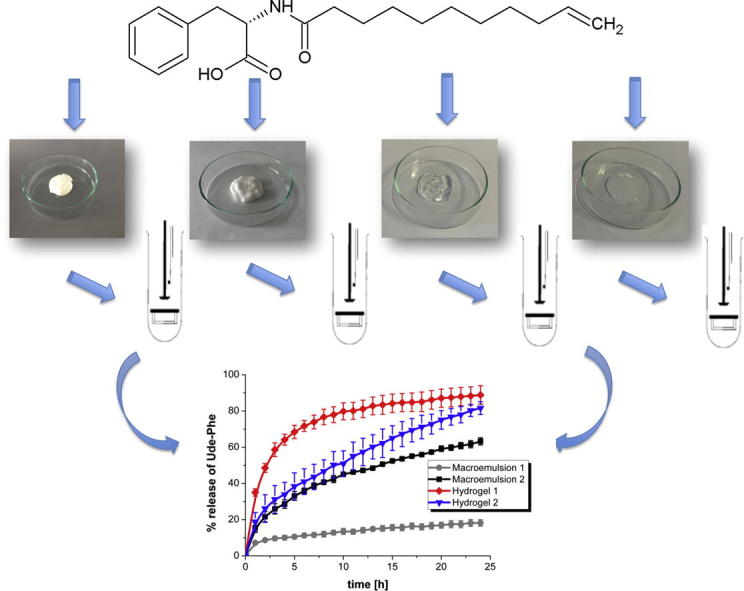
Graphical abstract
Keywords: Semi-solid formulations, Release studies, Synthetic membranes, Stability, Amino acid derivative
Abstract
The aim of this study was to characterize the stability of new vehicles for the undecylenoyl phenylalanine that is used as skin-lightening agent in the melasma treatment. The purpose of this research was also to analyse the release kinetics of phenylalanine derivative from topical preparations through different synthetic membranes. Topical formulations such as two different macroemulsions, hydrogels (based on carbomer and hydroxyethylcellulose) and microemulsions were characterized in terms of stability by laser diffraction method. Additionally, multiple light scattering assessed the stability of macroemulsions. The release rates of active substance through different membranes (such as Cuprophan, nitrocellulose, cellulose acetate and Strat-M) were determined using enhancer cell. In order to explain the mechanism of release process the results were fitted with different kinetic models. New stable vehicles for Ude-Phe were successfully obtained. The results proved that the membrane structure had the influence on the release rate of undecylenoyl phenylalanine. The slowest release rate of Ude-Phe was observed when Strat-M membrane was applied. The highest amount of active substance was released from the hydrogel based on carbomer. The release of undecylenoyl phenylalanine from both macroemulsions and hydrogel based on hydroxyethylcellulose followed the Higuchi model. Whereas the release results of Ude-Phe from both microemulsion-based hydrogels and carbomer hydrogel can be described by using Korsmeyer-Peppas model. Hydrogels and microemulsion-based hydrogels could be recommended as proper vehicles for the derivative of phenylalanine.
1. Introduction
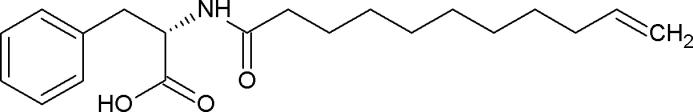
The active substance is one of the most crucial factors that influence the effectiveness of pharmaceutical products because it is responsible for their therapeutic effect. In recent years cosmetics and pharmaceutical industries have focused much attention on the application of amino acids and their derivatives as active substances (Burnett et al., 2017). Amino acids are components of the natural moisturizing factor as well as exhibit regenerative and anti-inflammatory activity (Lintner, 2007, Robinson et al., 2010). Additionally, they exhibit anti-free radical activity by the inhibiting the breakdown of collagen and elastin, which prevents skin aging. Amino acid derivatives are present in the preparations devoted for skin and hair applications (Burnett et al., 2017). In recent years N-undec-10-enoyl-l-phenylalanine (Ude-Phe, Fig. 1) has been used in dermatology to treat melasma. This derivative of phenylalanine inhibits the activity of alpha-melanocyte-stimulating hormones (MSH) that function is to stimulate skin cells to produce melanin, which is the pigment responsible for skin color. The stimulation of MSH induces the production of melanin, whereas inhibition of melanotropin can be an effective method for skin depigmentation. In dermatology, phenylalanine derivative is used in the treatment of melasma, skin problem associated with the appearance of brown spots on the face, commonly occurring in women (Katoulis et al., 2014). In order to minimize the skin hyperpigmentation the formulations containing a phenylalanine derivative in combination with niacinamide were tested in in vivo studies. The results showed that the combination of the emulsion containing 5% niacinamide and 1% phenylalanine derivative were much more effective in reducing the hyperpigmentation than vehicle control and preparations without Ude-Phe (Bisset et al., 2009). The formulations containing Ude-Phe were also used to minimize the solar lentigo. The study proved that the Ude-Phe could be used as a novel depigmenting agent because a significant lightening of the lesions was observed (Katoulis et al., 2009). In other study formulations containing Ude-Phe turned out to be more effective in reducing lesions than the pristine vehicle (Katoulis et al., 2014). So far the formulations containing Ude-Phe were tested only in in vivo studies, however to our best knowledge there has been no research concerning the stability of these preparations and in vitro release test of active compound from these topical semi-solids. Moreover, in all publications mentioned above the efficiency of Ude-Phe were tested only when emulsion was used as a vehicle for the active compound. We suggest that the application of other formulations can influence the release rate of Ude-Phe. Hydrogels could be a proper candidate for delivery of active compound (Vashist and Ahmad, 2013). On the other hand it is believed that microemulsion-based hydrogels enhance topical delivery of poorly soluble drugs (Shalviri et al., 2011).
Fig. 1.
Chemical structure of the N-undec-10-enoyl-L-phenylalanine.
Therefore, the aim of this study was to develop and characterize the stability of new vehicles (macroemulsions, hydrogels and microemulsion-based hydrogels) for the undecylenoyl phenylalanine and to compare the release rate of phenylalanine derivative from topical preparations through different synthetic membranes.
2. Materials and methods
The chemical composition of the macroemulsions and hydrogels is presented in Table 1.
Table 1.
The chemical composition of semisolid formulations.
