Abstract
Background
Clinical guidelines vary in determining optimal blood pressure targets in adults with diabetes mellitus.
Methods
We systematically searched PubMed, EMBASE, Cochrane Library, and clinicaltrials.gov in March 2018; conducted random effects frequentist meta-analyses of direct aggregate data; and appraised the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.
Results
From eligible 14 meta-analyses and 95 publications of randomized controlled trials (RCT), only 6 RCTs directly compared lower versus higher blood pressure targets; remaining RCTs aimed at comparative effectiveness of hypotensive drugs. In adults with diabetes mellitus and elevated systolic blood pressure (SBP), direct evidence (2 RCTs) suggests that intensive target SBP <120–140 mmHg decreases the risk of diabetes-related mortality [relative risk (RR) =0.68; 95% confidence interval (CI), 0.50–0.92], fatal (RR =0.41; 95% CI, 0.20–0.84) or nonfatal stroke (RR =0.60; 95% CI, 0.43–0.83), prevalence of left ventricular hypertrophy and electrocardiogram (ECG) abnormalities, macroalbuminuria, and non-spine bone fractures, with no differences in all-cause or cardiovascular mortality or falls. In adults with diabetes mellitus and elevated diastolic blood pressure (DBP) ≥90 mmHg, direct evidence (2 RCTs) suggests that intensive DBP target ≤80 versus 80–90 mmHg decreases the risk of major cardiovascular events. Published meta-analyses of aggregate data suggested a significant association between lower baseline and attained blood pressure and increased cardiovascular mortality.
Conclusions
We concluded that in adults with diabetes mellitus and arterial hypertension, in order to reduce the risk of stroke, clinicians should target blood pressure at 120–130/80 mmHg, with close monitoring for all drug-related harms.
Keywords: Quality of evidence, cardiovascular morbidity, diabetes mellitus, arterial hypertension, blood pressure targets
Introduction
One of the main goals in managing type 2 diabetes in adults is prevention of cardiovascular morbidity or mortality by controlling blood glucose, normalizing blood pressure, and reducing other cardiovascular risk factors (1,2). Despite extensive review of literature, clinical practice guidelines vary in determining the optimal blood pressure targets in patients with diabetes (3-5).
To support clinical decisions at point of care with all available evidence, we conducted a rapid review of the published and unpublished data from recently completed randomized controlled trials (RCT), meta-analyses of RCTs, and primary observational studies that compared different blood pressure targets in adults with diabetes.
Methods
We used a standard recommended methodology in conducting systematic literature reviews and meta-analyses from the Cochrane Collaboration and the Agency for Healthcare Research and Quality (6,7). We developed a priori protocol (available by request) for a systematic literature review to answer the clinical question about the comparative effectiveness of blood pressure targets on mortality and cardiovascular morbidity in adults with diabetes mellitus.
Eligible studies directly compared lower versus higher blood pressure targets or examined the association between baseline or attained blood pressure with patient outcomes in people treated with hypotensive medications. Eligible outcomes included all-cause and underlying cause-specific mortality, cardiovascular morbidity, stroke, heart failure, renal failure, and all drug harms.
We conducted a comprehensive search in PubMed, EMBASE, the Cochrane Library, and www.clinicaltrials.gov up to March 2018 to find systematic reviews, published and unpublished RCTs, and nationally represented controlled observational studies that reported adjusted effect estimates (6,7). The data were extracted from the Clinical Trials Transformation Initiative (https://www.ctti-clinicaltrials.org/aact-database), checked for quality, and stored in the High-Performance Computing Cluster platform (https://hpccsystems.com).
We tested the null hypotheses of no differences in patient outcomes after more versus less extensive blood pressure lowering (6). We abstracted the information about study population, interventions, comparators, and outcomes (6). We abstracted minimum datasets (e.g., number of the subjects in treatment groups and events) to estimate absolute risk difference, relative risk, and number needed to treat for categorical variables (6). Statistical significance was evaluated at a 95% confidence level (including the use of P values).
We conducted a rapid review following the framework of the AHRQ (8). We used the AHRQ recommended methodological approach in the integration of existing systematic reviews into our comprehensive synthesis of evidence (9). Our goal was the integration of previously published high quality reviews and consistent ranking of the quality of evidence using GRADE methodology.
We performed meta-analyses when definitions of active and control interventions and patient outcomes deem similar (10). We examined consistency in results across studies with chi-square tests and I2 statistics and concluded statistically significant heterogeneity if I2 was >50% (6). Statistically significant heterogeneity did not preclude statistical pooling (10). However, we planned exploring heterogeneity with a priori defined patient baseline hypertensive status (10).
We defined harms as the totality of all possible adverse consequences of an intervention.
We calculated absolute risk difference, number needed to treat, and the number of attributable events based on data from the published randomized trials, using STATA software (StataCorp LP, College Station, TX, USA) (11). Correction coefficients for zero events were used as a default option, and intention to treat was used for evidence synthesis (10). Superiority of interventions under comparison was hypothesized (12). We used consensus method guidelines for systematic review and meta-analyses that do not recommend conducting post hoc analyses of statistical power (13-15). Instead, we downgraded our confidence in true treatment effects based on calculated optimal information size as the number of patients required for an adequately powered individual trial (16). Since power is more closely related to number of events than to sample size, we concluded imprecision in treatment effects if less than 250 patients experienced the event (16).
We assessed reporting bias as a proportion of published among all registered studies, unreported outcomes compared with published protocols, or unreported minimum data sets for reproducibility of the results (17). We did not conduct formal statistical tests for publication bias due to the questionable validity of such tests (18).
We evaluated the quality of the primary studies using the Cochrane risk of bias tool on a 3-point scale: high bias, low bias, and unclear (6). We upgraded the risk of bias in the body of evidence from low to high if at least 1 RCT had high risk of bias (19,20). We defined indirectness in outcomes from intermediate outcomes (21).
Treatment effect estimates were defined as precise when pooled estimates had reasonably narrow 95% confidence intervals (CI) and the number of events were greater than 250 (22). Justification of the sample size was not included in grading of the evidence.
In assessing the quality of evidence in all studies, the authors looked for the strength of association and evidence of any reporting bias (23). The strength of the association was evaluated, defining a priori a large effect when the relative risk was greater than 2 and a very large effect when the relative risk was greater than 5 (23). A small treatment effect was construed when the relative risk was significant but less than 2 (23).
The authors assigned the quality of evidence ratings as high, moderate, low, or very low, according to risk of bias in the body of evidence, directness of comparisons, precision and consistency in treatment effects, and the evidence of reporting bias, using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (Supplementary file) (23). A high quality of evidence was assigned to well-designed RCTs with consistent findings. The quality of evidence was downgraded to moderate if at least 1 of 4 quality of evidence criteria were not met; for example, moderate quality of evidence was assigned if there was a high risk of bias in the body of evidence or if the results were not consistent or precise (23). The quality of evidence was downgraded to low if 2 or more criteria were not met.
A low quality of evidence was assigned to nonrandomized studies and upgraded for the rating if there was a strong association. Evidence was defined as insufficient when no studies provided valid information about treatment effects. This approach was applied regardless of whether the results were statistically significant.
Results
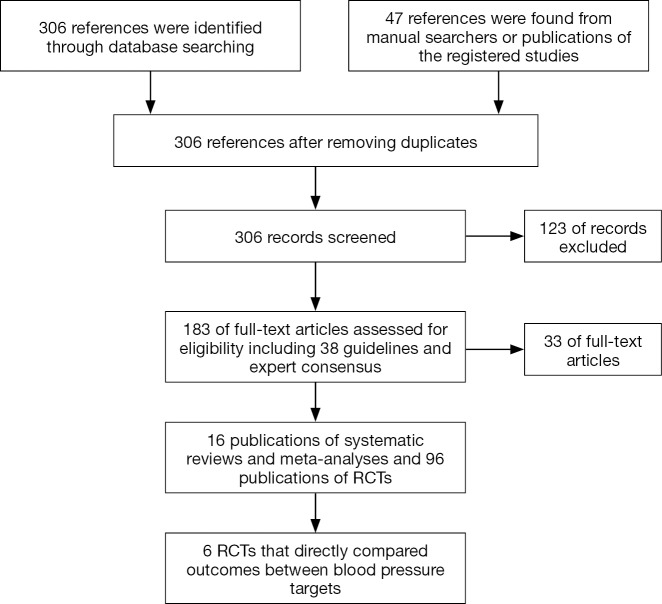
Our comprehensive search in PubMed, EMBASE, the Cochrane Library, and clinicaltrials.gov up to March 2018 retrieved 306 references and identified 16 publications of systematic reviews and meta-analyses and 96 publications of RCTs that enrolled adults primarily with type 2 diabetes mellitus (Supplementary file, Figure S1). We excluded 124 irrelevant references at the screening of the titles and abstracts and 33 publications after full text review because the studies did not address blood pressure targets in patients with diabetes (Supplementary file, Figure S1). Only 6 primary RCTs randomly assigned patients to lower versus higher blood pressure targets and compared systolic blood pressure (SBP) targets in patients with baseline arterial hypertension (24,25) or diastolic blood pressure (DBP) targets in patients with normal (26,27) or elevated baseline blood pressure (28,29). All other RCTs compared hypotensive drugs with placebo or with each other. Meta-analyses of such trials explored the association of baseline or achieved blood pressure with patient outcomes (3-5,30-39).
In adults with diabetes and normal arterial blood pressure, low-quality evidence suggests that there are no differences in all-cause and cardiovascular mortality or morbidity between intensive (10 mmHg below baseline DBP) and moderate blood pressure control (DBP goal 80–89 mmHg; Table 1). Intensive blood pressure control prevents cerebrovascular events and progression or retinopathy in some patients (Table 1).
Table 1. The benefits and harms of intensive versus moderate diastolic blood pressure control in normotensive adults with diabetes mellitus.
| Outcome | Risk with intervention/comparator per 1,000 | Attributable avoided events per 1,000 treated (95% CI) | Relative measure of association; number needed to treat (95% CI) | No. of participants (studies) |
|---|---|---|---|---|
| All-cause mortality** | 63/65 | NS | RR: 0.96 (0.53–1.75); HR: 0.96 (0.53–1.75) | 609 (2 RCTs) (26,27) |
| Cardiovascular mortality* | 55/37 | NS | RR: 1.48 (0.65–3.40) | 480 (1 RCT) (27,40) |
| Non-cardiovascular mortality* | 21/45 | NS | RR: 0.47 (0.16–1.32) | 480 (1 RCT) (27,40) |
| Cardiovascular event** | 73/56 | NS | RR: 1.31 (0.71–2.42) | 609 (2 RCTs) (26,27) |
| Congestive heart failure* | 51/45 | NS | RR: 1.12 (0.50–2.49) | 480 (1 RCT) (27,40) |
| Myocardial infarction* | 80/62 | NS | RR: 1.30 (0.68–2.49) | 480 (1 RCT) (27,36) |
| Cerebrovascular accident* | 17/53 | 37 [4–69] | RR: 0.32 (0.10–0.95); NNTp: 27 [14–255]# | 480 (1 RCT) (27,36) |
| Retinopathy progression** | 269/369 | NR | RR: 0.74 (0.60–0.93)# | 609 (2 RCTs) (26,27) |
| Neuropathy progression** | 349/337 | NS | RR: 1.04 (0.83–1.29) | 609 (2 RCTs) (26,27) |
Population: adults with diabetes and normal arterial blood pressure; Settings: outpatient; Intervention: intensive blood pressure control (10 mmHg below baseline DBP); Comparator: moderate blood pressure control (DBP goal 80–89 mmHg). #, favors lower blood pressure target; *, very low quality evidence; **, low quality evidence. CI, confidence interval; DBP, diastolic blood pressure; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HR, hazard ratio; NNTp, number needed to treat to prevent an outcome in one patient; RCT, randomized controlled trial; RR, relative risk; NS, no statistically significant difference; NR, not reported.
In adults with diabetes and elevated arterial blood pressure (DBP ≥90 mmHg), low-quality evidence suggests that there are no differences in all-cause and cardiovascular mortality or stroke between intensive (DBP ≤85–75 mmHg) and moderate blood pressure control (DBP goal 80–90 mmHg; Table 2). A single RCT suggests that a reduction of DBP ≤80 mmHg results in a lower risk of major cardiovascular events but higher risk of progressing neuropathy (Table 2).
Table 2. The benefits and harms of intensive versus moderate diastolic blood pressure control in adults with diabetes mellitus and arterial hypertension.
| Outcome | Risk with intervention/comparator per 1,000 | Attributable avoided events per 1,000 treated (95% CI) | Relative measure of association; number needed to treat (95% CI) | No. of participants (studies) |
|---|---|---|---|---|
| All-cause mortality** | 45/71 | NS | RR: 0.63 (0.38–1.05) | 1,971 (2 RCTs) (28,29,40) |
| Cardiovascular mortality** | 27/44 | NS | RR: 0.63 (0.39–1.03) | 1,971 (2 RCTs) (28,29,40) |
| Congestive heart failure* | 38/39 | NS | RR: 0.98 (0.40–2.43) | 470 (1 RCT) (28,40) |
| Major cardiovascular events, DBP ≤80* | 44/90 | Avoided 46 [15–77] | RR: 0.49 (0.30–0.80); NNTp: 22 [13–67]# | 1,000 (1 RCT) (29) |
| Major cardiovascular events, DBP ≤85* | 68/90 | NS | RR: 0.76 (0.49–1.16) | 1,002 (1 RCT) (29) |
| Any cardiovascular event* | 63/60 | NS | RR: 1.05 (0.52–2.13) | 470 (1 RCT) (28,41) |
| Myocardial infarction* | 25/38 | NS | RR: 0.78 (0.38–1.61) | 1,971 (2 RCTs) (28,40) |
| Stroke** | 27/35 | NS | RR: 0.81 (0.49–1.33) | 1,971 (2 RCTs) (28,29,40) |
| Neuropathy progression* | 400/310 | Excessive 92 [6–178] | RR: 1.30 (1.01–1.66); NNT: 11 [6–174]† | 470 (1 RCT) (28) |
| Retinopathy progression* | 300/340 | NS | RR: 0.88 (0.68–1.15) | 470 (1 RCT) (28) |
Population: adults with diabetes and elevated arterial blood pressure (DBP ≥90 mmHg); Settings: outpatient; Intervention: intensive blood pressure control (DBP ≤75–85 mmHg); Comparator: moderate blood pressure control (DBP goal 80–90 mmHg). #, favors lower blood pressure target; †, favors higher blood pressure target; *, very low quality evidence; **, low quality evidence. CI, confidence interval; DBP, diastolic blood pressure; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NNT, number needed to treat; NNTp, number needed to treat to prevent an outcome in one patient; RCT, randomized controlled trial; RR, relative risk.
