Abstract
The role and contributions of natural products chemistry in advancements of the physical and biological sciences, its interdisciplinary domains, and emerging of new avenues by providing novel applications, constructive inputs, thrust, comprehensive understanding, broad perspective, and a new vision for future is outlined. The developmental prospects in bio-medical, health, nutrition, and other interrelated sciences along with some of the emerging trends in the subject area are also discussed as part of the current review of the basic and core developments, innovation in techniques, advances in methodology, and possible applications with their effects on the sciences in general and natural products chemistry in particular. The overview of the progress and ongoing developments in broader areas of the natural products chemistry discipline, its role and concurrent economic and scientific implications, contemporary objectives, future prospects as well as impending goals are also outlined. A look at the natural products chemistry in providing scientific progress in various disciplines is deliberated upon.
Keywords: Emerging trends, Natural products chemistry, Perspective, Prospects
1. Introduction: sources, impacts, scope, and outreach
An unprecedented revitalization has been taking place in the interest for natural products, and natural product’s chemistry with their impact on various fields of scientific knowledge, technical advancements, and the economic activity. The thrust has been through a revival after the near loss of appeal for the subject. The decline of interests due to lack of prioritization, non-availability of precise tools and techniques with resulting inhibition, lack of interest, unavailability of upgraded analytical tools, and lack of wider academic and industrial programs along with the poor financial resources for advancements and research and developmental opportunities for new discoveries and applications impacted the (i) advancement of knowledge in understanding of physical and biological science avenues and its interrelation of wider disciplines with economic impacts, (ii) progression of technological advancements, especially in analytical, biotechnical, and pharmaceutical domains, (iii) importance, outreach, and the discipline’s role as a tool of modern research into chemical sciences for its broader applications, and (iv) later advances in mechanistic of natural world’s biotic and abiotic processes, environment and ecosystems with their natural products, metabolites, and the involved chemistries, all impacted the discipline. The natural products and its chemistries understanding led approaches and analyses in purifications, characterization, structure determination, functions, inter-relational chemical and metabolic dynamics as well as vigorously diversified characteristics and pharmacology-based usage and roles led to streamlining of the advancements in the farther understanding and contributions to the discipline which is seeing an unprecedented growth in terms of knowledge of the more complex products and their implications in understanding and unraveling the superior analytical, bioengineering and medicinal uses. The finer details in natural resources ecological settings and impact on the constituents’ generation, and its variations in design and diversification as well as levels of products’ involvement in biomechanistics of the cellular and ultra-cellular processes opened up the developmental horizon for finer details and broader applications in the chemistry of terrestrial species and marine organisms (Atanasov et al., 2015). Although, concurrent approaches have significantly impacted phytomedicines, synthetic chemistry aspects, purification methods development, ecological understanding, agrarian sciences, the chemo-environmental outlook from the natural products’ perspective and related technological advancements and economies. The finer understandings of the microbial chemistry, plant-microbe interactions, the metabolites biogenesis, importance and elicitations of secondary metabolites, symbiosis, and photochemistry have further opened up the intricacies of the subject. The understandings of the functions of the evolutionary-interrelated biosystems, the developments and expansions into spheres which encompasses inter-relational science areas for the natural products chemistry, chemical biology, systematics, metabolic engineering is beginning to become a chemical instrument to comprehend the biological facet of the natural resources and its chemistry, biology, ecology, and environment (Pucheault, 2008, Concha and Long, 2003, Kwan and Schmidt, 2013). Moreover, on the ground scale, the difference of approaches between the academia and industry in natural products resources utilization, products, and its development, raw materials sustenance, and plan for future actions also defined the pace and shape of the development of the subject (Amirkia and Heinrich, 2015). The widening perception-voids between the academic and industry experts curtailed the natural products discovery endeavors because of bottlenecks in approach, methodology, and design for resources’ sustainable use which included the new drug discovery, and other areas. The absence of proper technical means and other analytical tools, and probably, the resultant de-motivation for solving the encountered problems in natural products chemistry research due to shortcomings in applying of the existing knowledge to its utmost use, the intellectual queries resolutions slowly and steadily dampened at various times and locations. The multiple reasons of technology and tools, finances, supports and sponsorships culminated in lack of pace of the desired developments in the subject, and the underlying disciplines, if not severely, but significantly (Li and Vederas, 2009). Nonetheless, the advent of alternative therapies, interests in ethnobotanical herbs, nutraceuticals, procurement of technical products, newer drugs and their discovery templates, discoveries and understanding into chemical ecology and systematics, genetic profiling of the human genome, search for therapeutic templates for the chronic and genetic diseases, details into biomechanics, biogenesis and templated biosynthesis, marine templates and marine drugs including marine toxins, plant-microbe symbiosis and microbe-dependent chemistries, fermentation process and products, alternative foodstuff and designer foods, biocompatible and biodegradable polymers for use in broader disciplines of health sciences provided a renewed, conspicuous and well-accepted vibe and later a pace to the natural products and its interlinked chemistries. Furthermore, the recent debates on priorities and reports of natural products isolation from contrasting bio-diversified areas (Fitzpatrick, 2004, Bull, 2003), identification of traditional remedies’ active constituents (Mollo et al., 2015, Oberlies et al., 2003), and search for new compound-templates (Banerji, 2000) significantly helped to broaden the reach for new chemical entities and target templates for structurally and pharmacologically diverse bioactive compounds (Li and Vederas, 2009), thus serving the discipline in an effective manner.
The foremost capacity of natural products as a problem and solutions providing reservoir in structure, function, reactivity and pathway identification, synthetic route generation through retro-biogenetic concepts and solutions for other chemical challenges in phytochemistry, marine chemistry, chemical ecology, microbial chemistry, functional biochemistry and macromolecular chemistry contributions (Table 1) substantiated (de Smet, 1997, Berman and Flanery, 2001, Kayne, 2002, Fowler and Stepan-Sarkissian, 1983, Fowler, 1993, Scheuler, 1995, Lachance et al., 2012, Cordell and Colvard, 2012, Busch et al., 2012, Nguyen et al., 2008) the notion that the discipline still holds the key to future developments in all aspects of chemo-biological, pharmaceutical, health, and life sciences areas as well as in the biotechnology developments and progression (Newman and Cragg, 2009, Yao, 2004).
Table 1.
Contributions and advancements offered through the natural products chemistry discipline.
| Drug discovery & development, semi-synthetics | Drugs and other medicaments started with herbal origins, majority of drugs herbals before advent of synthetic drugs, feasible and cost-effective approach to drugs of semi-synthetic origin, contemporarily ∼50% drugs of natural origins, new templates in drug design and new chemical entities being continuously discovered |
| Chemical synthesis | Retro-approach to biogenetic synthesis, total synthesis in a bio-mimetic pattern, new routes, new templates, and challenging molecular frameworks availability |
| Therapeutic agents | Taxol, Cephalosporins, Penicillins, Tetracyclines, Camptothecin, Etoposides, Podophyllotoxin, Combretastatin, Homoharringtonine analogs, Marine-sourced Bryostatins, Ecteinascidin, Kahalalide F, etc. |
| Bioactives | Insect-repellents, herbicides, natural pesticides, larvicidal, auxins, phytoalexins, antihelminth, anti-filarial, neuro-active, cardiovascular, anti-hyperlipidemic, liver-protective, anti-inflammatory, anti-microbial, anti-cancers, anti-oxidants, anti-diabetics, pain-killers, anti-spasmodics, digestives, purgative, anti-ulcers, aphrodisiacs, tonics, anti-allergens, etc. |
| Economics | Developing world economy, economic up gradation in plant resource-rich nations, global imports, in-house native consumption, forest-economies, long-lasting sustenance, etc. |
| Pharmacopoeias, medical treatise, and alternative medicines | Ayurveda, Unani (Greco-Arab-Indian), Chinese as major plant-based healing systems, African, Shaman, Red-Indian and other ethnobotanical medicinal usage traditions and treatise. Aromatherapy, Homeopathy, and Siddha systems of medicinal usage |
| Consumables | Plant Pigments, Dyes, Essential oils, Fatty Acids & Lipids, Raisins and Gum, Aromatics, Alkaloids and Tannins, Neutraceuticals, Spices, Condiments, etc. |
| Plant-based drugs of abuse | Hallucinogens, Narcotics, Cannabis, Opium, Semi-synthetics derivatives thereof |
| Technical products, fibers, and plant polymers | Jute fibers, Paper, Paper-boards, etc., Pectins, Alginates, Cellulose, Marine-Sourced Products, etc. |
1.1. Drugs and drug discovery
The underlying interests in natural resources for its pharmacologically active constituents, structurally diversified and synthetically-challenging templates for development towards medicinal uses with an impact on the broader pharmaceutical interests and related economic activities have played a major role in advancing the discipline. The rush for small molecules drug discovery from templates and designs based on bio-diversified as well as chemically diversified natural products pools (O’Keefe, 2001, Mabry, 2001), development of new techniques in separation, purification and, characterization of ligand-bound and free substrates, generation and establishment of test scaffolds helped well to advance the separation of drug templates and newer drugs from terrestrial and aquatic sources as the reservoir of new leads and hits (Vuorelaa et al., 2004, Newman and Cragg, 2016). Again, the procurement of chemo-biological products for medicinal uses, the fast-paced scientific progress in the applications in technological domain with evolution of better methodologies with their impact on economic potentials has considerably up-scaled the static and regressive periods of interest in natural products, especially for the agrarian societies thereby serving as a tool of training, technological progress and economic gains (Harvey, 2010, Butler et al., 2014) on a renewed levels.
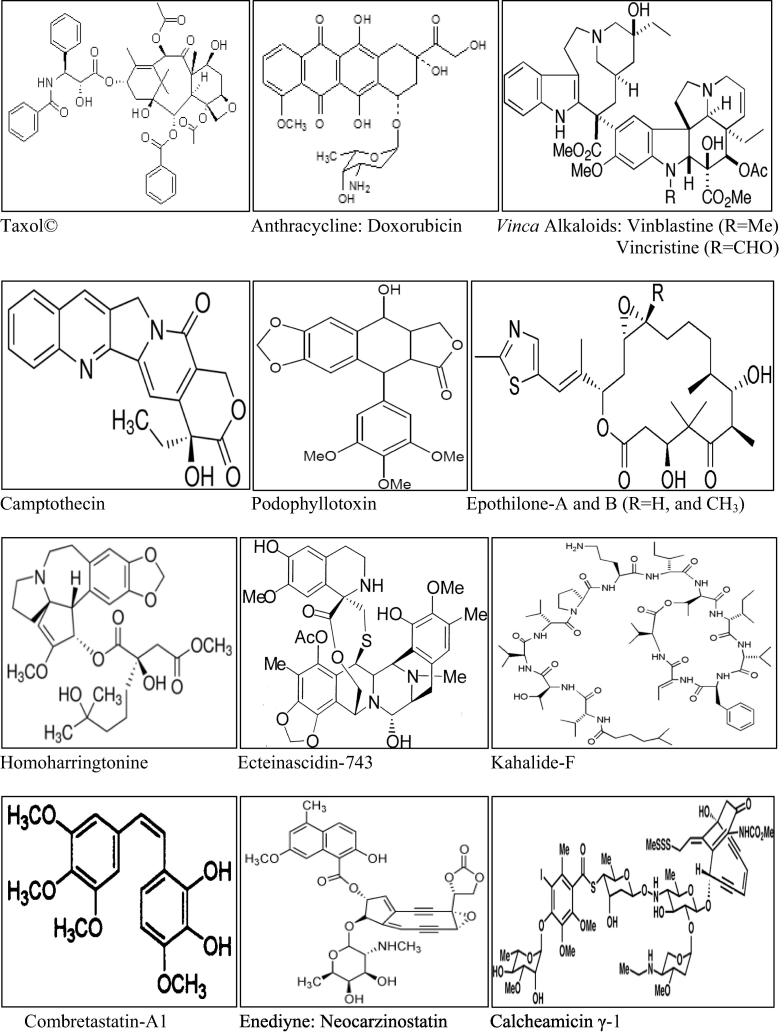
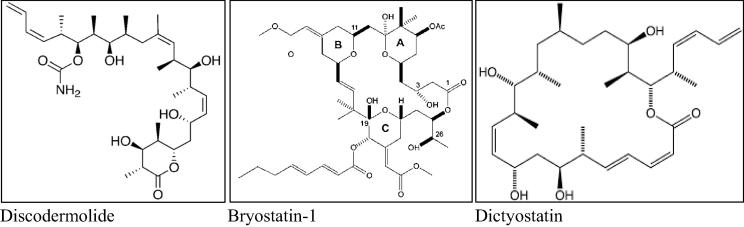
Furthermore, the ethnobotanical and ethnopharmacological prestige of the traditional herbals have provided the chemists with new avenues and challenges in drug discovery, and other health-related products discovery (Tan et al., 2015, Azam et al., 2016). Recent interest in natural products has yielded numerous interesting results (Cho et al., 2008, Qureshi et al., 2016), including the phenotypic and virtual screening based advances (Chang and Kwon, 2016, Shaikh and Siu, 2016, Cao et al., 2013) and it also saw a number of diversely-sourced products’ remarkable discovery with anti-microbial (Moloney, 2016), anti-inflammatory (Bezerra et al., 2013), anti-helminth (Das et al., 2010, Mali and Mehta, 2008), anti-diabetic (Zhu et al., 2013), and anti-cancer activity especially taxol® (paclitaxel), anthracyclines, Vinca alkaloids, camptothecin, podophyllotoxins, epothilones, enediynes, combretastatin, homoharringtonine analogs, marine-sourced bryostatins, ecteinascidin, and kahalalide F (Fig. 1) among several other natural products at various stages of development as anti-cancer agents (Kinghorn et al., 2009) leading the mainstream pharmacy’s acceptance of the natural products as part of modern and effective tools for new drugs, and new drug discovery templates’ reservoir (Von Nussbaum et al., 2006, Molinari, 2009, Butler, 2004, Quality, 1998, Hagino, 2000, Fauzi et al., 2013a, Donia et al., 2011a, Conforti et al., 2008). Nowadays, the ratio of natural products versus synthetic leads puts the natural products derived anti-cancer agents ∼60% for the successful leads.
Fig. 1.
Structures of some of the diversified natural products from various natural sources.
Again, the perspective of natural products re-examination, especially for much contested biological activity in a single component versus multi-component products from plant(s) with or, without the synergistic bioactivity profile, and the trace of no-activity or, watered-down biological activity of natural products of secondary metabolite’s origin in drug-template screening versus the bioactivity due to the presence of high molecular weight non-polymeric, conjugated/clustered components capable of receptor interactions is important for multi-facet drug discovery today.
