Abstract
Mitochondrial Sirtuin 5 (SIRT5) is an NAD+-dependent demalonylase, desuccinylase, and deglutarylase that controls several metabolic pathways. A number of recent studies point to SIRT5 desuccinylase activity being important in maintaining cardiac function and metabolism under stress. Previously, we described a phenotype of increased mortality in whole-body SIRT5KO mice exposed to chronic pressure overload compared with their littermate WT controls. To determine whether the survival phenotype we reported was due to a cardiac-intrinsic or cardiac-extrinsic effect of SIRT5, we developed a tamoxifen-inducible, heart-specific SIRT5 knockout (SIRT5KO) mouse model. Using our new animal model, we discovered that postnatal cardiac ablation of Sirt5 resulted in persistent accumulation of protein succinylation up to 30 weeks after SIRT5 depletion. Succinyl proteomics revealed that succinylation increased on proteins of oxidative metabolism between 15 and 31 weeks after ablation. Heart-specific SIRT5KO mice were exposed to chronic pressure overload to induce cardiac hypertrophy. We found that, in contrast to whole-body SIRT5KO mice, there was no difference in survival between heart-specific SIRT5KO mice and their littermate controls. Overall, the data presented here suggest that survival of SIRT5KO mice may be dictated by a multitissue or prenatal effect of SIRT5.
Keywords: sirtuin, protein acylation, proteomics, stress, cardiac hypertrophy
Introduction
Sirtuin 5 (SIRT5) is an NAD+-dependent demalonylase (1, 2), desuccinylase (2), and deglutarylase (3) that resides primarily in mitochondria. Previous studies demonstrated that SIRT5 desuccinylase activity is important in maintaining cardiac function and metabolism in response to stress. In a model of ischemia–reperfusion injury, infarct size in SIRT5KO4 mouse hearts was greater than in WT littermates (4). Additionally, SIRT5 depletion was detrimental to cardiac function at 39 weeks of age, with defects in cardiac fatty acid oxidation (5). Finally, we recently observed increased mortality and impaired oxidative metabolism in whole-body SIRT5KO mice, compared with WT littermates, with the stress of chronic pressure overload–induced cardiac hypertrophy (6). Each of these three studies on the role of SIRT5 in the heart performed succinyl proteomics measurements in WT and SIRT5KO heart tissue to identify enzymes and pathways potentially regulated by SIRT5. In all three studies, key pathways in mitochondrial metabolism, including oxidative phosphorylation, TCA cycle, and fatty acid oxidation, were identified as being regulated by SIRT5, given the increase in succinylation on many enzymes in these pathways with SIRT5 depletion (6). Together, these data suggest that SIRT5 is important in maintaining cardiac function by desuccinylating key enzymes in oxidative metabolism. Although these studies suggest that SIRT5 has a cardioprotective role, whether the effect of SIRT5 is cardiomyocyte-specific or due to an effect of SIRT5 outside of the heart remains unknown.
Most characterizations of the physiological roles of mitochondrial sirtuins have been conducted in whole-body sirtuin KO mice, including our previous work on the role of SIRT5 in cardiac function (6). However, differing degrees of acetylation in SIRT3KO tissues (7) and malonylation and succinylation in SIRT5KO tissues (8) suggest that the roles of sirtuins may differ between tissues. More recently, understanding the tissue-specific effects of mitochondrial sirtuins has emerged as an important area of investigation to determine the contribution of individual tissues to the phenotypes described in germline depletion models. For example, whole-body SIRT3KO mice on a high-fat diet have accelerated development of obesity, insulin resistance, hyperlipidemia, and steatohepatitis (9). However, in a liver- or skeletal muscle–specific SIRT3KO model (two tissues with a strong influence on whole-body metabolism), no metabolic differences compared with WT controls were observed despite hyperacetylation profiles similar to the whole-body SIRT3KO model (10). Either a tissue beyond liver/skeletal muscle or a coordinated tissue response was responsible for the phenotypes observed in the whole-body SIRT3KO mouse. Another major phenotype in the whole-body SIRT3KO mouse is the development of spontaneous cardiac hypertrophy (11, 12). A recent study found that this phenotype is recapitulated in a skeletal muscle- and heart-specific SIRT3KO model (13). However, it is not yet known whether this is exclusively an effect of SIRT3 in cardiomyocytes; indeed, SIRT3 has also been shown to play a direct role in fibrosis (14), a key component in the development of cardiac hypertrophy. So far, no studies have been published on the tissue-specific roles of SIRT4 or SIRT5. Thus, there is a need to develop tissue-specific sirtuin KO models to better understand the tissues contributing to the phenotypes revealed in whole-body KO models. To this end, we set out to determine whether the phenotypes described in the whole-body SIRT5KO mouse under chronic pressure overload–induced hypertrophy (6) were recapitulated in a heart-specific SIRT5KO mouse model.
A well-established experimental tool to knock out genes in an inducible and cardiomyocyte-specific manner is the α-MHC-MerCreMer mouse model. In this model, two mutated estrogen receptors (Mer) flank a Cre transgene located upstream of the cardiomyocyte-specific myosin heavy chain α (α-MHC) promoter (15). This system requires delivery of tamoxifen to bind to the mutated estrogen receptors, translocate to the nucleus, and induce expression of Cre. One benefit of using an inducible system is that the gene can be depleted postnatally, circumventing problems with embryonic lethality or compensation during early development. However, a major caveat of this mouse model is Cre toxicity in the heart, as evidenced by heart failure with high amounts of tamoxifen (16) and transient inflammation and hypertrophy with lower doses of tamoxifen (17). Here we present data on the development and characterization of an inducible cardiomyocyte-specific SIRT5KO mouse model. Further, we analyze the cardiac morphological and functional changes with chronic TAC and survey the succinylation profile in this novel mouse model.
Results
Characterization of the heart-specific, tamoxifen-inducible SIRT5KO mouse model
To test whether the cardiac phenotypes observed in the whole-body SIRT5KO mouse (6) were heart-intrinsic, we developed a heart-specific, tamoxifen-inducible SIRT5KO mouse model. To generate this mouse, we crossed SIRT5fl/fl (18) mice with αMHC-MerCreMer mice to generate littermates with the following genotypes: SIRT5fl/fl; αMHC-MerCreMer−/− (hereafter referred to as fl/fl) and SIRT5fl/fl; αMHC-MerCreMer+/− (hereafter referred to as fl/fl;MCM). Additionally, the floxed alleles were crossed out of this line to generate a second line of αMHC-MerCreMer−/− and αMHC-MerCreMer+/− mice to generate αMHC-MerCreMer+/− (hereafter referred to as MCM) mice to control for Cre toxicity.
Two methods of delivering tamoxifen were tested to determine optimal SIRT5 depletion in the heart. First, adult mice were fed a commercially available tamoxifen citrate diet (Envigo Tekland Diets, TD.130860) for 3 weeks. In a second method, tamoxifen was dissolved in corn oil and given to adult mice by oral gavage at a dose of 80 mg/kg for 3 consecutive days. We found that with either method of delivery, depletion of SIRT5 took more than 2 weeks (Fig. 1). Feeding tamoxifen citrate for 3 weeks followed by 2 weeks of regular chow resulted in ∼75% depletion of SIRT5 (Fig. 1, A and B), whereas 2 weeks after the oral gavage regimen, we observed about 50% depletion of SIRT5 (Fig. 1, D and E). Because expression of Cre in the heart has been shown to be toxic and results in acute cardiomyopathy (17), we measured cardiac hypertrophy by calculating the ratio of heart weight to body weight at multiple time points throughout these pilot studies. Although there was only a small sample size at each time point (n = 1 or 2), there were trends of increased hypertrophy in the fl/fl;MCM mice compared with the fl/fl mice after 3 weeks of the tamoxifen diet (Fig. 1C). This hypertrophy appeared to normalize after 2 weeks of regular chow following the tamoxifen diet. No differences in heart weight to body weight were observed between the fl/fl controls and fl/fl;MCM mice in the oral gavage pilot study (Fig. 1F). Finally, we used RT-qPCR to further measure the transcript levels of Sirt5 and markers of Cre toxicity, including Nppa (Anf), Nppb (Bnp), and Il6. Although SIRT5 protein levels were not diminished until weeks after tamoxifen dosing, Sirt5 transcript levels were depleted at earlier time points (Fig. 1G). Maximal depletion of Sirt5 transcript levels occurred after 2 weeks of the tamoxifen diet (Fig. 1G, left) and 1 day after the oral gavage dosing regimen (Fig. 1G, right). The transcript levels of Cre toxicity markers were highest after 3 weeks of tamoxifen diet feeding or 1 day to 1 week after oral gavage dosing (Fig. 1, H–J). In the tamoxifen diet pilot group, transcript levels of Anf, Bnp, and Il6 returned to control levels after 2 weeks of regular chow feeding. Anf and Bnp remained elevated in the oral gavage pilot group 2 weeks after oral gavage dosing (Fig. 1, H and I). Based on these data, we proceeded with optimization of the tamoxifen diet feeding regimen because greater depletion of SIRT5 was achieved, and we predicted that a shorter time on the diet would be sufficient to achieve depletion of SIRT5 and would result in less Cre toxicity. In studies described below, tamoxifen diet was fed for 8–10 days, and we observed decreased evidence of Cre toxicity (data not shown).