| Sample name | Composition | Company | Quantity (%, ±0.01) |
|---|---|---|---|
| Macroemulsion 1 | 1. Tego Care 450 (polyglyceryl-3 methylglucose distearate) | Evonik | 4.5 |
| 2. Tego Alkanol 1618 (cetearyl alcohol) | Evonik | 3.5 | |
| 3. Tegin 4100 Pellets (glyceryl stearate) | Evonik | 3.5 | |
| 4. Cetiol 868 (ethylhexyl stearate) | Cognis | 10.5 | |
| 5. Glycerin | Chempur | 5.0 | |
| 6. Distilled water | 72.5 | ||
| 7. Ude-Phe | Seppic | 0.5 | |
| Macroemulsion 2 | 1. Creagel EZ IN (sodium polyacrylate/sodium acryloyldimethyl taurate copolymer, isononyl isononanoate) | Créations Couleurs | 10.0 |
| 2. Alphaflow 20 (hydrogenated polydecene) | Créations Couleurs | 16.5 | |
| 3. Distilled water | 73.0 | ||
| 4. Ude-Phe | Seppic | 0.5 | |
| Hydrogel 1 | 1. Tego Carbomer 134 FD (carbomer) | Evonik | 0.5 |
| 2. Sodium hydroxide (10% solution) | Chempur | 1.0 | |
| 3. Isopropanol | Sigma Aldrich | 25.0 | |
| 4. Distilled water | – | 73.0 | |
| 5. Ude-Phe | Seppic | 0.5 | |
| Hydrogel 2 | 1. Hydroxyethylcellulose | Sigma Aldrich | 2.5 |
| 2. Glycerin | Sigma Aldrich | 10.0 | |
| 3. Distilled water | – | 87.0 | |
| 4. Ude-Phe | Seppic | 0.5 | |
| Microemulsion 1A | 1. Isopropyl myristate | Sigma Aldrich | 40.0 |
| 2. Tween 80 | Sigma Aldrich | 37.5 | |
| 3. Isopropyl alcohol | Sigma Aldrich | 12.5 | |
| 4. Distilled water | – | 9.0 | |
| 5. Ude-Phe | Seppic | 0.5 | |
| Microemulsion 2A | 1. Oleic acid | Sigma Aldrich | 10.0 |
| 2. Tween 80 | Sigma Aldrich | 37.5 | |
| 3. Isopropyl alcohol | Sigma Aldrich | 12.5 | |
| 4. Distilled water | – | 39.5 | |
| 5. Ude-Phe | Seppic | 0.5 | |
2.1. Preparation of topical formulations
2.1.1. Preparation of macroemulsion 1
The ingredients of the oil phase (1–4) were heated to 70 °C. At the same time water with the active substance was heated to 70 °C. When all of the oil phase ingredients were melted, water phase was added. The obtained mixture was stirred and cooled to 50 °C. Afterwards the emulsion was homogenized (Yellow Line DI 25 basic, IKA-Werk, Germany) and cooled to 30 °C. Afterwards glycerin was added and the formulation was homogenized.
2.1.2. Preparation of macroemulsion 2
Creagel EZIN and Alphaflow 20 were mixed in a beaker until the phase becomes homogenous. Water and Ude-Phe were heated to 70 °C. Afterwards, the water phase was added slowly to the oil phase and mixed, until the proper viscosity was fully developed.
2.1.3. Preparation of hydrogel 1
Tego Carbomer 134 was dispersed for 2 h in water by a magnetic stirrer. Then isopropanol with Ude-Phe was introduced and next NaOH was added until a pH of 6.5 was reached.
2.1.4. Preparation of hydrogel 2
Water was mixed with glycerin and with Ude-Phe. The mixture was heated to 80 °C. Afterwards hydroxyethylcellulose was added and stirred until the hydrogel formulation was obtained.
Additionally, the base of each formulation without the active compound was also prepared.
2.1.5. Preparation of Ude-Phe loaded microemulsions
The microemulsion composition was selected based on phase diagrams previously developed by Malakar et al. (2011). The composition of the microemulsions is shown in Table 1. Ude-Phe was dissolved in isopropyl alcohol. Then Tween 80 was added to the tube and mixed on a magnetic stirrer for 15 min at a speed of 2500 rpm. Afterwards isopropyl myristate (1A) / oleic acid (2A) was added and stirred for 15 min. Finally, water was introduced to the tube and mixed until the desired consistency was reached.
In order to increase the viscosity of the formulations, that was necessary for the release tests of Ude-Phe, Tego Carbomer 134 in an amount of 0.75% was added to the obtained microemulsions and the semisolid formulations were left for 24 h to reach the consistency of a hydrogel. Additionally, the microemulsions without the active compound were also prepared.
2.2. Characterization of the obtained topical formulations
2.2.1. Determination of pH
The pH values were determined by a pH-meter (pH 10 Pen, VWR International, USA). The measurements were performed at RT three times.
2.2.2. Centrifugation test
Centrifugation tests were performed by MPW-360R Centrifuge (MPW MED Instruments, Poland) for macro- and microemulsions directly after preparation. Physical stabilities of formulations were evaluated by centrifugation at 4000 rpm for 10 min and then the phase separation was examined.
2.2.3. Stability test by multiple light scattering
The stability measurements were conducted directly after preparation of the macroemulsions and at different times for 60 days by Turbiscan Lab Expert (Formulaction, France). Multiple light scattering was used to measure the stability of the macroemulsions at 4, 25 and 37 °C. Between the measurements the samples were stored at 4 °C, 25 °C. Additionally, the macroemulsions were also stored at 37 °C and 80% relative humidity in climate chamber (Binder GmbH, Germany). All of the emulsions were compared using Turbiscan Stability Index (TSI) that gives information regarding the general behavior of emulsions obtained. TSI is calculated as the sum of all of the destabilization processes occurring in the sample (Zhao et al., 2014, Carbone et al., 2015).
2.2.4. Particle size distribution analysis by laser diffraction
The particle size distributions of the formulations were determined by Mastersizer 2000 (Malvern, UK) equipped with a hydro dispersion unit. The measurements were carried out five times at RT in distilled water. The mean droplet diameter was presented as d3,2 known as the Sauter diameter that gives information about the average of particle size of formulation (Pérez-Mosqueda et al., 2015, Olejnik et al., 2015a).
2.2.5. The zeta potential of microemulsions
The zeta potential of microemulsions was measured in triplicate using a Zetasizer Nano ZS (Malvern, UK) at 25 °C. 100 µl of microemulsion was diluted in 10 ml of distilled water and then the zeta potential was determined.
2.3. Release studies of Ude-Phe
The release studies of Ude-Phe were performed by the USP Apparatus 2 (Agilent Technologies DS 708, USA) connected with the UV–Vis spectrophotometer (Cary 50 Bio, Varian, USA). Each formulation was placed into the enhancer cell. The analysis was performed in the medium mixture of potassium phosphate buffer at pH 5.8 (J.T.Baker, USA) and ethanol in the ratio of 65:35. The medium was maintained at 32.0 °C ± 0.5 °C and stirred at 100 rpm. In these studies different synthetic membranes such regenerated cellulose – Cuprophan (Agilent Technologies, USA), nitrocellulose (GE Whatman, USA), cellulose acetate (GVS Filter Technology, UK) and Strat-M (Merck Millipore, Germany) were used. The concentration of the released Ude-Phe was spectrophotometrically monitored at 258 nm.
2.4. Kinetics calculations
The release results were fitted with different kinetics models such as zero order (% Ude-Phe release vs. time), first order (log of% Ude-Phe remaining vs. time), Higuchi’s model (% Ude-Phe release vs. square root of time) and Korsmeyer-Peppas model (log of% Ude-Phe release vs. log time). For each model R2 values were calculated. The n value determined on the basis of Korsmeyer-Peppas model enables to characterise the release mechanism of Ude-Phe as described – n < 0.5 – quasi-Fickian diffusion, n = 0.5 – diffusion mechanism, 0.5 < n < 1 – non Fickian diffusion, n = 1 (0.89) – case II transport (zero order release), n > 1 (0.89) – super case II transport (Sahoo et al., 2012, Dash et al., 2010, Goscianska et al., 2016).
2.5. Comparison of release profiles – Model independent method
The release profiles were compared using the dissimilarity factor (f1) and the similarity factor (f2) which were calculated using the following equations:
where n is a number of points, R is the dissolution value of reference at time and T is the dissolution value of the test (Moore and Flanner, 1996). These factors provide a measure of similarity between pairs of release profiles. According to the FDA guidance f1 values of 0–15 and f2 values of 50–100 ensure sameness or equivalence of the two dissolution profiles (FDA, 1997). When the f2 value is 100 the test and reference profiles are treated as identical. As the f2 value becomes smaller, the dissimilarity between release profiles increases (Pillay and Fassihi, 1998).