1.2. Synthetic and semi-synthetic drugs
The entry of synthetic products as pharmacological agents laid down the foundations of modern drugs and drug discovery in a more conceptual and rational way. The natural products and their derived drugs dominated till the 1980s when over one-third of the drugs were originated from the natural resources. The synthetically originated drugs continued to surge and covered about half of all the drugs, while the other half is still dominated by the natural resource-based medicines. However, the claimed adverse effects and perceived toxicities, requirements for advanced facilities and infrastructure set-up, adherence to stricter regulatory mechanism and expertise in the design and up-scale preparation of synthetic drugs of semi-natural and purely synthetic origins led to the growth of phyto- and marine–sourced drugs at steady pace with development of drug templates for various biological activities. The conjunction of the synthetic approaches with the naturally-sourced drugs and drug templates, of late, has been a significant contributor to the natural products chemistry discipline’s progression and gradual advancements. The exercise realized the inter-dependence, molecular connectivity and structure-activity relationships (SAR) for several natural templates, particularly, anti-cancers and anti-biotics drugs in several ways with leads from different research groups and institutions worldwide (Lal et al., 2013). However, till recently, in major parts of the world, the natural products that were viewed as prophylactic agents as being nutraceuticals, food supplements, and herbal drugs, or as complementary and alternative medicinal agents, have started to reshape its appeal and perception. The mild and severe levels of adverse side effects of synthetic drugs for various chronic diseases of genetic, life-style-acquired, and occupational and other chronic conditions generated an opportunity to look out for safer and effective drugs from natural sources. The nature’s products which constituted the major portions of the global tribal population’s medicament also provided an opportunity to explore the traditional medicament resources and the knowledge for further development through systematic approach in bio-assay guided fractionation and activity location from the active constituent(s) as well as it also contributed towards synthesis development, semi-synthesis, and drug design avenues involving the obtained natural product(s) templates. Moreover, the semi-synthetic analogs of natural product also focused on intricately modifying the structural features with inputs from SAR and QSAR, and with or without disturbing the starting molecular template for improving the existing drug-like features for the biological activity. This made possible the cross-overs of the original natural product templates in terms of clinical significance and generating a new biological activity in the redesigned entity through comparisons and predictions, also involving the use of in silico activity predictors. The natural products have also served as feasible starting material to generate substances with improved and new therapeutic efficacy that are, at times, unrelated to the known biological functions of the starting material itself (DeCorte, 2016, Banwell, 2008, Galloway et al., 2009, Kombarov et al., 2010, Tanaka et al., 2009).
1.3. Separation sciences and structure elucidations
The advent of finer separation, assaying and detection techniques have, played significant roles in advancing the natural products research. The stable and chemically non-reactive techniques of isolation, purifications (Kloss et al., 2013, Donia et al., 2011a, Schmidt and Donia, 2009, Freeman et al., 2012, Taylor et al., 2007, Tianero et al., 2012), with improved technologies led to the discovery of minor-yield products on an unprecedented scale. The secondary metabolites mixture, molecular associations, polarity based mixture, and the preservation by biological molecules’ surroundings along with ionic and non-ionic interactions and deposits (especially in marine products), with chemical conjugation as well as presence of physicochemical phenomenon, were some of the challenging aspects in isolation chemistry (Bedir et al., 2002, Khan et al., 1996). The analytical techniques development in isolation and structure determination helped to examine the bio-diversified natural resources and products for their novel structures and bioactivities (Berkov et al., 2014). The development and more frequent use of non-conventional chromatographic techniques, especially, Flash®, UHPLC (Ultra High-Pressure Liquid Chromatography), MPLC (Medium Pressure Liquid Chromatography), Electrokinetic chromatography, droplet countercurrent, super-critical fluid, and circular chromatographies along with gel filtration techniques solved many mysteries in detection, isolation-purification, instant constituents screening or dereplication, and chemical profiling, finger printing of the natural products from various sources (Nielsen, 2014, Stege et al., 2011, Hu et al., 2010).
Recently, a neural-network-based descriptor model for predicting the chromatographic sequence of natural compounds in a gradient chromatographic procedure has been developed, which successfully facilitated the compounds identification in the chromatographic finger-print (Hou et al., 2016). Among the spectro-analytical techniques, the use of mass spectroscopy with other hyphenated techniques like GC–MS, LC-MS, LC-DAD-TOF-MS (Luzzatto-Knaan et al., 2015, Wolfender et al., 2006), circular dichroism, NMR and NMR based correlational spectroscopies have changed the approach to structure determination thereby abandoning the earlier necessity of chemical transformations nearly out of existence (Sprogoe et al., 2008). Furthermore, the purification and identification of active ingredient(s) from the aggregated, molecularly clustered or, conjugated and physicochemically bound natural products have now started getting more attention in the realm of natural products isolation dealing with terrestrial, marine, and microbial products-linked chemistries for the purpose, but the needs for faster and efficient techniques to isolate and characterize larger and hetero- and homo-polymeric molecules of natural origins from various sources seems to be more imminent and on the larger scale as well as on more complex problem-solving capabilities levels.
1.4. Combichem and HT screenings
The naturally templated biogenesis on the chosen molecular frame-work, especially, for the larger and repetitive units like tannins, biopolymers and small molecules (with different substitutions also) apparently pointed out a combinatorial or near combinatorial approach in the biogenetic scheme of the production. The phenomenon is more pronounced in prokaryotic entities because of chemical profiling of their products. The existence of several natural products in a single species exhibiting drug-likeliness along with the most potent natural ingredient pointed to the templated and a library-like synthesis have been observed. The purified natural products libraries can be regarded as a part of the combinatorial set-up with a view to find the increased biological activity and pharmacological diversity for further development of the hit template (Clardy and Walsh, 2004, Koehn and Carter, 2005, Tianero et al., 2012, Pelish et al., 2001). On a more practical approach, the diversity-oriented synthesis and combinatorial libraries of natural-products like substances’ synthesis have been reported which utilized strategies developed for the synthesis of skeletally diverse small molecules, including folding, the branching pathways, and oligomer-based approaches (Koehn and Carter, 2005, Trabocchi et al., 2013, Singh and Culberson, 2009, Galloway et al., 2009). A series of reports on the combinatorial synthesis of natural products based, and natural-products like molecules, bacterial and fungal origins constituent’s templated molecular frameworks have been reported that have broadened the outreach and applicability of the natural products chemistry in a more diversified ways (Davis et al., 2007, Georgiades and Clardy, 2008, Tanaka et al., 2010, Olano et al., 2010, Behnken et al., 2012).
The use of high-throughput (HT) screenings, utilization of various analytical techniques for purified natural products and natural products based combinatorial libraries for template selection (Poulsen et al., 2006, Frearson and Collie, 2009), including HT screening of the crude extracts holds promise for further applications (Donia et al., 2011a, Trindade et al., 2015, Donia et al., 2008). Moreover, the HT screening information and the existing database (NAPRALERT) have also been successfully utilized in sorting out pan-assay interference compounds (PAINS) responsible for false leads from a plethora of synthetic and natural products wherein the products had exhibited manifold bioactivities not adhering to the synthetic libraries characteristics, thereby designated as invalid metabolic panaceas (IMPs). The phenomenon followed the power law characteristics for nearly half of the 200,000 products put under analysis which returned the occurrence–bioactivity–effort space hyperbolic black hole with IMPs populating the high-effort base (Bisson et al., 2016, Georgiades and Clardy, 2008).
1.5. Biomechanistcs and the bioactivity
The chemical biology approaches as a tool for understanding the finer biomechanistics of the traditional medicine has provided insights into the action in details. The pharmacophoric model of bioactivity exhibition have been further detailed and the competing and/or synergistic constituents, targets, and pathways details into the mechanism have been outlined with its help. The mechanistic details helped to improve the understanding for developing the complex diseases therapies (Bai et al., 2016). The anti-cancer agents and their analogs work on several pathways whereby recent mechanism of targeting Sp transcription factor has also been proposed and according to that, the downregulation of specificity protein 1 (Sp1), Sp3, and Sp4 are highly expressed in tumors and tumor cell lines, and pro-oncogenic Sp-regulated genes are supposedly involved in cell growth (cyclin D1 and growth factor receptors), including in survival (bcl-2 and survivin), angiogenesis and migration (MMP-9, vascular endothelial growth factor and its receptors), and inflammation (NF-kB). Thus, the confirmation of the exact biomechanics may further help to optimize the clinical applications of natural products and their combination drugs (Safe and Kasiappan, 2016), if necessary. Towards elucidating the interaction of the drug, an in silico model and in vitro inhibition testings of aldehyde oxidase (AO), responsible for the biotransformation of drugs and xenobiotics, was carried out by several flavonoids resulting in potent behaviors of myricetin, quercetin, and epicatechin (Hamzeh-Mivehroud et al., 2014). Another flavonoid, butein has been shown to inhibit cervical anti-cancer growth through PI3K/AKT/mTOR pathway in an experimental HeLa human cervical cancer cell lines. It also increased the reactive oxygen species (ROS) formation along with reduced phosphorylation of PI3K, AKT, and mTOR expressions contributing to the inhibition of the tumor growth and reduction of the oxidative stress for the test subject (Bai et al., 2015). In another example, the heat-shock protein (Hsp90) inhibiting natural products and natural product derived templates as anti-cancer agents (Khandelwal et al., 2016), the antitumor activity of Hypericum hookerianum (Dongre et al., 2008), and polyketide inhibitors of eukaryotic protein synthesis are lately reported (Taylor, 2008) which points to the importance of biomechanics in assessing the bioactivity of the natural compounds.
1.6. Genome sequence impacts
The key to the understanding of the principles of disease lies in the genomic information whereas the therapeutics development is incumbent upon the ability to accurately diagnose and identify the causes that are routed through the established biomarkers to target the causative entity or, the function. This applies both to human and other genomes. In this detail, the use of human genome sequence information for particular cellular and other bioactions, and the biochemical information coded therein for the specific physiological conditions/ailments needs translation into diverse, step-wise responses to contain and cure. The information needs to be mined and used as arbitrator in the receptor-ligand binding studies; mode of action and bioactivity, site-directed drug delivery, and confirmation on other main and peripheral actions mediated by the genomics, which is much required. The production, functions, and fate of secondary plants and microbial metabolites (Donia et al., 2006, Schmidt et al., 2004, Schmidt, 2008, Partida-Martinez and Hertweck, 2005, Kwan et al., 2012, Kwan and Schmidt, 2013, Kroiss et al., 2010, Walter et al., 2011, Fauzi et al., 2013b,53), the small and big molecules as bioactive components filling the disease versus natural products interaction gap(s) together with the help from the natural product probe-compounds (Siegl et al., 2011, Zimmermann et al., 2009, Piel et al., 2004a, Sumner et al., 2003, Concha and Long, 2003) may lead to newer horizons in genome-based natural product-derived drugs and functional entities of natural origins. The understanding may also help in designing better templates and bio-functions inhibitors or supporters for various uses. The studies on genomics-based natural products identification have been reported (Franke et al., 2012, Hentschel et al., 2012, Banskota and McAlpine, 2006), and the genomic-identified and genomics-enabled targets may play a leading role in providing new therapeutic agents in future. The developments in anti-bacterial therapies are encouraging due to the understanding of the bacterial genome, the gene’s expression, mechanism of resistance to antibacterial agents, and the ability to succeed as a pathogen in human subjects (Punina et al., 2015). The genomics is here to play a more significant role and the advent of personalized natural products-based medicine is not far behind.
1.7. Enzymatic, enzyme-mimetics, fermentations, and bio-engineering
The identification of fermentation broth compounds, generation of new broth products from terrestrial and aquatic plant/organisms (Piel et al., 2000, Cai et al., 2013), the induced microbial transformations, cell suspension culture based products and their variants (Davidson, 1995), symbiotic conditions and their products (Tse and Boger, 2004, Pidot et al., 2014, Moore et al., 2002, Schmidt, 2005, Schmidt et al., 2005, Wilson et al., 2013, Peraud et al., 2009, Nützmann et al., 2013, Musiol et al., 2011, Wilson and Piel, 2013, Kampa et al., 2013, Brachmann et al., 2012) along with other biotechnological areas applying physiological knowledge and bio-engineering is getting more attention from natural products chemists (Rohm et al., 2010, Scharf et al., 2010, Bergmann et al., 2010, Vödisch et al., 2011). The use of large, medium, and small sized enzymes and enzyme-mimetic templates in applicable areas of chemistry, phyto and other natural chemicals, studies into the structural and, physicochemical property modulations in the enzyme and changes in the function and structural characteristics of the substrate(s) have started unraveling in more details the impact of the enzymology. The presence of various bio-molecular plant structures, response(s), and biochemical properties in defining the functional outlines and, if involved, product outcome from the biochemical processes, their reactions and levels of reactivity, holds the key to unravel the mysteries of biochemical bioengineering, which is being approached through broader natural products chemistry applications in enzyme, proteins, peptides and peptide-mimecs (Franke et al., 2013, Scharf et al., 2013, Partida-Martinez and Hertweck, 2007, Nguyen et al., 2008, Sudek et al., 2006, Scherlach et al., 2011, Sims and Schmidt, 2008, Schümann and Hertweck, 2006, Sarkar et al., 2012, He and Hertweck, 2004, Tillett et al., 2000, Piel et al., 2004a, Ortholand and Ganesan, 2004, Lackner et al., 2007, Gilbert et al., 1999, McIntosh et al., 2009, McIntosh et al., 2013, Arnison et al., 2013).
The use of enzymes and enzyme-mimetics manufacturing operations at bulk scales, the establishment of protocols, and the biocatalytic applicability for structurally-defined substrates is crucial in advancing the industrial operations to next levels of performance. The role of naturally-sourced catalytic products, the biocatalyst’s comparable reactivity in natural and non-natural settings along with the enzymatic co-factor utilization in synthesis and self-assembly of structures is another important area of thrust into the field (Ishida et al., 2010, Ding et al., 2011, Regueira et al., 2011, Weiz et al., 2011, Coyne et al., 2013, Scherlach and Hertweck, 2009, Schroeckh et al., 2009, Bretschneider et al., 2013, Piasecki et al., 2014).
The comparative analysis of the functional efficiency of the fermented and normal biogenetic products obtained from natural sources (Cvetanović et al., 2015), biochemically engineered natural products (Lopez et al., 2007), metabolomics (Yuliana et al., 2011), enzymatic synthesis and its optimization studies (Ahmad et al., 2010), glyco bioengineering (Thibodeaux et al., 2007), substrate specificity bioengineering (Dunn and Khosla, 2013), and probes mechanism have lately been reported (Takeo, 2007).