Figure 1.
Characterization of the tamoxifen-inducible, heart-specific SIRT5KO mouse model. A–C, tamoxifen diet pilot. A, Western blotting of SIRT5 over time (TD, tamoxifen diet; RC, regular chow; w, week). B, quantification of the blot. C, heart weight to body weight ratio. D–F, tamoxifen oral gavage pilot. D, Western blotting of SIRT5 over time (PG, post-gavage; d, day. E, quantification of the blot. F, heart weight to body weight ratio. Blots were normalized to total protein using Bio-Rad stain-free technology. G–J, transcript levels of Sirt5 (G), Anf (H), Bnp (I), and Il6 (J) normalized to 36B4 over time in the tamoxifen diet (left) and tamoxifen oral gavage (right) pilots. Adult males were used, with n = 1–2 per group.
Given that SIRT5 is a protein lysine desuccinylase and that succinylation in the whole-body SIRT5KO heart is abundant, we looked at succinylation over time after tamoxifen-induced ablation of Sirt5. Adult female mice were fed a tamoxifen citrate diet for 10 days, followed by a regular chow diet. Mice were sacrificed immediately after the tamoxifen diet regimen and 3, 6, 12.5, and 32.5 weeks after the tamoxifen diet regimen. Western blotting was used to analyze succinylation and SIRT5 protein expression in whole-heart lysates. Interestingly, we found that it took several weeks for protein succinylation to accumulate to levels that were comparable with succinylation in the whole-body SIRT5KO mouse (Fig. 2, A and B). Specifically, 32.5 weeks after depletion of Sirt5, succinylation in fl/fl;MCM mouse hearts was about 3-fold greater than succinylation in fl/fl mouse hearts. Although this was the maximum succinylation achieved in this time course, the whole-body SIRT5KO mouse heart has an approximate 5.5-fold increase in succinylation over the WT control (6). Thus, it is possible that sites of lysine succinylation would continue to accumulate when the time course is taken past 32.5 weeks. Interestingly, SIRT5 protein appeared to be maximally depleted 3 weeks after the tamoxifen diet feeding regimen (Fig. 2C). Together, these data show that, in our heart-specific, inducible SIRT5KO mouse model, SIRT5 is depleted after 3 weeks on the tamoxifen diet, but succinylation continues to increase for at least 30 weeks. This important finding provides a unique tool to assess the effects of SIRT5 ablation with varying degrees of protein lysine hypersuccinylation, which might allow us to disentangle the effects of SIRT5 from effects of protein succinylation.
Figure 2.
Succinylation accumulates over time after Sirt5 ablation. A, Western blotting of protein succinylation and SIRT5 weeks after feeding of tamoxifen diet (RC, regular chow; TD, tamoxifen diet; d, day; w, week). B and C, quantification of succinylation (B) and SIRT5 (C). Blots were normalized to total protein using Bio-Rad stain-free technology. Adult females were used in this study with n = 1 or 3 per group. Error bars represent S.E.
Heart-specific SIRT5KO succinyl proteomics
To further explore the increase in protein lysine succinylation after Sirt5 ablation, we performed succinyl proteomics in fl/fl and fl/fl;MCM mice 15 weeks and 31 weeks after tamoxifen diet feeding. From the previous time course experiment, we predicted that we would observe a small increase in protein succinylation in fl/fl;MCM mice 15 weeks after tamoxifen diet feeding and a large increase in protein succinylation in fl/fl;MCMC mice 31 weeks after tamoxifen diet feeding. Investigation of the cardiac succinylome at these time points would allow us to determine how the cardiac succinylome landscape changes with time after Sirt5 depletion. Immediately after weaning, fl/fl and fl/fl;MCM mice were fed tamoxifen for 8 days and then returned to a regular chow diet. One group of mice was sacrificed 15 weeks after tamoxifen-induced ablation of Sirt5 (n = 2 fl/fl and n = 3 fl/fl;MCM), and a second group of mice was sacrificed 31 weeks after tamoxifen-induced ablation of Sirt5 (n = 2 fl/fl and n = 2 fl/fl;MCM) (Fig. 3A). Left ventricles of hearts were pulverized and processed at the same time for succinyl proteomics analysis using a workflow leveraging peptide labeling with tandem mass tag (TMT) 10-plex reagents (Thermo Fisher Scientific), immunoprecipitation with anti-succinyl lysine antibody (Cell Signaling Technology), and nanoflow LC-MS/MS analysis on a Q Exactive Plus Orbitrap. See Table S1 for quantitative data on relative succinylpeptide and protein abundances.
Figure 3.
Overview of succinyl proteomics and comparison with whole-body SIRT5KO cardiac succinylome. A, study design. Newly weaned males were fed the tamoxifen diet for 8 days and then switched to regular chow for the remainder of the study. Group 1 was sacrificed 15 weeks after tamoxifen feeding, and group 2 was sacrificed 31 weeks after tamoxifen feeding. Proteomics on heart tissue from animals in groups 1 and 2 were performed simultaneously. B, proteome with proteins ranked according to log2 FC (KO/WT) 15 weeks post-tamoxifen feeding. C, Venn diagrams showing overlap of quantified succinylated peptides and proteins in whole-body SIRT5KO succinylome (orange) (6) and heart-specific SIRT5KO succinylome (purple). D, top 50 highest succinylated peptides identified in the heart-specific succinylome. The corresponding KO/WT log2 FC in the whole body SIRT5KO is shown in light blue. The shaded light blue columns indicate top 50 sites in the whole-body SIRT5KO.
First we analyzed changes in the proteome with respect to time and genotype. Using the parameters of log2 FC ≥ 1 and an adjusted p ≤ 0.1 (Benjamini–Hochberg correction, 10% FDR), there existed only one significant change in the proteome. Esr1 (estrogen receptor) was significantly increased in the 15-week SIRT5KO compared with the 15-week WT control (Fig. 3B). Esr1 induction is due to the constitutive transgenic overexpression of mutated estrogen receptors under the control of the MHC promoter and is thus not an effect of Sirt5 ablation. We interrogated the pathways represented in the top 5% (Fig. 3B, red) and bottom 5% (Fig. 3B, blue) of proteins when ranked according to -fold change and found pathways that were significantly overrepresented in these groups. Among the proteins that increased the most in hearts with Sirt5 ablation, the pathways of muscle contraction and response to stress were increased, whereas, among the proteins that decreased the most in hearts with Sirt5 ablation, pathways of muscle organ development, sensory perception of sound (an irrelevant pathway in heart), muscle contraction, and cellular component of morphogenesis decreased. Given that proteins in muscle contraction both increased and decreased, this is likely because a large number of these proteins were identified in heart tissue. These pathways were also represented in a pathway analysis comparing the log2 FC of SIRT5KO/WT 31 weeks after tamoxifen feeding (data not shown). Importantly, proteins in oxidative metabolism (the main targets of protein lysine succinylation) are not significantly changed with ablation of Sirt5 in the heart.
Next we compared the succinylome generated in the heart-specific SIRT5KO and the succinylome we previously generated from the hearts of whole-body SIRT5KO mice (6). Given the different proteomic techniques and study designs, we expected to identify a smaller number of sites of succinylation in the heart-specific SIRT5KO succinylome compared with the whole-body SIRT5KO heart succinylome. In the heart-specific SIRT5KO succinylome, we identified (at 1% FDR) and quantified 1,631 succinylated peptides (an additional 504 succinylated peptides were identified but not quantified) that mapped to 462 unique proteins (Fig. 3C). Interestingly, only about half of these succinylated peptides were also identified in the whole-body SIRT5KO heart succinylome. We ranked the identified succinylated peptides in descending order in both succinylome datasets and found that, of the top 50 peptides increased in heart-specific SIRT5KO compared with WT hearts 31 weeks after tamoxifen feeding, half were also in the top 50 peptides increased in the whole-body SIRT5KO heart compared with the WT heart (Fig. 3D, light blue columns). Additionally, the top 50 succinylated peptides in the heart-specific dataset were all identified in the whole-body SIRT5KO heart succinylome. This comparison illustrates that the SIRT5 targets identified in these proteomic datasets had strong overlap and that the two models of SIRT5 deficiency have similar effects on the succinylome of the heart relative to their respective controls.
