Abstract
Background
Indocyanine green (ICG) fluorescence imaging is a promising tool for intraoperative decision-making during surgical procedures, in particular to assess organs perfusion.
Methods
We used the ICG fluorescence during liver transplantations in six cirrhotic patients to help assessing the graft biliary duct perfusion in order to identify the appropriate level to perform the anastomosis. We also used ICG fluorescence also in five patients receiving kidney-pancreas transplantation to evaluate the perfusion levels of the duodenal stump of the pancreas graft.
Results
Follow-up period for the patients was 12 months. The perioperative period was uneventful, no biliary complications such as leaks or stenosis were reported after liver transplantation, no complications of the entero-enteric anastomoses occurred after pancreatic transplantation.
Conclusions
ICG fluorescence seems to safely provide important objectifiable perfusion information during organ transplantation procedures that can integrate surgeon’s expertise. In fact, detecting intra-operatively perfusion defects, it allows real time modifications on technical strategies potentially useful to reduce the feared risk of anastomotic leakage and consequent severe complications.
Keywords: Indocyanine green (ICG), liver, pancreas, transplantation, perfusion
Introduction
Indocyanine green (ICG) fluorescence is a useful real time imaging technique developed during the last few years to improve surgeon performance and, consequently, patient safety. ICG dye is a molecule with a mass of 776 Daltons, approved for clinical use in 1959 by the Food and Drugs Administration (1,2). Following intravenous injection, ICG first binds the albumin. Quickly after, it is removed from the blood and uptake by the hepatic cells. Without any modifications the dye is nearly exclusively excreted into bile, in about 8 min of time frames from the injection, depending on the liver vascularisation and function (1-3). ICG dye becomes fluorescent once excited either using a near-infrared (NIR) light. Specifically, designated scopes can visualize the fluorescence emitted. The standard clinical dose (0.1–0.5 mg/mL/kg) is safe and well below the toxic level.
The dye that can be injected intravenous with practically no adverse effects (1), has been used in medicine since the late 50s to assess liver function before major liver resections in cirrhotic patients (4). The Japanese first described the applicability of the fluorescence in the diagnosis of hepatic tumours as hepatocellular carcinoma and, afterwards, the identifiable patterns of fluorescence that result from differentiation-dependant hepatocyte dysfunction. Moreover, fluorescence has been extensively employed in the gynaecologic, endocrine and colorectal surgeries (1,5). Fluorescence, in fact, can provide detailed anatomical and functional information during surgery (1,5,6). The applicability and the possible advantages using ICG fluorescence during organ transplantation is limited and not well assessed. Herein, we present our initial experience of ICG-enhanced fluorescence applied during liver and pancreas transplants.
Methods
The study was approved by the institutional review board of the Montpellier University Hospital (No. 00115/2016) and informed consent was obtained from all patients.
We applied the ICG fluorescence for evaluating perfusion level during 6 livers and 5 pancreas transplantation procedures performed at Montpellier University Hospital (St. Eloi Hospital) between January and April 2016.
Fluorescence was identified using a full high definition camera system (VITOM®, KARL STORZ GmhH & Co. KG, Tuttlingen, Germany), connected to a 10-mm scope with 0° field, equipped with a specific filter for NIR fluorescence detection. The surgeon controls the switching from white light to NIR using a pedal. Visualization, moreover, is improved by a system of professional image enhancement (IMAGE1 STM system, KARL STORZ GmhH & Co. KG, Tuttlingen, Germany), which offers adjustable visualization modalities selected according to surgeon’s preferences.
This camera is specifically developed for open surgery procedures and it is fixed with a flexible arm to the operating room table between the first surgeon and the assistant. The surgeon is able to modify the camera position during the entire procedure and the position of the camera does not interfere with the surgeon work.
Primary end-points of the study
Liver transplantation: the aim is to assess the graft bile duct perfusion in order to identify the appropriate area of duct transection before performing the anastomosis;
Pancreas transplantation: to evaluate the perfusion level of the duodenal graft stump in order to identify some perfusion defect that could be transected before the anastomosis.
ICG fluorescence applied in liver transplantation
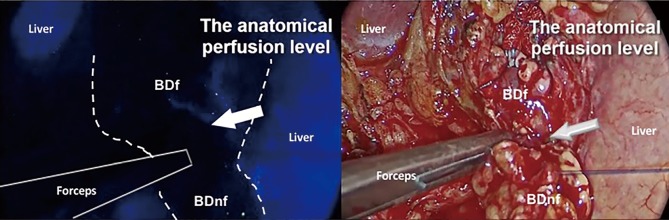
Once performed standard procedures for liver transplantation and provided the revascularization of the organ, we injected intravenously 0.5 mg/kg of ICG dye. After just 47 seconds, the perfusion level between the proximal vascularized part (fluorescent) and the distal non-vascularized part (non-fluorescent) of the graft bile duct was clearly recognized (Figures 1,2). At this precise level, we performed an end-to-end bile duct anastomosis with two running sutures of PDS 5/0. An IGC fluorescent angiography was re-performed after the anastomosis to evaluate the vascularization.
