of postoperative sore throat in adults undergoing tracheal intubation for elective surgery: a systematic review and meta-analysis. Anaesthesia 2018;73:889-900. 10.1111/anae.14224 [DOI] [PubMed] [Google Scholar]
- 37.Kuriyama A, Endo K. Goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer 2018;26:1051-9. 10.1007/s00520-017-4028-6 [DOI] [PubMed] [Google Scholar]