2.6. Statistical methods
Statistical analysis was performed using Statistica v. 12.0 PL software (StatSoft). The results were considered significant for p < 0.05. The comparison of the release results between formulations was performed using the Kruskal-Wallis test followed by the Multiple Comparison Test. The non-parametric test was used because the variables were not normally distributed as confirmed by Shapiro-Wilk’s test.
2.7. ESI mass spectrometry
The ESI-MS measurements of Ude-Phe were performed by the Waters/Micromass (Manchester, UK) ZQ equipped with a Harvard Apparatus syringe pump. ESI mass spectra were acquired in both positive and negative mode by scanning over the m/z range 100–500 with unit mass resolution.
3. Results and discussion
The study was conducted on six different formulations constituted by macroemulsions, hydrogels and microemulsions containing Ude-Phe. It was recommended by the producer of Ude-Phe, that the active compound should be introduced into the aqueous or oil phase at temperature higher than 60 °C. In both macroemulsions Ude-Phe was soluble after cooling the formulations. After preparing each semisolid pH values were measured. The pH of macroemulsion 1 and macroemulsion 2 was 5.00 ± 0.05 and 6.40 ± 0.05, respectively. The pH value of both hydrogels and microemulsions + carbomer was 6.50 ± 0.05. The pH values of all formulations resemble the skin’s pH, therefore they can be intended for topical applications.
In order to check the stability of formulations the centrifugation test was performed for macroemulsions and microemulsions. It was found that all macroemulsions and microemulsions were stable.
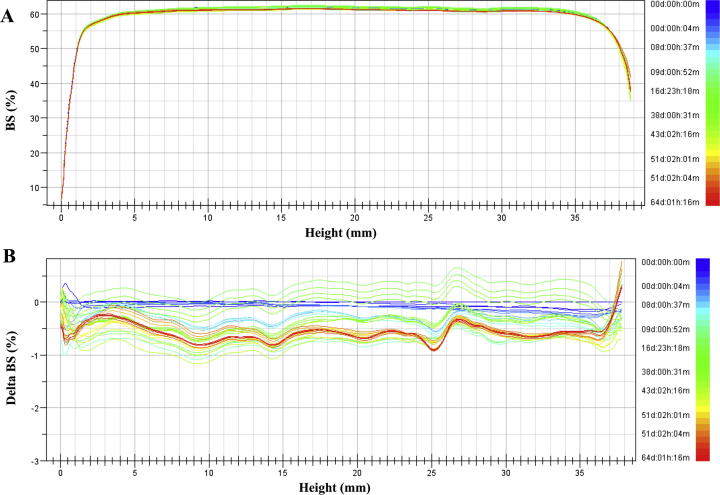
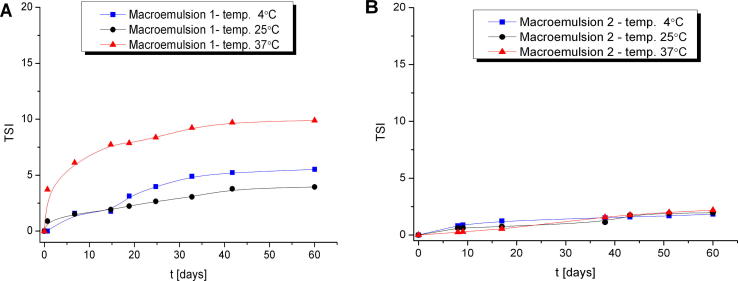
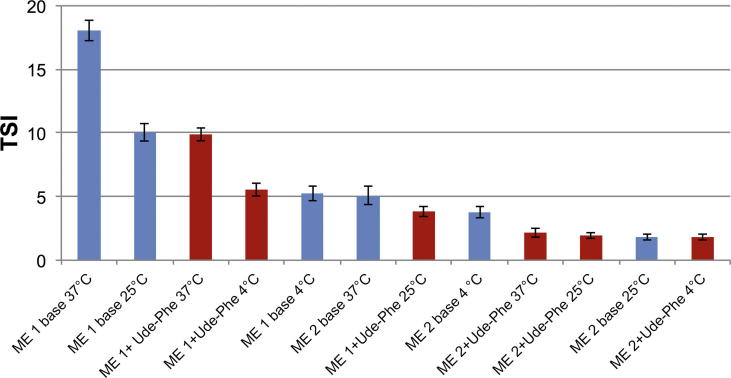
Additionally, stability studies of the macroemulsions obtained were performed by multiple light scattering measurements (MLS) which enable to detect the destabilization process that occur in formulation much faster than by human eye without sample dilution (Trujillo-Cayado et al., 2014, Santos et al., 2013). This technique enables to observe phenomena associated with migration (creaming, sedimentation) and the changes in the particle size such as coalescence and flocculation (Olejnik et al., 2015b). As a result of the experiment transmission and backscattering profiles versus sample height were obtained. For all macroemulsions only backscattering profiles were analyzed (Fig. 2A) due to the high concentration of formulations. In order to monitor changes in the sample, the results are analyzed in the reference mode in which each measurement is compared with the first collected data. Then the graph shows the relationship between the delta backscattering (ΔBS) and sample height. (Fig. 2B). In all of the macroemulsions stored at different temperature conditions no migration phenomena were detected. Only the changes in the backscattering intensity along the whole sample height were observed that could be related to the slight particle size variation such as flocculation. On the basis of all changes occurring in the samples TSI was determined which is an essential tool to compare the stability of different emulsions. The lower the values of the TSI, the more stable the analyzed sample (Olejnik et al., 2015b). The Fig. 3 shows the graph TSI over time for the macroemulsions 1 and 2 stored at different conditions. The results proved that macroemulsion 2 was much more stable than macroemulsion 1 irrespective of the storage conditions. For macroemulsion 1 the smallest changes in the TSI value were observed when the sample was stored at RT (Fig. 3A). Slightly higher TSI value was reached when macroemulsion 1 was stored at 4 °C. However, the highest variation in TSI value was observed for macroemulsion 1 stored at 37 °C. On the other hand, for macroemulsion 2 stored at different conditions (Fig. 3B) only slight changes in TSI value were observed. According to Celia et al. no changes in particles size occur when the backscattering profiles are within the interval of ±2% (Celia et al., 2009). Additionally, samples are treated as unstable when the variation of BS is higher than 10% (Mengual et al., 1999). Therefore, the macroemulsions 1 and 2 stored at different conditions can be considered as stable. Fig. 4 presents the results of TSI value of formulations with and without Ude-Phe stored at different conditions after 60 days. On the basis of the analysis it can be concluded that the macroemulsions 2 prepared at RT were more stable than macromulsions 1 obtained at high temperature. The preparation method and composition of the emulsion could have an impact on the better stability of the macroemulsions 2. The greater stability of this formulation can be explained by the presence of the auto-emulsifier (Creagel EZIN). In addition, the results of TSI value proved that the addition of Ude-Phe to both the macroemulsion 1 and macroemulsion 2 contributed to better stabilization of the preparations obtained. In particular, it was observed for macroemulsion 1 subjected to accelerate stability tests. After 60 days TSI for macroemulsion 1 with Ude-Phe was two times lower than in the case of the base of macroemulsion 1. A similar pattern was also observed for macroemulsion 2 stored at elevated temperature. It could be explained by the structure of Ude-Phe, which consists of amino acid and lipid residue.