Figure 1.

Intraoperative ICG fluorescence assessment of the graft bile duct perfusion during liver transplantation (7). ICG, indocyanine green. Available online: http://www.asvide.com/article/view/25317
Figure 2.
Intraoperative ICG evaluation of graft bile duct perfusion. The proximal fluorescent part is vascularized while the distal one is non-vascularized (non fluorescent), guiding the level of transection. BDf, bile duct fluorescent; BDnf, bile duct not fluorescent; ICG, indocyanine green.
ICG fluorescence applied in pancreatic transplantation
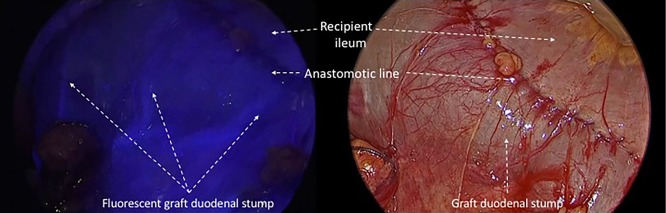
After performing the vascular anastomoses and subsequently provide the reperfusion of the pancreatic parenchyma, we intravenously injected 0.5 mg/kg of ICG dye to evaluate enteric diversion. After a time lapse of 35 seconds from the injection, we observed the arterial vascularisation of the graft duodenal stump, particularly at the level of the lateral portions previously resected using a linear stapler (Figures 3,4). We could evaluate, furthermore, the vascularization of the head of the pancreas and the site of the anastomoses between the graft duodenal stump and the recipient terminal ileum. We normally perform a side-to-side entero-enteric anastomosis with two running sutures of PDS 4/0. The patient receives a therapeutic dose of intravenous heparin for the first week after transplantation.
Figure 3.

Intraoperative ICG fluorescence assessment of the perfusion of the intestinal anastomosis during pancreas transplantation (8). ICG, indocyanine green. Available online: http://www.asvide.com/article/view/25319
Figure 4.
Intraoperative evaluation of arterial perfusion of the graft duodenal stump provided by ICG fluorescence. ICG, indocyanine green.
Results
Liver transplants
In two patients out of six we changed the intraoperative strategy performing a new graft bile duct resection before performing the anastomosis. In these two patients, we inserted a trans-anastomotic T-tube. The fluorescence procedure prolonged the operative time of about 10 minutes. No adverse events occurred in the perioperative and postoperative periods.
Follow-up period for the six patients having undergone liver transplantation was 12 months. All these patients have been regularly evaluated. The postoperative period was uneventful, liver function tests [(alanine transaminase (ALT), aspartate aminotransferase (AST), prothrombin time (PT), international normalized ratio (INR), total bilirubin)] were normalized and, above all, no biliary complications as leaks or stenosis were reported (Table 1).
Table 1. Patients data for liver transplants.
| Type of organ transplantation (Tx) | Patient number | Graft bile duct ICG fluorescence anomaly | Graft bile duct resection | Reconstruction method | Bile duct complications | Liver function tests (ALT, AST, PT, INR, total bilirubin) | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| Liver Tx | 1 | Not | Not | End-to-end | Not | NLF | 11 |
| 2 | Yes | Yes (12 mm of the distal part) | End-to-end (T-tube) | Not | NLF | 13 | |
| 3 | Not | Not | End-to-end | Not | NLF | 13 | |
| 4 | Not | Not | End-to-end | Not | NLF | 13 | |
| 5 | Yes | Yes (9 mm of the distal part) | End-to-end (T-tube) | Not | NLF | 14 | |
| 6 | Not | Not | End-to-end | Not | NLF | 14 |
ALT, alanine transaminase; AST, aspartate aminotransferase; PT, prothrombin time; INR, international normalized ratio; NLF, normal liver function; Tx, transplant.
Pancreas transplants
A duodenal stump resection was re-performed due to ischemia in a single case. Furthermore, in this case the reconstruction was performed by a duodenal-jejunum Roux en Y anastomosis. In the others cases a side-to-side duodenal-ileal anastomosis were performed. The fluorescence procedure prolonged the operative time of almost 8 minutes. No adverse events occurred in the perioperative period.
We employed ICG fluorescence to evaluate enteric diversion in the pancreatic-renal transplantation in others four patients without experiencing complications.