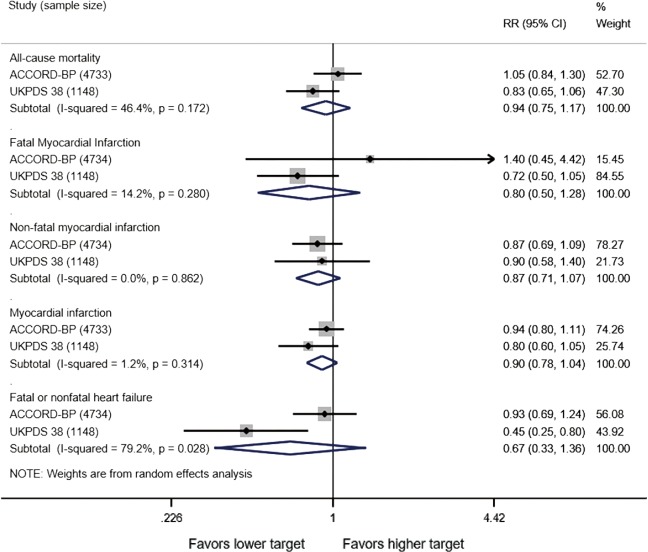
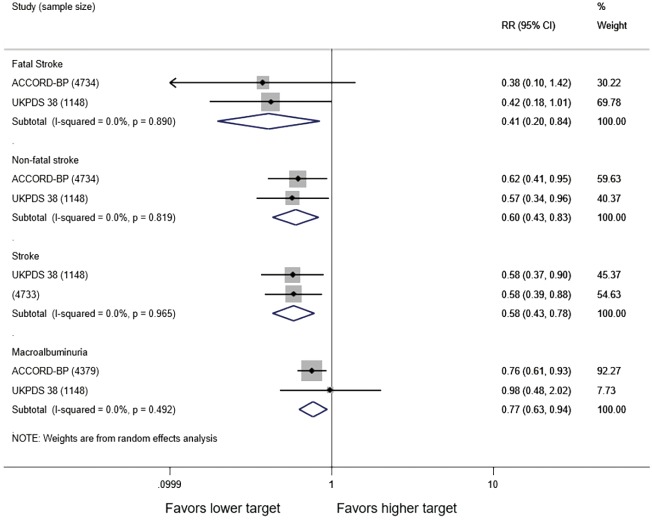
In adults with diabetes and elevated arterial blood pressure (SBP 130–190 mmHg), moderate-quality evidence suggests that there are no differences in all-cause or cardiovascular mortality between intensive and standard blood pressure control (Table 3 and Figure 1). However, intensive blood pressure control decreases the risk of diabetes-related mortality, fatal or nonfatal stroke (Figure 2), prevalence of left ventricular hypertrophy and electrocardiogram (ECG) abnormalities, macroalbuminuria, and non-spine bone fractures (Table 3). A single RCT (ACCORD) suggests that the risk of a composite outcome (nonfatal myocardial infarction, nonfatal stroke, and death from cardiovascular causes) is lower after intensive blood pressure and good glycemic control but higher in adults with poorly controlled diabetes (hemoglobin A1c >8.0; Table 3). The benefits from intensive blood pressure control sustain at 9 years of follow-up in older adults with 15% or greater 10-year coronary heart risk in the standard glucose control arm of ACCORD trial (Table 3). The same study reported an increased risk of adverse effects from hypotensive medications, including hypotension or hyperkalemia, after intensive blood pressure control (Table 3).
Table 3. The benefits and harms of intensive versus standard systolic blood pressure control in adults with diabetes mellitus and arterial hypertension.
| Outcome | Risk with intervention/comparator per 1,000 | Attributable avoided events per 1,000 treated (95% CI) | Relative measure of association; number needed to treat (95% CI) | No. of participants (studies) |
|---|---|---|---|---|
| All-cause mortality*** | 91/82 | NS | RR: 0.94 (0.75–1.18) | 5,881 (2 RCTs) (25,36) |
| All-cause mortality, 9 years of follow-up* | 224/234 | NS | RR: 0.96 (0.78–1.17) | 1,284 (1 RCT) (42) |
| Cardiovascular death, nonfatal MI, nonfatal stroke, 9 years of follow-up* | 199/259 | Avoided 60 [14–106] | RR: 0.77 (0.63–0.94); NNTp: 17 [9–70]# | 1,284 (1 RCT) (42) |
| Coronary death, nonfatal MI, unstable angina, 9 years of follow-up* | 201/267 | Avoided 66 [20–113] | RR: 0.75 (0.62–0.92); NNTp: 15 [9–49]# | 1,284 (1 RCT) (42) |
| Cardiovascular death, 9 years of follow-up* | 58/76 | NS | RR: 0.77 (0.51–1.16) | 1,284 (1 RCT) (42) |
| Fatal myocardial infarction** | 21/17 | NS | RR: 0.80 (0.50–1.28) | 5,882 (2 RCTs) (24,25) |
| Fatal stroke** | 4/7 | NR | RR: 0.41 (0.20–0.84)# | 5,882 (2 RCTs) (24,25) |
| Any stroke** | 24/35 | Avoided 16 [7–24] | RR: 0.58 (0.43–0.78); NNTp: 63 [42–143]# | 5,881 (2 RCTs) (25,36) |
| Nonfatal stroke** | 20/29 | Avoided 12 [4–20] | RR: 0.60 (0.43–0.83); NNTp: 83 [50–250]# | 5,882 (2 RCTs) (24,25) |
| Nonfatal myocardial infarction** | 57/63 | NS | RR: 0.87 (0.71–1.07) | 5,882 (2 RCTs) (24,25) |
| Non-fatal MI, 9 years of follow-up* | 100/147 | Avoided 47 [12–83] | RR: 0.68 (0.50–0.91); NNTp: 21 [12–87]# | 1,284 (1 RCT) (42) |
| Myocardial infarction, any*** | 115/123 | NS | RR: 0.90 (0.78–1.04) | 5,881 (2 RCTs) (25,36) |
| Cancer death** | 23/19 | NS | RR: 1.17 (0.74–1.84) | 5,882 (2 RCTs) (24,25) |
| Fatal or nonfatal heart failure* | 33/41 | NS | RR: 0.67 (0.34–1.36) | 5,882 (2 RCTs) (24,25) |
| MACE**; subgroup: HbA1c ≤8.0 | 444/489 | Avoided 45 [17–73] | RR: 0.91 (0.85–0.97); NNTp: 22 [14–61]# | 4,734 (1 RCT) (24) |
| MACE**; subgroup: HbA1c >8.0 | 554/507 | Excessive 47 [76–19] | RR: 1.09 (1.04–1.15); NNT: 21 (53–13)† | 4,734 (1 RCT) (24) |
| Mortality due to congestive heart failure* | 5/4 | NS | RR: 1.10 (0.47–2.59) | 4,734 (1 RCT) (24) |
| Mortality due to fatal arrhythmia* | 1/1 | NS | RR: 1.00 (0.14–7.12) | 4,734 (1 RCT) (24) |
| Mortality related to diabetes* | 108/159 | Avoided 51 [8–93] | RR: 0.68 (0.50–0.92); NNTp: 20 [11–120]# | 1,148 (1 RCT) (25) |
| Adverse Events from blood-pressure medications* | 33/13 | Excessive 20 [11–28] | RR: 2.58 (1.70–3.91); NNT: 50 [35–87]† | 4,733 (1 RCT) (24) |
| Abnormal Q waves in ECG* | 175/231 | Avoided 55 [5–105] | RR: 0.76 (0.60–0.96); NNTp: 18 [10–182]# | 1,148 (1 RCT) (25) |
| Abnormal Q, ST, or T waves in ECG* | 38/77 | Avoided 39 [9–68] | RR: 0.50 (0.30–0.82); NNTp: 26 [15–112]# | 1,148 (1 RCT) (25) |
| Angina* | 59/56 | NS | RR: 1.05 (0.64–1.73) | 1,148 (1 RCT) (25) |
| Left ventricular hypertrophy* | 17/30 | Avoided 13 [4–22] | RR: 0.58 (0.39–0.86); NNTp: 79 [46–273]# | 4,331 (1 RCT) (43) |
| Any diabetes-related end point** | 342/436 | Avoided 94 [35–154] | RR: 0.78 (0.67–0.91); NNTp: 11 [6–29]# | 1,148 (1 RCT) (25) |
| Macroalbuminuria** | 56/78 | NR | RR: 0.77 (0.63–0.94)# | 5,527 (2 RCTs) (24,25) |
| Microalbuminuria*** | 253/296 | NS | RR: 0.92 (0.85–1.01) | 5,527 (2 RCTs) (24,25) |
| Renal failure* | 4/3 | NS | RR: 1.38 (0.18–10.81) | 5,881 (2 RCTs) (24,25) |
| Peripheral vascular disease* | 11/21 | NS | RR: 0.51 (0.19–1.36) | 1,148 (1 RCT) (25) |
| Cataract extraction* | 47/36 | NS | RR: 1.32 (0.72–2.42) | 1,148 (1 RCT) (25) |
| Vision preventing driving * | 42/62 | NS | RR: 0.69 (0.41–1.15) | 1,148 (1 RCT) (25) |
| Vitreous hemorrhage* | 4/13 | NS | RR: 0.31 (0.07–1.29) | 1,148 (1 RCT) (25) |
| Falls* | 200/206 | NS | RR: 0.97 (0.84–1.11) | 3,099 (1 RCT) (44) |
| Fatal accident* | 1/3 | NS | RR: 0.51 (0.03–8.20) | 1,148 (1 RCT) (25) |
| Fatal accident/trauma* | 2/1 | NS | RR: 2.51 (0.49–12.92) | 4,734 (1 RCT) (24) |
| All non-spine bone fractures* | 76/98 | Avoided 23 [3–43] | RR: 0.77 (0.61–0.97); NNTp: 44 [23–337]# | 3,099 (1 RCT) (44) |
| Ankle fractures* | 16/24 | NS | RR: 0.67 (0.41–1.11) | 3,099 (1 RCT) (44) |
| Distal forearm fractures* | 8/8 | NS | RR: 0.94 (0.43–2.06) | 3,099 (1 RCT) (44) |
| Foot fractures* | 6/13 | NS | RR: 0.46 (0.21–1.01) | 3,099 (1 RCT) (44) |
| Hip fractures* | 3/8 | NS | RR: 0.43 (0.15–1.20) | 3,099 (1 RCT) (44) |
| Proximal humerus fractures* | 10/12 | NS | RR: 0.81 (0.41–1.58) | 3,099 (1 RCT) (44) |
| Hives or swelling* | 88/88 | NS | RR: 1.00 (0.67–1.51) | 969 (1 RCT) (24) |
| Hyperkalemia* | 4/0 | Excessive 3 [1–6] | RR: 9.03 (1.15–71.25); NNT: 295 [166–1,299]† | 4,733 (1 RCT) (24) |
| Hypotension* | 7/0 | Excessive 7 [3–10] | RR: 17.06 (2.27–128.12); NNT: 148 [97–306]† | 4,733 (1 RCT) (24) |
Population: adults with diabetes and elevated arterial blood pressure (SBP: 130–190 mmHg); Settings: outpatient; Intervention: intensive blood pressure control [target SBP <120 versus <140 mmHg in ACCORD study and 144/82 versus 154/87 mmHg in UKPDS 38 (66) study]; Comparator: standard blood pressure control. *, very low quality evidence; **, low quality evidence; ***, moderate quality evidence; #, favors lower blood pressure target; †, favors higher blood pressure target; CI, confidence interval; ECG, electrocardiogram; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NNT, number needed to treat; NNTp, number needed to treat to prevent an outcome in one patient; RCT, randomized controlled trial; RR, relative risk; SBP, systolic blood pressure; MACE, major cardiovascular events including nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes.
Figure 1.
Cardiovascular outcomes after intensive versus standard systolic blood pressure control in adults with diabetes mellitus and arterial hypertension. Target systolic blood pressure <120 versus <140 mmHg in ACCORD study and 144/82 versus 154/87 mmHg in UKPDS 38 study. RR, relative risk.
Figure 2.
Stroke after intensive versus standard systolic blood pressure control in adults with diabetes mellitus and arterial hypertension. Target systolic blood pressure <120 versus <140 mmHg in ACCORD study and 144/82 versus 154/87 mmHg in UKPDS 38 study.
Primary studies did not address circadian fluctuations in blood pressure or the risk of orthostatic hypotension after intensive versus standard blood pressure targets. For the record, the ACCORD study found no differences in patient falls or trauma after intensive blood pressure control (Table 3).
Published meta-analyses of aggregate data from RCTs aimed at efficacy or comparative effectiveness of specific hypotensive drugs in adults with comorbid diabetes and arterial hypertension (baseline blood pressure >150 mmHg) agree that attained SBP 130–139 and DBP 75–80 mmHg is associated with lower risk of all-cause mortality and stroke (Table S1) (5,31,33,37). Systematic reviews and guidelines vary in determining the balance between benefits and harms of lowering blood pressure below 130 mmHg (4,5). We looked at the pooled relative risk of all reported outcomes in published reviews (Table S1) and found 2 meta-analyses that reported a statistically significant increase in the risk of cardiovascular mortality in association with lower baseline blood pressure (Table S2) (31,37). Anti-hypertensive treatments are associated with a higher risk of cardiovascular mortality per each 10 mmHg lower baseline SBP and DBP (Table S2).
Discussion
Our review found moderate direct quality evidence that in adults with diabetes and elevated SBP, intensive blood pressure control (target SBP <120–140 mmHg) decreases the risk of diabetes-related mortality, fatal or nonfatal stroke, prevalence of left ventricular hypertrophy and ECG abnormalities, macroalbuminuria, and non-spine bone fractures, with no differences in all-cause or cardiovascular mortality or falls.
We downgraded the quality of evidence due to risk of bias, small number of events in the studies, and heterogeneity in treatment effects across the studies. We did not conduct meta-regression of few RCTs that directly compared patient outcomes after intensive versus standard blood pressure targets (6). Instead, we reviewed published meta-analyses of RCTs aimed at comparative effectiveness of blood lowering drugs in adults with diabetes that concluded no benefits from lowering blood pressure below 130 mmHg. Such publications employed meta-regression of aggregated data that can generate hypotheses of potential harms from extensive lowering blood pressure control specifically in adults with normal baseline blood pressure (45). Published meta-analyses that included the data from the Systolic Blood Pressure Intervention Trial (SPRINT) suggest similar reduction in the risk of major cardiovascular events from intensive blood pressure lowering in adults with and without diabetes (20,38,46). Individual rather than aggregate patient data meta-analyses would provide better evidence about the association between specific drugs, baseline and achieved blood pressure, and patient outcomes independent of drug effects (47).
We found no studies that addressed the risk of hospitalization or long-term quality of life in relation to blood pressure targets in adults with diabetes. Although orthostatic hypotension is associated with poor patient outcomes, the evidence regarding the risk of this complication after intensive or standard blood pressure control is insufficient (3,48,49). Primary studies and meta-analyses did not discuss the importance of pulse pressure in reducing morbidity and mortality in adults with diabetes (50).