Fig. 2.
The backscattering (A) and delta backscattering (B) profiles of macroemulsion 2 stored at 4 °C.
Fig. 3.
Turbiscan Stability Index over time of macroemulsion 1 and 2 stored at different conditions.
Fig. 4.
Turbiscan Stability Indexes of macroemulsions (ME) after 60-days of storage at different conditions (red – formulations containing Ude-Phe, blue – formulations without Ude-Phe). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
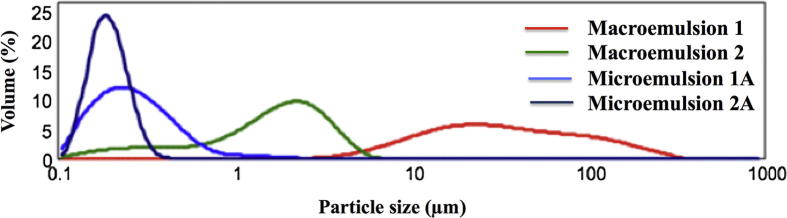
A significant impact on emulsions stability has the particle size distribution. All formulations were analyzed by laser diffraction method. Additionally, ultrasounds were applied to break up the potentially existing agglomerates and to obtain the dispersion of the primary particles. In the case of macroemulsion 1 after ultrasound treatment the volume fraction of particles of smaller size increased which means that the existing agglomerates were broken down. However, hardly any changes in particle size were detected in the case of macroemulsion 2. The formation of agglomerates was not observed. This indicates the greater stability of macroemulsion 2 compared to macroemulsion 1. These results are in accordance with the experiments performed by MLS. Additionally after ultrasound treatment no variations in particle size distribution was observed in the case of microemulsions. The introduction of Ude-Phe to microemulsions did not change the monomodal distribution pattern. The determination of particle size distribution of formulations provides important information not only about its stability, but it can be also used in the planning the release experiments. A comparative analysis of the particle size distribution of macro- and microemulsions containing Ude-Phe was presented in Fig. 5. Macroemulsion 1 had broad particle size distribution in the range of 5–500 μm, while in the case of macromulsion 2 the particle size distribution was in the range of 0.1–9.0 μm. The particles with the smallest size in the range of 0.1 and 0.5 µm were detected in microemulsion 2A. A monomodal data distribution between 0.1 and 1.0 μm was observed for microemulsion 1A. The particle sizes in the case of microemulsions partially overlapped and were in the range 0.1–5.0 μm, whereas for macroemulsions they were included in the range of 0.1–500 µm. The obtained results of both macro- and microemulsions are consistent with literature reports (Talegaonkar et al., 2008).
Fig. 5.
The particle size distribution for the marcoemulsion 1, macroemulsion 2, microemulsions 1A and microemulsion 2A.
Zeta potential is an important parameter indicating the stability of colloidal systems (Mikolajczyk et al., 2015). When zeta potential value is greater than ±25 mV the colloidal system is treated as stable (Lee et al., 2007). Zeta-potential value of microemulsion 1A was −28.4 ± 0.3 mV. It was observed the addition of Ude-Phe increased the zeta potential value. However, in the case of microemulsions 2A the zeta potential value was −22.5 ± 0.7 mV. The addition of Ude-Phe changed only slightly the zeta potential value in comparison with microemulsion 2 without the active compound.
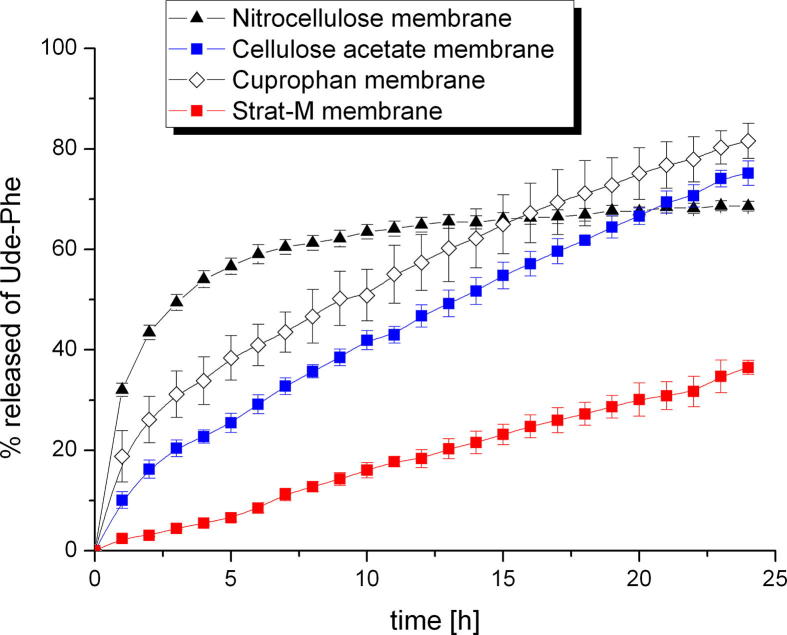
After determining the physicochemical parameters of the formulations the release studies of Ude-Phe were performed. It should be mentioned that the release experiments are important for development of novel formulations and are applied for screening of products prior to in vivo testing (Olejnik et al., 2012). Furthermore, the selection of the appropriate membrane is a crucial step in the development of methodology of release studies. There is no standard membrane, which is recommended for each active compound (Olejnik and Nowak, 2015, Shah and Elkins, 1995, Hg and Rouse, 2010). Therefore, the influence of four different membranes on the Ude-Phe release from the selected formulation such as hydrogel 2 was studied. Depending on the applied membrane the release profiles of the active compound were different (Fig. 6). The highest amount of Ude-Phe was released through Cuprophan membrane within 24 h about 80% was transferred to the receptor fluid. Slightly slower release rate with similar profile was observed when phenylalanine derivative was released through cellulose acetate membrane. Whereas, when Ude-Phe penetrated through the nitrocellulose membrane after 15 h 70% of active compound was released and this level remained constant until the experiment was finished. On the other hand the slowest release rate of Ude-Phe was observed when Strat-M membrane was applied. This membrane is used as non-animal based model in transdermal experiments to predict the diffusion of active compound in human skin. The obtained results proved that the structure of synthetic membranes could influence the release rate of Ude-Phe. Strat-M is a skin-mimic artificial membrane, which composed of double-layer structure (Uchida et al., 2015), therefore the release profile of Ude-Phe through this membrane was completely different than thorough other membranes. On the other hand according to our previous studies Cuprophan membrane consists of longitudinal cellulose fibres separated by irregular and inhomogenous pores (Olejnik and Nowak, 2015). It is believed that this kind of organization resembles the disordered structure of stratum corneum (Donnelly et al. , 2006). The pores of nitrocellulose and cellulose acetate membrane are distributed inhomogeneously across the surface (Olejnik and Nowak, 2015) that can also have an influence on the release rate of Ude-Phe. The experimental data were fitted with various kinetics models. Depending on the membrane type the results could be described by different models (Table 2). The data obtained from the release of Ude-Phe through Cuprophan membrane can be fitted with Higuchi equation (Higuchi, 1961, Higuchi, 1963). On the other hand when the phenylalanine derivative permeated though nitrocellulose and cellulose acetate membrane the release results could be described by using Korsmeyer-Peppas model. While for Strat-M membrane the highest correlation coefficient was observed for zero and first kinetics order.