1.8. Biochemical set-ups, physiology, biosystematics, and natural products chemistry
The biochemical interactions in physiological settings with its course of the reaction(s) and the understanding of the effects in the production of response-mediated phytochemicals, secondary metabolites, molecular templates, ion-clustered molecular systems, as well as ionic and non-ionic entities are important. The physiological effects on biogenesis, cellular signaling, and channels’ operating roles for natural products entities of various sizes and nature, and other intended chemicals, can be best understood from the generated clues within the biochemical set-up with the possibility of further applications in designing plants-based specific chemical’s production in parallel to the microbial bio-engineering for metabolites production by understanding the comparative levels of the physiology’s roles in various organism and species involved. The bio-experimental release of the formed templates in the cellular systems, and finding an analogy with instances in the interdisciplinary sciences could provide a required understanding of these interactions for pathway break-through at various stages of the natural and induced production of natural products in various biochemical and biogenetic settings. The understanding could further be applied to different levels including drugs-receptor, and xenobiotic material interactions at in vivo and in vitro conditions. The cell-based signaling assays and their activator (McCulloch et al., 2009), roles of TRP (Transient receptor potential) channels and natural products in bioactivity/drug discovery (Appendino et al., 2008, Pillon and Fogliani, 2009), bioactivity enhancements (Singh et al., 2015), and the inter and intra-molecular bio-chemical, physicochemical interactions mechanisms (Nicolaou et al., 2004) of different chemo-biological entities in the natural biosystems are fast emerging with implications and effects on the discipline and interrelated subject areas. The steps in mechanistic pathways in physicochemical and biochemical proceedings with identification and utilization of channels (Marko, 2003, Mori, 1997), and their feedback in biomechanisms at functions will shed more light on the broader understanding of the natural products role in these areas, and would in turn facilitate the understanding of various biological aspects affecting the biogenesis, biosynthetic plans, drug likeliness and drug discovery, drug action, pharmacodynamics, ADME, and synergism in biological activity evaluations to various extents.
1.9. Biogenesis, biochemical pathways, chemical ecology and biodiversity
The discovery of biogenetic pathways of the marine and microbial sources’ constituents’ biogenesis, production of designated compounds (Xu et al., 2012, Dückert et al., 2012, Potowski et al., 2012, Suo, 1999, Weiz et al., 2014, Hennicke et al., 2013, Kusebauch et al., 2011, Arya et al., 2002, Tyler, 1999, Hertweck, 2009) as well as symbiosis details on the constituents’ structure and functions are of prime importance in the chemistry of natural products. The discovery of novel and aberrant biogenesis, the advent of conceptual biosynthetic proposals including in self-assembly and biosystems-based combinatorial methodologies (Newman et al., 2003a, Tulp and Solvay, 1999, Winter et al., 2011, Lin et al., 2013) with comparable outcomes (Brooks, 1986, Newman et al., 2003b, Cordell, 2003) to normal, ecologically elicitated, and designed constituents is another facet of the chemical ecology and natural products chemistry interface (Singh et al., 2014). Further, the understanding of the ecological interactions, and involved bioengineering of natural products compounds has lately started getting more attention. The roles of external, and internal chemical entities in the main and supportive pathways, and their effects on the biogenesis of the naturally produced constituents and the biocatalysis will put the biogenetic agenda forward, also with a view on the establishment of genes roles in the process (Gatte-Picchi et al., 2014, He and Hertweck, 2003, Park et al., 2010). This area holds promise for generating newer biosynthetic templates and product as well as process outcomes in natural products chemistry to encompass other outlying disciplines especially chemical ecology, combinatorial chemistry, and retrosynthetic analysis along with the total and semi-syntheses techniques and designs in organic chemistry approaches and developments of new routes and reactions (Roessner and Scott, 1996, Calixto et al., 2005, König et al., 2013, Singh and Culberson, 2009, Spiteller, 2008, Spiteller, 2015, Demain, 1992, Romeo et al., 2001, Sariaslani and Rosazza, 1984, Buckland et al., 2000, Patel, 2000, Busch et al., 2012, Verdine, 1996).
The chemical nature of host-guest relationships, and their applications in the marine and amphibious environment's natural products production, toxicological behavior and toxicokinetics of natural toxins, toxic marine products, and other harmful plants, microbes, and fungi as well as the chemical defense, symbiosis, and metabolic byproducts, including the ways for sustainable utilization of the natural resources with broad green chemistry developments (Partida-Martinez and Hertweck, 2005, Tu and Gaffield, 2000, Scharf et al., 2010, Siegl et al., 2011, Schmidt, 2005, Schmidt et al., 2005, Piel et al., 2004b, Uria and Piel, 2009, Garson, 2001, Hentschel et al., 2012, Piel, 2002, Scherlach et al., 2006, Busch et al., 2013) applied over the natural products chemistry template holds the key to future directions of research and advancements in the discipline to generate new space in technology development/advancements, also with related up-scale economic activities.
The deeper understanding of the steps of diversified biochemical processes (including signal transduction impacts and implications within the chemical ecology domains and the exact roles with the natural signal regulators role in ecological interactions with their geometric, stereo-electronic and physicochemical properties impact in new/altered ecological conditions), all as a sources of information for decoding of pathways, including the role of its reactive and participative substrates, hopefully will yield new templates generation outlines, future designs and protocols, organization, and challenges in obtaing new and novel structures and functional variability hereto unknown.
The natural products from terrestrial and marine sources having gradual structural and functional complexity with advances in species evolution, effects of the acquired and evolved genome derived patterns in guiding biochemical activities for species and their near and distant inter-relationships in the genomic tree in producing their molecular constituents will determine the future concepts, foundations and details in chemical ecology, plant evolution, biodiversity, chemical constituents, surroundings induced secondary metabolic production with influence from human interactions in details (Piel, 2011, Spiteller, 2015, Demain, 1992, Bedir et al., 2002, Nielsen, 2014, Donia et al., 2011b, Klitgaard et al., 2014, Donia et al., 2011c, Donia et al., 2008, Heinstein, 1985, Liu, 1996, Costello and Ward, 2006) and will impact the plant-derived economies in near future (Ma et al., 2013, Brown, 1994) which certainly needs conservation on the ground for the resources and habitat.
1.10. Ecology, environment, and chemical perspective
The ecological and environmental alterations, the changes in geographical areas along with the phylogenetic cross-overs, and the species purity has considerably altered the resources’ authentic raw materials availability, herbal formulations validity, chemical profiling outcome, quality of the natural products, and of the essential oils along with other plant-derived products, as well as the presence and absence of active mono/poly-constituents in various natural sources at adverse interaction with the environment. The levels of contamination from man-made adulteration of the natural resources raw materials has translocated into the counterfeit herbal formulations with little to offer in terms of genuine products, usage safety and efficacy. Additionally, the chemical contamination of the surroundings, pollutant and toxic effects of xenobiotic products and other factors including the up-reach of man-made chemical entities in the ecological system, and ultimately to the end-product natural resources including food-chains have played its downsize part. The natural ways to look for alternatives and remediation have contributed towards the resources protection, cultivation, and promotion of bio-diversity maintenance on a larger scale to sustain the resources. From the chemical viewpoint, the ecological outcome has started getting attention from chemists and biologists alike. Thus, a template borrowed from phyto, marine, and microbial chemistry settings can push the understanding in the biochemical, and bio-mechanistic aspects dealing with the ecology in more effective ways in future (Larsen et al., 2005, Costello and Ward, 2006, Arnold and Targett, 2002, Garson, 2001).
1.11. Microbial, marine, and photo-chemistries
The microbial chemistry output has dominated the antibiotic spectrum for over half a century (Genilloud, 2014). The genus Streptomyces has produced useful metabolites, although the genus has produced same molecular framework compounds from various contrasting environmental sources possibly owing to the genetic non-exchanges between the species. Thus, necessitating a look out for new molecular templates with improved, and diversified biological properties which is eminent. For example, the anaerobic and rare actinobacteria from mangrove ecosystem have been proved to be a potential source of antibiotics, antimicrobials, antibacterials, antifungal, anticancer, and antimitotic (Behnken and Hertweck, 2012, Paterson et al., 2009, Azman et al., 2015) products. The metabolic products’ profiling (Larsen et al., 2005, Abdallah, 2011), and roles of new and integrated interdisciplinary approaches for unmet clinical needs has future stake in microbial metabolites development as a source of new and frequently-resisted major known antibacterials for the concurrently evolving super-resistant microbial species. New techniques in stereochemical structure determination of myxobacterial metabolites (Menche, 2008), fungal polyketide bioengineering (Agarwal and Moore, 2010, Yin et al., 2013), dereplication using spectro-analytical techniques (Klitgaard et al., 2014, Nielsen and Larsen, 2015) and interesting combinatorial biosynthesis of plant-specific products including certain coumarin in bacteria (Lin et al., 2013, Olano et al., 2010, Bechthold et al., 1999) have been reported and has the imprints of changing chemical approaches.
The developments in marine constituents’ isolation, characterization and diversified biological activity changed the scope of natural products chemistry forever. The discovery of structurally diversified novel molecular frameworks added new entities to a plethora of available templates for a wide range of screening against major pharmacological classes. These advancements also helped to understand the marine symbiosis between different species, and also revealed the constituents of marine-located fungi and other bacterium (Schmidt et al., 2005, Schmidt, 2005, Penesyan et al., 2010, Gundersen, 2013, Davis et al., 2012, Li et al., 2014, Lin et al., 2012, Uria and Piel, 2009) in the process of discipline’s advancements.
Furthermore, the natural products reactivity, and end-products variations of the photochemical set-up, the phototransformational diversity and the final product(s) formation with regards to pathways selection and its alternate execution may shed more light on the solar effects on the plants, their products, and the micro details of the chemistry undertaking in the plant system (Schümann and Hertweck, 2007, Nelson et al., 2007) which could be applied in green chemistry settings for future industrial techniques and know-how development whichin will extend the environmental carbon chemistry designs for viable templates from the ecology and the solar domains. The new photo-product(s) from the new, novel and known substrates under the influence of solar and non-solar sources could provide new molecular framework for drug discovery. An interesting observation on the curcumin photo-behavior in the zwitterionic micellar system has recently appeared (Banerjee et al., 2014) and supports the notion.
1.12. Toxicological, and immunomodulation evaluations, toxico-kinetics
The protective and toxicity ameliorating effects of naturally derived preparations play important role in ethnobotanical pharmacology. The toxicological profiling of natural formulations in use by the populace is necessary for the safety confirmation and further development of effective and safe drugs as well as better and superior acting antidotes and antivenins. The plants with the capacity to protect against organ toxicity, especially nephro and liver toxicity are abundantly reported with their screening results (Lustosa et al., 2016).
Some of the plants producing severe toxicity, poisonous in nature may also serve as a template for further study into the toxicological effects and toxicokinetics of the constituents, fractions and the plant parts including toxicity by other naturally occurring raw sources from terrestrial and aquatic situations (Ouédraogo et al., 2013, Tsuboy et al., 2010, Tu and Gaffield, 2000). Moreover, the toxicological profiling, the broad contamination in soil, air and water with pollutions in natural resources' immediate surroundings with the required containment and chemical and biochemical remediation of the causes and amelioration of harmful products by the natural resources and natural products sources may provide deeper understanding and applications of chemical toxicology, inter-linked immunological broad-base and natural products chemistry (Lee et al., 2009).
1.13. Bioactive and designer foods
The preparation and production of alternate and designer natural foodstuff and its formulations including the development of super-nutritious foods, designer-food components and their procurement, purification and formulations for selected nutritional value, and metabolic characteristics for a personalized prescription is another area getting attention which will add up to the leads for agrochemical, neutraceuticals and food industries dependent on natural resources. The traditional foods' therapeutic values confirmation, and dietary plants' bioactivity is also getting attention (Liu et al., 2016, FAO, 2011, Miroddi et al., 2013, Alfermann and Petersen, 1995, Luoma-aho, 2004, de Silva and Atal, 1995, Cseke et al., 2006).
1.14. Technical and polymeric products
The search for new templates and natural answers to the designer molecules like nanofibers, dendrimers, crown ethers, polymers, and natural gels for use in biomedical sciences, biofuels, biomass availability, and as technical, industrial and biomedical materials for eco-friendly thrust in economies is continuously going on encompassing various biodiversities and genetic resources for the purpose (Anonymous, 2003, Tcheknavorian-Asenbauer, 1993).
The agro-forestry based phytochemicals, designer afforestation, and development of genetically engineered renewable plant resources, human foodstuffs’ genetic alterations, microorganisms’ self and induced genetic changes are getting focus for their overall effects from the phytochemical viewpoint needing further elaboration on constituents, biological foundations as well as their bioactivity. The functions, chemical products reactivity levels and their extent of activity, and the environmental outreach through various biotic and abiotic means will help in defining the food constituents, biomass and bio-energy for the new millennia.
1.15. Computational methods and data mining
The evolution of faster advances in the enormously expanding plant sciences and natural products chemistry discipline demands high-end technological advancements in computational methods, data minning and data management. The envisioned leads, drug discovery and development, diversity in the broader natural products chemistry towards understanding of the complete influence and impact on the interdisciplinary sciences, broader subject area’s structural, functional and various other applications in several domains including medicine and veterinary medicine needs better handling of informational repository, data mining, retrieval as well as the safety, proper, benevolent and beneficial handling of the generated data. The impact of chemical understandings in various inter-linked sciences is setting new goals for challenges in bio-computing and computational resources management. The immense help from the contributions of natural products chemistry and natural products chemists have started playing its part. The prediction strategies and tools for various natural resources interactions for its probable pathways, products, biomechanics properties, software development, and other advancements in computation methods hold enormous promise for the future (Lopez-Perez et al., 2007, Nakamura et al., 2014, Huang et al., 2007).
1.16. Economic impacts
The industrial, civilizational, and economic impact from the view-point of the natural products chemists and chemistry, in general, is ready to take shape more deeply in the long and short-term future. The industrial utilization of plant resources, scope of phytopharmaceuticals, essential oils, food supplements, natural sources derived drugs, sustainable medicinal plants use, and technological impacts are gaining everyday grounds (Rouhi, 2003, UNIDO, 1991, Meinwald and Eisner, 2001, Wagner, 2004, Wijesekera, 1991, Kingston, 2011, de Silva, 1997a, de Silva, 1997b). Importantly, the role of natural product chemists in new methods and diversified fields is inevitable and will immensely affect the direction of the chemistry and related sciences and sub-disciplines in all its branches and applications, and will broadly affect the economic scenario more deeply in the coming years (Harvey et al., 2015, Mullane et al., 2014).
2. Discussion and conclusion
The natural products as discovery scaffolds, comparative as well as co-products of biochemical settings and physiological conditions product outcome, designer food and its constituents, ecological and environmental probes compounds, and secondary-metabolite products of aberrant biogenesis, metabolic and marine products, revisions on age-old traditional herbal uses and ethnobotany/ethnopharmacological understandings of underdeveloped regions, have helped to reverse the unfavorable and unflattering views of the natural products. The natural products as a source of diversified products of technical grades as well as preferential method of economic gains and as tools of scientific activity, and biomedical, especially, phytopharmaceuticals importance have been a boon for us.
The scientific and technological progress in the subject area of natural products chemistry and the prominent roles it played in up-scaling of the scientific knowledge, as well as the techniques and technological advancements which are an outcome of direct and indirect impacts of the understanding, maturing up and inter-relationships of the discipline with other scientific disciplines in the physicochemical sciences, medicine, and related bio- and techno-industrial technologies strongly led to economic gains and growth over the decades. This was in addition to the advantages the field of natural products provided on the basis of usage of traditional and local technologies and methods of procurement, and the processing of natural resources in the preceding years.