Guidelines recommend healthy diet, weight normalization, and physical activity for all adults with diabetes (Table S3) (51-58).
In addition to healthy lifestyle, recent guidelines recommend antihypertensive drug treatments in patients with diabetes and baseline blood pressure ≥130/80 mmHg (54-56,59-61). The American Heart Association recommends a treatment goal of <130/80 mmHg, while the American Diabetes Association recommends a treatment goal of <140/90 mmHg with lower targets in individuals at high risk of cardiovascular disease (54-56,59-61). Other guidelines also recommend baseline cardiovascular disease risk assessment and evaluation of kidney, eye, or cerebrovascular damage in determining individual treatment goals (51,62-65). The American Geriatrics Society guidelines acknowledge the potential harm from arterial hypotension in older adults with diabetes mellitus (66). Guidelines generally agree that high quality care for patients with diabetes include normalization of HbA1C without hypoglycemia (67-69). This definition of high quality care for patients with diabetes should include normalization of blood pressure including pulse pressure without hypotension.
Regardless of the intended blood pressure goal, the ability to maintain a lower blood pressure threshold in a real-world setting outside of a controlled trial is an important disease management consideration (70). It is often reported that more than half of treated patients are not able to maintain blood pressure control, even at a threshold of <140/85 mmHg (71). Findings from the DIALOGUE study (72), a multicenter prospective registry among patients with hypertension and type II diabetes, demonstrated that patients with a “strict” SBP target (≤130 mmHg) had more contacts with general practitioners than any other patient group. In addition, among patients with a lower blood pressure target, only half actually maintained this threshold over 6 months. More specifically, 53% of patients in the “strict” target group (≤130 mmHg) were able to maintain this blood pressure goal over time, and 55% of patients in the “medium” target group (130 to ≤135 mmHg) were able to do so.
The majority of the studies relied on office measurements of blood pressure rather than ambulatory blood pressure monitoring. However, ambulatory blood pressure monitoring improves baseline and post-treatment risk assessment (73-86). Evidence-based guidelines recommend ambulatory blood pressure monitoring for diagnosis and individualization of treatment goals in adults with arterial hypertension (51,56,87-89).
Our review has several limitations. We analyzed direct evidence from RCTs that randomly assigned patients to more versus less intensive blood pressure goals and did not abstract the data from RCTs aimed at efficacy or comparative effectiveness of hypotensive drugs. We could not reproduce the results from meta-regression, because the authors did not provide sufficient data (30,33,35-38,90). We did not contact authors of meta-analyses requesting reproducible data. We do not know how many unregistered and unpublished studies analyzed the association between baseline and attained blood pressure and patient outcomes.
Despite this limitation, we present conflicting evidence from all published and unpublished studies appraised with consistent GRADE methodology. In contrast with previous meta-analyses of direct evidence, we grouped studies by baseline hypertension status and by targeted diastolic and SBP targets (38,40).
Our review has implications for clinical practice. Clinicians should assess baseline cardiovascular risk, recommend behavioural and pharmacological treatments aiming at blood pressure normalization without hypotension or orthostatic hypotension (91). They should engage patients in life style optimization, blood pressure self-monitoring, and monitoring of drug adverse effects (92).
Our review has policy implications. High quality care in patients with diabetes and arterial hypertension should be defined as achievement of normal blood pressure without episodes of hypotension and with minimal risk of orthostatic hypotension or other serious harms from recommended drugs.
Our review has research implications. Future research should determine the optimal blood pressure targets in subpopulations with diabetes and various demographic, socioeconomic, and behavioral factors, as well as comorbidities. Composite outcomes should be avoided. Trials should use blood pressure monitoring and examine pulse pressure, the risk of orthostatic hypotension and other drug-related harms in determining optimal choice of drugs and blood pressure targets in individual patients.
Conclusions
Based on our review, we conclude that in adults with diabetes and arterial hypertension, in order to reduce the risk of stroke, left ventricular hypertrophy and ECG abnormalities, macroalbuminuria, and non-spine bone fractures, clinicians should encourage healthy lifestyle choices and antihypertensive medications targeting blood pressure of 120–130/80 mmHg, with close monitoring of daily blood pressure fluctuations, episodes of orthostatic hypotension, and other drug-related harms.
Acknowledgements
We thank David R. Goldmann, MD for his contribution to the development of the clinical question and review protocol. This work is supported by Elsevier Evidence-based Medicine Center.
Supplementary
PICO question this report is addressing:
What are the benefits and harms of “lower” blood pressure targets compared to “standard” blood pressure targets in high-risk diabetic patients?
| Population | Adults with type 2 diabetes |
| Baseline blood pressure | |
| Patient demographics, socioeconomic status, smoking, physical activity, diet (sodium intake), prior treatment and response to medications for hypertension, comorbidities (e.g., cardiac arrhythmias, obesity, diabetes, asthma, chronic obstructive pulmonary disease), concomitant and concurrent medications | |
| Intervention | Lower blood pressure targets as defined in the studies |
| Comparator | Higher blood pressure targets as defined in the studies |
| Primary outcome(s) | All-cause mortality |
| Cardiovascular disease (CVD) events (stroke, myocardial infarction) and mortality | |
| All adverse events | |
| Setting | Outpatient |
Study eligibility
Inclusion criteria
| Participants | Adults with type 2 diabetes |
| Language restrictions | English |
| Publication dates (from and to) for searching | 2010–2018 (published high-quality reviews should address early publications of randomized trials) |
| Inclusion of guidelines | ECRI institute (formerly the “Emergency Care Research Institute”) appraised, published since 2010 |
| Meeting Institute of Medicine criteria for trustworthy guidelines | |
| Inclusion of clinical performance measures | Yes |
| Inclusion for systematic reviews (review quality, reviews with quantitative analyses) | Yes |
| Inclusion of randomized trials | Yes, published since 2010 |
| Inclusion of observational studies for harms (study characteristics, design, applicability, sample size, statistical methods to reduce bias) | Nationally representative prospective cohort studies of adverse effects with multivariate adjustment of adverse effects |
Exclusion criteria
| Interventions | We exclude trials of interventions at adults without diabetes |
| Outcomes | Intermediate outcomes such as hemodynamic characteristics |
| Study design | Uncontrolled case series or uncontrolled clinical trials Meeting abstracts presenting the results of randomized controlled trials (RCT) that have been published in peer-reviewed journals or have results in clinicaltrials.gov |
Search strategy
The medical librarian develops specific search strategies based on the PICOs formulated by our clinical and epidemiology staff. We search for all relevant articles published in English from 2010 up to March 2018 in PubMed, EMBASE, and the Cochrane Library. To identify grey unpublished data, we conduct a search of the trial registry clinicaltrials.gov.
We conduct the following searches:
PubMed searches for:
RCTs;
Observational studies of harms (multivariate adjusted estimates from nationally representative cohorts or administrative databases) (6,7);
Clinical practice guidelines.
EMBASE searches for full publications of:
RCTs;
Observational studies (multivariate adjusted estimates from nationally representative cohorts or administrative databases).
The bibliographies of identified articles are scanned, and study investigators are contacted for additional publications.
Study selection
The study epidemiologist and an author-subject matter expert contribute equally to resolving differences and decide the determination of eligibility collaboratively.
The study epidemiologist and an author-subject matter expert determine eligibility for full text review, first screen title, and abstracts. All citations found during the searches are stored in a reference database.
Data extraction and strategy for data synthesis
Data extraction
The data was extracted from the Clinical Trials Transformation Initiative (https://www.ctti-clinicaltrials.org/aact-database), checked for quality, and stored in the HPCC platform (High-Performance Computing Cluster, https://hpccsystems.com/).
We manually abstracted the data from published articles into the abstraction form. We checked the data for ambiguity (i.e., data reported in percentiles conflicting with unit data and vice versa; values outside a normal range) and mismatch with the published data. Identified errors have been discussed and corrected.
We abstract the information about study population, interventions, comparators, and outcomes. We abstract minimum datasets (e.g., number of the subjects in treatment groups and events) to estimate absolute risk difference, relative risk, and number needed to treat for categorical variables.
Means and standard deviations of continuous variables, e.g., total scores from the quality of life scales are abstracted. Statistical significance is evaluated at a 95% confidence level (including the use of P values). All authors have access to the data.
We conduct an overview of the reviews following the framework of the Cochrane Collaboration. We perform meta-analyses or update published meta-analyses. Pooling criteria include the exact same definitions of the active and control intervention, patient outcomes, and similar follow-up time (10).
We define harms as the totality of all possible adverse consequences of an intervention. Investigators sometimes defined harmful effects as unrelated to examined treatments. Harms are analyzed regardless of how investigators related them to treatments.
We calculate absolute risk difference, number needed to treat, and the number of attributable events based on data from the published randomized trials, using STATA software. Correction coefficients for zero events are used as a default option in both software programs, and intention to treat is used for evidence synthesis. Superiority of interventions under comparison is hypothesized.
We assess reporting bias as a proportion of published among all registered studies, unreported outcomes compared with published protocols, or unreported minimum data sets for reproducibility of the results. We did not conduct formal statistical tests for publication bias due to the questionable validity of such tests (18).
To examine the role of patient characteristics, a search is undertaken for subgroup analyses by patient demographics, baseline and achieved blood pressure, prior treatment response, and comorbidities in systematic reviews and randomized trials, including significant interaction effects.
Methodological assessment of the included studies
For systematic reviews (QIRs), we use the Assessment of Multiple Systematic Reviews (AMSTAR) scale to determine the methodological strength of the systematic reviews (99).
For randomized studies, we apply the Cochrane risk of bias tool. Risk of bias is assessed on a 3-point scale: high bias, low bias, and unclear (100,101). A low risk of bias is assumed when RCTs met all the risk-of-bias criteria, a medium risk of bias if at least 1 of the risk-of-bias criteria is not met, and a high risk of bias if 2 or more risk-of-bias criteria are not met. An unknown risk of bias is assigned for the studies with poorly reported risk-of-bias criteria. We assign high risk of bias to all observational studies.
For clinical practice guidelines, we use the Appraisal of Guidelines for Research and Evaluation (AGREE) II (2009) tool, which covers 23 items in 6 domains and 2 overall global ratings (102,103).
Quality assessment of the included studies and the body of evidence by outcome according to the GRADE framework
The authors assign the quality of evidence ratings as high, moderate, low, or very low, according to risk of bias in the body of evidence, directness of comparisons, precision and consistency in treatment effects, and the evidence of reporting bias, using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (23). We upgrade the risk of bias from low to high if at least 1 RCT had high risk of bias. We define indirectness in outcomes from intermediate outcomes. We review published network meta-analyses but do not conduct indirect comparisons.
Treatment effect estimates are defined as precise when pooled estimates have reasonably narrow 95% confidence intervals and the number of events are greater than 250. Justification of the sample size is not included in grading of the evidence. We do not conduct post hoc statistical power analyses.
In assessing the quality of evidence in all studies, the authors look for a dose response association, the strength of association, and evidence of any reporting bias. The strength of the association is evaluated, defining a priori a large effect when the relative risk is greater than 2 and a very large effect when the relative risk is greater than 5. A small treatment effect is construed when the relative risk was significant but less than 2. For standardized continuous measures of secondary and intermediate outcomes, the magnitude of the effect is defined according to Cohen et al. as small, moderate, and large, corresponding to mean differences in standard deviation units of 0 to 0.5, 0.5 to 0.8, and greater than 0.8, respectively.
A high quality of evidence is assigned to well-designed RCTs with consistent findings. The quality of evidence is downgraded to moderate if at least 1 of 4 quality of evidence criteria is not met; for example, moderate quality of evidence is assigned if there was a high risk of bias in the body of evidence or if the results are not consistent or precise. The quality of evidence is downgraded to low if 2 or more criteria are not met.
A low quality of evidence is assigned to nonrandomized studies and upgraded for the rating if there was a strong or dose-response association. Evidence is defined as insufficient when no studies provided valid information about treatment effects. This approach is applied regardless of whether the results were statistically significant.
The authors assign strength of the recommendations based on overall quality of evidence, balances between benefits and harms, healthcare consumers’ and clinicians’ values and preferences, and cost-effectiveness studies using the GRADE methodology.