The goal of this study was to determine how the protein succinylation landscape changes over time after SIRT5 depletion, specifically in the heart. To begin to answer this question, we performed pathway analyses on a group of peptides that had increased succinylation, compared both with their respective time control (KO/WT at 15 weeks and KO/WT at 31 weeks; Fig. 4, A and B) and with their genotype control (KO 31 weeks/KO 15 weeks and WT 31 weeks/WT 15 weeks, Fig. 4C). We used parameters of log2 FC ≥ 1 and an adjusted p ≤ 0.1 (Benjamini–Hochberg correction, 10% FDR) to make a list of proteins for each given comparison that had increased succinylation. These lists were uploaded to PANTHER (Protein ANalysis THrough Evolutionary Relationships) to perform statistical overrepresentation tests (19) to determine pathways that were particularly susceptible to succinylation in this model. The top three significantly overrepresented pathways in each comparison (except WT 31 weeks/WT 15 weeks, where we saw no change in protein succinylation) were fatty acid β-oxidation, tricarboxylic acid cycle, and oxidative phosphorylation or cellular amino acid catabolic process. Together, these data suggest that proteins in the same metabolic pathways are succinylated in the SIRT5KO heart at 15 weeks and 31 weeks after SIRT5 depletion; indeed, these pathways are significantly overrepresented in this comparison (Fig. 4D). There were no peptides with changes in succinylation when comparing the succinylation profile of WT hearts 15 and 31 weeks after tamoxifen feeding (data not shown), demonstrating that few changes in the sites of protein succinylation occur under basal conditions in this time frame.
Figure 4.
Proteins in pathways of oxidative metabolism are targets of lysine succinylation. A–C, volcano plots of log2 FC (KO/WT) 15 weeks (A), log2 FC (KO/WT) 31 weeks (B), and log2 FC (31W/15W) KO (C). Log2 FC values are plotted against Benjamini–Hochberg–corrected p values with a 10% FDR. The text indicates the number of peptides and proteins in each group that have decreased succinylation (top left) and increased succinylation (top right). D, pathway analysis of proteins significantly increased in the 31 week KO to 15 week KO condition. E and F, all sites of lysine succinylation identified in fatty acid oxidation (E) and the TCA cycle (F). Dark red circles represent the log2 FC (KO/WT) 31 weeks after SIRT5 depletion, and light red circles represent the log2 FC (KO/WT) 15 weeks after SIRT5 depletion. Sites of lysine succinylation are aligned vertically. G–I, comparison of log2 FC (KO/WT) of SDHA:K179 (G), SDHA:K335 (H), and HADHA:K351 (I) in 15 week, 31 week, and whole-body SIRT5KO hearts. The p values are adjusted for multiple comparisons within the same experiment. Data for the whole body were published previously (6).
We hypothesized that the increase in succinylation that occurs in the SIRT5KO heart between 15 and 31 weeks after SIRT5 depletion was due to either an increase in the number of unique sites of succinylation or a further increase in succinylation at lysine residues already succinylated. The succinyl proteomics performed here do not allow us to fully address the former possibility because of the multiplexed nature of our workflow (i.e. qualitative identifications are made on a pool of succinylpeptides from nine samples mixed together for which relative quantitative comparisons are made). To explore the latter possibility, we plotted the -fold change of all sites of lysine succinylation at 31 weeks (KO/WT) and 15 weeks (KO/WT) in fatty acid oxidation (Fig. 4E) and the TCA cycle (Fig. 4F). We found that the -fold change of at least 50% of succinylation sites on proteins in fatty acid oxidation and the TCA cycle increased from 15 weeks (Fig. 4F, light red circles) to 31 weeks (Fig. 4F, dark red circles) after SIRT5 depletion. Only a few SIRT5 succinylation targets have been validated in the heart: lysine 179 and 335 on SDHA (4) and lysine 351 on HDHA (hydroxyacyl-CoA dehydrogenase, subunit A) (5). When we compared the -fold change of these sites at 15 weeks and 31 weeks and the whole-body SIRT5KO (6) (Fig. 4, G–I), we found that the log2 fold change is about the same in the 31-week and whole-body conditions but trends lower in the 15-week samples. These data suggest that succinylation of SIRT5 targets may not occur immediately after depletion of SIRT5; indeed, these data suggest that maximal succinylation on validated targets of SIRT5 occurs 31 weeks after Sirt5 ablation.
Together, our data reveal that deletion of Sirt5 postnatally in the heart results in accumulation of protein lysine succinylation over the course of several weeks to months, whereas SIRT5 protein expression is maximally depleted about 3 weeks after Sirt5 deletion by Cre. These findings provide a novel model to study the effect of loss of SIRT5 under conditions of basal succinylation and/or hypersuccinylation.
Heart-specific SIRT5KO mouse response to pressure overload–induced hypertrophy
To determine whether the phenotype of increased mortality with pressure overload–induced hypertrophy in SIRT5KO mice compared with WT mice is a cardiomyocyte or whole-body effect of SIRT5 (6), we induced left ventricle cardiac hypertrophy by performing TAC in heart-specific SIRT5KO mice. In this study, fl/fl control mice and fl/fl;MCM mice were fed a tamoxifen diet for 8 days immediately after weaning. The mice were fed a regular chow diet for 14 weeks to normalize any effects of Cre toxicity and to age-match the animals in the whole-body SIRT5KO TAC study. TAC surgery was performed on all animals in the study, and serial echocardiograms were taken at 0, 2, 4, 8, 12, and 16 weeks to investigate cardiac morphology and function (Fig. 5C). The most striking phenotype in whole-body SIRT5KO mice we reported previously was decreased survival over the course of 16 weeks of TAC. We repeated this study in fl/fl and fl/fl;MCM mice and, to our surprise, did not observe a difference in survival between the two groups with TAC (Fig. 5B). Within the first week, there was an overall 30% mortality, which was likely due to surgery (∼20% mortality is expected with TAC). Over the remaining 15 weeks of observation, there was only one instance of mortality in each group. At the end of 16 weeks, gradients were measured on surviving mice, and both groups had gradients well above the accepted 20 mm Hg cutoff (data not shown). Wall thickness significantly increased in the fl/fl and fl/fl;MCM groups (compared with their respective pre-TAC wall thickness) within the first 2 weeks of TAC (Fig. 5C), indicative of development of hypertrophy. However, we observed no difference between genotypes. Left ventricle weight to body weight was measured at the end of the study (data not shown), and no differences in genotype were observed. Additionally, we observed that cardiac function is maintained over the course of this TAC study, as fractional shortening was not significantly changed over time or between genotypes (Fig. 5D). These morphological and functional changes are consistent with the development of left ventricle hypertrophy with no apparent cardiac dysfunction. Together, these data show that the phenotype observed in the whole-body SIRT5KO mouse of increased mortality compared with WT controls with TAC does not repeat in the inducible, heart-specific SIRT5KO mouse model under these conditions.
Figure 5.

Heart-specific SIRT5KO response to pressure overload induced cardiac hypertrophy. A, schematic of the study. Males 4–5 weeks of age were fed tamoxifen citrate for 8 days, followed by 14.5 weeks of regular chow. Mice were 20–21 weeks of age when TAC surgery was performed. Mice were followed for 16 weeks after TAC. B, percent survival (n = 10 fl/fl; n = 10 fl/fl;MCM). Serial echocardiography was performed 0, 2, 4, 8, 12, and 16 weeks after TAC. C and D, wall thickness (C; *, p ltequ] 0.05, effect of time, two-way ANOVA with multiple comparisons, Bonferroni correction) and fractional shortening (D). At the end of the study, n = 7 fl/fl; n = 5 fl/fl;MCM. Error bars represent mean ± S.E.
Effect of pressure overload–induced hypertrophy on metabolism and protein lysine succinylation
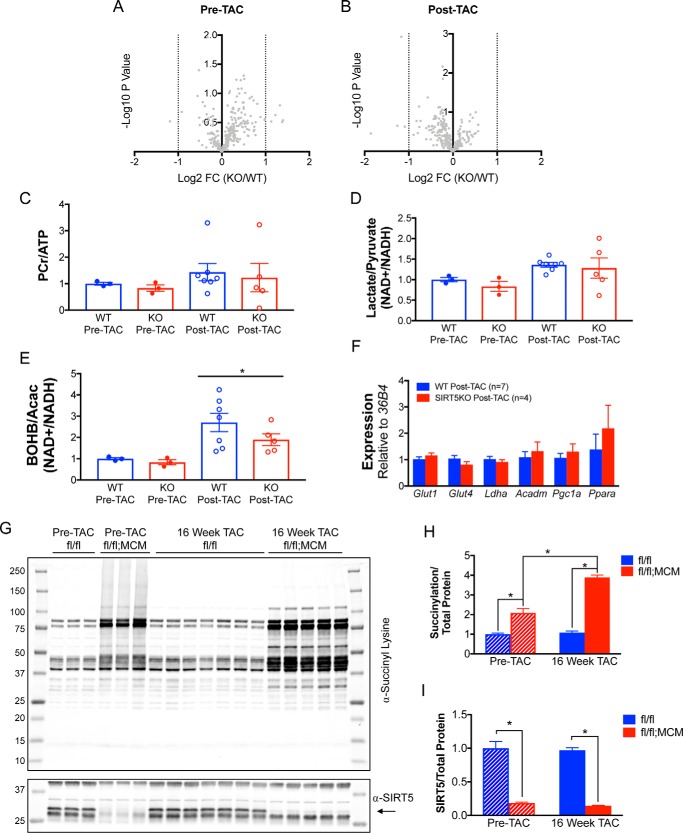
Although we saw no differences in cardiac function by measurements of fractional shortening, we probed the possibility that there could be a molecular signature of a trajectory toward cardiac dysfunction as we observed in the whole-body SIRT5KO heart under TAC stress (6). We examined the metabolite profiles of fl/fl and fl/fl;MCM hearts isolated from mice before and after 16 weeks of TAC by using high-resolution MS and saw no differences in metabolite profiles between genotypes in either the pre-TAC (Fig. 6A) or post-TAC (Fig. 6B) group. This suggests that cardiac metabolic pathways between WT and heart-specific SIRT5KO are similar under both basal and post-TAC conditions. In contrast, we saw evidence of reduced oxidative metabolism via metabolic profiling in the whole-body SIRT5KO mouse heart compared with the WT heart after 4 weeks of TAC (6). To further investigate the possibility of differential cardiac energetics between genotypes, we examined the ratio of PCr/ATP, lactate/pyruvate, and β-hydroxybutyrate/acetoacetate (Fig. 6, C–E). We found no differences in these ratios in either the pre-TAC or post-TAC groups. Interestingly, β-hydroxybutyrate/acetoacetate was significantly increased under the TAC condition, suggesting that oxidative metabolism may be increased under the post-TAC condition, consistent with the development of cardiac hypertrophy and maintenance of cardiac function. Additionally, mRNA expression of genes that regulate cardiac metabolism were the same in the fl/fl and fl/fl;MCM mice after TAC (Fig. 6F), providing further evidence of no additional effect of TAC on metabolism in the heart-specific SIRT5KO mice compared with their littermate controls.