Thus, the contributions, and the role played by the natural products and its chemistry well over a century into the initial developments of chemistry, medicine, and technology leading to the expansions in the interdisciplinary sciences, especially in biology, biochemical and microbiological sciences, chemical ecology, in life sciences, as a tool and as a partner, seemed to be upscaling after slipping away from significant leads for the future now. The discipline of natural products chemistry holds immense promise and a broad arena in biochemical, ecological and, medicinal fields to work for further understanding, and advancements of the knowledge. It will provide a plethora of solutions to problems in understanding nature and will certainly gift us queries, and problems to work on (Table 2).
Table 2.
Some of the emerging trends, and prospective goals in natural products chemistry.
| Isolation, and Purification | Priorities for natural products isolation from contrasting bio-diversified areas, identification of traditional remedies’ active constituents, search for new compound-templates |
| Development of new techniques in identification & characterization of ligand-bound and free substrates in plants, | |
| Fine separation methods and the need for finer, stable and chemically non-reactive techniques in isolation procedures, need for faster and efficient techniques to isolate and characterize larger molecules | |
| Purification and preservation of biological molecules’ surroundings and associations- chemical and physical during isolation | |
| Leading to safe, easily available and affordable plant-derived products; thrust and scientific revival for natural products chemistry | |
| Designer and Alien Molecules, Structure Elucidation | Versatile, and variable structures of new atomic compositions and elemental combinations from marine and harsh terrestrial plants |
| Natural answers to the designer molecules like carbon nanotube, fullerenes, crown ethers, etc., non-reactive fibers, natural gels for industrial use in food and non-food purposes, herbal fuel, plastics from plants, New techniques, and tools for structure elucidation, tagged spectro-analytical techniques | |
| Drug Discovery, and Development | Currently ∼50% of modern drugs of plant origin, share of synthetic drug steadily increasing over the previous decades, plant-based products in prophylactic role, perception of subdued and zero toxicity driving push in phytochemicals as new chemical entity (NCE) for drug development |
| Small molecules drug discovery led to natural pool with plant pharmaceuticals and bioactive products reservoir from nature; new templates and designs based on nature | |
| Antibiotics and anti-cancers major class of plant-based drugs; prioritization, advancements in strategy for newer techniques, and insight into the future drug discovery providing the trends and impact on the phytopharmaceuticals worldwide | |
| Bioactivity, Synergism, and HTS | Slowed and divided or partly ineffective biological activity, perceived ecological and environmental alterations, purity and originality of major product and constituents, the level of contaminations and man-made adulteration of the herbal products. |
| Biological activity in single product versus multi-component products from plant(s) in synergy with no trace or watered-down activity of natural products, secondary metabolite versus the higher molecular weight non-polymeric/polymeric active natural component capable of receptor interactions, its identification as well as the experimental replication of synergies associated in these single/multi-components | |
| High-throughput screening in purified natural products for template selection and HT screening of natural products as crude extracts holds promise | |
| Biomechanics | The discovery of new pathways in biosynthesis, novel conceptual biogenetic proposals including in self-assembly and combinatorial methodologies and its compatibility and comparability to other natural pathways |
| Use of large and small enzymes and enzyme-mimetic templates in applicable areas of phytochemistry and studies into the structural and physicochemical property changes in the enzyme and changes in the functional stage substrate, structures, behavior/response and biochemical properties | |
| Spectrum of biochemical processes- including signal transduction and structures of natural signal regulators and their electrochemical properties, all at first in smaller systems, plants and or animals and their corresponding organs/tissues and systems in primitive animal and essentially into the plant kingdom as sources of information decoding of pathways and its reactive substrates | |
| The natural order of chemical evolution, advancements in structures, biogenesis, chemical dynamics in the biosystem, the paradigm of optimum for the time-bound key substrate or other molecular structure, the mobility of natures’ macromolecular and micromolecular entity and other present products in biochemical systems of life - both in vivo and in vitro settings | |
| Photochemistry | Photochemical aspects of natural products reactivity and end-products variations, photo-transformation diversity and definitively of the final product formation with regards to pathways selection and its execution |
| Solar effects on plants, their products and the micro details of the chemistry undertaking in the plant system | |
| Proteomics | The use of plant-based peptidomimetic, enzymes, and enzyme mimics as chemozymes at large scale and in more defined substrates and functions, the biocatalyst’s comparable reactivity in natural and non-natural reaction settings, enzymes and enzymatic co-factor utilization in self-assembly of structures in nature and especially in biodegradable polymeric materials and life-chemicals e.g., proteins, amino sugars, proteoglycans/glycoproteins, polypeptides and saccharide polymeric formations |
| Specific reactivity of a natural product and its applications to functional proteomics | |
| Genomics | Use of human genome sequences for particular site oriented action and reactivity and the chemical information needing translation into diverse and stepwise and non-step-wise responses coded therein for the specific ailments need to be used as arbitrator in the receptor-ligand binding studies |
| The level of genetic advancement, ecology-assisted subtle, permanent, non-permanent and ongoing changes of pathways, products and plant system organizations in terrestrial, and marine plants, and other organisms, the gradual complexity of the products and concerned effects on acquired genome and filial-genome derived evolution patterns for species in perspective and their distant relationship in the genomic tree | |
| Designer forest and development of genetically engineered renewable plant resources, human foods’ genetic alterations, microorganisms’ self and induced genetic changes | |
| Metabolomics | Site-directed drug delivery and action, the fate of metabolites, the small and big molecule as an active component and prodrugs, all somewhat filling the disease versus plant products interaction gap together with the help from natural product probe-compounds |
| Plant Metabolomics and large-scale phytochemicals in the functional genomics arena | |
| Toxicology | Toxicological behavior and toxicokinetics of natural toxins and other plants products, biochemical oxidation, biochemical reduction processes and their byproducts |
| Constituents, biological and non-biological functions and reactivity, toxicological profile, broad contamination and its eventual containment of the identified product/cause | |
| Food Science, Designer Foods | Alternate natural foodstuff formulation, development of designer food, designer-food components -their purification, procurement, and formulation based on nature’s design and required an individual and personalized prescription |
| Plant Microbe Relations | Identification of fermentation broth compounds, generation of new broth products from terrestrial and aquatic plant/organism pool, the variant and induced microbial transformation, cell suspension culture products, other biotechnological areas application to the discipline |
| The variant and induced microbial transformation, cell suspension culture products, other biotechnological areas application | |
| Secondary Metabolites: Their Function and Evolution, Biocatalysis in Natural Products Chemistry | |
| Computation, Data Mining | The advancement of computation methods, prediction strategies for various kinds of interactions, and biological activities, software platforms and techniques required for data handling, retrieval, and data mining |
| Ecology and Environment | Chemical contamination of surroundings, pollutant effects of xenobiotic factors/entities, reach of man-made products in the ecological system and ultimately to the food chain |
| Sustainable utilization of natural resources, broad green chemistry developments over the natural products chemistry template | |
| Ecological interaction of natural products compounds, and entities including their effects on the biogenesis of products in the plant, in guest-hosts, and also in independent marine and amphibious environments | |
| Economic Impacts | Herbal supplements, botanical drugs, organically grown products, alternative medicines, traditional uses, and ethnobotany of underdeveloped countries |
Thus, again, the emerging trends and promise for future from these advancements, and deeper understanding of the natural products chemistry domains with its interrelationship with natural and biological sciences and with the hereto hidden concepts/understandings in biotechnology, drug discovery, pharmaceutical sciences, genetics, and chemical ecology will help to race ahead, and set the agenda for further work with promise of new economic, and technical growth so intricately linked. The current scenario of the developing fronts in the chemical, biochemical, and chemical ecology fields, as well as the commercial side of the research and developments with possibilities in finding newer phytochemically-templated food-stuff, drugs, and all chemically viable items in an era of economic turndown, holds immense promise and importance on global scale. The advancements in science, technology, and economic turnarounds also pass through the realm of natural products chemistry and natural products chemists’ contributions which are acquiring focus and importance on an increasing levels.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdallah E.M. Plants: an alternative source for antimicrobials. J. App. Pharm. Sci. 2011;01:16–20. [Google Scholar]
- Agarwal V., Moore B.S. Fungal polyketide engineering comes of age. Proc. Natl. Acad. Sci. U.S.A. 2010;111:12278–12279. doi: 10.1073/pnas.1412946111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F.B.H., Moghaddam M.G., Basri M., Rahman M.B.A. Enzymatic synthesis of betulinic acid ester as an anticancer agent: optimization study. Biocat. Biotrans. 2010;28:192–200. [Google Scholar]
- Alfermann A.W., Petersen M. Natural product formation by plant cell biotechnology. Plant Cell, Tissue Organ Cult. 1995;43:199–205. [Google Scholar]
- Amirkia V., Heinrich M. Natural products, and drug discovery: a survey of stakeholders in industry and academia. Front. Pharmacol. 2015 doi: 10.3389/fphar.2015.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous, 2003. In: 1st International Conference on Bio-Based Polymers, 12–14 Nov. 2003, Saitama, Japan. [DOI] [PubMed]
- Appendino G., Minassi A., Pagani A., Ech-Chahad A. The role of natural products in the ligand deorphanization of TRP channels. Curr. Pharm. Des. 2008;14:2–17. doi: 10.2174/138161208783330781. [DOI] [PubMed] [Google Scholar]
- Arnison P.G., Bibb M.J., Bierbaum G., Bowers A.A., Bugni T.S., Bulaj G. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013;30:108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T.M., Targett N.M. Marine tannins: the importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002;28:1919–1934. doi: 10.1023/a:1020737609151. [DOI] [PubMed] [Google Scholar]
- Arya P., Joseph R., Chou D.T.H. Towards high-throughput synthesis of complex natural product-like compounds in the genomics and proteomics age. Chem. Biol. 2002;9:145–156. doi: 10.1016/s1074-5521(02)00105-9. [DOI] [PubMed] [Google Scholar]
- Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotech. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam, M.N.K., Rahman, M.M., Biswas, S., Ahmed, M.N., 2016. Appraisals of Bangladeshi Medicinal Plants Used by Folk Medicine Practitioners in the Prevention and Management of Malignant Neoplastic Diseases. Inter Schol Res Notices. ID 7832120. 10.1155/2016/7832120. [DOI] [PMC free article] [PubMed]
- Azman A.-S., Othman I., Velu S.S., Chan K.G., Lee L.-H. Mangrove rare actinobacteria: taxonomy, natural compound, and discovery of bioactivity. Front. Microbiol. 2015;6:856. doi: 10.3389/fmicb.2015.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai G., Hou Y., Jiang M., Gao J. Integrated systems biology and chemical biology approach to exploring mechanisms of traditional chinese medicines. Chin. Herbal Med. 2016;8:99–106. [Google Scholar]
- Bai X., Ma Y., Zhang G. Butein suppresses cervical cancer growth through the PI3K/AKT/mTOR pathway. Oncol. Rep. 2015;33:3085–3092. doi: 10.3892/or.2015.3922. [DOI] [PubMed] [Google Scholar]
- Banerjee C., Ghosh S., Mandal S., Kuchlyan J., Kundu N., Sarkar N. Exploring the photophysics of curcumin in zwitterionic micellar system: an approach to control ESIPT process in the presence of Room Temperature Ionic Liquids (RTILs) and anionic surfactant. J. Phys. Chem., B. 2014;118:3669–3681. doi: 10.1021/jp411778q. [DOI] [PubMed] [Google Scholar]
- Banerji A. Resurgence of natural product research: a phoenix act. Proc. Indian Nat. Sci. Acad., Part A: Phys. Sci. 2000;66(3&4):383–392. [Google Scholar]
- Banskota A.H., McAlpine J.B., Dan Sørensen, Aouidate M., Piraee M., Alarco A.M., Omura S., Shiomi K., Farnet C.M., Zazopoulos E. Isolation and identification of three new 5-Alkenyl-3,3(2H)- furanones from two streptomyces species using a genomic screening approach. J. Antibiot. 2006;59:168–176. doi: 10.1038/ja.2006.24. [DOI] [PubMed] [Google Scholar]
- Banwell M. Research in natural product synthesis: a vital and dynamic global enterprise. Tetrahedron. 2008;64:4669–4670. [Google Scholar]
- Bechthold A., Domann S., Faust B., Hoffmeister D., Stockett S., Trefzer A., Weitnauer G., Westrich L. Glycosylated natural products: perspectives for combinatorial biosynthesis. Chemo J. 1999;8:130–135. [Google Scholar]
- Bedir E., Tatli I.I., Khan R.A., Zhao J., Takamatsu S., Walker L.A., Goldman P., Khan I.A. Biologically active secondary metabolites from Ginkgo biloba. J. Agric. Food Chem. 2002;50:3150–3155. doi: 10.1021/jf011682s. [DOI] [PubMed] [Google Scholar]
- Behnken S., Hertweck C. Anaerobic bacteria as producers of antibiotics. App. Microbiol. Biotech. 2012;96:61–67. doi: 10.1007/s00253-012-4285-8. [DOI] [PubMed] [Google Scholar]
- Behnken S., Lincke T., Kloss F., Ishida K., Hertweck C. Antiterminator-mediated unveiling of cryptic polythioamides in an anaerobic bacterium. Angew. Chem. Int. Ed. 2012;51:2425–2458. doi: 10.1002/anie.201108214. [DOI] [PubMed] [Google Scholar]
- Bergmann S., Funk A.N., Scherlach K., Schroeckh V., Shelest E., Horn U., Hertweck C., Brakhage A.A. Activation of a silent fungal polyketide biosynthesis pathway through regulatory cross-talk with a cryptic non-ribosomal peptide synthetase gene cluster. Appl. Environ. Microbiol. 2010;76:8143–8149. doi: 10.1128/AEM.00683-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkov S., Mutafova B., Christen P. Molecular biodiversity and recent analytical developments: a marriage of convenience. Biotech. Adv. 2014;32:1102–1110. doi: 10.1016/j.biotechadv.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Berman, A., Flanery, M.A., 2001. America’s Botanico-Medical Movements: Vox Populi. The Haworth Press Inc., Binghamton, New York, pp. 19101–19147. ISSN# 0-7890-1235-9.