Fig. 6.
The influence of membrane type on the release of Ude-Phe from hydrogel 2.
Table 2.
Kinetics release models used to describe the release of Ude-Phe from hydrogel 2 through different membranes.
| Membrane type | Zero order |
First order |
Higuchi model |
Korsmeyer–Peppas model |
|
|---|---|---|---|---|---|
| Regression coefficient | n | ||||
| Nitrocellulose | 0.683 | 0.776 | 0.824 | 0.990 | 0.20 |
| Cellulose acetate | 0.993 | 0.990 | 0.991 | 0.997 | 0.63 |
| Cuprophan | 0.979 | 0.995 | 0.999 | 0.997 | 0.47 |
| Strat-M | 0.996 | 0.996 | 0.972 | 0.984 | 0.94 |
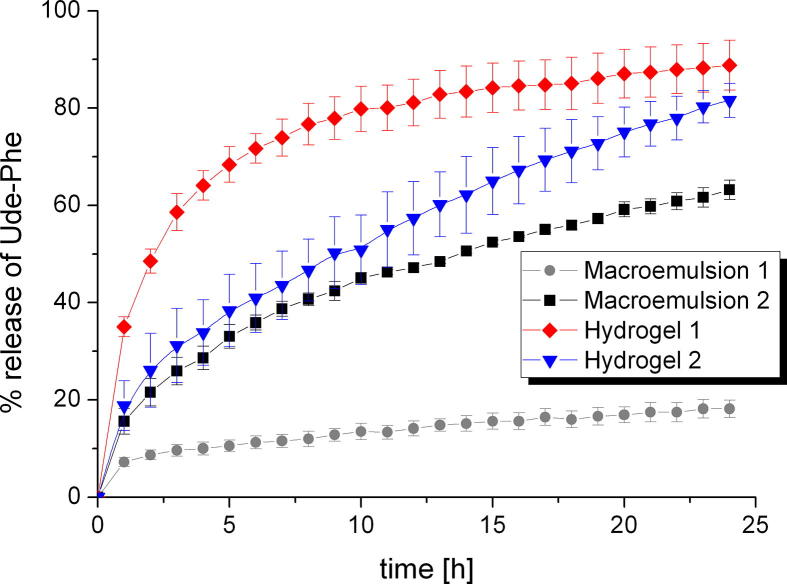
The release kinetics of the active compound can be also influenced by the type of formulation (de Oliveira Gomes et al., 2004). Moreover, the effectiveness of topical products is dependent on drug release from the formulation (Shah and Skelly, 1993). Therefore, the release of Ude-Phe was performed from different semi-solids through one selected membrane (Cuprophan membrane) (Fig. 7) to check which formulation will be the best vehicle for the phenylalanine derivative. A comparative analysis showed that the highest amount of active substance was released from the hydrogel 1. After 10 h about 80% of the Ude-Phe was permeated through the membrane. Then, the percentage of phenylalanine derivative slightly increased to reach a constant level ca. 90%. However, for hydrogel 2 a continuous increase of active compound released was observed which after 24 h reached about 80%. Additionally, it can be noted that much more Ude-Phe was released from macroemulsion 2 than 1. After 24 h 60% of total mass of active compound permeated to the receptor medium while in the case of macroemulsion 1 only 20% of Ude-Phe was released. The differences in release rates of phenylalanine derivative from various semi-solids through the membrane to the receptor fluid could be explained by the different physicochemical parameters of the formulations. The release profiles of Ude-Phe are influenced by particle size distribution and also by solubility of the active substance in the formulation. The largest amount of active substance in the shortest time was released from the hydrogel 1 based on carbomer, while the smallest amount of Ude-Phe was diffused from macroemulsion 1. We suggest that it could be attributed to better availability of Ude-Phe in hydrogel than in macroemulsion. However, further in vivo studies are required.
Fig. 7.
The influence of formulation type on the release profiles of Ude-Phe through Cuprophan membrane.
The results obtained for macroemulsion 1 could be explained by the fact that the diffusion of active compound through oily phase might be a limiting step for undecylenoyl phenylalanine release. It should be added that it is generally known that emulsions such as creams are mid-to-high viscosity (Kimball, 2016) and the viscosity of hydrogels is smaller than that of o/w emulsions (Eros et al., 2003). It could be suggested that due to the lower viscosity of hydrogels compared to emulsions, higher amount of active substance can be released and therefore the effectiveness of these formulations could be better. On this basis of these findings, it can be concluded that hydrogels can be better vehicle for the Ude-Phe than macroemulsions. The release profiles from hydrogels were different and higher amount of active compound was release from hydrogel 1. This could be also explained by different viscosity of these formulations. According to Kulkarni and Shaw aqueous gels (in our study hydrogel 2) have a higher viscosity than hydroalcoholic gels (in our study hydrogel 1) (Kulkarni and Shaw, 2015).
In order to perform the release tests of Ude-Phe from microemulsions, it was necessary to introduce a thickener (Carbomer) (Špiclin et al., 2003) to increase the viscosity. During the release experiments of Ude-Phe from the microemulsions-based hydrogel, it was observed that at the same wavelength as the active substance absorbs other component of the formulation. For this reason, spectroscopic analysis of each component of microemulsion was conducted. The studies proved that peak at the same wavelength, as the Ude-Phe is present only in Tween 80. Polysorbate 80 is a fully saturated molecule with one carbon-carbon double bond with a λmax at 195 nm (Gordon and Ford, 1972) and an alkyl ester group with a λmax between 195 and 210 nm and polyethylene glycol chain with a λmax in the range of 180–185 nm. Therefore, the presence of peaks in the range of 250–300 nm indicates the presence of impurities in the reagent. According to literature the spectrum of Tween 80 may be different depending on the supplier of the reagent (Wuelfing et al. 2006). In order to overcome this problem, the release test was performed on the microemulsions with and without the active compound. Taking into account both results the percentage release of Ude-Phe was calculated. Additionally, it should be mentioned that it was possible to perform the release studies from microemulsions only by using nitrocellulose membrane. When other membranes were applied the solution was not clear therefore the determination of release rate of Ude-Phe was impeded.
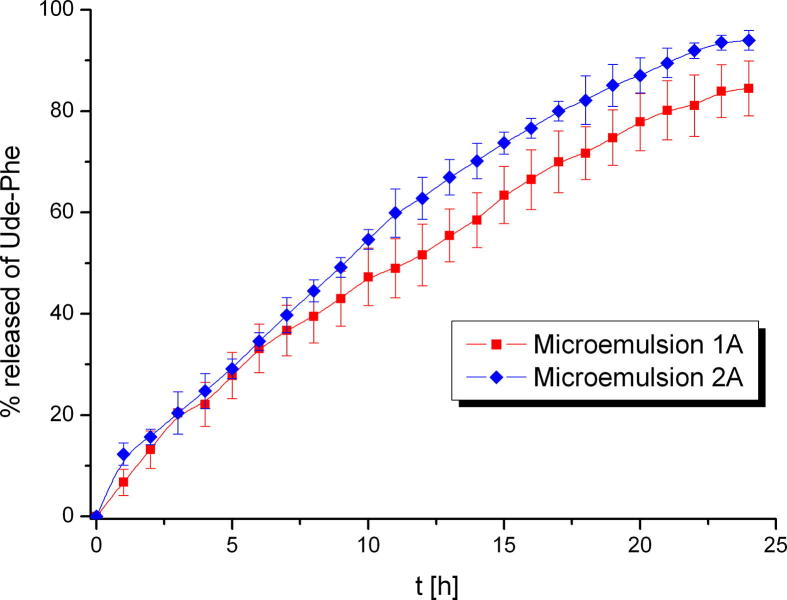
In Fig. 8 the results of release of Ude-Phe from microemulsions-based hydrogel is presented. Initially, both release profiles were similar, however, the significant change can be observed after 10 h. The% release from microemulsion 2A was higher and reached about 95% after 24 h. In the case of microemulsion 1A 85% of Ude-Phe was released. This can be explained by the different particle size distribution of both formulations. Additionally, in Table 3 the kinetics models used to describe the release of Ude-Phe from different formulations are shown. For both macroemulsions and hydrogel 2 the highest value of regression coefficient was observed for the Higuchi model, which means that the release of Ude-Phe followed this kinetics equation. On the other hand the release results of Ude-Phe from both microemulsions and hydrogel 1 can be described by using Korsmeyer-Peppas model.