Figure 6.
Metabolite profiles of WT and heart-specific SIRT5KO mouse hearts are similar despite differences in protein lysine succinylation. Males 4–5 weeks of age were fed tamoxifen citrate for 8 days followed by 17.5 weeks of regular chow and sacrificed (pre-TAC group; n = 3 fl/fl, n = 3 fl/fl;MCM). Males 4–5 weeks of age were fed tamoxifen citrate for 8 days followed by 14.5 weeks of regular chow, underwent TAC or sham surgery for 16 weeks, and were sacrificed (16-week TAC group; n = 7 fl/fl, n = 5 fl/fl;MCM). A and B, volcano plots comparing WT and heart-specific SIRT5KO cardiac metabolites before TAC surgery (A) and after TAC surgery (B). Uncorrected p values are represented because Benjamini–Hochberg correction resulted in p values greater than 1. No metabolites were significantly different with Benjamini–Hochberg correction and a 10% FDR. C–E, ratios of PCr/ATP (C), lactate/pyruvate (D), and BOHB/Acac (β-hydroxybutyrate/acetoacetate) (E). *, p ≤ 0.05; effect of TAC, two-way ANOVA. F, cardiac mRNA expression of Glut1, Glut4, Ldha, Acadm, Pgc1α, and Pparα in WT (n = 7) and heart-specific SIRT5KO (n = 4) hearts post-TAC. G, Western blotting of succinylation and SIRT5 pre-TAC and post-TAC. H and I, quantification of succinylation (H) and SIRT5 (I). Blots were normalized to total protein using Bio-Rad stain-free technology. Values are mean ± S.E. *, p ≤ 0.05; two-way ANOVA with multiple comparison, Bonferroni correction.
Given the surprisingly similar metabolite profiles in the fl/fl and fl/fl;MCM hearts, we decided to interrogate the effect of TAC surgery (and development of left ventricle hypertrophy) on protein lysine succinylation. In the whole-body SIRT5KO heart, succinylation decreased in 4 week post-TAC compared with pre-TAC SIRT5KO animals (6). A decrease in succinylation over the course of a 16-week study could explain the similar metabolite profiles. However, we found that protein succinylation was 2-fold higher in fl/fl;MCM mice compared with fl/fl mice in the pre-TAC group and that protein succinylation was 4-fold higher in fl/fl;MCM mice compared with fl/fl mice in the post-TAC group (Fig. 6, G and H). Additionally, there was an increase in protein succinylation when comparing the fl/fl;MCM 16-week post-TAC group and fl/fl;MCM pre-TAC group (Fig. 6, G and H). In contrast to whole-body SIRT5KO mice, where protein succinylation decreases with 4 weeks of TAC, we find that succinylation increases with 16 weeks of TAC in heart-specific SIRT5KO mice. In both models, there is no change in succinylation with TAC in the control groups (WT or fl/fl). SIRT5 protein expression was the same in the fl/fl;MCM pre-TAC and 16-week TAC groups (Fig. 6, G and I). Thus, there is a difference in protein succinylation in the whole-body SIRT5KO mouse model and heart-specific SIRT5KO mouse model both at the time of TAC surgery and in response to TAC, although Sirt5 is ablated in the heart in both models. Together, these data suggest that the phenotype observed in the whole-body SIRT5KO mouse model with TAC may be influenced by a role of SIRT5 in tissues outside of the heart and is likely not a direct effect of the accumulation of lysine succinylation on cardiac proteins in adulthood.
Discussion
A major goal of this study was to determine whether increased mortality in whole-body SIRT5KO mice exposed to pressure overload–induced cardiac hypertrophy was a cardiac-intrinsic effect or an effect of SIRT5 in extracardiac tissues. To answer this question, we developed a novel, tamoxifen-inducible, cardiomyocyte-specific SIRT5KO mouse model and characterized the cardiac succinylome. Similar to our previous study (6), we used TAC surgery to initiate cardiac hypertrophy by pressure overload.
To our knowledge, prior studies involving tissue-specific sirtuin KO mouse models have not been inducible but driven by constitutive expression of Cre. A study characterizing a liver-specific SIRT3KO mouse model and a skeletal muscle–specific SIRT3KO mouse model reported similar acetylation profiles compared with the whole-body SIRT3KO mouse model (10). In contrast, in the heart-specific SIRT5KO mouse model, we found that succinylation accumulated over a time course of months after Sirt5 ablation, with no change in succinylation in fl/fl controls after feeding the tamoxifen diet (Fig. 2).
One emerging model suggests that acylation is a form of carbon stress and that sirtuins function to remove acyl-lysine modifications as a part of the protein quality control network (20). Acylation could occur nonenzymatically, and recent studies from our laboratory demonstrate that succinyl-CoA undergoes intramolecular catalysis to form a highly reactive cyclic anhydride intermediate (21). In support of this notion, incubating cell protein lysates ex vivo with increasing concentrations of acyl-CoA increases the acyl-lysine signal, as measured by Western blotting (22). Our observations here suggest that accumulation of succinylation in this model is a result of availability of reactive succinyl-CoA coupled with the loss of SIRT5, the only known lysine desuccinylase. Given the reactive anhydride that forms during intramolecular catalysis of succinyl-CoA and the extended length of time to see any observable differences in lysine succinylation after SIRT5 depletion, we predict that the succinyl-CoA available for protein lysine succinylation would be relatively low in abundance under basal conditions. Previous studies have shown that acetylation accumulates under disease conditions such as heart failure when acetyl-CoA homeostasis is altered (23). To further test this model of succinylation in the heart-specific SIRT5KO mouse model, it would be of interest to increase succinyl-CoA concentrations and determine the effect on the time of increases in protein lysine succinylation.
Inducible, heart-specific SIRT5KO mice were exposed to chronic pressure overload via TAC surgery for 16 weeks to determine whether this mouse model recapitulated the whole-body SIRT5KO mouse model's response to the same stress. Previously, we described a potential mechanism of increased mortality because of impaired oxidative metabolism in cardiomyocytes with a primary lesion at oxidative phosphorylation (OXPHOS) and an accelerated trajectory of cardiac dysfunction (6). Surprisingly, we saw no difference in mortality between heart-specific SIRT5KO mice and their littermate controls in response to TAC. Additionally, we observed no differences in cardiac hypertrophy or function between genotypes (Fig. 5). This was in contrast to the whole-body SIRT5KO TAC mouse model, where we observed exaggerated hypertrophy compared with WT TAC mice. The exaggerated hypertrophic response to TAC in SIRT5KO mice was based on echocardiogram readings of wall thickness, although we did not observe any genotypic differences in left ventricle weight at the end of the TAC study. We interpreted these data at the time as indicative of two different morphologies of cardiac hypertrophy; the SIRT5KO mice developed primarily concentric hypertrophy, whereas the WT controls developed a mix of concentric and eccentric hypertrophy. Considering the observations made in the heart-specific SIRT5KO model in response to TAC, we re-examined the whole-body SIRT5KO echocardiogram data and considered that another interpretation of these data could be pseudohypertrophy based on a multitissue effect of SIRT5 that affects echocardiogram readings. Physiological factors, including heart rate, preload, and afterload, combined with environmental factors such as stress can influence echocardiogram readings (24). Preload can be changed by dehydration (resulting in a lower blood volume) and could also cause the impression of hypertrophy (25). Regardless of this new interpretation of the exaggerated hypertrophy in whole-body SIRT5KO mice in response to TAC, reduced survival in whole-body SIRT5KO compared with WT controls in response to TAC was the most salient finding from this previous study and is clearly not repeated in the inducible, heart-specific SIRT5KO model. One major caveat of this study was the differences in degree of succinylation in the heart-specific SIRT5KO mouse at the time of TAC surgery (Fig. 6) compared with the degree of succinylation in the whole-body SIRT5KO mouse at the time of TAC surgery. Based on these data, we consider two models to describe the effect of SIRT5 on cardiac function.