| Grade | Definition |
|---|---|
| High | We are very confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has few or no deficiencies. We believe that the findings are stable, i.e., another study would not change the conclusions |
| Moderate | We are moderately confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has some deficiencies. We believe that the findings are likely to be stable, but some doubt remains |
| Low | We have limited confidence that the estimate of effect lies close to the true effect for this outcome. The body of evidence has major or numerous deficiencies (or both). We believe that additional evidence is needed before concluding either that the findings are stable or that the estimate of effect is close to the true effect |
| Very low | We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. Any estimate of effect is very uncertain |
| Insufficient | We have no evidence, we are unable to estimate an effect, or we have no confidence in the estimate of effect for this outcome. No evidence is available or the body of evidence has unacceptable deficiencies, precluding reaching a conclusion |
PubMed search of record:
(((((((((((((((diabet*[Title/Abstract]) OR "Diabetes Mellitus/drug therapy"[Majr])) AND (((((hypertensi*[Title/Abstract]) OR (("Blood Pressure/drug effects"[Mesh] OR "Blood Pressure/pharmacology"[Mesh] OR "Blood Pressure/therapy"[Mesh]))) OR (((("systolic blood pressure"[Title/Abstract]) OR "systolic pressure"[Title/Abstract]) OR "diastolic blood pressure"[Title/Abstract]) OR "diastolic pressure"[Title/Abstract])) OR normotensive[Title/Abstract]) OR "Hypertension/drug therapy"[Majr]))) AND ((((((strict*[Title/Abstract]) OR target*[Title/Abstract]) OR tight*[Title/Abstract]) OR intens*[Title/Abstract]) OR below[Title/Abstract]) OR moderat*[Title/Abstract])))) AND (((((("Antihypertensive Agents"[Mesh]) OR "Antihypertensive Agents" [Pharmacological Action]) OR "Angiotensin II Type 1 Receptor Blockers"[Mesh])) OR antihypertensive[Title/Abstract]) OR "angiotensin II"[Title/Abstract]))) NOT ((((((("Letter"[Publication Type]) OR "News"[Publication Type]) OR "Patient Education Handout"[Publication Type]) OR "Comment"[Publication Type]) OR "Editorial"[Publication Type])) OR "Newspaper Article"[Publication Type]))) NOT (("Animals"[Mesh]) NOT (("Animals"[Mesh]) AND "Humans"[Mesh])))) AND (((((((((random*[Title/Abstract]) OR placebo*[Title/Abstract]) OR "double blind"[Title/Abstract]) OR "triple blind"[Title/Abstract]) OR prospective[Title/Abstract]) OR multicenter[Title/Abstract])) OR "Multicenter Study" [Publication Type]) OR "Randomized Controlled Trial" [Publication Type:NoExp])))
PubMed search of record for CPGs:
(((((((((((diabet*[Title/Abstract]) OR "Diabetes Mellitus/drug therapy"[Majr])) AND (((((hypertensi*[Title/Abstract]) OR (("Blood Pressure/drug effects"[Mesh] OR "Blood Pressure/pharmacology"[Mesh] OR "Blood Pressure/therapy"[Mesh]))) OR (((("systolic blood pressure"[Title/Abstract]) OR "systolic pressure"[Title/Abstract]) OR "diastolic blood pressure"[Title/Abstract]) OR "diastolic pressure"[Title/Abstract])) OR normotensive[Title/Abstract]) OR "Hypertension/drug therapy"[Majr]))) AND ((((((strict*[Title/Abstract]) OR target*[Title/Abstract]) OR tight*[Title/Abstract]) OR intens*[Title/Abstract]) OR moderat*[Title/Abstract])))) AND (((((("Antihypertensive Agents"[Mesh]) OR "Antihypertensive Agents" [Pharmacological Action]) OR "Angiotensin II Type 1 Receptor Blockers"[Mesh])) OR antihypertensive[Title/Abstract]) OR "angiotensin II"[Title/Abstract]))) AND (((((((((((((((((((((((("Guideline"[Publication Type]) OR "Practice Guideline"[Publication Type])) OR "Consensus Development Conference"[Publication Type]) OR "Consensus Development Conference, NIH"[Publication Type]) OR "Practice Guideline"[Publication Type]) OR "Guideline"[Publication Type]) OR ((clinical[Title]) AND guideline*[Title])) OR (((clinical*[Title]) AND guide*[Title]) AND manage*[Title])) OR ((best[Title]) AND practice*[Title])) OR ((evidence[Title]) AND synthes*[Title])) OR ((consensus[Title]) AND develop*[Title])) OR ((practice[Title]) AND guideline*[Title])) OR (("evidence based"[Title]) AND guideline*[Title])) OR ((consensus[Title]) AND statement*[Title])) OR ((committee[Title]) AND opinion*[Title])) OR ((practice[Title]) AND bulletin*[Title])) OR ((clinical[Title]) AND recommendation*[Title])) OR ((("U.S. Preventive Services Task Force"[Title/Abstract]) OR USPSTF[Title/Abstract]) OR "United States Preventive Services Task Force"[Title/Abstract])) OR ACR Appropriateness Criteria[Title])) NOT ((((((("Letter"[Publication Type]) OR "News"[Publication Type]) OR "Patient Education Handout"[Publication Type]) OR "Comment"[Publication Type]) OR "Editorial"[Publication Type])) OR "Newspaper Article"[Publication Type]))) NOT (("Animals"[Mesh]) NOT (("Animals"[Mesh]) AND "Humans"[Mesh])))))
| Embase |
| No. |
| Query |
| Results |
| 273 |
| #64 |
| #53 AND #63 |
| 2,015,375 |
| #63 |
| #54 OR #56 OR #57 OR #58 OR #59 OR #62 |
| 1,509,234 |
| #62 |
| #61 OR 'double blind procedure'/de OR 'multicenter study'/de OR 'prospective study'/de OR 'randomized controlled trial'/de |
| 1,130,198 |
| #61 |
| random*:ab,ti OR placebo*:ab,ti OR (double NEXT/1 blind):ab,ti OR (triple NEXT/1 blind):ab,ti |
| 33,224 |
| #59 |
| 'hazard ratio'/de |
| 53,805 |
| #58 |
| 'proportional hazards model'/de |
| 151,708 |
| #57 |
| nation*:ab,ti OR registr* AND cohort OR 'cox regression':ab,ti OR 'hazard ratio':ab,ti |
| 274,206 |
| #56 |
| 'multivariate analysis'/exp |
| 324,727 |
| #54 |
| multivar*:ab,ti |
| 638 |
| #53 |
| #14 AND #52 |
| 1,471 |
| #52 |
| #47 NOT #51 |
| 4,776,528 |
| #51 |
| #48 NOT #50 |
| 15,578,685 |
| #50 |
| #48 AND #49 |
| 15,578,685 |
| #49 |
| 'human'/exp |
| 20,265,320 |
| #48 |
| 'animal'/exp |
| 1,488 |
| #47 |
| #45 NOT #46 |
| 5,188,835 |
| #46 |
| 'letter'/de OR 'editorial'/de OR 'note'/de OR 'conference paper'/de OR 'short survey'/exp OR 'conference abstract'/it |
| 2,124 |
| #45 |
| #43 AND #44 |
| 671,989 |
| #44 |
| #1 OR #3 OR #4 |
| 14,384 |
| #43 |
| #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33OR #34 OR #36 OR #37 OR #38 OR #41 OR #42 |
| 19 |
| #42 |
| (aggressive NEAR/3 systolic):ab,ti |
| 4 |
| #41 |
| (aggressive NEAR/3 diastolic):ab,ti |
| 464 |
| #38 |
| (below NEAR/3 systolic):ab,ti |
| 352 |
| #37 |
| (below NEAR/3 diastolic):ab,ti |
| 214 |
| #36 |
| (intensi* NEAR/3 systolic):ab,ti |
| 81 |
| #34 |
| (intensi* NEAR/3 diastolic):ab,ti |
| 12 |
| #33 |
| (tight* NEAR/3 diastolic):ab,ti |
| 23 |
| #32 |
| (tight* NEAR/3 systolic):ab,ti |
| 17 |
| #31 |
| (strict* NEAR/3 systolic):ab,ti |
| 4 |
| #30 |
| (strict* NEAR/3 diastolic):ab,ti |
| 208 |
| #29 |
| (target* NEAR/3 diastolic):ab,ti |
| 403 |
| #28 |
| (target* NEAR/3 systolic):ab,ti |
| 522 |
| #27 |
| (moderate NEAR/3 systolic):ab,ti |
| 451 |
| #26 |
| (moderate NEAR/3 diastolic):ab,ti |
| 161 |
| #25 |
| (standard NEAR/3 diastolic):ab,ti |
| 324 |
| #24 |
| (standard NEAR/3 systolic):ab,ti |
| 1,635 |
| #23 |
| (standard NEAR/3 pressure):ab,ti |
| 1,226 |
| #22 |
| (moderate NEAR/3 pressure):ab,ti |
| 394 |
| #21 |
| (aggressive NEAR/3 pressure):ab,ti |
| 3,343 |
| #20 |
| (below NEAR/3 pressure):ab,ti |
| 1,492 |
| #19 |
| (intensi* NEAR/3 pressure):ab,ti |
| 452 |
| #18 |
| (tight* NEAR/3 pressure):ab,ti |
| 420 |
| #17 |
| (strict* NEAR/3 pressure):ab,ti |
| 3,756 |
| #16 |
| (target* NEAR/3 pressure):ab,ti |
| 117,953 |
| #14 |
| #12 OR #13 |
| 53,840 |
| #13 |
| antihypertensive:ab,ti |
| 78,644 |
| #12 |
| 'antihypertensive agent'/exp/mj/dd_dt |
| 504,814 |
| #9 |
| hypertensi*:ab,ti |
| 61,009 |
| #8 |
| 'hypertension'/exp/mj/dm_dt |
| 83,927 |
| #4 |
| #3 AND 'drug therapy'/lnk |
| 664,883 |
| #3 |
| diabet*:ab,ti |
| 74,733 |
| #1 |
| 'diabetes mellitus'/exp/mj/dm_dt |
Figure S1.
Study flow diagram. Publications of primary RCTs of blood pressure targets (24-29,42-44,104-107); publications of RCTs that examined efficacy or comparative effectiveness of antihypertensive drugs in 1970–1989 (108-114), 1990–1999 (41,115-135), 2000–2002 (136-152), 2003–2005 (153-174), 2006–2009 (175-190), 2010–2014 (191-207); publications of reviews (3-5,30,31,33-38,40,50,90,93,208,209); publications of guidelines published in 2010–2014 (59,60,62-66,94,95,97,210-213) and in 2015–2018 (51-56,61,87,96,98,214-217).
Table S1. Relative risk of patient outcomes depending on baseline or attained blood pressure in adults with diabetes, the results from meta-analyses of aggregate data from randomized controlled clinical trials.
| Outcome | Blood pressure | Relative risk | No. of participants (studies) |
|---|---|---|---|
| Meta-regression of aggregate data by baseline blood pressure | |||
| All-cause mortality | 10 mmHg lower baseline SBP | 1.04 (0.98–1.10) | 73,738 (49 RCTs) (31) |
| All-cause mortality | 10 mmHg lower baseline DBP | 1.08 (0.99–1.18) | 73,738 (49 RCTs) (31) |
| Cardiovascular mortality | 10 mmHg lower baseline SBP | 1.15 (1.03–1.29) | 73,738 (49 RCTs) (31) |
| Cardiovascular mortality | 10 mmHg lower baseline DBP | 1.28 (1.05–1.55) | 73,738 (49 RCTs) (31) |
| End-stage renal disease | 10 mmHg lower baseline SBP | 1.05 (0.90–1.22) | 73,738 (49 RCTs) (31) |
| End-stage renal disease | 10 mmHg lower baseline DBP | 1.13 (0.88–1.44) | 73,738 (49 RCTs) (31) |
| Heart failure | 10 mmHg lower baseline SBP | 1.05 (0.93–1.20) | 73,738 (49 RCTs) (31) |
| Heart failure | 10 mmHg lower baseline DBP | 1.11 (0.90–1.36) | 73,738 (49 RCTs) (31) |
| Myocardial infarction | 10 mmHg lower baseline SBP | 1.12 (1.03–1.22) | 73,738 (49 RCTs) (31) |
| Myocardial infarction | 10 mmHg lower baseline DBP | 1.11 (0.98–1.26) | 73,738 (49 RCTs) (31) |
| Stroke | 10 mmHg lower baseline SBP | 1.07 (0.98–1.18) | 73,738 (49 RCTs) (31) |
| Stroke | 10 mmHg lower baseline DBP | 1.09 (0.93–1.27) | 73,738 (49 RCTs) (31) |
| Subgroup meta-analysis of aggregate data by baseline blood pressure | |||
| All-cause mortality | Baseline SBP >150 mmHg | 0.89 (0.80–0.99) | 12,824 (16 RCTs) (31) |
| Cardiovascular mortality | Baseline SBP >150 mmHg | 0.75 (0.57–0.99) | 9,073 (11 RCTs) (31) |
| Myocardial infarction | Baseline SBP >150 mmHg | 0.74 (0.63–0.87) | 9,914 (13 RCTs) (31) |
| Stroke | Baseline SBP >150 mmHg | 0.77 (0.65–0.91) | 11,444 (15 RCTs) (31) |
| Heart failure | Baseline SBP >150 mmHg | 0.73 (0.53–1.01) | 6,510 (7 RCTs) (31) |
| End-stage renal disease | Baseline SBP >150 mmHg | 0.82 (0.71–0.94) | 4,814 (5 RCTs) (31) |
| All-cause mortality | Baseline SBP 140–150 mmHg | 0.87 (0.78–0.98) | 24,652 (10 RCTs) (31) |
| All-cause mortality | Baseline SBP >140mmHg | 0.73 (0.64–0.84) | 30,998 (13 RCT) (93) |
| Cardiovascular mortality | Baseline SBP 140–150 mmHg | 0.87 (0.71–1.05) | 24,243 (9 RCTs) (31) |
| Cardiovascular disease | Baseline SBP >140mmHg | 0.74 (0.65–0.85) | 29,044 (11RCT) (93) |
| Myocardial infarction | Baseline SBP 140–150 mmHg | 0.84 (0.76–0.93) | 23,286 (7 RCTs) (31) |
| Stroke | Baseline SBP 140–150 mmHg | 0.92 (0.83–1.01) | 30,135 (9 RCTs) (31) |
| Stroke | Baseline SBP >140mmHg | 0.74 (0.74–0.86) | 36,934 (14 RCT) (93) |
| Heart failure | Baseline SBP 140–150 mmHg | 0.80 (0.66–0.97) | 12,723 (7 RCTs) (31) |
| End-stage renal disease | Baseline SBP 140–150 mmHg | 0.91 (0.74–1.12) | 21,376 (6 RCTs) (31) |
| All-cause mortality | Baseline SBP <140 mmHg | 1.05 (0.95–1.16) | 24,350 (14 RCTs) (31) |
| All-cause mortality | Baseline SBP <140 mmHg | 1.07 (0.92–1.26) | 12,559 (7 RCTs) (93) |
| Cardiovascular mortality | Baseline SBP <140 mmHg | 1.15 (1.00–1.32) | 22,439 (10 RCTs) (31) |
| Cardiovascular disease | Baseline SBP <140 mmHg | 0.96 (0.88–1.05) | 21,574 (6 RCTs) (93) |