The five patients who have undergone the combined kidney-pancreas transplantation were alive and fine at 12 months of follow-up. One of five patients was re-operated at the 10th postoperative day for drainage of an intra-abdominal infected hematoma causing fever and abdominal pain. No complications of the duodenal-enteric anastomosis or the graft duodenal stump occurred.
At the evaluation after one year from surgery, the mean serum creatinine was 1.35 mg/dL (1.2–1.5 mg/dL), while glycaemia was in the normal range, the mean serum C-peptide was 1.9 ng/mL (range, 0.62–3.01 ng/mL) without exogenous insulin administration (Table 2).
Table 2. Patients data for pancreas transplants.
| Type of organ transplantation (Tx) | Patient number | Graft duodenum ICG fluorescence anomaly | Duodenal graft resection | Reconstruction method | Duodenal graft complications | Pancreas function tests (fast blood sugar, c-peptide) | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| Pancreas Tx | 1 | Not | Not | Side-to-side (duodeno-ileal) | Not | Normal | 11 |
| 2 | Not | Not | Side-to-side (duodeno-ileal) | Not | Normal | 12 | |
| 3 | Not | Not | Side-to-side (duodeno-ileal) | Not | Normal | 12 | |
| 4 | Yes (stump*) | Yes | Roux-en-Y | Not | Normal | 13 | |
| 5 | Not | Not | Side-to-side (duodeno-ileal) | Not | Normal | 14 |
Tx, transplant; (*), duodenal stump.
Discussion
Bowel and biliary anastomoses have historically been, respectively, the Achilles heel of pancreas and liver transplantation. In our experience during the last 5 years we reported about 7.5% of bile duct leaks and 22.5% of bile duct stenosis in case of liver transplantation, and about 10% of duodenal graft leaks in case of pancreas transplantation. Improvements in the surgical technique and donor management have mitigated many of these issues. Despite this, still a considerable amount of postoperative complications are attributable to various causes, particularly, to an ischemic damage.
As the application of ICG fluorescence angiography could probably add important real time information on territories of vascularization, influencing the intraoperative decision-making, we employed it during open organ transplantation procedures.
The purpose of testing this emerging imaging technique is to identify a method of increased sensibility compared to personal experience and the standard intraoperative Doppler ultrasound, that can help improving surgeon performance and, consequently, patient safety (1,3,5,9).
Hopefully therefore ICG introduction might lead to a reduction of post-operative complication’s rate attributable to ischemia.
The ICG dye was already applied in solid organ transplantation. In the University of Illinois surgeons used ICG dye to demonstrate a good graft revascularization during pancreas transplantation (10). Furthermore, Japanese tested ICG dye to evaluate the portal reperfusion in a case of living donor liver transplantation (11,12). Moreover, the application of ICG fluorescence is currently under-evaluation to predict the short-terms outcomes after liver transplantation (13-15).
However, one of the most feared complications after liver transplantation concerns the bile duct vascularization and the biliary anastomoses (16,17).
Biliary complications occur approximately in 10–20% of the patients who have undergone liver transplantation and are responsible of graft loss and increased mortality (16,17).
Causes of biliary complications are various as graft/recipient anatomical, surgical or immunological issues (16-19). Correct organ procurement and bile duct dissection play a key-role to avoid complications reported as well (18).
Up to now, the graft bile duct vascularization is extremely difficult to estimate and only the surgeon’s intraoperative evaluation (observing brisk bleeding from the proximal stump) and experience are currently employed (20).
The emerging technique of the ICG fluorescence may fill up this gap, giving an objective real time visualization of the bile duct microvascular perfusion without considerably lengthening the operation time.
In the same manner, pancreas transplantation procedure could be affected by severe complications causing graft loss and severely increased mortality as the digestive leak, in case of enteric diversion (21). The main cause of this severe complication is a defective duodenal graft vascularization (21,22). In fact, the duodenal stump vascularization is far from being optimal due to gastroduodenal artery ligation during the organ procurement (22).
The interest of an objective, quantifiable, real time information on vascularization of the duodenal stump as provided by IGC fluorescence is, in this contest, extremely high to the intraoperative decision-making.
In conclusion, the ICG fluorescence seems to safely provide important objectifiable perfusion information during organ transplantation procedures that can integrate surgeon’s expertise. In fact, detecting intra-operatively perfusion defects, it allows real time modifications on technical strategies potentially useful to reduce the feared risk of anastomotic leakage and consequent severe complications.
Acknowledgements
None.