- Bezerra R.J.S., Calheiros A.S., Ferreira N.C.S., Frutuoso V.D.S., Alves L.A. Natural products as a source for new anti-inflammatory and analgesic compounds through the inhibition of Purinergic P2X receptors. Pharmaceuticals. 2013;6:650–658. doi: 10.3390/ph6050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson, J., McAlpine, J.B., Friesen, J.B., Chen, S.-N., Graham, J., Pauli, G.F, 2016. Can invalid bioactives undermine natural product-based drug discovery? J. Med. Chem. 59, 1671–1690. [DOI] [PMC free article] [PubMed]
- Brachmann A.O., Reimer D., Lorenzen W., Alonso E.A., Kopp Y., Piel J., Bode H.B. Reciprocal cross-talk between fatty acid and antibiotic biosynthesis in a nematode symbiont. Angew. Chem. Int. Ed. 2012;51:12086–12089. doi: 10.1002/anie.201205384. [DOI] [PubMed] [Google Scholar]
- Bretschneider T., Heim J.B., Heine D., Winkler R., Busch B., Kusebauch B., Stehle T., Zocher G., Hertweck C. Vinylogous chain branching catalysed by a dedicated polyketide synthase module. Nature. 2013;502(7469):124–128. doi: 10.1038/nature12588. [DOI] [PubMed] [Google Scholar]
- Brooks, D.W, 1986. Applications of microbial transformations: total synthesis of natural products. NATO ASI Ser. C: Math. Phys. Sci. 178 (Enzy Catal Org Syn), 142–169. ISSN# 0258-2003.
- Brown K. Approaches to valuing plant medicines: the economics of culture or the culture of economics? Biodiver. Conserv. 1994;3:734–750. [Google Scholar]
- Buckland B.C., Robinson D., Chartrain M. Biocatalysis for the pharmaceuticals-status and prospects for a key technology. Metabol. Eng. 2000;2:42–48. doi: 10.1006/mben.1999.0138. [DOI] [PubMed] [Google Scholar]
- Bull, J.R. (Ed.), 2003. Special topic issue: theme of natural products. Pure Appl. Chem. 75, 141–19.
- Busch B., Ueberschaar N., Behnken S., Sugimoto Y., Werneburg M., Traitcheva N., He J., Hertweck C. Multifactorial control of iteration events in a modular polyketide assembly line. Angew. Chem. Int. Ed. 2013;52:5285–5289. doi: 10.1002/anie.201301322. [DOI] [PubMed] [Google Scholar]
- Busch B., Ueberschaar N., Sugimoto Y., Hertweck C. Interchenar retro transfer of aureothin intermediates in an iterative polyketide synthase module. J. Am. Chem. Soc. 2012;134:12382–12385. doi: 10.1021/ja304454r. [DOI] [PubMed] [Google Scholar]
- Butler M.S., Robertson A.A., Cooper M.A. Natural product and natural product-derived drugs in clinical trials. Nat. Prod. Rep. 2014;31:1612–1661. doi: 10.1039/c4np00064a. [DOI] [PubMed] [Google Scholar]
- Butler M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- Cai X.F., Teta R., Kohlhaas C., Crüsemann M., Ueoka R., Mangoni A., Freeman M.F., Piel J., Wilson M.C. Manipulation of regulatory genes reveals complexity and fidelity in hormaomycin biosynthesis. Chem. Biol. 2013;20:839–846. doi: 10.1016/j.chembiol.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Calixto J.B., Kassuya C.A., André E., Ferreira J. Contribution of natural products to the discovery of the Transient Receptor Potential (TRP) channels family and their functions. Pharmacol. Ther. 2005;106:179–208. doi: 10.1016/j.pharmthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Cao X., Jiang J., Zhang S., Zhu L., Zou J., Diao Y., Xiao W., Shan L., Sun H., Zhang W., Huang J., Li H. Discovery of natural estrogen receptor modulators with structure-based virtual screening. Bioorg. Med. Chem. Lett. 2013;23:3329–3333. doi: 10.1016/j.bmcl.2013.03.105. [DOI] [PubMed] [Google Scholar]
- Chang J., Kwon H.J. Discovery of novel drug targets and their functions using phenotypic screening of natural products. J. Ind. Microbiol. Biotech. 2016;43:221–231. doi: 10.1007/s10295-015-1681-y. [DOI] [PubMed] [Google Scholar]
- Cho H.J., Yu F., Sun R., Lee D., Song M.J. Lytic induction of Kaposi’s sarcoma-associated herpesvirus in primary effusion lymphoma cells with natural products identified by a cell-based fluorescence moderate-throughput screening. Arch. Virol. 2008;153:1517–1525. doi: 10.1007/s00705-008-0144-4. [DOI] [PubMed] [Google Scholar]
- Clardy J., Walsh C. Lessons from natural molecules. Nature (London) 2004;432(7019):829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- Concha N.P., Long P.F. Mining the microbial metabolome: a new Frontier for natural product lead discovery. Drug Dis. Today. 2003;8:1078–1084. doi: 10.1016/s1359-6446(03)02901-5. [DOI] [PubMed] [Google Scholar]
- Conforti F., Ioele G., Statti G.A., Marrelli M., Ragno G., Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008;46:3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Cordell G.A., Colvard M.D. Natural products and traditional medicine: turning on a paradigm. J. Nat. Prod. 2012;75:514–525. doi: 10.1021/np200803m. [DOI] [PubMed] [Google Scholar]
- Cordell G.A. Natural products in drug discovery-creating a new vision. Phytochem. Rev. 2003;1:261–273. [Google Scholar]
- Costello C., Ward M. Search, bioprospecting and biodiversity conservation. J. Environ. Econ. Manag. 2006;52:615–626. [Google Scholar]
- Coyne S., Chizzali C., Khalil M.N.A., Litomska A., Richter K., Beerhues L., Hertweck C. Biosynthesis of the antimetabolite 6-thioguanine in Erwinia amylovora plays a key role in fire blight pathogenesis. Angew. Chem. Int. Ed. 2013;52 doi: 10.1002/anie.201305595. 1. 0564-68. [DOI] [PubMed] [Google Scholar]
- Cseke, L., Kirakosyan, A., Kaufman, P.B., Warber, S.L., Duke, J.A., Brielmann, H.L., 2006. Natural Products from Plants. CRC Press, Boca Raton, FL, USA. 611 pp. ISBN 0-8493-2976-0.
- Cvetanović A., Gajić J.S., Zeković Z., Savić S., Vulić J., Mašković P., Ćetković G. Comparative analysis of anti-oxidant, anti-microbiological and cytotoxic activities of native and fermented chamomile ligulate flower extracts. Planta. 2015;242:721–732. doi: 10.1007/s00425-015-2308-2. [DOI] [PubMed] [Google Scholar]
- Das S., Sasmal D., Basu S.P. Antispasmodic and anthelmintic activity of Nyctanthes arbortristis LINN. Inter. J. Pharm. Sci. Res. 2010:51–55. [Google Scholar]
- Davidson B.S. New dimensions in natural products research: culture marine microorganisms. Curr. Opin. Biotech. 1995;6:284–291. [Google Scholar]
- Davis R.A., Buchanan M.S., Duffy S., Avery V.M., Charman S.A., Charman W.N., White K.L., Shackleford D.L., Edstein M.D., Andrews K.T., Camp D., Quinn R.J. Antimalarial activity of pyrroloiminoquinones from the australian marine sponge Zyzzya sp. J. Med. Chem. 2012;55:5851–5858. doi: 10.1021/jm3002795. [DOI] [PubMed] [Google Scholar]
- Davis R.A., Pierens G.K., Parsons P.G. Synthesis and spectroscopic characterization of a combinatorial library based on the fungal natural product 3-chloro-4-hydroxyphenylacetamide. Mag. Reson. Chem. 2007;45:442–445. doi: 10.1002/mrc.1984. [DOI] [PubMed] [Google Scholar]
- de Silva, T., Atal, C.K., 1995. Processing, Refinement and Value Addition of Non-wood Forest Products. FAO report of the International Expert Consultation on Non-wood Forest Products. pp. 167–193.
- de Silva, T., 1997a. A manual on the essential oil industry. United Nations Industrial Development Organisation, Vienna, Austria. 10.1002/(SICI)1099-1026(199705) 12:3,222::AID-FFJ684>3.0.CO;2-Q.
- de Silva, T., 1997b. Industrial utilization of medicinal plants in developing countries. Medicinal plants for forest conservation and health care. FAO report, UNIDO development programs on industrial utilization of medicinal and aromatic plants. Acta Horticul. 333, 47–54.
- de Smet P.A. The role of plant-derived drugs and herbal medicine in healthcare. Drugs. 1997;54:801–840. doi: 10.2165/00003495-199754060-00003. [DOI] [PubMed] [Google Scholar]
- DeCorte B.L. Underexplored opportunities for natural products in drug discovery. J. Med. Chem. 2016 doi: 10.1021/acs.jmedchem.6b00473. [DOI] [PubMed] [Google Scholar]
- Demain, A.L., 1992. Microbial secondary metabolism: a new theoretical frontier for academia, a new opportunity for industry. In: Ciba Foundation Symposium, 171 (Secondary Metabolites: Their Function, and Evolution). pp. 3–23. ISSN# 0300-5208. [DOI] [PubMed]
- Ding, L., Maier, A., Fiebig, H.H., Görls, H., Lin, W.H., Peschel, G., Hertweck, C., 2011. Divergolides A-D from a mangrove endophyte reveal an unparalleled plasticity in a macrolide biosynthesis. Angew. Chem. Int. Ed. 50, 1630–1664. [DOI] [PubMed]
- Dongre S.H., Badami S.S., Godavarthi A. Antitumor activity of Hypericum hookerianum against DLA induced tumor in mice and its possible mechanism of action. Phytother. Res. 2008;22:23–29. doi: 10.1002/ptr.2248. [DOI] [PubMed] [Google Scholar]
- Donia M., Hathaway B.J., Sudek S., Haygood M.G., Rosovitz M.J., Ravel J., Schmidt E.W. Natural combinatorial peptide libraries in cyanobacterial symbionts of marine ascidians. Nat. Chem. Biol. 2006;2:729–735. doi: 10.1038/nchembio829. [DOI] [PubMed] [Google Scholar]
- Donia M.S., Fricke F., Ravel J., Schmidt E.W. Variation in tropical reef symbiont metagenomes defined by secondary metabolism. PLoS One. 2011;6:e17897. doi: 10.1371/journal.pone.0017897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia M.S., Ravel J., Schmidt E.W. A global assembly line for cyanobactins. Nat. Chem. Biol. 2008;4:341–343. doi: 10.1038/nchembio.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia M.S., Ruffner D.E., Cao S., Schmidt E.W. Accessing the hidden majority of marine natural products through metagenomics. ChemBioChem. 2011;12:1230–1236. doi: 10.1002/cbic.201000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia M.S., Fricke W.F., Partensky F., Cox J., Elshahawi S.I., White J.R., Phillippy A.M., Schatz M.C., Piel J., Haygood M.G., Ravel J., Schmidt E.W. Complex microbiome underlying secondary and primary metabolism in the tunicate-Prochloron symbiosis. Proc. Nat. Acad. Sci. USA. 2011;108:E1423–E1432. doi: 10.1073/pnas.1111712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dückert H., Pries V., Khedkar V., Menninger S., Bruss H., Bird A.W. Natural product-inspired cascade synthesis yields modulators of centrosome integrity. Nat. Chem. Biol. 2012;8:179–184. doi: 10.1038/nchembio.758. [DOI] [PubMed] [Google Scholar]
- Dunn B.J., Khosla C. Engineering the acyltransferase substrate specificity of assembly line polyketide synthases. J. R. Soc. Interface. 2013;10:20130297. doi: 10.1098/rsif.2013.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauzi F.M., Koutsoukas A., Lowe R., Joshi K., Fan T.P., Glen R.C., Bender A. Linking Ayurveda and Western medicine by integrative analysis. J Ayur. Integ. Med. 2013;4:117–119. doi: 10.4103/0975-9476.113882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauzi F.M., Koutsoukas A., Lowe R., Joshi K., Fan T.P., Glen R.C., Bender A. Chemogenomics approaches to rationalizing the mode-of-action of traditional chinese and ayurvedic medicines. J. Chem. Inf. Model. 2013;53(3):661–673. doi: 10.1021/ci3005513. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, K., 2004. Au Naturel: Feature Article. Canadian Chemical News. Chemical Institute of Canada, Ottawa. vol. 56. pp. 18–20.
- Food and Agriculture Organization (FAO) of the United Nations, 2011. Second Global Plan of Action, Commission on Genetic Resources for Food and Agriculture, 29th November 2011, Rome, Italy. ISBN 978-92-5-107163-2.
- Fowler, M.W., Stepan-Sarkissian, G., 1983. Chemicals from plant cell fermentation. In: Mizrahi, A., van Wezel, A.L. (Eds.), Advances in Biotechnological Processes, vol. 2, pp. 135–158.
- Fowler, M.W., 1993. Plant Cell Culture as a Source of Novel Medicinal Agents: New Drugs from Natural Sources. In: Conference Proceed, 1991; p 131–140 Abstracts, CAN 118:35787, AN 1993:35787.
- Franke J., Ishida K., Hertweck C. Genomics-driven discovery of burkholderic acid, a non-canonical, cryptic polyketide from human pathogenic Burkholderia species. Angew. Chem. Int. Ed. 2012;51:11611–11615. doi: 10.1002/anie.201205566. [DOI] [PubMed] [Google Scholar]
- Franke, J., Ishida, K., Ishida-Ito, M., Hertweck, C., 2013. Nitro versus hydroxamate in siderophores of pathogenic bacteria: effect of missing hydroxylamine protection in malleobactin biosynthesis. Angew. Chem. Int. Ed. 52, 8271–75. [DOI] [PubMed]
- Frearson J.A., Collie I.T. HTS and hit finding in academia – from chemical genomics to drug discovery. Drug Discov. Today. 2009;14:1150–1158. doi: 10.1016/j.drudis.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M.F., Gurgui C., Helf M.J., Morinaka B.I., Uria A.R., Oldham N.J., Sahl H.G., Matsunaga S., Piel J. Metagenome mining reveals polytheonamides as modified ribosomal peptides. Science. 2012;338:387–390. doi: 10.1126/science.1226121. [DOI] [PubMed] [Google Scholar]
- Galloway W.R.J.D., Bender A., Welch M., Spring D.R. The discovery of antibacterial agents using diversity-oriented synthesis. Chem. Comm. 2009;18:2446–2462. doi: 10.1039/b816852k. [DOI] [PubMed] [Google Scholar]
- Garson, M.J., 2001. Ecological perspectives on marine natural products biosynthesis. In: McClintock, J.B., Baker, B.J. (Eds). Marine Chem Biol. CRC Press, Boca Raton, FL, USA, pp. 71–114. ISBN: 978-0-8493-9064-7.
- Gatte-Picchi D., Weiz A., Ishida K., Hertweck C., Dittmann E. Functional analysis of environmental DNA-derived microviridins provides new insights into the diversity of the tricyclic peptide family. Appl. Environ. Microbiol. 2014;80:1380–1387. doi: 10.1128/AEM.03502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genilloud O. The re-emerging role of microbial natural products in antibiotic discovery. Antonie Van Leeuwenhoek. 2014;106:173–188. doi: 10.1007/s10482-014-0204-6. [DOI] [PubMed] [Google Scholar]
- Georgiades S.N., Clardy J. Synthetic libraries of tyrosine-derived bacterial metabolites. Bioorg. Med. Chem. Lett. 2008;18:3117–3121. doi: 10.1016/j.bmcl.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, H.J., Davies, G.J., Henrissat, B., Svensson, B., (Eds.), 1999. Recent Advances in Carbohydrate Bioengineering. The Royal Society of Chemistry, Cambridge, UK. pp. 302–308. ISBN# 0-85404-774-3.