One model that could explain the different response to TAC in the whole-body and heart-specific SIRT5KO models is that the effect is due to a whole-body or multitissue role of SIRT5. Although succinylation increases dramatically in the heart in whole-body SIRT5KO mice, it also increases greatly in the kidney, brain, and brain adipose tissue. Cardiac function is intricately linked to kidney function, and thus increased mortality with TAC in whole-body SIRT5KO mice could be a result of Sirt5 ablation in both the heart and kidneys. Indeed, SIRT5 plays a role in regulating ammonia production by desuccinylation (and activation) of glutaminase; loss of SIRT5 leads to increased ammonia production in hepatic and extrahepatic tissues (26). Increased ammonia production can contribute to kidney disease (27) and could play a role in the response of whole-body SIRT5KO mice to TAC. Additionally, the cardiac hypertrophic response is not exclusive to cardiomyocytes but also includes an inflammatory response with the infiltration of macrophages and increased fibrosis (28). A role for SIRT3 in preventing fibrosis by activity in fibroblasts has recently been described (14), and several studies suggest that sirtuins may reduce inflammation (29). The specific role of SIRT5 in the inflammatory response is a nascent area of investigation, but recent studies suggest that SIRT5 suppresses the pro-inflammatory response in macrophages (30). Therefore, a possible explanation for the lack of difference in response to TAC in cardiomyocyte-specific SIRT5KO mice compared with WT controls could be a reduced inflammatory response in noncardiomyocyte tissues so that it is not suppressed in whole-body SIRT5KO mice with TAC; this hypothesis needs to be tested directly.
In another model, we predict that the degree of succinylation may influence the response to TAC. Given the differences in succinylation at the time of TAC surgery in the whole-body SIRT5KO model (6) and the heart-specific SIRT5KO model, it is possible that succinylation of enzymes is not sufficient to alter overall oxidative metabolism in response to TAC. Indeed, we observed no differences in metabolite profiles or metabolic representations of cardiac energetics between fl/fl and fl/fl;MCM after 16 weeks of TAC (Fig. 6). However, when we looked at specific targets of SIRT5 in the heart, it appeared that succinylation continued to increase between 15 and 31 weeks after SIRT5 depletion (Fig. 4). To address this caveat, mice could be aged for more than 30 weeks after Sirt5 ablation before TAC surgery to increase protein lysine succinylation. However, this would increase the age of the mouse at the time of surgery from 12–20 weeks to close to 40 weeks of age. This itself could pose problems in comparing the heart-specific and whole-body models of the same age, as whole-body SIRT5KO mice aged to 39 weeks exhibit cardiac dysfunction compared with controls (5).
Together, these data point to a role of SIRT5 and protein succinylation in the developing heart, either prenatally or during early postnatal development. Importantly, a key difference in this cardiomyocyte-specific SIRT5KO model compared with the whole-body SIRT5KO model is the time when Sirt5 is ablated. In the whole-body SIRT5KO mouse, Sirt5 is ablated in the germline, and thus the protein is never present in the developing mouse. In contrast, Sirt5 was ablated at 3 weeks post-birth in the heart-specific SIRT5KO mouse model described here. Our data show that depletion of SIRT5 protein took a number of weeks even after gene ablation. As a result, succinylation was maximally increased in the whole-body SIRT5KO mouse heart compared with WT controls at the earliest time points we tested (6 weeks of age, data not shown), but succinylation was not maximal in the heart-specific SIRT5KO model until 30 weeks after Sirt5 ablation. This observation provides insight into the kinetics of succinylation and suggests that free succinyl-CoA available for succinylation could be different (in amount, localization, etc.) in the developing heart compared with the adult heart. As a result, it takes several weeks for the highly reactive succinyl-CoA to succinylate available lysine residues. In the heart-specific SIRT5KO model, SIRT5 is present, and succinylation is at baseline levels during embryogenesis and early post-natal development but depleted in early adulthood; the cardiac stress of chronic pressure overload does not result in increased mortality compared with controls. In contrast, in the whole-body SIRT5KO model, Sirt5 is ablated from the germline, and the stress of chronic pressure overload results in increased mortality compared with WT controls. Although we suggest that, in some ways, this could be due to a multitissue effect of SIRT5, it will be important to test the effect of SIRT5 in the prenatal heart by using a noninducible heart-specific SIRT5KO mouse model. Conversely, an inducible, whole-body SIRT5KO model would be informative. Together, these models would certainly provide insight into the role of cardiac succinylation and SIRT5 in the heart.
From this study, we conclude that the phenotype of increased mortality with chronic TAC in whole-body SIRT5KO mice does not translate to heart-specific SIRT5KO mice when ablation of Sirt5 is induced 3 weeks postnatally. Given the differences in these models, specifically the time of cardiac SIRT5 depletion and subsequent hypersuccinylation, future studies are required to determine the role of SIRT5 in the developing heart. These studies will be focused on understanding the kinetics of Sirt5 ablation and succinylation in the developing heart and how it influences the response to cardiac stress and will further clarify the role of SIRT5 as an emerging regulator of survival under cardiac stress.
Experimental procedures
Animals
All animal research has been reviewed by the Duke University Institutional Animal Care and Use Committee and approved under protocol registry number A091-17-04. To generate the tamoxifen-inducible cardiomyocyte-specific SIRT5KO mouse, we crossed SIRT5fl/fl females (18) (a generous gift from Johan Auwerx, École Polytechnique Fédéral de Lusanne (EPFL), Lausanne, Switzerland) with αMHC-MerCreMer+/− males (obtained from The Jackson Laboratory, Bar Harbor, ME; stock no. 005657) to generate littermates with the following genotypes: SIRT5fl/fl; αMHC-MerCreMer−/− (hereafter referred to as fl/fl) and SIRT5fl/fl; αMHC-MerCreMer+/− (hereafter referred to as fl/fl;MCM). Additionally, the floxed alleles were crossed out of this line by crossing SIRT5fl/fl; αMHC-MerCreMer+/− males with C57BL/6J females from The Jackson Laboratory (Bar Harbor, ME, stock no. 000664) to generate a line of nonfloxed αMHC-MerCreMer−/− and αMHC-MerCreMer+/− mice to generate αMHC-MerCreMer+/− (hereafter referred to as MCM) mice to control for Cre toxicity. All of these mice are on the C57BL/6J background. Mice were group-housed on a 12-h light/dark cycle with free access to water and PicoLab Rodent Diet 20 (LabDiet 5053, St. Louis, MO). To induce MerCreMer expression, a tamoxifen citrate diet (Envigo Tekland Diets, Madison, WI; TD.130860) was fed for 8–10 days. Tamoxifen citrate pellets were moistened to encourage eating, and regular chow was fed over the weekends (after 3–5 days of tamoxifen citrate feeding) to decrease weight loss with the new diet. The age, sex, genotype, and number of animals used per study are provided in the corresponding figure legends. All in vivo procedures were performed on healthy animals in accordance with the Duke Institutional Animal Care and Use Program.
Western blots
Left ventricle tissue (whole or part) was dissected and flash-frozen. Tissue was homogenized in radioimmunoprecipitation assay buffer assay buffer with protease inhibitors using a rotor. A Teflon pestle rotated at 1000 rpm was used to homogenize tissues with ∼10 strokes. Tissue was spun down for 10 min at 10,000 × g at 4 °C. The supernatant was collected, and a BCA assay (Sigma) was run to determine protein concentration. Protein concentrations for each sample were normalized to 2 μg/μl in 4× Laemmli sample buffer (Bio-Rad). Whole-cell protein extracts were resolved by SDS-PAGE using stain free Bio-Rad gels and transferred to nitrocellulose membranes using Bio-Rad's Trans-Blot Turbo. Total protein was quantified with the Gel Doc XR+ (Bio-Rad) using stain-free technology. The membranes were blocked in 5% milk in TBS-T (TBS containing 0.1% Tween 20) for 1 h at room temperature and probed with primary antibodies in TBS-T. For anti-acyl-K blots, 3% BSA was added to the primary antibody solution. After incubation with IR dye-conjugated antibodies, the blots were developed using the Odyssey IR imaging system (LI-COR Biosciences). The commercial antibody anti-succinyl-K from PTM (401) was used. Anti-SIRT5 was a generous gift from Leonard Guarente (Massachusetts Institute of Technology, Cambridge, MA).