| Myocardial infarction | Baseline SBP <140 mmHg | 1.00 (0.87–1.15) | 18,051 (9 RCTs) (31) |
| Stroke | Baseline SBP <140 mmHg | 0.81 (0.53–1.22) | 17,911 (8 RCTs) (31) |
| Stroke | Baseline SBP <140 mmHg | 0.69 (0.69–0.92) | 17,127 (5 RCT) (93) |
| Heart failure | Baseline SBP <140 mmHg | 0.90 (0.79–1.02) | 17,392 (8 RCTs) (31) |
| End-stage renal disease | Baseline SBP <140 mmHg | 0.97 (0.80–1.17) | 19,973 (7 RCTs) (31) |
| Cardiovascular mortality | Baseline SBP <130 mmHg | 2.95 (0.43–20.20) | 4,946 (2 RCTs) (37) |
| All-cause mortality | Baseline DBP >90 mmHg | 0.85 (0.73–1.00) | 6,591 (9 RCTs) (31) |
| Cardiovascular mortality | Baseline DBP >90 mmHg | 0.70 (0.55–0.89) | 4,452 (6 RCTs) (31) |
| Myocardial infarction | Baseline DBP >90 mmHg | 0.79 (0.62–1.00) | 3,681 (6 RCTs) (31) |
| Stroke | Baseline DBP >90 mmHg | 0.74 (0.58–0.94) | 5,211 (8 RCTs) (31) |
| Heart failure | Baseline DBP >90 mmHg | 0.50 (0.29–0.85) | 1,259 (2 RCTs) (31) |
| End-stage renal disease | Baseline DBP >90 mmHg | 0.96 (0.14–6.76) | 1,259 (2 RCTs) (31) |
| All-cause mortality | Baseline DBP 80–90 mmHg | 0.90 (0.82–0.99) | 25,779 (16 RCTs) (31) |
| Cardiovascular mortality | Baseline DBP 80–90 mmHg | 0.91 (0.78–1.07) | 24,842 (13 RCTs) (31) |
| Myocardial infarction | Baseline DBP 80–90 mmHg | 0.85 (0.76–0.95) | 24,861 (13 RCTs) (31) |
| Stroke | Baseline DBP 80–90 mmHg | 0.92 (0.83–1.03) | 30,604 (14 RCTs) (31) |
| Heart failure | Baseline DBP 80–90 mmHg | 0.81 (0.67–0.97) | 13,322 (11 RCTs) (31) |
| End-stage renal disease | Baseline DBP 80–90 mmHg | 0.83 (0.72–0.94) | 20,912 (8 RCTs) (31) |
| All-cause mortality | Baseline DBP <80 mmHg | 0.97 (0.89–1.06) | 29,456 (15 RCTs) (31) |
| Cardiovascular mortality | Baseline DBP <80 mmHg | 1.08 (0.82–1.41) | 27,091 (11 RCTs) (31) |
| Myocardial infarction | Baseline DBP <80 mmHg | 0.90 (0.79–1.02) | 22,709 (10 RCTs) (31) |
| Stroke | Baseline DBP <80 mmHg | 0.86 (0.70–1.05) | 23,675 (10 RCTs) (31) |
| Heart failure | Baseline DBP <80 mmHg | 0.89 (0.80–1.00) | 22,044 (9 RCTs) (31) |
| End-stage renal disease | Baseline DBP <80 mmHg | 0.97 (0.83–1.13) | 23,992 (8 RCTs) (31) |
| Meta-regression of aggregate data by attained blood pressure | |||
| All-cause mortality | Each 10 mmHg lower attained SBP | 0.94 (0.89–0.99) | 53,344 (20 RCTs) (33) |
| All-cause mortality | Each 10 mmHg lower attained SBP | 1.02 (0.93–1.12) | 73,738 (49 RCTs) (31) |
| All-cause mortality | Each 10 mmHg lower attained DBP | 1.07 (0.93–1.23) | 73,738 (49 RCTs) (31) |
| Cardiovascular mortality | Each 10 mmHg lower attained SBP | 1.20 (0.99–1.44) | 73,738 (49 RCTs) (31) |
| Cardiovascular mortality | Each 10 mmHg lower attained DBP | 1.35 (0.98–1.86) | 73,738 (49 RCTs) (31) |
| Coronary heart disease | Each 10 mmHg lower attained SBP | 0.88 (0.80–0.97) | 52,129 (19 RCTs) (33) |
| Major cardiovascular events | Each 10 mmHg lower attained SBP | 0.88 (0.82–0.94) | 59,773 (23 RCTs) (33) |
| Myocardial infarction | Each 10 mmHg lower attained SBP | 1.09 (0.98–1.21) | 73,738 (49 RCTs) (31) |
| Myocardial infarction | Each 10 mmHg lower attained DBP | 1.13 (0.96–1.33) | 73,738 (49 RCTs) (31) |
| Heart failure | Each 10 mmHg lower attained SBP | 0.90 (0.83–0.98) | 41,960 (13 RCTs) (33) |
| Heart failure | Each 10 mmHg lower attained SBP | 1.05 (0.90–1.22) | 73,738 (49 RCTs) (31) |
| Heart failure | Each 10 mmHg lower attained DBP | 1.11 (0.90–1.36) | 73,738 (49 RCTs) (31) |
| End-stage renal disease | Each 10 mmHg lower attained SBP | 1.02 (0.85–1.24) | 73,738 (49 RCTs) (31) |
| End-stage renal disease | Each 10 mmHg lower attained DBP | 1.11 (0.88–1.41) | 73,738 (49 RCTs) (31) |
| Renal failure | Each 10 mmHg lower attained SBP | 0.92 (0.84–1.01) | 28,190 (9 RCTs) (33) |
| Stroke | Each 10 mmHg lower attained SBP | 0.74 (0.65–0.84) | 58,064 (21 RCTs) (33) |
| Stroke | Each 10 mmHg lower attained SBP | 0.97 (0.82–1.13) | 73,738 (49 RCTs) (31) |
| Stroke | Each 10 mmHg lower attained DBP | 0.95 (0.75–1.21) | 73,738 (49 RCTs) (31) |
| Subgroup meta-analysis of aggregate data by attained blood pressure | |||
| All-cause mortality | – | 0.92 (0.87–0.96) | 66,130 (45 RCTs) (31) |
| All-cause mortality | Attained SBP >140 | 0.96 (0.86–1.06) | 21,876 (13 RCTs) (31) |
| All-cause mortality** | Attained SBP ≥140 | 0.98 (0.89–1.07) | 11,559 (9 RCTs) (37) |
| All-cause mortality** | Attained SBP 130–139 | 0.85 (0.78–0.92) | 23,714 (11 RCTs) (37) |
| All-cause mortality* | Attained SBP 130–139 | 0.81 (0.49–1.35) | 1,654 (4 RCTs) (37) |
| All-cause mortality | Attained SBP 130–140 | 0.86 (0.79–0.93) | 28,900 (18 RCTs) (31) |
| All-cause mortality** | Attained SBP <130 | 1.00 (0.82–1.21) | 6,117 (2 RCTs) (37) |
| All-cause mortality* | Attained SBP <130 | 1.44 (0.81–2.57) | 4,946 (2 RCTs) (37) |
| All-cause mortality | Attained SBP <130 | 1.10 (0.91–1.33) | 11,050 (9 RCTs) (31) |
| All-cause mortality | Attained DBP >80 mmHg | 0.95 (0.86–1.06) | 13,092 (13 RCTs) (31) |
| All-cause mortality | Attained DBP 75–80 mmHg | 0.86 (0.75–0.98) | 14,059 (13 RCTs) (31) |
| All-cause mortality | Attained DBP <75 | 0.97 (0.89–1.04) | 34,675 (14 RCTs) (31) |
| Cardiovascular mortality | – | 0.92 (0.82–1.03) | 59,956 (33 RCTs) (31) |
| Cardiovascular mortality | Attained SBP >140 | 0.87 (0.71–1.07) | 20,703 (11 RCTs) (31) |
| Cardiovascular mortality** | Attained SBP ≥140 | 0.92 (0.75–1.13) | 10,386 (10 RCTs) (37) |
| Cardiovascular mortality** | Attained SBP 130–139 | 0.79 (0.67–0.93) | 22,942 (7 RCTs) (37) |
| Cardiovascular mortality* | Attained SBP 130–139 | 0.61 (0.15–2.47) | 2459 (4 RCTs) (37) |
| Cardiovascular mortality | Attained SBP 130–140 | 0.86 (0.72–1.04) | 25,095 (12 RCTs) (31) |
| Cardiovascular mortality** | Attained SBP <130 | 1.12 (0.77–1.63) | 6,117 (2 RCTs) (37) |
| Cardiovascular mortality | Attained SBP <130 mmHg | 1.26 (0.89–1.77) | 10,587 (7 RCTs) (31) |
| Cardiovascular mortality | Attained DBP >80 mmHg | 0.71 (0.53–0.97) | 11,229 (10 RCTs) (31) |
| Cardiovascular mortality | Attained DBP 75–80 mmHg | 0.85 (0.69–1.05) | 13,040 (10 RCTs) (31) |
| Cardiovascular mortality | Attained DBP <75 mmHg | 1.16 (0.92–1.47) | 32,116 (10 RCTs) (31) |
| Coronary heart disease** | Attained SBP ≥140 | 0.72 (0.60–0.85) | 11,559 (9 RCTs) (37) |
| Coronary heart disease** | Attained SBP 130–139 | 0.86 (0.78–0.94) | 22,942 (10 RCTs) (37) |
| Coronary heart disease* | Attained SBP 130–139 | 0.67 (0.29–1.55) | 1,274 (3 RCTs) (37) |
| Coronary heart disease* | Attained SBP <130 | 0.67 (0.40–1.14) | 4,946 (2 RCTs) (37) |
| Myocardial infarction | – | 0.87 (0.81–0.94) | 53,512 (31 RCTs) (31) |
| Myocardial infarction | Attained SBP >140 mmHg | 0.82 (0.72–0.92) | 21,286 (12 RCTs) (31) |
| Myocardial infarction | Attained SBP 130–140 mmHg | 0.88 (0.79–0.97) | 23,828 (11 RCTs) (31) |
| Myocardial infarction | Attained SBP <130 mmHg | 0.94 (0.76–1.15) | 6,137 (6 RCTs) (31) |
| Myocardial infarction | Attained DBP >80 mmHg | 0.76 (0.63–0.93) | 9,608 (8 RCTs) (31) |
| Myocardial infarction | Attained DBP 75–80 mmHg | 0.81 (0.72–0.91) | 13,650 (12 RCTs) (31) |
| Myocardial infarction | Attained DBP <75 mmHg | 0.95 (0.84–1.07) | 27,993 (9 RCTs) (31) |
| Heart failure | – | 0.82 (0.75–0.89) | 40,196 (25 RCTs) (31) |
| Heart failure | Attained SBP >140 mmHg | 0.83 (0.68–1.00) | 19,060 (9 RCTs) (31) |
| Heart failure** | Attained SBP ≥140 | 0.86 (0.70–1.05) | 8,743 (5 RCTs) (37) |
| Heart failure | Attained SBP 130–140 mmHg | 0.81 (0.70–0.94) | 11,568 (8 RCTs) (31) |
| Heart failure** | Attained SBP 130–139 | 0.82 (0.69–0.97) | 20,952 (8 RCTs) (37) |
| Heart failure | Attained SBP <130 mmHg | 0.93 (0.71–1.21) | 5,997 (5 RCTs) (31) |
| Heart failure** | Attained SBP <130 | 0.89 (0.65–1.23) | 6,117 (2 RCTs) (37) |
| Heart failure | Attained DBP >80 mmHg | 0.72 (0.45–1.15) | 7,656 (5 RCTs) (31) |
| Heart failure | Attained DBP 75–80 mmHg | 0.79 (0.65–0.96) | 11,135 (9 RCTs) (31) |
| Heart failure | Attained DBP <75 mmHg | 0.90 (0.79–1.01) | 17,834 (8 RCTs) (31) |
| Stroke | – | 0.87 (0.79–0.96) | 59,490 (32 RCTs) (31) |
| Stroke** | Attained SBP ≥140 mmHg | 0.89 (0.74–1.07) | 11,730 (10 RCTs) (37) |
| Stroke | Attained SBP >140 mmHg | 0.90 (0.76–1.06) | 22,045 (14 RCTs) (31) |
| Stroke | Attained SBP 130–140 mmHg | 0.91 (0.83–1.00) | 30,342 (12 RCTs) (31) |
| Stroke** | Attained SBP 130–139 | 0.85 (0.75–0.96) | 28,685 (11 RCTs) (37) |
| Stroke* | Attained SBP 130–139 | 0.89 (0.45–1.78) | 1,274 (3 RCTs) (37) |
| Stroke** | Attained SBP <130 | 0.66 (0.49–0.88) | 5,839 (3 RCTs) (37) |
| Stroke* | Attained SBP <130 | 1.36 (0.55–3.39) | 4,946 (2 RCTs) (37) |
| Stroke | Attained SBP <130 mmHg | 0.65 (0.42–0.99) | 7,103 (6 RCTs) (31) |
| Stroke | Attained DBP >80 mmHg | 0.87 (0.73–1.04) | 11,011 (10 RCTs) (31) |
| Stroke | Attained DBP 75–80 mmHg | 0.86 (0.75–0.97) | 19,380 (12 RCTs) (31) |
| Stroke | Attained DBP <75 mmHg | 0.87 (0.70–1.08) | 29,099 (10 RCTs) (31) |
| Stroke + CHD** | Attained SBP ≥140 | 0.80 (0.71–0.90) | 11,568 (9 RCTs) (37) |
| Stroke + CHD** | Attained SBP 130–139 | 0.85 (0.78–0.91) | 25,060 (11 RCTs) (37) |
| Stroke + CHD* | Attained SBP 130–139 | 0.73 (0.51–1.04) | 2,858 (5 RCTs) (37) |
| Stroke + CHD* | Attained SBP <130 | 0.80 (0.51–1.26) | 4,946 (2 RCTs) (37) |
| Stroke + CHD + HF** | Attained SBP ≥140 | 0.85 (0.74–0.97) | 9,003 (7 RCTs) (37) |
| Stroke + CHD + HF** | Attained SBP 130–139 | 0.87 (0.80–0.94) | 30,032 (12 RCTs) (37) |
| Stroke + CHD + HF* | Attained SBP <130 | 0.84 (0.60–1.19) | 862 (2 RCTs) (37) |
| End-stage renal disease | – | 0.88 (0.80–0.97) | 47,439 (18 RCTs) (31) |
| End-stage renal disease | Attained SBP >140 mmHg | 0.88 (0.76–1.03) | 18,287 (7 RCTs) (31) |
| End-stage renal disease | Attained SBP 130–140 mmHg | 0.84 (0.66–1.07) | 17,912 (6 RCTs) (31) |
| End-stage renal disease | Attained SBP <130 mmHg | 1.01 (0.71–1.43) | 9,964 (5 RCTs) (31) |
| End-stage renal disease | Attained DBP >80 mmHg | 0.77 (0.39–1.52) | 6,171 (3 RCTs) (31) |
| End-stage renal disease | Attained DBP 75–80 mmHg | 0.81 (0.71–0.93) | 8,437 (7 RCTs) (31) |
| End-stage renal disease | Attained DBP <75 mmHg | 0.98 (0.84–1.14) | 31,555 (8 RCTs) (31) |
*, low/moderate cardiovascular risk; **,high/very high cardiovascular risk. CHD, coronary heart disease; DBP, diastolic blood pressure; HF, heart failure; RCT, randomized controlled trial; RR, relative risk; SBP, systolic blood pressure.
Table S2. GRADE summary of findings. Harms of blood pressure control in adults with diabetes mellitus (low-quality evidence from published aggregate data meta-analyses of any antihypertensive treatments).
| Outcome | Risk with intervention per 1,000 (95% CI) | Risk with comparator per 1,000 | Relative measure of association | No. of participants (studies) |
|---|---|---|---|---|
| Cardiovascular mortality; baseline SBP <130 mmHg | 6 | 2 | RR: 3.96 (1.33–11.84); 2.95 (0.43–20.20) | 4,946 (2 RCTs) (37) |
| Cardiovascular mortality; baseline SBP <140 mmHg | NR | NR | RR: 1.15 (1.00–1.32) | 22,439 (10 RCTs) (31) |
| Cardiovascular mortality; each 10 mmHg lower baseline SBP | NR | NR | RR: 1.15 (1.03–1.29) | 73,738 (49 RCTs) (31) |
| Cardiovascular mortality; each 10 mmHg lower baseline DBP | NR | NR | RR: 1.28 (1.05–1.55) | 73,738 (49 RCTs) (31) |
| Myocardial infarction; each 10 mmHg lower baseline SBP | NR | NR | RR: 1.12 (1.03–1.22) | 73,738 (49 RCTs) (31) |
Population: adults with diabetes; Settings: outpatient; Intervention: antihypertensive treatment; Comparator: control as no active antihypertensive treatment; CI, confidence interval; DBP, diastolic blood pressure; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NR, not reported; RCT, randomized controlled trial; RR, relative risk; SBP, systolic blood pressure.