- Gundersen L.L. Synthesis and biological activities of marine terpene-adenine hybrids and synthetic analogs. Phytochem. Rev. 2013;12:467–486. [Google Scholar]
- Hagino, N., 2000. West Meets East. Looking for the interphase of western medicine and, traditional oriental medicine in future. Rinsho Byori 48, 764–70 (Jap). [PubMed]
- Hamzeh-Mivehroud M., Rahmani S., Feizi M.A.H., Dastmalchi Rashidi M.R. In vitro and in silico studies to explore structural features of flavonoids for aldehyde oxidase inhibition. Arch. Pharm. 2014;347:738–747. doi: 10.1002/ardp.201400076. [DOI] [PubMed] [Google Scholar]
- Harvey A. The role of natural products in drug discovery and development in the new millennium. Drugs. 2010;13:70–72. [PubMed] [Google Scholar]
- Harvey A.L., Edrada-Ebel R.A., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- He J., Hertweck C. Biosynthetic origin of the rare nitroaryl moiety of the polyketide antibiotic aureothin: involvement of an unprecedented N-Oxygenase. J. Am. Chem. Soc. 2004;126:3694–3695. doi: 10.1021/ja039328t. [DOI] [PubMed] [Google Scholar]
- He J., Hertweck C. Iteration as programmed event during polyketide assembly; molecular analysis of the aureothin biosynthesis gene cluster. Chem. Biol. 2003;10:1225–1232. doi: 10.1016/j.chembiol.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Heinstein P.F. Future approaches to the formation of secondary natural products in plant cell suspension cultures. J. Nat. Prod. 1985;48:1–9. [Google Scholar]
- Hennicke F., Grumbt M., Lehmann U., Ueberschaar N., Palige K., Böttcher B. Factors supporting cysteine tolerance and sulfite production in Candida albicans. Eukaryot. Cell. 2013;12:604–613. doi: 10.1128/EC.00336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel U., Piel J., Degnan S., Taylor M. Genomic insights into the marine sponge microbiome. Nat. Rev. Microbiol. 2012;10:641–654. doi: 10.1038/nrmicro2839. [DOI] [PubMed] [Google Scholar]
- Hertweck C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009;48:4688–4716. doi: 10.1002/anie.200806121. [DOI] [PubMed] [Google Scholar]
- Hou S., Wang J., Li Z., Wang Y., Wang Y., Yang S., Xu J., Zhu W. Five-descriptor model to predict the chromatographic sequence of natural compounds. J. Sep. Sci. 2016;39:864–872. doi: 10.1002/jssc.201501016. [DOI] [PubMed] [Google Scholar]
- Hu R., Lu Y., Dai X., Pan Y. Screening of antioxidant phenolic compounds in Chinese Rhubarb combining fast counter-current chromatography fractionation and liquid chromatography/mass spectrometry analysis. J. Sep. Sci. 2010;33:1595–1603. doi: 10.1002/jssc.201000045. [DOI] [PubMed] [Google Scholar]
- Huang Q., Qiao X., Xu X. Potential synergism and inhibitors to multiple target enzymes of Xuefu Zhuyu Decoction in cardiac disease therapeutics: a computational approach. Bioorg. Med. Chem. Lett. 2007;17:1779–1783. doi: 10.1016/j.bmcl.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Ishida K., Lincke T., Behnken S., Hertweck C. Induced biosynthesis of cryptic polyketide metabolites in a Burkholderia thailandensis quorum sensing mutant. J. Am. Chem. Soc. 2010;132:13966–13968. doi: 10.1021/ja105003g. [DOI] [PubMed] [Google Scholar]
- Kampa, A., Gagunashvili, A.N., Gulder, T.A.M., Daolio, C., Godejohann, M., Miao, V.P.W., Piel, J., Andrésson, O.S., 2013. Metagenomic natural product discovery in lichen provides evidence for specialized biosynthetic pathways in diverse symbioses. Proc. Natl. Acad. Sci. USA 110, E 3129-37. [DOI] [PMC free article] [PubMed]
- Kayne, S.B. (Ed.), 2002. Complementary Therapies for Pharmacists. Pharmaceutical Press, London, UK. ISSN# 0-85369-430-3.
- Khan R.A., Agrawal P.K., Kapil R.S. Puetuberosanol, an epoxychalcanol from Pueraria tuberosa. Phytochem. 1996;42:42–44. [Google Scholar]
- Khandelwal A., Crowley V.M., Blagg B.S.J. Natural product inspired N-terminal Hsp90 inhibitors: from bench to bedside? Med. Res. Rev. 2016;36:92–118. doi: 10.1002/med.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn A.D., Young-Won C., Swanson S.M. Discovery of natural product anticancer agents from biodiverse organisms. Curr. Opin. Drug Discov. Devel. 2009;12:189–196. [PMC free article] [PubMed] [Google Scholar]
- Kingston D.G.I. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011;74:496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard A., Iversen A., Andersen M.R., Larsen T.O., Frisvad J.C., Nielsen F. Aggressive dereplication using UHPLC–DAD–QTOF: screening extracts for up to 3000 fungal secondary metabolites. Anal. Bioanal. Chem. 2014;406:1933–1943. doi: 10.1007/s00216-013-7582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss F., Pidot S., Goerls H., Friedrich T., Hertweck C. Formation of a dinuclear copper (I) complex from the clostridium-derived antibiotic closthioamide. Angew. Chem. Int. Ed. 2013;52:10745–10748. doi: 10.1002/anie.201304714. [DOI] [PubMed] [Google Scholar]
- Koehn F.E., Carter G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- Kombarov R., Altieri A., Dmitry G., Kirpichenok M., Kochubey V., Rakitina N., Titarenko Z. BioCores: identification of a drug/natural product-based privileged structural motif for small-molecule lead discovery. Mol. Diver. 2010;14:193–200. doi: 10.1007/s11030-009-9157-5. [DOI] [PubMed] [Google Scholar]
- König C.C., Scherlach K., Schroeckh V., Horn F., Nietzsche S., Brakhage A.A., Hertweck C. Bacterium induces cryptic meroterpenoid pathway in the pathogenic fungus Aspergillus fumigatus. ChemBioChem. 2013;14:938–942. doi: 10.1002/cbic.201300070. [DOI] [PubMed] [Google Scholar]
- Kroiss J., Kaltenpoth M., Schneider B., Schwinger M.G., Hertweck C. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 2010;6:261–263. doi: 10.1038/nchembio.331. [DOI] [PubMed] [Google Scholar]
- Kusebauch B., Brendel N., Kirchner H., Dahse H.M., Hertweck C. Assessing oxazole bioisosteres as muta synthons on the rhizoxin assembly line. ChemBioChem. 2011;12:2284–2288. doi: 10.1002/cbic.201100387. [DOI] [PubMed] [Google Scholar]
- Kwan J.C., Donia M.S., Han A., Hirose E., Haygood M.G., Schmidt E.W. Genome streamlining for chemical defense in a coral reef symbiosis. Proc. Nat. Acad. Sci. USA. 2012;109:20655–20660. doi: 10.1073/pnas.1213820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan J.C., Schmidt E.W. Bacterial endosymbiosis in a chordate host: long-term co-evolution and conservation of secondary metabolism. PLoS ONE. 2013;201(8):e80822. doi: 10.1371/journal.pone.0080822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance H., Wetzel S., Kumar K., Waldmann H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 2012;55:5989–6001. doi: 10.1021/jm300288g. [DOI] [PubMed] [Google Scholar]
- Lackner G., Schenk A., Xu Z., Reinhardt K., Yunt Z.S., Piel J., Hertweck C. Biosynthesis of pentangular polyphenols: deductions from the benastatin and griseorhodin pathways. J. Am. Chem. Soc. 2007;129:9306–9312. doi: 10.1021/ja0718624. [DOI] [PubMed] [Google Scholar]
- Lal J., Gupta S.K., Thavaselvam D., Agarwal D.D. Biological activity, design, synthesis and structure-activity relationship of some novel derivatives of curcumin containing sulfonamides. Euro. J. Med. Chem. 2013;64:579–588. doi: 10.1016/j.ejmech.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Larsen T.O., Smedsgaard J., Nielsen K.F., Hansen M.E., Frisvad J.C. Phenotypic taxonomy and metabolite profiling in microbial drug discovery. Nat. Prod. Rep. 2005;22:672–695. doi: 10.1039/b404943h. [DOI] [PubMed] [Google Scholar]
- Lee J., McIntosh J., Hathaway B., Schmidt E.W. Using marine natural products to discover a protease that catalyzes peptide macrocyclization of diverse substrates. J. Am. Chem. Soc. 2009;131:2122–2124. doi: 10.1021/ja8092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.W., Vederas J.C. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325(5937):161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- Li Y., Wu C., Liu D., Proksch P., Guo P., Lin W., Chartarlactams A.-P. Phenylspirodrimanes from the sponge-associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014;77:138–147. doi: 10.1021/np400824u. [DOI] [PubMed] [Google Scholar]
- Lin Y., Sun X., Yuan Q., Yan Y. Combinatorial biosynthesis of plant-specific coumarins in bacteria. Metabol. Eng. 2013;8:69–77. doi: 10.1016/j.ymben.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Lin Z., Flores M., Forteza I., Concepcion G.P., Rosenberg G., Haygood M.G., Olivera B.M., Light A., Schmidt E.W. Totopotensamides, polyketide-cyclic peptide hybrids from a mollusk-associated bacterium Streptomyces sp. J. Nat. Prod. 2012;75:644–649. doi: 10.1021/np200886x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C., Dai, L., Liu, Y., Rong, L., Dou, D., Sun, Y., Ma, L., 2016. Antiproliferative activity of triterpene glycoside nutrient from monk fruit in colorectal cancer and throat cancer. Nutrients 8, 360. 10.3390/nu8060360. [DOI] [PMC free article] [PubMed] [Retracted]
- Liu, J.K., 1996. New advances in the molecular natural compounds research. Youji Huaxue 16, 284–88 (Jap).
- Lopez S.N., Ramallo I.A., Sierra M.G., Zacchino S.A., Furlan R.L.E. Chemically engineered extracts as an alternative source of bioactive natural product-like compounds. Prod. Nat. Acad. Sci. USA. 2007;104:441–444. doi: 10.1073/pnas.0608438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Perez J.L., Theron R., del Olmo E., Diaz D. NAPROC-13: a database for the dereplication of natural product mixtures in bioassay-guided protocols. Bioinformatics. 2007;23:3256–3257. doi: 10.1093/bioinformatics/btm516. [DOI] [PubMed] [Google Scholar]
- Luoma-aho, T., 2004. International Plant Genetic Resources Institute, Forest Genetic Resources Conservation, and Management: Proceedings of the Asia Pacific Forest Genetic Resources Programme (APFORGEN), Inception Workshop. 15-18 July 2003, Kepong, Kuala Lumpur, Malaysia. Biodiversity International, 2004, 338 pp; ISBN 9290436247, 9789290436249.
- Lustosa A.K.M.F., Arcanjo D.D.R., Ribeiro R.G., Rodrigues K.A.F., Passos F.F.B., Piauilino C.A., Silva-Filho J.C., Araújo B.Q., Lima-Neto J.S., Costa-Júnior J.S., Carvalho F.A.A., Citó A.M.D.G.L. Immunomodulatory and toxicological evaluation of the fruit seeds from Platonia insignis, a native species from Brazilian Amazon Rainforest. Revista Brasile de Farmacog. 2016;26:77–82. [Google Scholar]
- Luzzatto-Knaan T., Melnik A.V., Dorrestein P.C. Mass spectrometry tools and workflows for revealing microbial chemistry. Analyst. 2015;140:4949–4966. doi: 10.1039/c5an00171d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J.K., Christou, P., Chikwamba, R., Haydon, H., Paul, M., Ferrer, M.P., Ramalingam, S., Rech, E., Rybicki, E., Wigdorowitz, A., Yang, D.C., Thangaraj, H., 2013. Realising the value of plant molecular pharming to benefit the poor in developing countries and emerging economies. Plant Biotechnol. J. 11, 1029–1033. [DOI] [PubMed]
- Mabry T.J. Selected topics from forty years of natural products research: betalains to flavonoids, antiviral proteins, and neurotoxic non-protein amino acids. J. Nat. Pro. 2001;64:1596–1604. doi: 10.1021/np010524s. [DOI] [PubMed] [Google Scholar]
- Mali R.G., Mehta A.A. A review on anthelmintic plants. Nat. Prod. Rad. 2008;7:466–475. [Google Scholar]
- Marko I.E. Some thoughts on the total synthesis of natural products. Are there still important challenges. Actual. Chim. 2003;4–5:143–144. [Google Scholar]
- McCulloch M.W.B., Coombs G.S., Banerjee N., Bugni T.S., Cannon K.M., Harper M.K., Veltri C.A., Virshup D.M., Ireland C.M. Psammaplin A as a general activator of cell-based signaling assays via HDAC inhibition and studies on some bromotyrosine derivatives. Bioorg. Med. Chem. 2009;17:2189–2198. doi: 10.1016/j.bmc.2008.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J.A., Donia M.S., Schmidt E.W. Ribosomal peptide natural products: bridging the ribosomal and non-ribosomal worlds. Nat. Prod. Rep. 2009;26:537–559. doi: 10.1039/b714132g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J.A., Lin Z., Tianero M.D.B., Schmidt E.W. Aestuaramides, a natural library of cyanobactin cyclic peptides resulting from isoprene-derived claisen rearrangements. ACS Chem. Biol. 2013;8:877–883. doi: 10.1021/cb300614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinwald J., Eisner T. Natural products chemistry: new opportunities, uncertain future. Helv Chim Acta. 2001;86:3633–3637. [Google Scholar]
- Menche D. New methods for stereochemical determination of complex polyketides: configurational assignment of novel metabolites from myxobacteria. Nat. Prod. Rep. 2008;25:905. doi: 10.1039/b707989n. [DOI] [PubMed] [Google Scholar]
- Miroddi M., Mannucci C., Mancari F., Navarra M., Calapai G. Research and development for botanical products in medicinals and food supplements market. Evid. Based Compl. Alter. Med. 2013:649720. doi: 10.1155/2013/649720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari G. Natural products in drug discovery: present status and perspectives. Chapter, pharmaceutical biotechnology. Vol 655. Adv. Exp. Med. Biol. 2009:13–27. doi: 10.1007/978-1-4419-1132-2_2. 10.1007/978-1-4419-1132-2-2. Print ISBN 978-1-4419-1131-5. ISBN 978-1-4419-1132-2. [DOI] [PubMed] [Google Scholar]
- Mollo E., Cimino G., Ghiselin M.T. Alien biomolecules: a new challenge for natural product chemists. Biol. Invas. 2015;17:941–950. [Google Scholar]
- Moloney M.G. Natural products as a source for novel antibiotics. Trends Pharm. Sci. 2016;37:1689–1701. doi: 10.1016/j.tips.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Moore B.S., Hertweck C., Hopke J.N., Izumikawa M., Kalaitzis J.A., Nilsen G. Plant-like biosynthetic pathways in bacteria: from benzoic acid to chalcone 1. J. Nat. Prod. 2002;65:1956–1962. doi: 10.1021/np020230m. [DOI] [PubMed] [Google Scholar]
- Mori, K., 1997. Organic synthesis in life sciences for the 21st century. Kagakuto Seibutsu 35, 157–61 (Jap). ISSN 0453-073X.