RT-qPCR
Animals were euthanized by exposure to CO2 for 5 min and the whole heart was excised immediately. The heart was washed in PBS, and the left ventricle was dissected. A small (∼50 mg) piece of the left ventricle apex was flash-frozen immediately for downstream RNA analysis. RNA was extracted from tissues using the RNeasy Mini Kit from Qiagen (74106). cDNA was made from 750 ng of RNA using the iScript cDNA Synthesis Kit from Bio-Rad (170-8890) and diluted 1:8 with nuclease-free water. Amplification was performed using iTaq Universal SYBR Green Supermix from Bio-Rad (1725121) on the QuantStudio 6 Flex (ThermoFisher Scientific). The RT-qPCR reaction mix contained 2.5 μl of 1:8 cDNA, 0.5 μl of 10 μm forward and reverse primer mixture, 4 μl of SYBR, and 1 μl of nuclease free water. 36B4 was used as a reference gene, and relative expression was calculated using the ΔΔCT method.
Proteomics
Protein digestion
Animals were euthanized by exposure to CO2 for 5 min and immediate excision of the whole heart. The heart was washed in PBS, and the left ventricle was dissected and immediately flash-frozen. The left ventricle tissue was pulverized using a Bessman tissue pulverizer (Spectrum Labs) in liquid nitrogen, and ∼20 mg of tissue was weighed out for further sample preparation. To each sample, 300 μl of urea lysis buffer (8 m urea, 50 mm (pH 8.0) Tris, 40 mm NaCl, 2 mm MgCl2, 10 mm nicotinamide, 10 μm trichostatin A, and protease inhibitors (Roche Complete Ultra)) was added, and the tissue was disrupted with a TissueLyzer (Qiagen) for 1 min at 30 Hz. Samples were frozen on dry ice and thawed at 32 °C for three freeze–thaw cycles. Samples were sonicated with a pencil tip probe sonicator at power level 3, 3 bursts for 5 s each. Samples were centrifuged at 10,000 × g for 10 min at 4 °C. Supernatant was placed in a clean tube, and a BCA assay (Sigma) was performed to determine protein concentration, after which 500 μg of each sample was diluted in 200 μl of urea lysis buffer. Samples were reduced by adding 5 mm DTT and incubating at 32 °C for 30 min, cooled to room temperature (RT) and alkylated by incubation with 15 mm iodoacetamide for 30 min at RT in the dark. This reaction was quenched by adding DTT to a final concentration of 15 mm. To each sample, 5 μg of LysC (Wako) was added, and digestion proceeded for 4 h at 32 °C. Samples were subsequently diluted to 1.5 m urea, and 10 μg of sequence-grade trypsin (Promega) was added. Samples were digested overnight at 32 °C. Samples were acidified to 0.5% v/v trifluoroacetic acid (TFA), spun down at room temperature, and desalted using a 50 mg tC18 Sep-Pak SPE column (Waters). Samples were dried using a SpeedVac.
Peptide isobaric labeling
Samples were resuspended in 100 μl of 200 mm triethylammonium bicarbonate. Labeling reagents (Thermo TMT10plex Kit, 90110) were resuspended in 50 μl of acetonitrile (ACN) and subsequently added to the sample tubes to incubate for 4 h at RT while shaking. Reactions were quenched by adding 0.8 μl of 50% hydroxylamine and shaking for 15 min at RT. All samples were combined into a single tube, which was vortexed, and dried in a SpeedVac. The sample was reconstituted in 0.5% TFA, desalted on a 100-mg tC18 Sep-Pak SPE column (Waters), and eluted. Subsequently, 5% of the “input” material was removed for quantification of unmodified peptides, leaving the remaining 95% in a second aliquot for succinylpeptide enrichment. Both aliquots of the desalted peptide mixture were dried on a SpeedVac.
Succinylpeptide enrichment
Beads conjugated with succinyl-lysine antibody (CST, PTMscan Succinyl-Lysine Motif, 13764) were washed twice in ice-cold PBS. All wash steps included spinning the bead slurry down at 2,000 × g at 4 °C for 30 s and carefully removing the supernatant. Beads were transferred to a new tube and washed twice more with ice-cold PBS. The TMT-labeled sample (the larger aliquot from above containing 95% of the peptide mixture) was resuspended in 1.4 ml of 1× immunoaffinity purification buffer (CST, PTMscan Succinyl-Lysine Motif, 13764) and centrifuged at 10,000 × g at 4 °C for 5 min. The supernatant was added to the washed succinyl-lysine antibody beads and incubated on a rotator overnight at 4 °C. The sample was centrifuged at 2,000 × g at 4 °C for 30 s, and the flow-through was saved. The beads were washed twice with 1× IAP with gentle mixing by inversion in between washes. Beads were washed three times with Milli-Q H2O and transferred to a new tube. Succinyl peptides were eluted from the beads by adding 100 μl of 0.15% TFA and letting it sit at room temperature for 10 min with gentle mixing every 2–3 min. The sample was centrifuged at 2,000 × g for 30 s, and the supernatant was transferred to a new tube. This elution step was repeated once, and the sample was brought to a final concentration of 0.5% TFA. The sample was cleaned by loading and eluting from a 50-mg tC18 Sep-Pak SPE column (Waters), dried on a SpeedVac, and resuspended in 22.5 μL0.1% formic acid (FA), loaded into an autosampler tube, and stored at −80 °C until ready for mass spectrometry analysis.
Input fractionation
The input sample was resuspended in 0.5 ml of 0.1% TFA and diluted to 0.33 mg/ml in 0.1% TFA. Fractionation columns (Pierce, High pH Reversed-Phase Peptide Fractionation Kit, 84868) were conditioned according to the manufacturer's instructions, and 300 μl of sample was loaded onto a fractionation column and centrifuged at 3,000 × g for 2 min at RT. The sample was washed with 300 μl water followed by 300 μl of 5% ACN, 0.1% TFA and eluted in eight fractions containing increasing amount of ACN. The fractions were dried in a SpeedVac. Sample fractions were resuspended in 10 μl of 0.1% FA. Peptide fractions were quantified using a Pierce Quantitative Colorimetric Peptide Assay (Thermo Scientific, 23275).
Nano-LC-MS/MS
All samples were subjected to nanoLC-MS/MS analysis using an EASY-nLC ultra-performance liquid chromatography system (Thermo Fisher Scientific) coupled to a Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific) via an EASY-Spray nanoelectrospray ionization source (Thermo Fisher Scientific). Prior to injection, the succinylpeptide sample was resuspended in 22.5 μl of 0.1% FA, and each of the high pH reversed-phase peptide fractions of the input material were resuspended in enough 0.1% FA to achieve a peptide concentration around 0.15 μg/μl (which was determined precisely using the peptide quantitation assay described above). The succinylpeptide sample was analyzed with technical triplicate runs, with 6.5 μl of sample injected for each. The eight input HPRP fractions were analyzed singly with 1-μg injections (roughly 7 μl for each based on the precise concentration). For each injection, the sample was first trapped on an Acclaim PepMap 100 C18 trapping column (3-μm particle size, 75 μm × 20 mm) with 18 μl of solvent A (0.1% FA) at a variable flow rate dictated by a maximum pressure of 500 bar, after which the analytical separation was performed over a 105-min gradient (flow rate of 300 nl/min) of 5% to 40% solvent B (90% ACN, 0.1% FA) using an Acclaim PepMap RSLC C18 analytical column (2-μm particle size, 75 μm × 500 mm column (Thermo Fisher Scientific) with a column temperature of 55 °C. MS1 (precursor ions) was performed at 70,000 resolution with an AGC target of 3 × 106 ions and a maximum injection time of 60 ms. MS2 spectra (product ions) were collected by data-dependent acquisition of the top 10 most abundant precursor ions with a charge greater than 1 per MS1 scan, with dynamic exclusion enabled for a window of 30 s. Precursor ions were filtered with a 0.7 m/z isolation window and fragmented with a normalized collision energy of 30. MS2 scans were performed at 35,000 resolution, with an AGC target of 1 × 105 ions and a maximum injection time of 60 ms.