Table S3. Guideline recommendations regarding blood pressure targets in adults with diabetes.
| Organization | Recommendations |
|---|---|
| World Health Organization (WHO); A Global Brief on Hypertension, 2013 (4,94) | This guideline recommends that target blood pressure in patients with diabetes should be <130/80 mmHg |
| Eighth Joint National Committee (JNC 8). Evidence-based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the JNC 8, 2014 (53,95) (AGREE II score: 78%) | This guideline recommends initiating pharmacologic treatment to lower blood pressure at SBP 140 mmHg or DBP 90 mmHg and treat to a goal of SBP <140/90 mmHg in all adults with diabetes |
| AHA/ASA Guidelines for the Prevention of Stroke in Patients With Stroke or Transient Ischemic Attack: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association, 2011–2017 (56,59) | This guideline recommends that in adults with diabetes and hypertension, antihypertensive drug treatment should be initiated at a blood pressure of 130/80 mmHg or higher with a treatment goal of <130/80 mmHg |
| American Diabetes Association. Position Statement on the Standards of Medical Care in Diabetes, 2018 (54,55,60,61) (AGREE II score not available) | This guideline recommends that most patients with diabetes and hypertension should be treated to an SBP goal of <140/90 mmHg; lower SBP and DBP targets, such as 130/80 mmHg, may be appropriate for individuals at high risk of cardiovascular disease, if they can be achieved without undue treatment burden |
| American Association of Clinical Endocrinologists and American College of Endocrinology. Clinical Practice Guidelines for Developing a Diabetes Mellitus Comprehensive Care Plan, 2015 (96) (AGREE II score: 53%) | The blood pressure goal for persons with diabetes mellitus or prediabetes should be individualized and should generally be about 130/80 mmHg; a more intensive goal (e.g., <120/80 mmHg) should be considered for some patients, provided this target can be reached safely without adverse effects from medication; more relaxed goals may be considered for frail patients with complicated comorbidities or those who have adverse medication effects |
| Clinical Practice Guidelines for the Management of Hypertension in the Community: A Statement by the American Society of Hypertension and the International Society of Hypertension Clinical Practice Guidelines for the Management of Hypertension in the Community: A Statement by the American Society of Hypertension and the International Society of Hypertension (97) | This guideline recommends that patients with diabetes should be treated to <140/90 mmHg; this guideline acknowledges that other guidelines have recommended diagnostic values of 130/80 mmHg for patients with diabetes or chronic kidney disease. However, the clinical benefits of this lower target have not been established; some experts recommend <130/80 mmHg if albuminuria is present in patients with comorbid chronic kidney disease |
| The American Geriatrics Society Guidelines for Improving the Care of Older Adults With Diabetes Mellitus, 2013 (66) | This guideline states that if an older adult has diabetes and requires medical therapy for hypertension, then the target blood pressure should be less than 140/90 mmHg if it is tolerated; there is potential harm in lowering SBP to less than 120 mmHg in older adults with type 2 diabetes mellitus (1B) |
| American Academy of Family Physicians, 2017 (98) | The AAFP continues to endorse the 2014 Evidence-Based Guidelines for the Management of High Blood Pressure in Adults, developed by panel members appointed to the Eighth Joint National Committee, with a blood pressure target of <140/80 mmHg |
| 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) (65) | This guideline recommends an SBP goal of <140/90 mmHg in patients with diabetes |
| The National Institute for Health and Care Excellence, 2011 (63); Type 2 Diabetes in Adults: Management, 2015 (51) | This guideline recommends treating adults with type 2 diabetes and arterial hypertension to achieve blood pressure <140/80 mmHg (<130/80 mmHg if there is kidney, eye, or cerebrovascular damage) |
| Scottish Intercollegiate Guidelines Network. Management of Diabetes: A National Clinical Guideline (62) (AGREE II score: 88%) | This guideline recommends target blood pressure <130/80 mmHg in patients with diabetes; in patients with diabetes and kidney disease, blood pressure should be reduced to the lowest achievable level to slow the rate of decline of glomerular filtration rate and reduce proteinuria |
| Canadian Hypertension Education Program (CHEP) Guidelines for Pharmacists, 2013 (64) | This guideline recommends that patients with kidney disease and concomitant diabetes should be treated to a target of <130/80 mmHg |
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr, et al. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 2016;164:542-52. 10.7326/M15-3016 [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Golden SH, Anderson C, et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care 2015;38:1777-803. 10.2337/dci15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solini A, Grossman E. What Should Be the Target Blood Pressure in Elderly Patients With Diabetes? Diabetes Care 2016;39 Suppl 2:S234-43. 10.2337/dcS15-3027 [DOI] [PubMed] [Google Scholar]
- 4.Tankeu AT, Noubiap JJ. Oscillating blood pressure therapeutic target in type 2 diabetes patients with hypertension. Ann Transl Med 2016;4:422. 10.21037/atm.2016.11.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunström M, Eliasson M, Nilsson PM, et al. Blood pressure treatment levels and choice of antihypertensive agent in people with diabetes mellitus: an overview of systematic reviews. J Hypertens 2017;35:453-62. 10.1097/HJH.0000000000001183 [DOI] [PubMed] [Google Scholar]
- 6.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane book series. London: The Cochrane Collaboration, 2011. [Google Scholar]
- 7.Slutsky J, Atkins D, Chang S, et al. AHRQ series paper 1: comparing medical interventions: AHRQ and the effective health-care program. J Clin Epidemiol 2010;63:481-3. 10.1016/j.jclinepi.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 8.Hartling L, Guise JM, Kato E, et al. EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews [Internet]. AHRQ Comparative Effectiveness Reviews 2015. [PubMed] [Google Scholar]
- 9.Robinson KA, Chou R, Berkman ND, et al. Integrating Bodies of Evidence: Existing Systematic Reviews and Primary Studies. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Methods for Effective Health Care. Rockville 2008. [PubMed] [Google Scholar]
- 10.Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011;64:1187-97. 10.1016/j.jclinepi.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Palmer TM, Sterne JA. Meta-Analysis in Stata: An Updated Collection from the Stata Journal. Second Edition. Texas: Stata Press, 2016. [Google Scholar]
- 12.Treadwell JR, Uhl S, Tipton K, et al. Assessing equivalence and noninferiority. J Clin Epidemiol 2012;65:1144-9. 10.1016/j.jclinepi.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Yuan KH, Maxwell S. On the post hoc power in testing mean differences. J Educ Behav Stat 2005;30:141-67. 10.3102/10769986030002141 [DOI] [Google Scholar]
- 14.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med 1994;121:200-6. 10.7326/0003-4819-121-3-199408010-00008 [DOI] [PubMed] [Google Scholar]
- 15.Levine M, Ensom MH. Post hoc power analysis: an idea whose time has passed? Pharmacotherapy 2001;21:405-9. 10.1592/phco.21.5.405.34503 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol 2011;64:1283-93. 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Balshem HS, Ansari M, Norris S, et al. Finding Grey Literature Evidence and Assessing for Outcome and Analysis Reporting Biases When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. Available online: www.effectivehealthcare.ahrq.gov/reports/final.cfm [PubMed]
- 18.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol 2011;64:407-15. 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 2011;64:1277-82. 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol 2011;64:1303-10. 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 22.Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719-25. 10.1016/j.jclinepi.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 23.Grading of Recommendations Assessment DaEGWG Available online: http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html#h.fueh5iz0cor4
- 24.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575-85. 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703-13. 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estacio RO, Coll JR, Tran ZV, et al. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006;19:1241-8. 10.1016/j.amjhyper.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 27.Schrier RW, Estacio RO, Esler A, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002;61:1086-97. 10.1046/j.1523-1755.2002.00213.x [DOI] [PubMed] [Google Scholar]
- 28.Estacio RO, Jeffers BW, Gifford N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23:B54-64. [PubMed] [Google Scholar]
- 29.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-62. 10.1016/S0140-6736(98)04311-6 [DOI] [PubMed] [Google Scholar]
- 30.Xie X, Atkins E, Lv J, et al. Intensive blood pressure lowering–Authors' reply. Lancet 2016;387:2291. 10.1016/S0140-6736(16)30366-X [DOI] [PubMed] [Google Scholar]
- 31.Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016;352:i717. 10.1136/bmj.i717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bundy JD, Li C, Stuchlik P, et al. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol 2017;2:775-81. 10.1001/jamacardio.2017.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957-67. 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 34.Jerums G, Panagiotopoulos S, Ekinci E, et al. Cardiovascular outcomes with antihypertensive therapy in type 2 diabetes: an analysis of intervention trials. J Hum Hypertens 2015;29:473-7. 10.1038/jhh.2014.117 [DOI] [PubMed] [Google Scholar]
- 35.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ 2013;185:949-57. 10.1503/cmaj.121468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBrien K, Rabi DM, Campbell N, et al. Intensive and Standard Blood Pressure Targets in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-analysis. Arch Intern Med 2012;172:1296-303. 10.1001/archinternmed.2012.3147 [DOI] [PubMed] [Google Scholar]
- 37.Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension. 11. Effects of total cardiovascular risk and achieved blood pressure: overview and meta-analyses of randomized trials. J Hypertens 2017;35:2138-49. 10.1097/HJH.0000000000001548 [DOI] [PubMed] [Google Scholar]
- 38.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387:435-43. 10.1016/S0140-6736(15)00805-3 [DOI] [PubMed] [Google Scholar]
- 39.Feldstein CA. Lowering blood pressure to prevent stroke recurrence: a systematic review of long-term randomized trials. J Am Soc Hypertens 2014;8:503-13. 10.1016/j.jash.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 40.Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev 2013;10:CD008277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estacio RO, Schrier RW. Antihypertensive therapy in type 2 diabetes: implications of the appropriate blood pressure control in diabetes (ABCD) trial. Am J Cardiol 1998;82:9R-14R. 10.1016/S0002-9149(98)00750-4 [DOI] [PubMed] [Google Scholar]
- 42.Buckley LF, Dixon DL, Wohlford GF, 4th, et al. Effect of Intensive Blood Pressure Control in Patients with Type 2 Diabetes Mellitus Over 9 Years of Follow-Up: A Subgroup Analysis of High-Risk ACCORDION Trial Participants. Diabetes Obes Metab 2018;20:1499-502. 10.1111/dom.13248 [DOI] [PubMed] [Google Scholar]
- 43.Soliman EZ, Byington RP, Bigger JT, et al. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Diabetes Mellitus: Action to Control Cardiovascular Risk in Diabetes Blood Pressure Trial. Hypertension 2015;66:1123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med 2014;29:1599-606. 10.1007/s11606-014-2961-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pigott T, Noyes J, Umscheid CA, et al. AHRQ series on complex intervention systematic reviews-paper 5: advanced analytic methods. J Clin Epidemiol 2017;90:37-42. 10.1016/j.jclinepi.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 46.Berlowitz DR, Foy CG, Kazis LE, et al. Effect of Intensive Blood-Pressure Treatment on Patient-Reported Outcomes. N Engl J Med 2017;377:733-44. 10.1056/NEJMoa1611179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones AP, Riley RD, Williamson PR, et al. Meta-analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clin Trials 2009;6:16-27. 10.1177/1740774508100984 [DOI] [PubMed] [Google Scholar]
- 48.Wu JS, Yang YC, Lu FH, et al. Population-based study on the prevalence and risk factors of orthostatic hypotension in subjects with pre-diabetes and diabetes. Diabetes Care 2009;32:69-74. 10.2337/dc08-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolters FJ, Mattace-Raso FU, Koudstaal PJ, et al. Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study. PLoS Med 2016;13:e1002143. 10.1371/journal.pmed.1002143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakris G, Sorrentino M. Redefining Hypertension - Assessing the New Blood-Pressure Guidelines. N Engl J Med 2018;378:497-9. 10.1056/NEJMp1716193 [DOI] [PubMed] [Google Scholar]
- 51.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guidelines 2015. [PubMed]
- 52.Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: A scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation 2015;131:e435-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [Internet] 2016. [DOI] [PubMed]
- 54.Standards of medical care in diabetes-2016: Summary of revisions. Diabetes Care 2016;39:S4-5. 10.2337/dc16-S003 [DOI] [PubMed] [Google Scholar]
- 55.de Boer IH, Bangalore S, Benetos A, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care 2017;40:1273-84. 10.2337/dci17-0026 [DOI] [PubMed] [Google Scholar]
- 56.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127-248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 57.American Diabetes Association 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S7-12. 10.2337/dc18-S001 [DOI] [PubMed] [Google Scholar]
- 58.American Diabetes Association 4. Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S38-50. 10.2337/dc18-S004 [DOI] [PubMed] [Google Scholar]
- 59.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:227-76. 10.1161/STR.0b013e3181f7d043 [DOI] [PubMed] [Google Scholar]
- 60.American Diabetes Association Standards of Medical Care in Diabetes - 2012. Diabetes Care 2012;35:S11-63. 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Summary of Revisions: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S4-6. 10.2337/dc18-Srev01 [DOI] [PubMed] [Google Scholar]
- 62.Network SI. Management of diabetes: a national clinical guideline, 2010.