- Mullane K., Winquist R.J., Williams M. Translational paradigms in pharmacology and drug discovery. Biochem. Pharmacol. 2014;87:189–210. doi: 10.1016/j.bcp.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Musiol E.M., Härtner T., Kulik A., Moldenhauer J., Piel J., Wohlleben W., Weber T. Supramolecular templating in polyketide biosynthesis - the role of the discrete acyltransferase KirCII in kirromycin production. Chem. Biol. 2011;18:438–444. doi: 10.1016/j.chembiol.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Afendi F.M., Parvin A.K., Ono N., Tanaka K., Hirai Morita A., Sato T., Sugiura T., Altaf-Ul-Amin M., Kanaya S. KNApSAcK Metabolite Activity Database for retrieving the relationships between metabolites and biological activities. Plant Cell Physiol. 2014;55:e7. doi: 10.1093/pcp/pct176. and references cited therein. [DOI] [PubMed] [Google Scholar]
- Nelson J.T., Lee J.H., Sims J.W., Schmidt E.W. Characterization of SafC, a catechol 4-O-methyltransferase involved in Saframycin biosynthesis. Appl. Environ. Microbiol. 2007;73:3575–3580. doi: 10.1128/AEM.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, D.J., Cragg, G.M., Kingston, D.G.I., 2003a. Natural Products as Pharmaceuticals & Sources for Lead Structures. Practice of Medicinal Chemistry, second ed., pp. 91–09.
- Newman D.J., Cragg G.M., Snader K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Newman, D.J., Cragg ,G.M., 2009. Natural products as drugs and leads to drugs: the historical perspective. In: Natural Products Chemistry for Drug Discovery. Royal Society of Chemistry Publications, UK. ISBN 978-1-84755-989-0.
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nguyen T.A., Ishida K., Jenke-Kodama H., Dittmann E., Gurgui C. Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection. Nat. Biotech. 2008;26:225–233. doi: 10.1038/nbt1379. [DOI] [PubMed] [Google Scholar]
- Nicolaou, K.C., Evans, R.M., Roecker, A.J., Hughes, R., Dowens, M., Pfefferkorn, J.A., 2004. Discovery of potent, non-steroidal fxr agonists originating from natural product-like libraries. In: 228thACS National Meeting, Philadelphia PA, USA. August 22–26. MEDI-171.
- Nielsen F. Aggressive dereplication using UHPLC–DAD–QTOF: screening extracts for up to 3000 fungal secondary metabolites. Ana Bioana Chem. 2014;406:1933–1943. doi: 10.1007/s00216-013-7582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K.F., Larsen L.O. The importance of mass spectrometric dereplication in fungal secondary metabolite analysis. Front. Microbiol. 2015 doi: 10.3389/fmicb.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nützmann H.W., Fischer J., Scherlach K., Hertweck C., Brakhage A.A. Distinct amino acids of histone H3 control secondary metabolism in Aspergillus nidulans. Appl. Environ. Microbiol. 2013;79:6102–6169. doi: 10.1128/AEM.01578-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe B.R. Biologically active proteins from natural product extracts. J. Nat. Prod. 2001;64:1373–1381. doi: 10.1021/np0103362. [DOI] [PubMed] [Google Scholar]
- Oberlies, N.H., Flora, S., Weaver, A.L., 2003. Camptothecin & Taxol: The Story Behind the Science. Chem International (An IUPAC Publication). p. 4.
- Olano C., Méndez C., Salas J.A. Post-PKS tailoring steps in natural product-producing actinomycetes from the perspective of combinatorial biosynthesis. Nat. Prod. Rep. 2010;27:571–616. doi: 10.1039/b911956f. [DOI] [PubMed] [Google Scholar]
- Ortholand J.Y., Ganesan A. Natural products and combinatorial chemistry: back to future. Curr. Opin. Chem. Biol. 2004;8:271–280. doi: 10.1016/j.cbpa.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Ouédraogo M., Lamien-Sanou A., Ramdé N., Ouédraogo A.S., Ouédraogo M., Zongo S.P., Goumbri O., Duez P., Guissou P.I. Protective effect of Moringa Oleifera leaves against gentamicin-induced nephrotoxicity in rabbits. Exp. Toxicol. Pathol. 2013;65:335–339. doi: 10.1016/j.etp.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Park S.R., Han A.R., Ban Y.H., Yoo Y.J., Kim E.J., Yoon Y.J. Genetic engineering of macrolide biosynthesis: past advances, current state, and future prospects. Appl. Microbiol. Biotechnol. 2010;85:1227–1239. doi: 10.1007/s00253-009-2326-8. [DOI] [PubMed] [Google Scholar]
- Partida-Martinez L.P., Hertweck C. A gene cluster encoding rhizoxin biosynthesis in Burkholderia rhizoxina, the bacterial endosymbiont of the fungus Rhizopus microsporus. ChemBioChem. 2007;8:41–45. doi: 10.1002/cbic.200600393. [DOI] [PubMed] [Google Scholar]
- Partida-Martinez L.P., Hertweck C. Pathogenic fungus harbors endosymbiotic bacteria for toxin production. Nature. 2005;437:884–888. doi: 10.1038/nature03997. [DOI] [PubMed] [Google Scholar]
- Patel R.N., editor. Stereoselective Biocatalysis. Marcel Dekker Inc; New York: 2000. [Google Scholar]
- Paterson I., Findlay A.D., Noti C. Total Synthesis of (-)-Spirangien A, an antimitotic polyketide isolated from the myxobacterium sorangium. Chem.- An Asian J. 2009;4:594–611. doi: 10.1002/asia.200800445. [DOI] [PubMed] [Google Scholar]
- Pelish H.E., Westwood N.J., Feng Y., Kirchhausen T., Shair M.D. Use of biomimetic diversity-oriented synthesis to discover galanthamine-like molecules with biological properties beyond those of the natural product. J. Am. Chem. Soc. 2001;123(27):6740–6741. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]
- Penesyan A., Kjelleberg S., Egan S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs. 2010;8:438–459. doi: 10.3390/md8030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraud O., Biggs J.S., Hughen R.W., Light A., Concepcion G.P., Olivera B.M., Schmidt E.W. Microhabitats within venomous cone snails yield diverse actinobacteria. Appl. Environ. Microbiol. 2009;75:6820–6826. doi: 10.1128/AEM.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki S.K., Zheng J., Axelrod A.J., Detelich M., Keatinge-Clay A.T. Structural and functional studies of a trans-acyltransferase polyketide assembly line enzyme that catalyzes stereoselective α- and β-ketoreduction. Proteins. 2014;82:2067–2077. doi: 10.1002/prot.24561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidot S.J., Coyne S., Kloss F., Hertweck C. Antibiotics from neglected bacterial sources. Int. J. Med. Microbiol. 2014;304:14–22. doi: 10.1016/j.ijmm.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Piel J., Hertweck C., Shipley P.R., Hunt D.M., Newman M.S., Moore B.S. Cloning, sequencing, and analysis of the enterocin biosynthesis gene cluster from the marine isolate Streptomyces maritimus: evidence for the derailment of an aromatic polyketide synthase. Chem. Biol. 2000;7:943–955. doi: 10.1016/s1074-5521(00)00044-2. [DOI] [PubMed] [Google Scholar]
- Piel J., Hui D., Höfer I. Evidence for a symbiosis island involved in horizontal transfer of pederin biosynthetic capabilities in bacterial symbionts of Paederus spp Beetles. J. Bacteriol. 2004;186:1280–1286. doi: 10.1128/JB.186.5.1280-1286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J., Hui D., Wen G., Butzke D., Platzer M., Fusetani N., Matsunaga S. Antitumor polyketide biosynthesis by a bacterial symbiont of the marine sponge Theonella swinhoei. Proc. Nat. Acad. Sci. USA. 2004;101:16222–16227. doi: 10.1073/pnas.0405976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J. A polyketide synthase - nonribosomal peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Nat. Acad. Sci. USA. 2002;99:14002–14007. doi: 10.1073/pnas.222481399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J. Approaches to capturing and designing biologically active small molecules produced by uncultured microbes. Annu. Rev. Microbiol. 2011;65:431–453. doi: 10.1146/annurev-micro-090110-102805. [DOI] [PubMed] [Google Scholar]
- Pillon Y., Fogliani B. Evidence for a correlation between systematics and bioactivity in new caledonian cunoniaceae and its implications for screening and conservation. Pacif. Sci. 2009;63:97–103. [Google Scholar]
- Potowski M., Schürmann M., Preut H., Antonchick A.P., Waldmann H. Programmable enantioselective one-pot synthesis of molecules with eight stereo-centers. Nat. Chem. Biol. 2012;8:428–430. doi: 10.1038/nchembio.901. [DOI] [PubMed] [Google Scholar]
- Poulsen S.A., Davis R.A., Keys T.G. Screening a natural product-based combinatorial library using FTICR mass spectrometry. Bioorg. Med. Chem. 2006;14:510–515. doi: 10.1016/j.bmc.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Pucheault M. Natural products: chemical instruments to apprehend biological symphony. Org. Biomol. Chem. 2008;6:424–432. doi: 10.1039/b713022h. [DOI] [PubMed] [Google Scholar]
- Punina N.V., Makridakis N.M., Remnev M.A., Topunov A.F. Whole-genome sequencing targets drug-resistant bacterial infections. Human Genom. 2015;9:19. doi: 10.1186/s40246-015-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quality control methods for herbal materials: updated edition of quality control methods for medicinal plant materials, 1998. WHO, Geneva, Switzerland. ISBN 978 92 4 150073 9.
- Qureshi, R., Ghazanfar, S.A., Obied, H., Vasileva, V., Tariq, M.A., 2016. Ethnobotany: a living science for alleviating human suffering. Evid. Based Complem. Alter. Med. Article ID 9641692. [DOI] [PMC free article] [PubMed]
- Regueira T.B., Kildegaard K.R., Hansen B.G., Mortensen U.H., Hertweck C., Nielsen J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl. Environ. Microbiol. 2011;77:3035–3043. doi: 10.1128/AEM.03015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner C.A., Scott A.I. Achieving natural product synthesis and diversity via catalytic networking ex vivo. Chem. Biol. 1996;3:325–330. doi: 10.1016/s1074-5521(96)90114-3. [DOI] [PubMed] [Google Scholar]
- Rohm B., Scherlach K., Hertweck C. Biosynthesis of the mitochondrial adenine nucleotide translocase (ATPase) inhibitor bongkrekic acid in Burkholderia gladioli. Org. Biomol. Chem. 2010;8:1520–1522. doi: 10.1039/b925483h. [DOI] [PubMed] [Google Scholar]
- Romeo, J.T., Ibrahim, R., Varin, L., DeLuca, V., (Ed.), 2001. Recent Advances in Phytochemistry. Vol 34: Evolution of Metabolic Pathways. Elsevier Science Publishers, The Netherlands. ISBN# 0-08-043860-1.
- Rouhi, A.M., 2003. Rediscovering Natural Products. Chem Eng News, 2003; p 77; Betting on Natural Products for Cures, ibid, idem, p 93. Moving Beyond Natural Products, ibid, idem, p 104.
- Safe S., Kasiappan R. Natural products as mechanism-based anticancer agents: sp transcription factors as targets. Phytother. Res. 2016 doi: 10.1002/ptr.5669. [DOI] [PubMed] [Google Scholar]
- Sariaslani F.S., Rosazza J.P.N. Biocatalysis in natural products chemistry. Enzyme Micro Tech. 1984;6:242–253. [Google Scholar]
- Sarkar A., Funk A.N., Scherlach K., Horn F., Schroeckh V., Chankhamjon P., Westermann M., Roth M., Brakhage A.A., Hertweck C., Horn U. Differential expression of silent polyketide biosynthesis gene clusters in chemostat cultures of Aspergillus nidulans. J. Biotech. 2012;160:64–71. doi: 10.1016/j.jbiotec.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Scharf D.H., Chankhamjon P., Scherlach K., Heinekamp T., Willing K., Brakhage A.A., Hertweck C. Epidithiodiketopiperazine biosynthesis: a four-enzyme cascade converts glutathione conjugates into transannular disulfide bridges. Angew. Chem. Int. Ed. 2013;52:11092–11095. doi: 10.1002/anie.201305059. [DOI] [PubMed] [Google Scholar]
- Scharf D.H., Remme N., Heinekamp T., Hortschansky P., Brakhage A.A., Hertweck C. Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus. J. Am. Chem. Soc. 2010;132:10136–10141. doi: 10.1021/ja103262m. [DOI] [PubMed] [Google Scholar]
- Scherlach K., Hertweck C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009;7:1753–1760. doi: 10.1039/b821578b. [DOI] [PubMed] [Google Scholar]
- Scherlach K., Partida-Martinez L.P., Dahse H.M., Hertweck C. Antimitotic rhizoxin derivatives from a cultured bacterial endosymbiont of the rice pathogenic fungus Rhizopus microsporus. J. Am. Chem. Soc. 2006;128:11529–11536. doi: 10.1021/ja062953o. [DOI] [PubMed] [Google Scholar]
- Scherlach K., Sarkar A., Schroeckh V., Dahse H.-M., Roth M., Brakhage A.A., Horn U., Hertweck C. Two induced fungal polyketide pathways converge into antiproliferative spiroanthrones. ChemBioChem. 2011;12:1836–1839. doi: 10.1002/cbic.201100132. [DOI] [PubMed] [Google Scholar]
- Scheuler P.J. Marine natural products research: a look into the dive bag. J. Nat. Prod. 1995;58:335–343. [Google Scholar]
- Schmidt E.W., Donia M.S. Cyanobactin ribosomal peptides - a case of deep metagenome mining. Methods Enzymol. 2009;458:575–596. doi: 10.1016/S0076-6879(09)04823-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E.W., Sudek S., Haygood M.G. Genetic evidence supports secondary metabolic diversity in Prochloron sp, the cyanobacterial symbiont of a tropical ascidian. J. Nat. Prod. 2004;67:1341–1345. doi: 10.1021/np049948n. [DOI] [PubMed] [Google Scholar]
- Schmidt, E.W., Nelson, J.T., Rasko, D.A., Sudek, S., Eisen, J.A., Haygood, M.G., Ravel, J., 2005. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc. Nat. Acad. Sci. USA 102, 7315–7320. [DOI] [PMC free article] [PubMed]
- Schmidt E.W. From chemical structure to environmental biosynthetic pathways: navigating marine invertebrate-bacteria associations. Trends Biotechnol. 2005;23:437–440. doi: 10.1016/j.tibtech.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Schmidt E.W. Trading molecules and tracking targets in symbiotic interactions. Nat. Chem. Biol. 2008;4:466–473. doi: 10.1038/nchembio.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeckh V., Scherlach K., Nützmann H.W., Shelest E., Schmidt-Heck W. Intimate bacterial–fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14558–14563. doi: 10.1073/pnas.0901870106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann J., Hertweck C. Advances in cloning, functional analysis and heterologous expression of fungal polyketide synthase genes. J. Biotech. 2006;124:690–703. doi: 10.1016/j.jbiotec.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Schümann J., Hertweck C. Molecular basis of cytochalasan biosynthesis in fungi: gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J. Am. Chem. Soc. 2007;129:9564–9565. doi: 10.1021/ja072884t. [DOI] [PubMed] [Google Scholar]
- Shaikh F., Siu S.W.I. Identification of novel natural compound inhibitors for human complement component 5a receptor by homology modeling and virtual screening. Med. Chem. Res. 2016;25:1564–1573. doi: 10.1007/s00044-016-1591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl A., Kamke J., Hochmuth T., Piel J., Richter M., Liang C., Dandekar T., Hentschel U. Single-cell genomics reveals the lifestyle of Poribacteria, a candidate phylum symbiotically associated with marine sponges. ISME J. 2011;5:61–70. doi: 10.1038/ismej.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J.W., Schmidt E.W. Thioesterase-like role for reductive domains in tetramic acid biosynthesis. J. Am. Chem. Soc. 2008;130:11149–11155. doi: 10.1021/ja803078z. [DOI] [PubMed] [Google Scholar]
- Singh S., Peltier-Pain P., Tonelli M., Thorson J.S. A general NMR-based strategy for in situ characterization of sugar-nucleotide-dependent biosynthetic pathways. Org. Lett. 2014;16:3220–3223. doi: 10.1021/ol501241a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S.B., Culberson, J.C., 2009. Chemical space and the difference between natural products and synthesis, in: Natural Products Chemistry for Drug Discovery, Royal Society of Chemistry Publications, UK. ISBN 978-1-84755-989-0. 10.1039/9781847559890-00028.