Fig. 8.
The release profiles of Ude-Phe from microemulsions 1A and 2A based hydrogel through nitrocellulose membrane.
Table 3.
Kinetics models used to describe the release of Ude-Phe from various formulations.
| Formulation | Zero order |
First order |
Higuchi model |
Korsmeyer-Peppas model |
Type of transport | |
|---|---|---|---|---|---|---|
| Regression coefficient | n | |||||
| Macroemulsion 1 | 0.970 | 0.975 | 0.994 | 0.984 | 0.30 | Fickian difussion |
| Macroemulsion 2 | 0.945 | 0.985 | 0.996 | 0.995 | 0.44 | Ficikan difussion |
| Hydrogel 1 | 0.733 | 0.914 | 0.865 | 0.921 | 0.25 | Ficikan difussion |
| Hydrogel 2 | 0.979 | 0.995 | 0.999 | 0.997 | 0.47 | Ficikan difussion |
| Microemulsion 1A | 0.985 | 0.983 | 0.994 | 0.995 | 0.77 | Non Fickian diffusion |
| Microemulsion 2A | 0.977 | 0.966 | 0.990 | 0.999 | 0.72 | Non Fickian diffusion |
The dissimilarity (f1) and similarity (f2) factors were presented in Table 4. It was observed that release profiles of hydrogel 2 and macroemulsion 2 could be considered equivalent because the value of f1 was less than 15 and f2 was more than 50 (62.93). According to the data presented in table 4 the release profiles of microemulsion 1A and microemulsion 2A also demonstrated similarity. Other compared pairs showed f2 values <50 which indicated dissimilarity in release profiles. Additionally, the results presented in table 5 indicate that there were no statistically significant differences in the release pattern between macroemulsion 2 × hydrogel 2 (p > 0.05) and microemulsion 1A × microemulsion 2A (p > −0.05). Other compared pairs demonstrated statistically highly significant difference in release results (p <0 .001).
Table 4.
The dissimilarity (f1) and similarity factors (f2) for each comparison.
| Comparison | f1a | f2 | Release profile |
|---|---|---|---|
| Hydrogel 1 × Macroemulsion 1 | 83.62 | 12.57 | Not similar |
| Hydrogel 1 × Macroemulsion 2 | 49.97 or 99.90 | 23.99 | Not similar |
| Hydrogel 1 × Hydrogel 2 | 41.87 or 72.03 | 27.81 | Not similar |
| Hydrogel 2 × Macroemulsion 1 | 71.83 | 27.21 | Not similar |
| Hydrogel 2 × Macroemulsion 2 | 13.94 or 16.20 | 62.93 | Similarity demonstrated |
| Macroemulsion 2 × Macroemulsion 1 | 67.26 | 31.68 | Not similar |
| Microemulsion 1A × Microemulsion 2A | 12.07 or 10.77 | 68.36 | Similarity demonstrated |
The first f1 is obtained when the first formulation on the left is set as reference.
Table 5.
The results of multiple comparison test.
| Comparison | p-value |
|---|---|
| Macroemulsion 1 × Macroemulsion 2 | p < 0.001 |
| Macroemulsion 1 × Hydrogel 1 | p < 0.001 |
| Macroemulsion 1 × Hydrogel 2 | p < 0.001 |
| Macroemulsion 2 × Hydrogel 1 | p <0 .001 |
| Macroemulsion 2 × Hydrogel 2 | p > 0.05 |
| Hydrogel 1 × Hydrogel 2 | p < 0.001 |
| Microemulsion 1A × Microemulsion 2A | p > 0.05 |
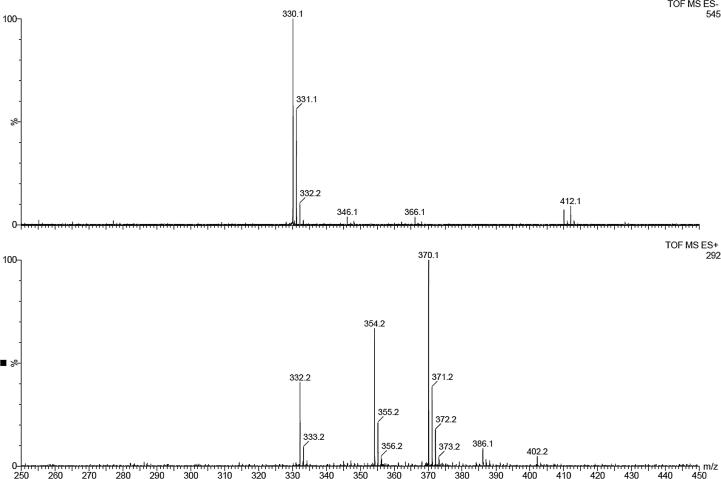
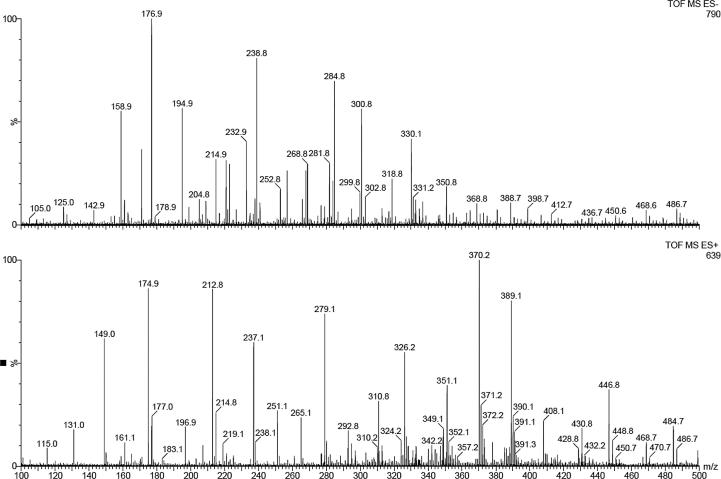
In order to check the stability of Ude-Phe in the formulations and after the release experiments the MS spectra of receptor medium were performed. First of all the active compound was characterized by ESI mass spectrometry (Fig. 9). The positive ion mode of Ude-Phe (MW-331.1) shows a protonated molecular ion [M+H]+ at m/z = 332.1 and a molecular ion associated with sodium [M+Na]+ at m/z = 354.2. An additional, signal at m/z = 370.1 corresponds to the potassium adduct [M+K]+. In the case of negative ion mode, mass a signal at m/z 330.1 corresponds to deprotonated molecular ion [M−H]−. After the release experiment the receptor medium was analyzed (see Fig. 10). In negative ion mode the deprotonated molecular ion of Ude-Phe [M−H]− can be observed. Additionally, in the positive ion mode a molecular ion with associated potassium at m/z 370.2 was also detected. This demonstrates that the phenylalanine derivative remained stable in the formulations and during the release studies.