- 63.Krause T, Lovibond K, Caulfield M, et al. Management of hypertension: Summary of NICE guidance. BMJ 2011;343:d4891. 10.1136/bmj.d4891 [DOI] [PubMed] [Google Scholar]
- 64.Houle SK, Padwal R, Tsuyuki RT. The 2012-2013 Canadian Hypertension Education Program (CHEP) guidelines for pharmacists: An update. Can Pharm J (Ott) 2013;146:146-50. 10.1177/1715163513487476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Task Force for the management of arterial hypertension of the European Society of Hypertension; Task Force for the management of arterial hypertension of the European Society of Cardiology. 2013 ESH/ESC guidelines for the management of arterial hypertension. Blood Pressure 2013;22:193-278. 10.3109/08037051.2013.812549 [DOI] [PubMed] [Google Scholar]
- 66.Moreno G, Mangione CM, Kimbro L, et al. Guidelines abstracted from the American Geriatrics Society guidelines for improving the care of older adults with diabetes mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020-6. 10.1111/jgs.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodriguez-Gutierrez R, Ospina NS, McCoy RG, et al. Inclusion of hypoglycemia in clinical practice guidelines and performance measures in the care of patients with diabetes. JAMA Intern Med 2016;176:1714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez-Gutierrez R, Lipska KJ, McCoy RG, et al. Hypoglycemia as an indicator of good diabetes care. BMJ 2016;352:i1084. 10.1136/bmj.i1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.American Diabetes Association 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S55-64. 10.2337/dc18-S006 [DOI] [PubMed] [Google Scholar]
- 70.Mills KT, Obst KM, Shen W, et al. Comparative Effectiveness of Implementation Strategies for Blood Pressure Control in Hypertensive Patients: A Systematic Review and Meta-analysis. Ann Intern Med 2018;168:110-20. 10.7326/M17-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahya Eren N, Harman E, Dolek D, et al. Rate of blood pressure control and antihypertensive treatment approaches in diabetic patients with hypertension. Turk Kardiyol Dern Ars 2014;42:733-40. 10.5543/tkda.2014.53384 [DOI] [PubMed] [Google Scholar]
- 72.Schmieder RE, Gitt AK, Koch C, et al. Achievement of individualized treatment targets in patients with comorbid type-2 diabetes and hypertension: 6 months results of the DIALOGUE registry. BMC Endocr Disord 2015;15:23. 10.1186/s12902-015-0020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Shi X, Ma C, et al. Visit-to-visit blood pressure variability is a risk factor for all-cause mortality and cardiovascular disease: a systematic review and meta-analysis. J Hypertens 2017;35:10-7. 10.1097/HJH.0000000000001159 [DOI] [PubMed] [Google Scholar]
- 74.Karmali KN, Persell SD, Perel P, et al. Risk scoring for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;3:CD006887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016;354:i4098. 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diaz KM, Tanner RM, Falzon L, et al. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: a systematic review and meta-analysis. Hypertension 2014;64:965-82. 10.1161/HYPERTENSIONAHA.114.03903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kollias A, Ntineri A, Stergiou GS. Association of night-time home blood pressure with night-time ambulatory blood pressure and target-organ damage: a systematic review and meta-analysis. J Hypertens 2017;35:442-52. 10.1097/HJH.0000000000001189 [DOI] [PubMed] [Google Scholar]
- 78.Salles GF, Reboldi G, Fagard RH, et al. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension 2016;67:693-700. 10.1161/HYPERTENSIONAHA.115.06981 [DOI] [PubMed] [Google Scholar]
- 79.Roush GC, Fagard RH, Salles GF, et al. Prognostic impact of sex-ambulatory blood pressure interactions in 10 cohorts of 17 312 patients diagnosed with hypertension: systematic review and meta-analysis. J Hypertens 2015;33:212-20. 10.1097/HJH.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 80.Xie JC, Yan H, Zhao YX, et al. Prognostic value of morning blood pressure surge in clinical events: a meta-analysis of longitudinal studies. J Stroke Cerebrovasc Dis 2015;24:362-9. 10.1016/j.jstrokecerebrovasdis.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 81.Sheppard JP, Hodgkinson J, Riley R, et al. Prognostic significance of the morning blood pressure surge in clinical practice: a systematic review. Am J Hypertens 2015;28:30-41. 10.1093/ajh/hpu104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nomura K, Asayama K, Thijs L, et al. Thresholds for conventional and home blood pressure by sex and age in 5018 participants from 5 populations. Hypertension 2014;64:695-701. 10.1161/HYPERTENSIONAHA.114.03839 [DOI] [PubMed] [Google Scholar]
- 83.Brguljan-Hitij J, Thijs L, Li Y, et al. Risk stratification by ambulatory blood pressure monitoring across JNC classes of conventional blood pressure. Am J Hypertens 2014;27:956-65. 10.1093/ajh/hpu002 [DOI] [PubMed] [Google Scholar]
- 84.Asayama K, Thijs L, Brguljan-Hitij J, et al. Risk stratification by self-measured home blood pressure across categories of conventional blood pressure: a participant-level meta-analysis. PLoS Med 2014;11:e1001591. 10.1371/journal.pmed.1001591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep 2013;15:413. 10.1007/s11886-013-0413-z [DOI] [PubMed] [Google Scholar]
- 86.Omboni S, Gazzola T, Carabelli G, et al. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens 2013;31:455-67; discussion 67-8. 10.1097/HJH.0b013e32835ca8dd [DOI] [PubMed] [Google Scholar]
- 87.American Diabetes Association 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S86-104. 10.2337/dc18-S009 [DOI] [PubMed] [Google Scholar]
- 88.Yannoutsos A, Kheder-Elfekih R, Halimi JM, et al. Should blood pressure goal be individualized in hypertensive patients? Pharmacol Res 2017;118:53-63. 10.1016/j.phrs.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 89.Wan EYF, Yu EYT, Fung CSC, et al. Do We Need a Patient-Centered Target for Systolic Blood Pressure in Hypertensive Patients With Type 2 Diabetes Mellitus? Hypertension 2017;70:1273-82. 10.1161/HYPERTENSIONAHA.117.10034 [DOI] [PubMed] [Google Scholar]
- 90.Brunström M, Carlberg B. Association of Blood Pressure Lowering With Mortality and Cardiovascular Disease Across Blood Pressure Levels: A Systematic Review and Meta-analysis. JAMA Intern Med 2018;178:28-36. 10.1001/jamainternmed.2017.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phillips RA, Xu J, Peterson LE, et al. Impact of Cardiovascular Risk on the Relative Benefit and Harm of Intensive Treatment of Hypertension. J Am Coll Cardiol 2018;71:1601-10. 10.1016/j.jacc.2018.01.074 [DOI] [PubMed] [Google Scholar]
- 92.McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet 2018;391:949-59. 10.1016/S0140-6736(18)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015;313:603-15. 10.1001/jama.2014.18574 [DOI] [PubMed] [Google Scholar]
- 94.World Health Organization. A global brief on Hypertension. World Health Day 2013. Available online: http://ish-world.com/downloads/pdf/global_brief_hypertension.pdf
- 95.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20. 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 96.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract 2015;21:1-87. 10.4158/EP15672.GLSUPPL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weber MA, Schiffrin EL, White WB, et al. Clinical Practice Guidelines for the Management of Hypertension in the Community: A Statement by the American Society of Hypertension and the International Society of Hypertension Clinical Practice Guidelines for the Management of Hypertension in the Community: A Statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014;16:14-26. 10.1111/jch.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chris Crawford. AAFP Decides to Not Endorse AHA/ACC Hypertension Guideline. Available online: https://wwwaafporg/patient-care/clinical-recommendations/non-endorsed.html
- 99.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013-20. 10.1016/j.jclinepi.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 100.Viswanathan M, Berkman ND, Dryden DM, et al. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 101.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ 2010;182:E472-8. 10.1503/cmaj.091716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ 2010;182:1045-52. 10.1503/cmaj.091714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ó Hartaigh B , Szymonifka J, Okin PM. Achieving target SBP for lowering the risk of major adverse cardiovascular events in persons with diabetes mellitus. J Hypertens 2018;36:101-9. 10.1097/HJH.0000000000001515 [DOI] [PubMed] [Google Scholar]
- 105.Barzilay JI, Howard AG, Evans GW, et al. Intensive blood pressure treatment does not improve cardiovascular outcomes in centrally obese hypertensive individuals with diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Blood Pressure Trial. Diabetes Care 2012;35:1401-5. 10.2337/dc11-1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen LY, Bigger JT, Hickey KT, et al. Effect of Intensive Blood Pressure Lowering on Incident Atrial Fibrillation and P-Wave Indices in the ACCORD Blood Pressure Trial. Am J Hypertens 2016;29:1276-82. 10.1093/ajh/hpv172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Margolis KL, O'Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014;37:1721-8. 10.2337/dc13-2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.The Hypertension Detection and Follow-up Program Cooperative Research Group Mortality findings for stepped-care and referred-care participants in the hypertension detection and follow-up program, stratified by other risk factors. Prev Med 1985;14:312-35. 10.1016/0091-7435(85)90059-3 [DOI] [PubMed] [Google Scholar]
- 109.Wright RA, Judson FN. Five-year findings of the hypertension detection and follow-up program. JAMA 1997;277:157-66. 10.1001/jama.1997.03540260071040 [DOI] [PubMed] [Google Scholar]
- 110.Gundersen T, Kjekshus J. Timolol treatment after myocardial infarction in diabetic patients. Diabetes Care 1983;6:285-90. 10.2337/diacare.6.3.285 [DOI] [PubMed] [Google Scholar]
- 111.Hypertension-Stroke Cooperative Study Group Effect of Antihypertensive Treatment on Stroke Recurrence. JAMA 1974;229:409-18. 10.1001/jama.1974.03230420021019 [DOI] [PubMed] [Google Scholar]
- 112.Hypertension Detection and Follow-up Program Cooperative Group Five-Year Findings of the Hypertension Detection and Follow-up Program: I. Reduction in Mortality of Persons With High Blood Pressure, Including Mild Hypertension. JAMA 1979;242:2562-71. 10.1001/jama.1979.03300230018021 [DOI] [PubMed] [Google Scholar]
- 113.Amery A, Brixko P, Clement D, et al. Mortality and morbidity results from the european working party on high blood pressure in the elderly trial. Lancet 1985;1:1349-54. 10.1016/S0140-6736(85)91783-0 [DOI] [PubMed] [Google Scholar]
- 114.Langford HG, Stamier J, Wassertheil-Smoller S, et al. All-cause mortality in the hypertension detection and follow-up program: Findings for the whole cohort and for persons with less severe hypertension, with and without other traits related to risk of mortality. Prog Cardiovasc Dis 1986;29:29-54. 10.1016/0033-0620(86)90033-2 [DOI] [PubMed] [Google Scholar]
- 115.Gustafsson I, Torp-Pedersen C, Køber L, et al. Effect of the angiotensin-converting enzyme inhibitor trandolapril on mortality and morbidity in diabetic patients with left ventricular dysfunction after acute myocardial infarction. J Am Coll Cardiol 1999;34:83-9. 10.1016/S0735-1097(99)00146-1 [DOI] [PubMed] [Google Scholar]
- 116.Yusuf S, Pitt B, Davis CE, et al. Effect of Enalapril on Mortality and the Development of Heart Failure in Asymptomatic Patients with Reduced Left Ventricular Ejection Fractions. N Engl J Med 1992;327:685-91. 10.1056/NEJM199209033271003 [DOI] [PubMed] [Google Scholar]
- 117.Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302. 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- 118.Moyé LA, Pfeffer MA, Wun CC, et al. Uniformity of captopril benefit in the SAVE study: Subgroup analysis. Survival and Ventricular Enlargement Study. Eur Heart J 1994;15:2-8. 10.1093/eurheartj/15.suppl_B.2 [DOI] [PubMed] [Google Scholar]
- 119.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-7. 10.1016/S0140-6736(99)04440-2 [DOI] [PubMed] [Google Scholar]
- 120.Swedberg K, Held P, Kjekshus J, et al. Effects of the Early Administration of Enalapril on Mortality in Patients with Acute Myocardial Infarction: Results of the Cooperative New Scandinavian Enalapril Survival Study II (Consensus II). N Engl J Med 1992;327:678-84. 10.1056/NEJM199209033271002 [DOI] [PubMed] [Google Scholar]
- 121.Dahlöf B, Hansson L, Lindholm LH, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991;338:1281-5. 10.1016/0140-6736(91)92589-T [DOI] [PubMed] [Google Scholar]
- 122.SHEP Cooperative Research Group Prevention of Stroke by Antihypertensive Drug Treatment in Older Persons With Isolated Systolic Hypertension: Final Results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255-64. 10.1001/jama.1991.03460240051027 [DOI] [PubMed] [Google Scholar]
- 123.Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of Captopril on Mortality and Morbidity in Patients with Left Ventricular Dysfunction after Myocardial Infarction: Results of the Survival and Ventricular Enlargement Trial. N Engl J Med 1992;327:669-77. 10.1056/NEJM199209033271001 [DOI] [PubMed] [Google Scholar]
- 124.Lewis EJ, Hunsicker LG, Bain RP, et al. The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy. N Engl J Med 1993;329:1456-62. 10.1056/NEJM199311113292004 [DOI] [PubMed] [Google Scholar]
- 125.Køber L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting–enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 1995;333:1670-6. 10.1056/NEJM199512213332503 [DOI] [PubMed] [Google Scholar]
- 126.Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. Am J Med 1995;99:497-504. 10.1016/S0002-9343(99)80226-5 [DOI] [PubMed] [Google Scholar]
- 127.Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA 1996;276:1886-92. 10.1001/jama.1996.03540230036032 [DOI] [PubMed] [Google Scholar]
- 128.Shindler DM, Kostis JB, Yusuf S, et al. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) trials and registry. Am J Cardiol 1996;77:1017-20. 10.1016/S0002-9149(97)89163-1 [DOI] [PubMed] [Google Scholar]
- 129.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757-64. 10.1016/S0140-6736(97)05381-6 [DOI] [PubMed] [Google Scholar]
- 130.UK Prospective Diabetes Study Group Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998;317:713-20. 10.1136/bmj.317.7160.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med 1998;338:645-52. 10.1056/NEJM199803053381003 [DOI] [PubMed] [Google Scholar]
- 132.Holman R, Turner R, Stratton I, et al. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998;317:713-20. 10.1136/bmj.317.7160.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. J Hypertens 1998;16:1823-9. 10.1097/00004872-199816120-00016 [DOI] [PubMed] [Google Scholar]
- 134.Ravid M, Brosh D, Levi Z, et al. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus: A randomized, controlled trial. Ann Intern Med 1998;128:982-8. 10.7326/0003-4819-128-12_Part_1-199806150-00004 [DOI] [PubMed] [Google Scholar]
- 135.Tuomilehto J, Rastenyte D, Birkenhager WH, et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med 1999;340:677-84. 10.1056/NEJM199903043400902 [DOI] [PubMed] [Google Scholar]
- 136.Schrier RW, Estacio RO. Additional follow-up from the ABCD trial in patients with type 2 diabetes and hypertension. N Engl J Med 2000;343:1969. 10.1056/NEJM200012283432614 [DOI] [PubMed] [Google Scholar]
- 137.Lindholm LH, Hansson L, Ekbom T, et al. Comparison of antihypertensive treatments in preventing cardiovascular events in elderly diabetic patients: Results from the Swedish trial in old patients with hypertension - 2. J Hypertens 2000;18:1671-5. 10.1097/00004872-200018110-00020 [DOI] [PubMed] [Google Scholar]