Ethical Statement: The study was approved by the institutional review board of the Montpellier University Hospital (No. 00115/2016) and informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Boni L, David G, Mangano A, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 2015;29:2046-55. 10.1007/s00464-014-3895-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diana M, Noll E, Diemunsch P, et al. Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 2014;259:700-7. 10.1097/SLA.0b013e31828d4ab3 [DOI] [PubMed] [Google Scholar]
- 3.Diana M, Halvax P, Dallemagne B, et al. Real-time navigation by fluorescence-based enhanced reality for precise estimation of future anastomotic site in digestive surgery. Surg Endosc 2014;28:3108-18. 10.1007/s00464-014-3592-9 [DOI] [PubMed] [Google Scholar]
- 4.Kudo H, Ishizawa T, Tani K, et al. Visualization of subcapsular hepatic malignancy by indocyanine-green fluorescence imaging during laparoscopic hepatectomy. Surg Endosc 2014;28:2504-8. 10.1007/s00464-014-3468-z [DOI] [PubMed] [Google Scholar]
- 5.Diana M, Agnus V, Halvax P, et al. Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 2015;102:e169-76. 10.1002/bjs.9725 [DOI] [PubMed] [Google Scholar]
- 6.Liu YY, Pop R, Diana M, et al. Real-time fluorescence angiography by intra-arterial indocyanine green injection to identify obscure gastrointestinal bleeding territory: proof of concept in the porcine model. Surg Endosc 2016;30:2143-50. 10.1007/s00464-015-4460-y [DOI] [PubMed] [Google Scholar]
- 7.Panaro F, Benedetti E, Habibeh H, et al. Intraoperative ICG fluorescence assessment of the graft bile duct perfusion during liver transplantation. Asvide 2018;5:562. http://www.asvide.com/article/view/25317
- 8.Panaro F, Benedetti E, Habibeh H, et al. Intraoperative ICG fluorescence assessment of the perfusion of the intestinal anastomosis during pancreas transplantation. Asvide 2018;5:563. Available online: http://www.asvide.com/article/view/25319
- 9.Ayloo SM, D'Amico G, West-Thielke P, et al. Combined Robot-assisted Kidney Transplantation and Sleeve Gastrectomy in a Morbidly Obese Recipient. Transplantation 2015;99:1495-8. 10.1097/TP.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Roca R, Walczak D, Tzvetanov I, et al. The application of indocyanine green to evaluate duodenal perfusion in pancreas transplantation. Am J Transplant 2014;14:226-8 10.1111/ajt.12542 [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi Y, Akamatsu N, Ishizawa T, et al. Evaluation of hepatic perfusion in the liver graft using fluorescence imaging with indocyanine green. Int J Surg Case Rep 2015;14:149-51. 10.1016/j.ijscr.2015.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hori T, Ogura Y, Onishi Y, et al. Systemic hemodynamics in advanced cirrhosis: Concerns during perioperative period of liver transplantation. World J Hepatol 2016;8:1047-60. 10.4254/wjh.v8.i25.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J Hepatol 2016;8:355-67. 10.4254/wjh.v8.i7.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolondi G, Moccheggiani F, Montalti R, et al. Predictive factors of short term outcome after liver transplantation: A review. World J Gastroenterol 2016;22:5936-49. 10.3748/wjg.v22.i26.5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olmedilla L, Lisbona CJ, Pérez-Peña JM, et al. Early Measurement of Indocyanine Green Clearance Accurately Predicts Short-Term Outcomes After Liver Transplantation. Transplantation 2016;100:613-20. 10.1097/TP.0000000000000980 [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman MA, Baker T, Goodrich NP, et al. Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: a report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl 2013;19:259-67. 10.1002/lt.23595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camacho JC, Coursey-Moreno C, Telleria JC, et al. Nonvascular post-liver transplantation complications: from US screening to cross-sectional and interventional imaging. Radiographics 2015;35:87-104. 10.1148/rg.351130023 [DOI] [PubMed] [Google Scholar]
- 18.Kim PT, Saracino G, Jennings L, et al. Ratio of hepatic arterial flow to recipient body weight predicts biliary complications after deceased donor liver transplantation. HPB (Oxford) 2014;16:1083-7. 10.1111/hpb.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simoes P, Kesar V, Ahmad J. Spectrum of biliary complications following live donor liver transplantation. World J Hepatol 2015;7:1856-65. 10.4254/wjh.v7.i14.1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karliczek A, Harlaar NJ, Zeebregts CJ, et al. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 2009;24:569-76. 10.1007/s00384-009-0658-6 [DOI] [PubMed] [Google Scholar]
- 21.Low G, Jaremko JL, Lomas DJ. Extravascular complications following abdominal organ transplantation. Clin Radiol 2015;70:898-908. 10.1016/j.crad.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Grochowiecki T, Gałązka Z, Madej K, et al. Surgical complications related to transplanted pancreas after simultaneous pancreas and kidney transplantation. Transplant Proc 2014;46:2818-21. 10.1016/j.transproceed.2014.08.012 [DOI] [PubMed] [Google Scholar]