Raw data processing
Raw LC-MS/MS data have been deposited to the ProteomeXchange Consortium via the PRIDE (31) partner repository with the dataset identifier PXD008728. Raw LC-MS/MS data were processed in Proteome Discoverer v2.2 (PD2.2, Thermo Fisher Scientific) using both the Sequest HT (32) and MS Amanda 2.0 (33) search engines. Data were searched against the UniProt mouse complete proteome database of reviewed (Swiss-Prot) and unreviewed (TrEMBL) proteins, which consisted of 52,015 sequences on the date of download (September 23, 2017). Default search parameters included oxidation (15.995 Da on M) as a variable modification and carbamidomethyl (57.021 Da on C) and TMT (229.163 Da on peptide N-term and K). To assess labeling efficiency as a quality control measure, the input fraction was researched with N-terminal TMT as a variable modification, confirming N-terminal labeling of 88% of all peptide spectral matches (PSMs). Succinyl runs added succinyl (100.01604 Da on K) as a variable modification and changed TMT to a variable modification on K (remaining fixed on peptide N-term). Data were searched with a 10 ppm precursor mass and 0.02 Da product ion tolerance. The maximum number of missed cleavages was set to a default value of 2 (but changed to 4 for succinyl runs), and enzyme specificity was trypsin (full). Considering each data type (succinyl, input) separately, PSMs from each search algorithm were filtered to a 1% false discovery rate (FDR) using the Percolator (34) node of PD2.2. For succinyl data, site localization probabilities were determined for succinyl lysines using the ptmRS algorithm (35). PSMs were grouped to unique peptides while maintaining a 1% FDR at the peptide level and using a 90% site localization threshold for succinyl lysines. Peptides from all samples (succinyl, input) were grouped to proteins using the rules of strict parsimony, and proteins were filtered to 1% FDR using the Protein FDR Validator node of PD2.2. Reporter ion intensities for all PSMs having co-isolation interference below 50% (of the ion current in the isolation window) and average reporter S/N > 2.5 were summed together at the peptide group and protein level but keeping quantification for each data type (succinyl, input) separate. Peptides shared between protein groups were excluded from protein quantitation calculations.
Statistical analysis
Protein and peptide groups tabs in the PD2.2 results were exported as tab delimited .txt files, opened in Microsoft Excel, and analyzed as described previously (36). First, peptide group reporter intensities for each peptide group in the input material were summed for each TMT channel, and each channel's sum was divided by the average of all channels' sums, resulting in channel-specific loading control normalization factors to correct for any deviation from equal protein/peptide input between each sample. Reporter intensities for peptide groups from the succinyl fraction and for proteins from the input fraction were divided by the loading control normalization factors for each respective TMT channel. Analyzing the acetylpeptide and protein datasets separately, all loading control–normalized TMT reporter intensities were converted to log2 space, and the average value from the six samples was subtracted from each sample-specific measurement to normalize the relative measurements to the mean. For each genotype–age condition, condition average and standard deviation were calculated. For each genotype comparison (Sirt5 KO versus control) at each age (15 and 31 weeks) and each age comparison (15 versus 31 weeks) within each genotype (Sirt5 KO and control), log2 -fold change, p value (two-tailed student's t test, assuming equal variance), and adjusted p value (Benjamini–Hochberg FDR correction) were calculated (37, 38). For protein-level quantification, only Master Proteins, or the most statistically significant protein representing a group of parsimonious proteins containing common peptides identified at 1% FDR, were used for quantitative comparison. Succinylpeptide measurements were calculated both alone (referred to as relative abundance) and with normalization to any change in the corresponding master protein (referred to as relative occupancy), calculated by subtracting log2 master protein values from succinylpeptide quantitation values on a sample-specific basis.
Transverse aortic constriction
Pressure overload in mice was performed by Lan Mao of the Duke Cardiovascular Physiology Core and was induced using methods described previously (39), except that the suture was placed between the left carotid and the left axillary arteries. Serial echocardiography was performed on conscious mice from all groups with a Vevo 2100 high-resolution imaging system (VisualSonics) as described previously.
Metabolite extraction
10 to 20 mg of frozen crushed heart tissue was weighed in an Eppendorf tube, and 200 μl of ice-cold 80% methanol was added. A glass bead was added and homogenized using a TissueLyzer for 2 min at 30 Hz. 300 μl of ice-cold 80% methanol was added, vortexed, and incubated on ice for 10 min. Tissue extract was centrifuged at 20,000 × g at 4 °C for 10 min. Supernatant containing 2 mg of tissue was transferred to a new Eppendorf tube and dried in vacuum concentrator at room temperature. The dry pellets were reconstituted into 30 μl (per 2 mg tissue) of sample solvent (water:methanol:acetonitrile, 2:1:1, v/v/v), and 3 μl was further analyzed by LC-MS.
LC-MS method
An Ultimate 3000 UHPLC (Dionex) was coupled to an Q Exactive Plus mass spectrometer (QE-MS, Thermo Scientific) for metabolite profiling. A hydrophilic interaction chromatography method (HILIC) employing an Xbridge amide column (100 × 2.1 mm inner diameter, 3.5 μm; Waters) was used for polar metabolite separation. The LC method was described previously in detail (40); mobile phase A was replaced with water containing 5 mm ammonium acetate (pH 6.8). The QE-MS is equipped with a heated electrospray ionization probe with related parameters set as follows: heater temperature, 120 °C; sheath gas, 30; auxiliary gas, 10; sweep gas, 3; spray voltage, 3.0 kV for the positive mode and 2.5 kV for the negative mode; capillary temperature, 320 °C; S-lens, 55; scan range (m/z), 70 to 900 for positive mode (1.31 to 12.5 min) and negative mode (1.31 to 6.6 min) and 100 to 1,000 for negative mode (6.61 to 12.5 min); resolution, 70,000; automated gain control (AGC), 3 × 106 ions. Customized mass calibration was performed before data acquisition.
Metabolomics data analysis
LC-MS peak extraction and integration were performed using the commercially available software Sieve 2.2 (Thermo Scientific). The peak area was used to represent the relative abundance of each metabolite in different samples. The missing values were handled as described in a previous study (40). Data were uploaded to Metaboanalyst for further downstream analysis (41, 42).
RT-qPCR
RNA was extracted from tissues using the RNeasy Mini Kit from Qiagen (74106). cDNA was made from 750 ng RNA using the iScript cDNA Synthesis Kit from Bio-Rad (170-8890). Amplification was performed using iTaq Universal SYBR Green Supermix from Bio-Rad (172-5121) on the QuantStudio 6 Flex (Thermo Fisher Scientific). 36B4 was used as a reference gene, and relative expression was calculated using the ΔΔCT method.
Author contributions
K. A. H. and M. D. H. conceptualization; K. A. H., D. M. A., J. L., and P. A. G. investigation; K. A. H., J. L., and P. A. G. methodology; K. A. H. and M. D. H. writing-original draft; K. A. H., D. M. A., P. A. G., and M. D. H. writing-review and editing; J. W. L., P. A. G., and M. D. H. supervision; M. D. H. funding acquisition; M. D. H. project administration.
Supplementary Material
Acknowledgments
We thank Lan Mao and Zhiqiang Chen for assistance with TAC surgeries and echocardiogram readings. We also thank Johan Auwerx (EPFL, Lausanne, Switzerland) for the generous gift of the SIRT5fl/fl mice and Howard Rockman and other members of K. A. H.'s dissertation committee for feedback. Cardiac phenotyping services were supported by the Edna and Fred L. Mandel Jr. Foundation for Hypertension and Atherosclerosis at Duke.
This work was supported by American Heart Association Grants 12SDG8840004 and 12IRG9010008; NIA, National Institutes of Health Grant R01AG045351; and NIDDK, National Institutes of Health Award P30DK096493. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Table S1.
The mass spectrometric raw data and spectral libraries associated with this manuscript are available from ProteomeXchange with the accession number PXD008728.
- KO
- knockout
- MHC
- myosin heavy chain
- TMT
- tandem mass tag
- FDR
- false discovery rate
- FC
- -fold change
- RT
- room temperature
- TFA
- trifluoroacetic acid
- ACN
- acetonitrile
- FA
- formic acid
- AGC
- automated gain control
- PSM
- peptide spectral match
- cDNA
- complementary DNA
- ANOVA
- analysis of variance
- TCA
- tricarboxylic acid
- TAC
- transverse aortic constriction
- RT-qPCR
- quantitative real-time PCR
- SDHA
- succinate dehydrogenase complex, subunit A
- SPE
- solid phase extraction
- PCr
- phosphocreatine.