Fig. 9.
ESI-MS spectrum of the undecylenoyl phenylalanine.
Fig. 10.
ESI-MS spectrum of Ude-Phe in receptor medium after the release studies.
4. Conclusions
Novel topical formulations containing the undecylenoyl phenylalanine were successfully obtained. The pH values of all semi-solid preparations resemble the skin’s pH, therefore they can be intended for dermal applications. Moreover, the obtained results proved that the storage conditions, preparation method and composition influenced the stability of formulations. The macroemulsion 2 prepared at RT was more stable than macroemulsion 1 obtained at high temperature. The results proved that macroemulsion 2 was much more stable than macroemulsion 1 irrespective of the storage conditions. The preparation method and composition of the emulsion could have an impact on the better stability of the macroemulsion 2. The greater stability of this formulation can be explained by the presence of the auto-emulsifier (Creagel EZIN). Additionally, the presence of phenylalanine derivative enhanced the stability of both macroemulsions regardless the storage conditions. The in vitro release studies proved that the membrane structure had the influence on the release rate of undecylenoyl phenylalanine. The release profile of Ude-Phe through Strat-M membrane was completely different than through other membranes.
Furthermore, it was observed that with the increase in viscosity of the formulation decrease the ability of Ude-Phe to diffuse through the membrane. Therefore, hydrogels and microemulsion-based hydrogels could be recommended as proper vehicles for this active compound. Additional studies are required to assess the influence of the formulation type on skin permeation of Ude-Phe and to determine its bioavailability.
It should be added that in vitro penetration studies could be a valid and easy test for formulations producer to employ during screening for the best product (Soares et al. 2017). Moreover, in vitro models presented in this study could be ideal in terms of cost and simplicity. However, till now they are not fully accepted by regulatory agencies to substitute in vivo bioavailability tests. It is generally known that the in vitro release studies do not reflect excipients interaction with uppermost skin structure that influences the drug penetration and its bioavailability. On the other hand there are numerous studies that present extremely good correlation between in vitro and in vivo protocols (Lehman et al., 2011). Recently, FDA has introduced draft guidance for topical acyclovir cream where in vitro skin penetration tests are considered to assess bioequivalence instead of clinical end-point study (FDA, 2016). Therefore, there is a hope that in the future in vitro models may replace in vivo studies.
Acknowledgement
Financial support from Polish Ministry of Science and Higher Education is acknowledged. The authors would like to thank Merck Millipore for supplying the Strat-M membrane.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bisset D.L., Robinson L.R., Raleigh P.S., Miyamoto K., Hakozaki T., Li J., Kelm G.R. Reduction in the appearance of facial hyperpigmentation by topical N-undecyl-10-enoyl-l-phenylalanine and its combination with niacinamide. J. Cosmet. Dermatol. 2009;8:260–266. doi: 10.1111/j.1473-2165.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- Burnett C., Heldreth B., Bergfeld W.F., Belsito D.V., Hill R.A., Klaassen C.D., Liebler D.C., Marks J.G., Shank R.C., Slaga T.J., Snyder P.W., Andersen F.A. Safety assessment of amino acid alkyl amides as used in cosmetics. Int. J. Toxicol. 2017;36(1_suppl):17S–56S. doi: 10.1177/1091581816686048. [DOI] [PubMed] [Google Scholar]
- Carbone C., Musumeci T., Lauro M.R., Puglisi G. Eco-friendly aqueous core surface-modified nanocapsules. Colloids Surf B: Biointerfaces. 2015;125:190–196. doi: 10.1016/j.colsurfb.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Celia C., Trapasso E., Cosco D., Paolino D., Fresta M. Turbiscan lab expert analysis of the stability of ethosomes and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf B: Biointerfaces. 2009;72:155–160. doi: 10.1016/j.colsurfb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Dash S., Murthy P.N., Nath L., Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010;67:217–223. [PubMed] [Google Scholar]
- de Oliveira Gomes S.F., de Aguiar Nunan E., Ferreira L.A.M. Influence of the formulation type (o/w, w/o/w emulsions and ointment) on the topical delivery of paromomycin. Braz. J. Pharm. Sci. 2004;40:345–352. [Google Scholar]
- Donnelly R.F., McCarron P.A., Zawislak A.A., Woolfson A.D. Design and physicochemical characterization of a bioadhesive patch for dose-controlled topical delivery of imiquimod. Int. J. Pharm. 2006;307:318–325. doi: 10.1016/j.ijpharm.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Eros I., Abu-Eida E.Y., Csoka I., Santa Z., Cserne A., Kover T. Optimization of drug release from dermatological semisolid preparations. Drug Dev. Res. 2003;59:316–325. [Google Scholar]
- FDA, 1997. Dissolution Testing of Immediate Release SolidOral Dosage Forms; Guidance for Industry; U.S. Department of Health and Human Services, Foodand Drug Administration, Center for Drug Evaluation and Research (CDER), U.S. Government Printing Office: Washington, DC. <http://www.fda.gov/ cder/guidance/1713bp1.pdf>.
- FDA. 2016. Draft Guidance on Acyclovir. US Food and Drug Admini- stration (FDA). Recommended Dec 2014; Revised Dec 2016. Available at: <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM428195.pdf>.