- 138.Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and β-blockers on cardiovascular morbidity and mortality in hypertension: The Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359-65. 10.1016/S0140-6736(00)02526-5 [DOI] [PubMed] [Google Scholar]
- 139.Lindholm LH, Ibsen H, Dahlöf B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002;359:1004-10. 10.1016/S0140-6736(02)08090-X [DOI] [PubMed] [Google Scholar]
- 140.Niskanen L, Hedner T, Hansson L, et al. Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/β-blocker-based treatment regimen: A subanalysis of the captopril prevention project. Diabetes Care 2001;24:2091-6. 10.2337/diacare.24.12.2091 [DOI] [PubMed] [Google Scholar]
- 141.Erdmann E, Philippe L, Patricia V, et al. Results from post-hoc analyses of the CIBIS II trial: Effect of bisoprolol in high-risk patient groups with chronic heart failure. Eur J Heart Fail 2001;3:469-79. 10.1016/S1388-9842(01)00174-X [DOI] [PubMed] [Google Scholar]
- 142.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002;288:2981-97. 10.1001/jama.288.23.2981 [DOI] [PubMed] [Google Scholar]
- 143.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355:253-9. 10.1016/S0140-6736(99)12323-7 [DOI] [PubMed] [Google Scholar]
- 144.Gerstein HC, Yusuf S, Mann JFE, et al. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253-9. 10.1016/S0140-6736(99)12323-7 [DOI] [PubMed] [Google Scholar]
- 145.O'Hare P, Bilous R, Mitchell T, et al. Low-dose ramipril reduces microalbuminuria in type 1 diabetic patients without hypertension: Results of a randomized controlled trial. Diabetes Care 2000;23:1823-9. 10.2337/diacare.23.12.1823 [DOI] [PubMed] [Google Scholar]
- 146.Wang JG, Staessen JA, Gong L, et al. Chinese trial on isolated systolic hypertension in the elderly. Systolic Hypertension in China (Syst-China) Collaborative Group. Arch Intern Med 2000;160:211-20. 10.1001/archinte.160.2.211 [DOI] [PubMed] [Google Scholar]
- 147.Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-9. 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 148.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75. 10.1056/NEJMoa010713 [DOI] [PubMed] [Google Scholar]
- 149.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851-60. 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 150.MacMahon S, Neal B, Tzourio C, et al. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41. 10.1016/S0140-6736(01)06178-5 [DOI] [PubMed] [Google Scholar]
- 151.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870-8. 10.1056/NEJMoa011489 [DOI] [PubMed] [Google Scholar]
- 152.Fogari R, Preti P, Zoppi A, et al. Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients. Am J Hypertens 2002;15:1042-9. 10.1016/S0895-7061(02)03017-0 [DOI] [PubMed] [Google Scholar]
- 153.Schrader J, Lüders S, Kulschewski A, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: Principal results of a prospective randomized controlled study (MOSES). Stroke 2005;36:1218-26. 10.1161/01.STR.0000166048.35740.a9 [DOI] [PubMed] [Google Scholar]
- 154.Yui Y, Sumiyoshi T, Kodama K, et al. Nifedipine retard was an effective as angiotensin converting enzyme inhibitors in preventing cardiac events in high-risk hypertensive patients with diabetes and coronary artery disease: The Japan Multicenter Investigation for Cardiovascular Diseases-B (JMIC-B) subgroup analysis. Hypertens Res 2004;27:449-56. 10.1291/hypres.27.449 [DOI] [PubMed] [Google Scholar]
- 155.Bakris GL, Gaxiola E, Messerli FH, et al. Clinical outcomes in the diabetes cohort of the international verapamil SR-trandolapril study. Hypertension 2004;44:637-42. 10.1161/01.HYP.0000143851.23721.26 [DOI] [PubMed] [Google Scholar]
- 156.Mancia G, Brown M, Castaigne A, et al. Outcomes with nifedipine GITS or Co-amilozide in hypertensive diabetics and nondiabetics in intervention as a goal in hypertension (INSIGHT). Hypertension 2003;41:431-6. 10.1161/01.HYP.0000057420.27692.AD [DOI] [PubMed] [Google Scholar]
- 157.Domanski M, Krause-Steinrauf H, Deedwania P, et al. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol 2003;42:914-22. 10.1016/S0735-1097(03)00856-8 [DOI] [PubMed] [Google Scholar]
- 158.Berl T, Hunsicker LG, Lewis JB, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med 2003;138:542-9. 10.7326/0003-4819-138-7-200304010-00010 [DOI] [PubMed] [Google Scholar]
- 159.Dens JA, Desmet WJ, Coussement P, et al. Long term effects of nisoldipine on the progression of coronary atherosclerosis and the occurrence of clinical events: The NICOLE study. Heart 2003;89:887-92. 10.1136/heart.89.8.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fox KM, Bertrand M, Ferrari R, et al. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: Randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782-8. 10.1016/S0140-6736(03)14286-9 [DOI] [PubMed] [Google Scholar]
- 161.Havranek EP, Esler A, Estacio RO, et al. Differential effects of antihypertensive agents on electrocardiographic voltage: results from the Appropriate Blood Pressure Control in Diabetes (ABCD) trial. Am Heart J 2003;145:993-8. 10.1016/S0002-8703(02)94780-0 [DOI] [PubMed] [Google Scholar]
- 162.Lithell H, Hansson L, Skoog I, et al. The study on cognition and prognosis in the elderly (SCOPE): Principal results of a randomized double-blind intervention trial. J Hypertens 2003;21:875-86. 10.1097/00004872-200305000-00011 [DOI] [PubMed] [Google Scholar]
- 163.Mehler PS, Coll JR, Estacio R, et al. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation 2003;107:753-6. 10.1161/01.CIR.0000049640.46039.52 [DOI] [PubMed] [Google Scholar]
- 164.Berthet K, Neal BC, Chalmers JP, et al. Reductions in the risks of recurrent stroke in patients with and without diabetes: the PROGRESS Trial. Blood Press 2004;13:7-13. 10.1080/08037050410029605 [DOI] [PubMed] [Google Scholar]
- 165.Braunwald E, Domanski MJ, Fowler SE, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 2004;351:2058-68. 10.1056/NEJMoa042739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marre M, Lievre M, Chatellier G, et al. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: Randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ 2004;328:495-9. 10.1136/bmj.37970.629537.0D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure. The CAMELOT study: A randomized controlled trial. JAMA 2004;292:2217-25. 10.1001/jama.292.18.2217 [DOI] [PubMed] [Google Scholar]
- 168.Poole-Wilson PA, Lubsen PJ, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): Randomised controlled trial. Lancet 2004;364:849-57. 10.1016/S0140-6736(04)16980-8 [DOI] [PubMed] [Google Scholar]
- 169.Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004;351:1941-51. 10.1056/NEJMoa042167 [DOI] [PubMed] [Google Scholar]
- 170.Daly CA, Fox KM, Remme WJ, et al. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: Results from the PERSUADE substudy. Eur Heart J 2005;26:1369-78. 10.1093/eurheartj/ehi225 [DOI] [PubMed] [Google Scholar]
- 171.Deedwania PC, Giles TD, Klibaner M, et al. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: Experiences from MERIT-HF. Am Heart J 2005;149:159-67. 10.1016/j.ahj.2004.05.056 [DOI] [PubMed] [Google Scholar]
- 172.Kowey PR, Dickson TZ, Zhang Z, et al. Losartan and end-organ protection - Lessons from the RENAAL study. Clin Cardiol 2005;28:136-42. 10.1002/clc.4960280307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) Study: A randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005;23:2157-72. 10.1097/01.hjh.0000194120.42722.ac [DOI] [PubMed] [Google Scholar]
- 174.Trenkwalder P, Elmfeldt D, Hofman A, et al. The study on Cognition and Prognosis in the Elderly (SCOPE) - Major CV events and stroke in subgroups of patients. Blood Pressure 2005;14:31-7. 10.1080/08037050510008823 [DOI] [PubMed] [Google Scholar]
- 175.Schrader J, Hammersen F, Lüders S, et al. Morbidity and mortality after stroke in patients with diabetes - Subgroup analysis from the MOSES study. Journal of Clinical and Basic Cardiology 2006;9:2-5. [Google Scholar]
- 176.Bilous R, Chaturvedi N, Sjølie AK, et al. Effect of Candesartan on Microalbuminuria and Albumin Excretion Rate in Diabetes Three Randomized Trials. Ann Intern Med 2009;151:11-20. 10.7326/0003-4819-151-1-200907070-00120 [DOI] [PubMed] [Google Scholar]
- 177.Östergren J, Poulter NR, Sever PS, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: Blood pressure-lowering limb: Effects in patients with type II diabetes. J Hypertens 2008;26:2103-11. 10.1097/HJH.0b013e328310e0d9 [DOI] [PubMed] [Google Scholar]
- 178.McLean RC, Mohler ER, Blumenthal RS. Vascular Disease, Hypertension, and Prevention. J Am Coll Cardiol 2006;47:D3-8. 10.1016/j.jacc.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 179.Schrier RW, Estacio RO, Mehler PS, et al. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: a summary of the ABCD trial. Nat Clin Pract Nephrol 2007;3:428-38. 10.1038/ncpneph0559 [DOI] [PubMed] [Google Scholar]
- 180.Wagener G. Calcium antagonists and hypertension: Role of co-existent coronary disease impaired renal function and diabetes. Rotterdam: Erasmus University Rotterdam, 2007. [Google Scholar]
- 181.Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372:1174-83. 10.1016/S0140-6736(08)61242-8 [DOI] [PubMed] [Google Scholar]
- 182.JATOS Study Group Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008;31:2115-27. 10.1291/hypres.31.2115 [DOI] [PubMed] [Google Scholar]
- 183.Chaturvedi N, Porta M, Klein R, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 2008;372:1394-402. 10.1016/S0140-6736(08)61412-9 [DOI] [PubMed] [Google Scholar]
- 184.Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: The SANDS randomized trial. JAMA 2008;299:1678-89. 10.1001/jama.299.14.1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lüders S, Schrader J, Berger J, et al. The PHARAO study: Prevention of hypertension with the angiotensin- converting enzyme inhibitor ramipril in patients with high-normal blood pressure - A prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens 2008;26:1487-96. 10.1097/HJH.0b013e3282ff8864 [DOI] [PubMed] [Google Scholar]
- 186.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456-67. 10.1056/NEJMoa0805450 [DOI] [PubMed] [Google Scholar]
- 187.Sjølie AK, Klein R, Porta M, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet 2008;372:1385-93. 10.1016/S0140-6736(08)61411-7 [DOI] [PubMed] [Google Scholar]
- 188.Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008;359:1225-37. 10.1056/NEJMoa0804593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.de Galan BE, Perkovic V, Ninomiya T, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 2009;20:883-92. 10.1681/ASN.2008070667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40-51. 10.1056/NEJMoa0808400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nakao K, Hirata M, Oba K, et al. Role of diabetes and obesity in outcomes of the candesartan antihypertensive survival evaluation in Japan (CASE-J) trial. Hypertens Res 2010;33:600-6. 10.1038/hr.2010.38 [DOI] [PubMed] [Google Scholar]
- 192.Weber MA, Bakris GL, Jamerson K, et al. Cardiovascular Events During Differing Hypertension Therapies in Patients With Diabetes. J Am Coll Cardiol 2010;56:77-85. 10.1016/j.jacc.2010.02.046 [DOI] [PubMed] [Google Scholar]
- 193.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233-44. 10.1056/NEJMoa1001288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61-8. 10.1001/jama.2010.884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Elliott HL, Lloyd SM, Ford I, et al. Improving blood pressure control in patients with diabetes mellitus and high cardiovascular risk. Int J Hypertens 2010;2010. [DOI] [PMC free article] [PubMed]
- 196.Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in elderly isolated systolic hypertension study. Hypertension 2010;56:196-202. 10.1161/HYPERTENSIONAHA.109.146035 [DOI] [PubMed] [Google Scholar]
- 197.Haller H, Ito S, Izzo JL, Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907-17. 10.1056/NEJMoa1007994 [DOI] [PubMed] [Google Scholar]
- 198.Imai E, Chan JC, Ito S, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: A multicentre, randomised, placebo-controlled study. Diabetologia 2011;54:2978-86. 10.1007/s00125-011-2325-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ruggenenti P, Fassi A, Ilieva AP, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: The BENEDICT-B randomized trial. J Hypertens 2011;29:207-16. 10.1097/HJH.0b013e32834069bd [DOI] [PubMed] [Google Scholar]
- 200.Ruggenenti P, Lauria G, Iliev IP, et al. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: The delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension 2011;58:776-83. 10.1161/HYPERTENSIONAHA.111.174474 [DOI] [PubMed] [Google Scholar]
- 201.Tillin T, Orchard T, Malm A, et al. The role of antihypertensive therapy in reducing vascular complications of type 2 diabetes. Findings from the DIabetic REtinopathy Candesartan Trials-Protect 2 study. J Hypertens 2011;29:1457-62. 10.1097/HJH.0b013e3283480db9 [DOI] [PubMed] [Google Scholar]
- 202.Zhang Y, Zhang X, Liu L, et al. Is a systolic blood pressure target <140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J 2011;32:1500-8. 10.1093/eurheartj/ehr039 [DOI] [PubMed] [Google Scholar]
- 203.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204-13. 10.1056/NEJMoa1208799 [DOI] [PubMed] [Google Scholar]
- 204.Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: The SPS3 randomised trial. Lancet 2013;382:507-15. 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892-903. 10.1056/NEJMoa1303154 [DOI] [PubMed] [Google Scholar]
- 206.Win S, Anand I, McMurray J, et al. Morbidity and mortality in diabetics with heart failure and a preserved ejection fraction: Results from the i-preserve trial. J Am Coll Cardiol 2013;61:E706 10.1016/S0735-1097(13)60706-8 [DOI] [Google Scholar]
- 207.Palacio S, McClure LA, Benavente OR, et al. Lacunar strokes in patients with diabetes mellitus: risk factors, infarct location, and prognosis: the secondary prevention of small subcortical strokes study. Stroke 2014;45:2689-94. 10.1161/STROKEAHA.114.005018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Reboussin DM, Allen NB, Griswold ME, et al. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:2176-98. 10.1016/j.jacc.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation 2011;123:2799-810. 10.1161/CIRCULATIONAHA.110.016337 [DOI] [PubMed] [Google Scholar]
- 210.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: A report of the American college of cardiology foundation task force on clinical expert consensus documents. J Am Coll Cardiol 2011;57:2037-114. 10.1016/j.jacc.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 211.Mancia G, Fagard R, Narkiewicz K, et al. The task force for the management ofarterial hypertension of the european society ofhypertension (esh) and of the european society of cardiology (esc). J Hypertens 2013;31:1281-357. 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 212.Aschner P, Beck-Nielsen H, Bennett P, et al. Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014;104:1-52. 10.1016/j.diabres.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 213.Go AS, Bauman MA, Coleman King SM, et al. An effective approach to high blood pressure control: A science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. J Am Coll Cardiol 2014;63:1230-8. 10.1016/j.jacc.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 214.American Diabetes Association 8. Cardiovascular disease and risk management. Diabetes Care 2015;38:S49-57. 10.2337/dc15-S011 [DOI] [PubMed] [Google Scholar]
- 215.Introduction: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S1-2. 10.2337/dc18-Sint01 [DOI] [PubMed] [Google Scholar]
- 216.American Diabetes A. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes 2018;36:14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.American Diabetes A. 11. Older Adults: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S119-25. 10.2337/dc18-S011 [DOI] [PubMed] [Google Scholar]