References
- 1. Peng C., Lu Z., Xie Z., Cheng Z., Chen Y., Tan M., Luo H., Zhang Y., He W., Yang K., Zwaans B. M. M., Tishkoff D., Ho L., Lombard D., He T. C., et al. (2011) The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics 10, M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Du J., Zhou Y., Su X., Yu J. J., Khan S., Jiang H., Kim J., Woo J., Kim J. H., Choi B. H., He B., Chen W., Zhang S., Cerione R. A., Auwerx J., et al. (2011) Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 10.1126/science.1207861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan M., Peng C., Anderson K. A., Chhoy P., Xie Z., Dai L., Park J., Chen Y., Huang H., Zhang Y., Ro J., Wagner G. R., Green M. F., Madsen A. S., Schmiesing J., et al. (2014) Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 19, 605–617 10.1016/j.cmet.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boylston J. A., Sun J., Chen Y., Gucek M., Sack M. N., and Murphy E. (2015) Characterization of the cardiac succinylome and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 88, 73–81 10.1016/j.yjmcc.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadhukhan S., Liu X., Ryu D., Nelson O. D., Stupinski J. A., Li Z., Chen W., Zhang S., Weiss R. S., Locasale J. W., Auwerx J., and Lin H. (2016) Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.1519858113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hershberger K. A., Abraham D. M., Martin A. S., Mao L., Liu J., Gu H., Locasale J. W., and Hirschey M. D. (2017) Sirtuin 5 is required for mouse survival in response to cardiac pressure overload. J. Biol. Chem. 10.1074/jbc.M117.809897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dittenhafer-Reed K. E., Richards A. L., Fan J., Smallegan M. J., Fotuhi Siahpirani A., Kemmerer Z. A., Prolla T. A., Roy S., Coon J. J., and Denu J. M. (2015) SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 21, 637–646 10.1016/j.cmet.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishida Y., Rardin M. J., Carrico C., He W., Sahu A. K., Gut P., Najjar R., Fitch M., Hellerstein M., Gibson B. W., and Verdin E. (2015) SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol. Cell. 10.1016/j.molcel.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirschey M. D., Shimazu T., Jing E., Grueter C. A., Collins A. M., Aouizerat B., Stančáková A., Goetzman E., Lam M. M., Schwer B., Stevens R. D., Muehlbauer M. J., Kakar S., Bass N. M., Kuusisto J., et al. (2011) SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell. 44, 177–190 10.1016/j.molcel.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandez-Marcos P. J., Jeninga E. H., Canto C., Harach T., de Boer V. C. J., Andreux P., Moullan N., Pirinen E., Yamamoto H., Houten S. M., Schoonjans K., and Auwerx J. (2012) Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci. Rep. 10.1038/srep00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pillai V. B., Sundaresan N. R., Kim G., Gupta M., Rajamohan S. B., Pillai J. B., Samant S., Ravindra P. V., Isbatan A., and Gupta M. P. (2010) Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J. Biol. Chem. 285, 3133–3144 10.1074/jbc.M109.077271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hafner A. V., Dai J., Gomes A. P., Xiao C.-Y., Palmeira C. M., Rosenzweig A., and Sinclair D. A. (2010) Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging 2, 914–923 10.18632/aging.100252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin A. S., Abraham D. M., Hershberger K. A., Bhatt D. P., Mao L., Cui H., Liu J., Liu X., Muehlbauer M. J., Grimsrud P. A., Locasale J. W., Payne R. M., and Hirschey M. D. (2017) Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich's ataxia cardiomyopathy model. JCI Insight 10.1172/jci.insight.93885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sundaresan N. R., Bindu S., Pillai V. B., Samant S., Pan Y., Huang J.-Y., Gupta M., Nagalingam R. S., Wolfgeher D., Verdin E., and Gupta M. P. (2015) SIRT3 Blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol. Cell. Biol. 36, 678–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sohal D. S., Nghiem M., Crackower M. A., Witt S. A., Kimball T. R., Tymitz K. M., Penninger J. M., and Molkentin J. D. (2001) Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 89, 20–25 10.1161/hh1301.092687 [DOI] [PubMed] [Google Scholar]
- 16. Bersell K., Choudhury S., Mollova M., Polizzotti B. D., Ganapathy B., Walsh S., Wadugu B., Arab S., and Kühn B. (2013) Moderate and high amounts of tamoxifen in αMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis. Model Mech. 6, 1459–1469 10.1242/dmm.010447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lexow J., Poggioli T., Sarathchandra P., Santini M. P., and Rosenthal N. (2013) Cardiac fibrosis in mice expressing an inducible myocardial-specific Cre driver. Dis. Model Mech. 6, 1470–1476 10.1242/dmm.010470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu J., Sadhukhan S., Noriega L. G., Moullan N., He B., Weiss R. S., Lin H., Schoonjans K., and Auwerx J. (2013) Metabolic characterization of a Sirt5 deficient mouse model. Sci. Rep. 3, 2806 10.1038/srep02806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mi H., Muruganujan A., Casagrande J. T., and Thomas P. D. (2013) Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 8, 1551–1566 10.1038/nprot.2013.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner G. R., and Hirschey M. D. (2014) Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol. Cell. 54, 5–16 10.1016/j.molcel.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner G. R., Bhatt D. P., O'Connell T. M., Thompson J. W., Dubois L. G., Backos D. S., Yang H., Mitchell G. A., Ilkayeva O. R., Stevens R. D., Grimsrud P. A., and Hirschey M. D. (2017) A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab. 25, 823–837.e8 10.1016/j.cmet.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner G. R., and Payne R. M. (2013) Widespread and enzyme-independent Nϵ-acetylation and Nϵ-succinylation in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 10.1074/jbc.M113.486753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horton J. L., Martin O. J., Lai L., Riley N. M., Richards A. L., Vega R. B., Leone T. C., Pagliarini D. J., Muoio D. M., Bedi K. C. Jr., Margulies K. B., Coon J. J., and Kelly D. P. (2016) Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2, e84897 10.1172/jci.insight.84897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeMaria A. N., Neumann A., Schubart P. J., Lee G., and Mason D. T. (1979) Systematic correlation of cardiac chamber size and ventricular performance determined with echocardiography and alterations in heart rate in normal persons. Am. J. Cardiol. 43, 1–9 10.1016/0002-9149(79)90036-5 [DOI] [PubMed] [Google Scholar]
- 25. Di Segni E., Preisman S., Ohad D. G., Battier A., Boyko V., Kaplinsky E., Perel A., and Vered Z. (1997) Echocardiographic left ventricular remodeling and pseudohypertrophy as markers of hypovolemia: an experimental study on bleeding and volume repletion. J. Am. Soc. Echocardiogr. 10, 926–936 10.1016/S0894-7317(97)80009-0 [DOI] [PubMed] [Google Scholar]
- 26. Polletta L., Vernucci E., Carnevale I., Arcangeli T., Rotili D., Palmerio S., Steegborn C., Nowak T., Schutkowski M., Pellegrini L., Sansone L., Villanova L., Runci A., Pucci B., Morgante E., et al. (2015) SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 11, 253–270 10.1080/15548627.2015.1009778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen W., and Abramowitz M. K. (2014) Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol. 15, 55 10.1186/1471-2369-15-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samak M., Fatullayev J., Sabashnikov A., Zeriouh M., Schmack B., Farag M., Popov A.-F., Dohmen P. M., Choi Y.-H., Wahlers T., and Weymann A. (2016) Cardiac hypertrophy: an introduction to molecular and cellular basis. Med. Sci. Monit. Basic. Res. 22, 75–79 10.12659/MSMBR.900437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winnik S., Auwerx J., Sinclair D. A., and Matter C. M. (2015) Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur. Heart J. 36, 3404–3412 10.1093/eurheartj/ehv290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang F., Wang K., Xu W., Zhao S., Ye D., Wang Y., Xu Y., Zhou L., Chu Y., Zhang C., Qin X., Yang P., and Yu H. (2017) SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. 19, 2331–2344 10.1016/j.celrep.2017.05.065 [DOI] [PubMed] [Google Scholar]
- 31. Vizcaíno J. A., Csordas A., del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., Xu Q.-W., Wang R., and Hermjakob H. (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 10.1093/nar/gkv1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yates J. R., 3rd (2015) Pivotal role of computers and software in mass spectrometry: SEQUEST and 20 years of tandem MS database searching. J. Am. Soc. Mass Spectrom. 26, 1804–1813 10.1007/s13361-015-1220-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dorfer V., Pichler P., Stranzl T., Stadlmann J., Taus T., Winkler S., and Mechtler K. (2014) MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. J. Proteome Res. 13, 3679–3684 10.1021/pr500202e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Käll L., Canterbury J. D., Weston J., Noble W. S., and MacCoss M. J. (2007) Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods. 4, 923–925 10.1038/nmeth1113 [DOI] [PubMed] [Google Scholar]
- 35. Taus T., Köcher T., Pichler P., Paschke C., Schmidt A., Henrich C., and Mechtler K. (2011) Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 10, 5354–5362 10.1021/pr200611n [DOI] [PubMed] [Google Scholar]
- 36. McDonnell E., Crown S. B., Fox D. B., Kitir B., Ilkayeva O. R., Olsen C. A., Grimsrud P. A., and Hirschey M. D. (2016) Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell Rep. 17, 1463–1472 10.1016/j.celrep.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benjamini Y., and Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. STOR. 57, 289–300 10.2307/2346101 [DOI] [Google Scholar]
- 38. Lesack K., and Naugler C. (2011) An open-source software program for performing Bonferroni and related corrections for multiple comparisons. J. Pathol. Inform. 2, 52 10.4103/2153-3539.91130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rockman H. A., Ross R. S., Harris A. N., Knowlton K. U., Steinhelper M. E., Field L. J., Ross J. Jr., and Chien K. R. (1991) Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 88, 8277–8281 10.1073/pnas.88.18.8277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu X., Ser Z., and Locasale J. W. (2014) Development and quantitative evaluation of a high-resolution metabolomics technology. Anal. Chem. 86, 2175–2184 10.1021/ac403845u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xia J., Sinelnikov I. V., Han B., and Wishart D. S. (2015) MetaboAnalyst 3.0: making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xia J., and Wishart D. S. (2016) Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinformatics 55, 14.10.1–14.10.91 10.1002/cpbi.11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.