- Gordon A.J., Ford R.J. The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References. Wiley and Sons; New York: 1972. Spectroscopy; pp. 162–342. [Google Scholar]
- Goscianska J., Nowak I., Olejnik A. Sorptive poperties of aluminium ions containing mesopoprous silica towards L-histidine. Adsortpion. 2016;22:571–579. [Google Scholar]
- Hg S.F., Rouse J. A comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using Franz diffusion cells. Pharmaceutics. 2010;2:209–223. doi: 10.3390/pharmaceutics2020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961;50:874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Katoulis A.C., Alevizou A., Bozi E., Makris M., Zafeiraki A., Mantas N., Kousta F., Mistidou M., Kanelleas A., Stavrianeas N.G. A randomized, double-blind, vehicle-controlled study of a prepartion containing undecylenoyl phenylalanine 2% in the treatment of solar lentignes. Clin. Exp. Dermatol. 2009;35:473–476. doi: 10.1111/j.1365-2230.2009.03605.x. [DOI] [PubMed] [Google Scholar]
- Katoulis A., Alevizou A., Soura E., Mantas N., Bozi E., Gregorious S., Makris M., Rigopoulos D. A double-blind vehicle-controlled study of a preparation containing undecylenoyl phenylalanine 2% in the treatment of melasma in females. J. Cosmet. Dermatol. 2014;13:86–90. doi: 10.1111/jocd.12089. [DOI] [PubMed] [Google Scholar]
- Kimball M. Manufacturing topical formulations: Scale-up from Lab to Pilot Production. In: Dayan N., editor. Handbook of formulating dermal applications: a definitive practical guide. John Wiley & Sons Inc.; Hoboken: 2016. pp. 167–232. [Google Scholar]
- Kulkarni V.S., Shaw C. Preparation stability testing Essential chemistry for formulators of semisolid and liquid dosagesPreparation stability testing Essential chemistry for formulators of semisolid and liquid dosages. Academic Press Inc Elsevier Science Publishing Co Inc; San Diego, USA: 2015. p. 135. [Google Scholar]
- Lee J., Kim M., Hong C.K., Shim S.E. Measurement of the dispersion stability of pristine and surface multiwalled carbon nanotubes in various nonpolar and polar solvents. Meas. Sci. Technol. 2007;18:3707–3712. [Google Scholar]
- Lehman P.A., Raney S.G., Franz T.J. Percutaneous absorption in man: in vitro-in vivo correlation. Skin Pharmacol. Physiol. 2011;24:224–230. doi: 10.1159/000324884. [DOI] [PubMed] [Google Scholar]
- Lintner K. Peptides, amino acids and proteins in skin care? Cosmetics & Toiletries. 2007;122:26–34. [Google Scholar]
- Malakar J., Sen S.O., Nayak A.K., Sen K.K. Development and evaluation of microemulsions for transdermal delivery of insulin. ISRN Pharm. 2011;780150:1–7. doi: 10.5402/2011/780150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengual O., Meunier G., Cayre K., Puech P., Snabre P. Turbiscan MA 2000: multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta. 1999;50:445–456. doi: 10.1016/s0039-9140(99)00129-0. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk A., Gajewicz A., Rasulev B., Schaeublin N., Maurer-Gardner E., Hussain S., Leszczynski J., Puzyn T. Zeta potential for metal oxide nanoparticles: a predictive model developed by a nano-quantitative structure−property relationship approach. Chem. Mater. 2015;27:2400–2407. [Google Scholar]
- Moore J.W., Flanner H.H. Mathematical comparison of dissolution profiles. Pharm. Tech. 1996;20:64–74. [Google Scholar]
- Olejnik A., Nowak I. Atomic force microscopy analysis of synthetic membranes applied in release studies. Appl. Surf. Sci. 2015;355:686–697. [Google Scholar]
- Olejnik A., Goscianska J., Nowak I. Active compounds release from semisolid dosage forms. J. Pharm. Sci. 2012;101:4032–4050. doi: 10.1002/jps.23289. [DOI] [PubMed] [Google Scholar]
- Olejnik A., Schoreder G., Nowak I. The tetrapeptide N-acetyl-Pro-Pro-Tyr-Leu in skin care formulations – Physicochemical and release studies. Int. J. Pharm. 2015;492:161–168. doi: 10.1016/j.ijpharm.2015.06.050. [DOI] [PubMed] [Google Scholar]
- Olejnik A., Goscianska J., Zielinska A., Nowak I. Stability determination of the formulations containing hyaluronic acid. Int. J. Cosmet. Sci. 2015;37:401–407. doi: 10.1111/ics.12210. [DOI] [PubMed] [Google Scholar]
- Pérez-Mosqueda L.M., Trujillo-Cayado L.A., Carrillo F., Ramierez P., Munoz J. Formulation and optimization by experimental design of eco-friendly emulsions based on D-limonene. Colloids Surf. B: Biointerfaces. 2015;128:127–131. doi: 10.1016/j.colsurfb.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Pillay V., Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: and alternative method. J. Control. Release. 1998;55:45–55. doi: 10.1016/s0168-3659(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Robinson M., Visscher M., Laruffa A., Wickett R. Natural moisturizing factors (NMF) in the stratum corneum (SC). I. Effects of lipid extraction and soaking. J. Cosmet. Sci. 2010;61:13–22. [PubMed] [Google Scholar]
- Sahoo S., Chakraborti C.K., Behera P.K. Development and evaluation of gastroretentive controlled release polymeric suspensions containing ciprofloxacin and carbopol polymers. J. Chem. Pharm. Res. 2012;4:2268–2284. [Google Scholar]
- Santos J., Trujillo L.A., Calero N., Alfaro M.C., Munoz J. Physical characterization of a commercial suspoemulsion as a reference for the development of suspoemulsions. Chem. Eng. Technol. 2013;36:1883–1890. [Google Scholar]
- Shah V.P., Elkins J.S. In vitro release form corticosteroid ointments. J. Pharm. Sci. 1995;84:1139–1140. doi: 10.1002/jps.2600840920. [DOI] [PubMed] [Google Scholar]
- Shah V.P., Skelly J.P. Practical considerations in developing a quality control (in vitro release) procedure for topical drug products. In: Shah V.P., Maibach H.I., editors. Topical drug bioavailability, bioequivalence and penetration. Plenum Press; New York: 1993. pp. 107–116. [Google Scholar]
- Shalviri A., Sharma A.C., Patel D., Sayani A. Low-surfactant microemulsions for enhanced topical delivery of poorly soluble drugs. J. Pharm. Pharm. Sci. 2011;14:315–324. doi: 10.18433/j38p4v. [DOI] [PubMed] [Google Scholar]
- Soares K.C.C., de Souza W.C., de Souza Texeira L., da Cunha-Filho M.S.S., Gelfuso G.M., Gratieri T. Comparison of clobetasol propionate generics using simplified in vitro bioequivalence method for topical drug products. Curr Drug Deliv. 2017 doi: 10.2174/1567201814666171120125333. Nov 20. [DOI] [PubMed] [Google Scholar]
- Špiclin P., Homar M., Zupancic-Valant A., Gasperlin M. Sodium ascorbyl phosphate in topical microemulsions. Int. J. Pharm. 2003;256:65–73. doi: 10.1016/s0378-5173(03)00063-2. [DOI] [PubMed] [Google Scholar]
- Talegaonkar S., Azeem A., Ahmad F.J., Khar R.K., Pathan S.A., Khan Z.I. Microemulsions: a novel approach to enhanced drug delivery. Recent Pat. Drug Deliv. Formul. 2008;2:238–257. doi: 10.2174/187221108786241679. [DOI] [PubMed] [Google Scholar]
- Trujillo-Cayado L.A., Ramirez P., Alfaro M.C., Ruiz M., Munoza J. Adsorption at the biocompatible a-pinene-water interface and emulsifying properties of two eco-friendly surfactants. Colloids Surf. B: Biointerfaces. 2014;122:623–629. doi: 10.1016/j.colsurfb.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Uchida T., Kadhum W.R., Kanai S., Todo H., Oshizaka T., Sugibayashi K. Prediction of skin permeation by chemical compound using the artificial membrane Strat-M. Eur. J. Pharm. Sci. 2015;25:113–118. doi: 10.1016/j.ejps.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Vashist A., Ahmad S. Hydrogels: smart materials for drug delivery. Orient. J. Chem. 2013;29:861–870. [Google Scholar]
- Wuelfing W.P., Kosuda K., Templeton A.C., Harman A., Mowery M.D., Reed R.A. Polysorbate 80 UV/vis spectral and chromatographic characteristics - defining boundary conditions for use of the surfactant in dissolution analysis. J. Pharm. Biomed. Anal. 2006;41:774–782. doi: 10.1016/j.jpba.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Zhao L., Zhang S., Uluko H., Liu L., Lu J., Xue H., Kong F., Lv J. Effect of ultrasound pretreatment on rennet-induced coagulation properties of goat's milk. Food Chem. 2014;165:167–174. doi: 10.1016/j.foodchem.2014.05.081. [DOI] [PubMed] [Google Scholar]