- Singh T.D., Meitei H.T., Sharma A.L., Robinson A., Singh L.S.K., Singh T.R. Anticancer properties and enhancement of therapeutic potential of cisplatin by leaf extract of Zanthoxylum armatum DC. Biol. Res. 2015;48:46–54. doi: 10.1186/s40659-015-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteller P. Chemical ecology of fungi. Nat. Prod. Rep. 2015;32:971–993. doi: 10.1039/c4np00166d. [DOI] [PubMed] [Google Scholar]
- Spiteller P. Chemical defence strategies of higher fungi. Chem. - A Euro. J. 2008;14:9100–9110. doi: 10.1002/chem.200800292. [DOI] [PubMed] [Google Scholar]
- Sprogoe K., Staerk D., Ziegler H.L., Jensen T.H., Holm-Moeller S.B., Jaroszewski J.W. Combining HPLC-PDA-MS-SPE-NMR with circular dichroism for complete natural product characterization in crude extracts: Levorotatory gossypol in Thespesia danis. J. Nat. Prod. 2008;71:516–519. doi: 10.1021/np800010r. [DOI] [PubMed] [Google Scholar]
- Stege P.W., Sombra L.L., Davicino R.C., Olsina R.A. Analysis of nordihydroguaiaretic acid in Larrea divaricata Cav. extracts by micellar electrokinetic chromatography. Phytochem. Anal. 2011;22:74–79. doi: 10.1002/pca.1255. [DOI] [PubMed] [Google Scholar]
- Sudek S., Haygood M.G., Youssef D.T.A., Schmidt E.W. Structure of trichamide, a cyclic peptide from the bloom-forming bacterium Trichodesmium erythraeum, predicted from the genome sequence. Appl. Environ. Microbiol. 2006;72:4382–4387. doi: 10.1128/AEM.00380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner L.W., Mendes P., Dixon R.A. Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochem. 2003;62:817–836. doi: 10.1016/s0031-9422(02)00708-2. [DOI] [PubMed] [Google Scholar]
- Suo Z., Walsh C.T., Miller D.A. Tandem heterocyclization activity of the multidomain 230 kDa HMWP2 subunit of Yersinia pestis yersiniabactin synthetase: interaction of the 1–1382 and 1383–2035 fragments. Biochem. 1999;38:14023–14035. doi: 10.1021/bi991574n. [DOI] [PubMed] [Google Scholar]
- Takeo U. Actin- and microtubule-targeting bioprobes: their binding sites and inhibitory mechanisms. Biosci. Biotech. Biochem. 2007;71:300–308. doi: 10.1271/bbb.60516. [DOI] [PubMed] [Google Scholar]
- Tan A.C., Konczak I., Li G., Roufogalis B.D., Sekhon B., Sze-Daniel M.-Y. An ethnopharmacological approach to the preliminary screening of native Australian herbal medicines for anticancer activity. J. Comp. Integ. Med. 2015;12:245–249. doi: 10.1515/jcim-2014-0017. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Yamaguchi S., Yoshizawa A., Takagi M., Shin-ya K., Takahashi T. Combinatorial synthesis of deoxyhexasaccharides related to the landomycin A sugar moiety, based on an orthogonal deprotection strategy. Chem. - An Asian J. 2010;5:1407–1424. doi: 10.1002/asia.200900640. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Yoshizawa A., Chijiwa S., Ueda J., Takagi M., Shin-ya K., Takahashi T. Efficient synthesis of the deoxysugar part of versipelostatin by direct and stereoselective glycosylation and revision of the structure of the trisaccharide unit. Chem. - An Asian J. 2009;4:1114–1125. doi: 10.1002/asia.200800448. [DOI] [PubMed] [Google Scholar]
- Taylor M.W., Hill R.T., Piel J., Thacker R.W., Hentschel U. Soaking it up: the complex life of sponges and their microbial associates. ISME J. 2007;1:187–190. doi: 10.1038/ismej.2007.32. [DOI] [PubMed] [Google Scholar]
- Taylor R.E. Tedanolide and the evolution of polyketide inhibitors of eukaryotic protein synthesis. Nat. Prod. Rep. 2008;25:854. doi: 10.1039/b805700c. [DOI] [PubMed] [Google Scholar]
- Tcheknavorian-Asenbauer A. Industrial utilization of medicinal and aromatic plant resources in developing countries. Acta Hortic. 1993;333:19–46. [Google Scholar]
- Thibodeaux, C.J., Liu, H.W., Thorson, J.S., 2007. Complementary routes to natural product glycodiversification: Pathway engineering and glycorandomization. In: Kamerling, J., Boons, G.-J., Lee, Y., Suzuki, A., Taniguchi, N., Voragen, A.G.J. (Ed.). Comprehensive Glycoscience. Elsevier, vol. 3. pp. 373–396.
- Tianero D., Donia M.S., Young T.S., Schultz P.G., Schmidt E.W. Ribosomal route to small molecule diversity. J. Am. Chem. Soc. 2012;134:418–425. doi: 10.1021/ja208278k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett D., Dittmann E., Erhard M., von Döhren H., Börner T., Neilan B.A. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem. Biol. 2000;7:753–764. doi: 10.1016/s1074-5521(00)00021-1. [DOI] [PubMed] [Google Scholar]
- Trabocchi, A., Dow, M., Marchetti, F., Nelson, A., 2013. Diversity-oriented synthesis of natural product-like libraries in diversity-oriented synthesis: basics and applications in organic synthesis. Drug Discov. Chem. Biol. ISBN: 978-1-118-14565-4. 10.1002/9781118618110.ch9.
- Trindade M., van Zyl L.J., Navarro-Fernández J., Abd Elrazak A. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates. Front. Microbiol. 2015;6:890. doi: 10.3389/fmicb.2015.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse W.C., Boger D.L. Sequence-selective DNA Recognition: natural products and nature’s lessons. Chem. Biol. 2004;11:1607–1617. doi: 10.1016/j.chembiol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Tsuboy M.S., Marcarini J.C., Luiz R.C., Barros I.B., Ferreira D.T., Ribeiro L.R., Mantovani M.S. In vitro evaluation of the genotoxic activity and apoptosis induction of the extracts of roots and leaves from the medicinal plant Coccoloba mollis (Polygonaceae) J. Med. Food. 2010;13:503–508. doi: 10.1089/jmf.2009.0119. [DOI] [PubMed] [Google Scholar]
- Tu A.T., Gaffield W., editors. Natural, and Synthetic Toxins: Biological Implications. American Chemical Society; Washington DC: 2000. [Google Scholar]
- Tulp M.T.M., Solvay D.B.V. The use of receptor binding, a very specific, high capacity screening method, in the identification of biologically active components from natural sources. Proc. Phytochem. Soc. Euro. 1999;43:53–65. [Google Scholar]
- Tyler V.E. Phytomedicines: back to the future. J. Nat. Prod. 1999;62:1589–1592. doi: 10.1021/np9904049. [DOI] [PubMed] [Google Scholar]
- UNIDO (United Nations Industrial Development Organization). 1991. Design Options for a Polyvalent Pilot Plant Unit for the Distillation and Extraction of Medicinal and Aromatic Plants. IPCT. 1991, 143 UNIDO/IPCI.143 (SPEC.), V-91-29116.
- Uria A., Piel J. Cultivation-independent approaches to investigate the chemistry of marine symbiotic bacteria. Phytochem. Rev. 2009;8:401–414. [Google Scholar]
- Verdine G.L. The combinatorial chemistry of nature. Nature. 1996;384(6604 Supplement):11–13. doi: 10.1038/384011a0. [DOI] [PubMed] [Google Scholar]
- Vödisch M., Scherlach K., Winkler R., Hertweck C., Braun H.-P., Roth M., Haas H., Werner E.R., Brakhage A.A., Kniemeyer O. Analysis of the Aspergillus fumigatus proteome reveals metabolic changes and the activation of the pseurotin A biosynthesis gene cluster in response to hypoxia. J. Proteome Res. 2011;10:2508–2524. doi: 10.1021/pr1012812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Nussbaum F., Brands M., Hinzen B., Weigand S., Häbich B.D. Antibacterial natural products in medicinal chemistry-exodus or revival? Angew. Chem. Inter. Ed. 2006;45:5072–5129. doi: 10.1002/anie.200600350. [DOI] [PubMed] [Google Scholar]
- Vuorelaa P., Leinonen B.M., Saikkuc P., Tammelaa P., Rauhad J.P., Wennberge T., Vuorela H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004;11:1375–1389. doi: 10.2174/0929867043365116. [DOI] [PubMed] [Google Scholar]
- Wagner, H., 2004. Natural Products Chemistry and, Phytomedicine in the New Millennium: New Developments and Challenges. ARKIVOC (Gainesville, FL, USA).vol. 7. pp. 277–844.
- Walter J., Britton R.A., Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. U.S.A. 2011;108(Suppl 1):4645–4652. doi: 10.1073/pnas.1000099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiz A.R., Ishida K., Makower K., Ziemert N., Hertweck C., Dittmann E. Leader peptide and a membrane protein scaffold guide the biosynthesis of the tricyclic peptide microviridin. Chem. Biol. 2011;18:1413–1421. doi: 10.1016/j.chembiol.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Weiz A.R., Ishida K., Quitterer F., Meyer S., Kehr J.C., Müller K.M., Groll M., Hertweck C., Dittmann E. Harnessing the evolvability of tricyclic microviridins to dissect protease-inhibitor interactions. Angew. Chem. Int. Ed. 2014;53:3735–3738. doi: 10.1002/anie.201309721. [DOI] [PubMed] [Google Scholar]
- Wijesekera R.O.B. Is there an industrial future for phytopharmaceutical drugs? An outline of UNIDO programs in the sector. J. Ethnopharmacol. 1991;32:217–224. doi: 10.1016/0378-8741(91)90121-s. [DOI] [PubMed] [Google Scholar]
- Wilson M.C., Mori T., Rückert C., Uria A.R., Helf M.J., Takada K. An environmental bacterial taxon with a large and distinct metabolic repertoire, Sponge bacteria as chemical factory. Nature. 2013;506:58–62. doi: 10.1038/nature12959. [DOI] [PubMed] [Google Scholar]
- Wilson M.C., Piel J. Metagenomic approaches for exploiting uncultivated bacteria as resource for novel biosynthetic enzymology. Chem. Biol. 2013;20:636–647. doi: 10.1016/j.chembiol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Winter J.M., Behnken S., Hertweck C. Genomics-inspired discovery of natural products. Curr. Opin. Chem. Biol. 2011;15:22–31. doi: 10.1016/j.cbpa.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Wolfender J.L., Queiroz E.F., Hostettmann K. The importance of hyphenated techniques in the discovery of new lead compounds from nature. Expert Opin. Drug Discov. 2006;1:237–260. doi: 10.1517/17460441.1.3.237. [DOI] [PubMed] [Google Scholar]
- Xu Z., Baunach M., Ding L., Hertweck C. Bacterial synthesis of diverse indole terpene alkaloids by an unparalleled cyclization sequence. Angew. Chem. Int. Ed. 2012;51:10293–10297. doi: 10.1002/anie.201204087. [DOI] [PubMed] [Google Scholar]
- Yao, X., 2004. Current Trends and, Future Prospects of Traditional Chinese Medicines in the 21st Century, Abstract of Papers, In: 227th ACS National Meeting, Anaheim, CA, USA, March 28-April 1, 2004; AGFD-1288.
- Yin W.B., Chooi Y.H., Smith A.R., Cacho R.A., Hu Y., White T.C., Tang Y. Discovery of cryptic polyketide metabolites from dermatophytes using heterologous expression in Aspergillus nidulans. ACS Synth. Biol. 2013;2:629–634. doi: 10.1021/sb400048b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuliana N.D., Khatib A., Choi Y.H., Verpoorte R. Metabolomics for bioactivity assessment of natural products. Phytother. Res. 2011;25:157–169. doi: 10.1002/ptr.3258. [DOI] [PubMed] [Google Scholar]
- Zhu J., Huang X., Gao H., Bao Q., Zhao Y., Hu J.-F., Xia G. A novel glucagon-like peptide 1 peptide identified from Ophisaurus harti. J. Peptide Sci. 2013;19:598–605. doi: 10.1002/psc.2538. [DOI] [PubMed] [Google Scholar]
- Zimmermann K., Engeser M., Blunt J., Munro M.H.G., Piel J. Pederin-type pathways of uncultivated bacterial symbionts: analysis of O-methyltransferases and generation of a biosynthetic hybrid. J. Am. Chem. Soc. 2009;131:2780–2781. doi: 10.1021/ja808889k. [DOI] [PubMed] [Google Scholar]