Abstract
Mutations in thin filament regulatory proteins that cause hypertrophic cardiomyopathy (HCM) increase myofilament Ca2+ sensitivity. Mouse models exhibit increased Ca2+ buffering and arrhythmias, and we hypothesized that these changes are primary effects of the mutations (independent of compensatory changes) and that increased Ca2+ buffering and altered Ca2+ handling contribute to HCM pathogenesis via activation of Ca2+-dependent signaling. Here, we determined the primary effects of HCM mutations on intracellular Ca2+ handling and Ca2+-dependent signaling in a model system possessing Ca2+-handling mechanisms and contractile protein isoforms closely mirroring the human environment in the absence of potentially confounding remodeling. Using adenovirus, we expressed HCM-causing variants of human troponin-T, troponin-I, and α-tropomyosin (R92Q, R145G, and D175N, respectively) in isolated guinea pig left ventricular cardiomyocytes. After 48 h, each variant had localized to the I-band and comprised ∼50% of the total protein. HCM mutations significantly lowered the Kd of Ca2+ binding, resulting in higher Ca2+ buffering of mutant cardiomyocytes. We observed increased diastolic [Ca2+] and slowed Ca2+ reuptake, coupled with a significant decrease in basal sarcomere length and slowed relaxation. HCM mutant cells had higher sodium/calcium exchanger activity, sarcoplasmic reticulum Ca2+ load, and sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) activity driven by Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylation of phospholamban. The ryanodine receptor (RyR) leak/load relationship was also increased, driven by CaMKII-mediated RyR phosphorylation. Altered Ca2+ homeostasis also increased signaling via both calcineurin/NFAT and extracellular signal–regulated kinase pathways. Altered myofilament Ca2+ buffering is the primary initiator of signaling cascades, indicating that directly targeting myofilament Ca2+ sensitivity provides an attractive therapeutic approach in HCM.
Keywords: cardiomyopathy, troponin, tropomyosin, Ca2+/calmodulin-dependent protein kinase II (CaMKII), extracellular-signal-regulated kinase (ERK), NFAT transcription factor, SERCA
Introduction
Hypertrophic cardiomyopathy (HCM)4 is an autosomal dominant disorder, usually caused by a single heterozygous genetic variant shared by all affected family members. It is the most common inherited cardiac disorder with a prevalence of 1 in 500 (1) and the leading cause of sudden death in young adults and athletes in particular (2). Mutations underlying the disease are principally found in genes that encode components of the contractile apparatus (3). The most commonly affected genes encode the thick filament proteins myosin-binding protein C (MyBPC) and β-myosin heavy chain (MyHC), whereas other HCM genes encode the thin filament regulatory proteins cardiac troponin T (cTnT), cardiac troponin I (cTnI), and α-tropomyosin (α-TM). We and others have established that HCM mutations in thin filament regulatory proteins increase myofilament Ca2+ sensitivity of actomyosin ATPase activity, in vitro motility and force of skinned muscle fibers (4–7). Furthermore, we have shown that this is due to an increase in actual Ca2+ affinity of the low-affinity regulatory Ca2+-binding site of cTnC (8, 9). Other HCM mutations (e.g. in MYH7 (10) and MYBPC3 (11)) are also predicted to increase troponin C Ca2+ binding caused by the effects of additional cross-bridge formation on cooperative thin filament activation. Thus, heightened myofilament Ca2+ sensitivity is likely to be a consistent feature of HCM mutations. Troponin C is the principal dynamic buffer of cytoplasmic Ca2+ and has been estimated to bind approximately half of the Ca2+ ions released by the sarcoplasmic reticulum (SR) during systole (12). We predict that the increased myofilament Ca2+ affinity will directly alter intracellular Ca2+ homeostasis in patients with HCM via increasing myofilament Ca2+ buffering. Increased buffering would cause deleterious changes to intracellular Ca2+ cycling, which may trigger Ca2+-dependent hypertrophic signaling and increase the probability of arrhythmic events. Recent work on transgenic mice containing troponin mutations has provided evidence of these outcomes, although whether this is due to primary effects of the mutant protein or compensatory changes is unclear. Some studies have shown profound increases in basal [Ca2+]i in the presence of increased Ca2+ buffering (13, 14), whereas others have found the opposite effect depending on the age of the mice (15, 16). In this study we have systematically tested the changes to both Ca2+ cycling and Ca2+-dependent signaling in a stable but short-term cardiomyocyte model of HCM. This approach allows evaluation of the direct cellular consequences of a HCM mutation, free of the secondary effects of pathological remodeling caused by compensatory (or maladaptive) gene expression. For example, reduction of SERCA levels (17) and myofilament protein isoform switching (18), both well known hallmarks of heart failure and cardiomyopathy, would be expected to confound electrophysiological and contractile changes caused by the primary mutation in animal models and patients. We have used guinea pig left ventricular cardiomyocytes to model human cardiomyocyte Ca2+ cycling more accurately than previous studies published in transgenic mice. Mouse cardiomyocytes fundamentally differ from both human and guinea pig in structure and function. For example, they contain predominantly fast α-MyHC versus slow β-MyHC in human and guinea pig (19). The generation of Ca2+ transients relies almost entirely on Ca2+-induced Ca2+ release from the SR with very little contribution from NCX current, whereas in humans and guinea pigs, the NCX contribution is ∼30% (20). Cardiac action potentials in mice lack any appreciable plateau and differ markedly in waveform (21), indicating a restructured electrophysiological regulation.
We have engineered adenoviruses to express WT and R92Q cTnT, WT, and R145G cTnI, and WT and D175N α-TM. By measuring the impact of mutations affecting three different sarcomeric proteins, we aimed to identify hallmark changes in Ca2+ handling in HCM. Furthermore, we present for the first time a novel analytical paradigm to fully incorporate the consequences of Ca2+ buffering on cardiomyocyte Ca2+ transients and Ca2+ handling protein activities. We consider the dynamics of total intracellular Ca2+ ([Ca2+]total) derived from NCX integral measurements rather than free Ca2+ ([Ca2+]i) from fura2 fluorescence when assessing SR load, fractional SR release, and SERCA activity. When applied, our data show that the primary effects of HCM mutant gene expression include altered Ca2+ transients and increased myofilament Ca2+ buffering, increased [Ca2+] in both the SR and cytoplasm, and activation of Ca2+-dependent signaling mediated by Ca2+/calmodulin-dependent protein kinase II (CaMKII), calcineurin/nuclear factor of activated T-cells (NFAT), and mitogen-activated protein kinase/ERK.
Results
Adenoviral expression of human troponin and tropomyosin in guinea pig cardiomyocytes
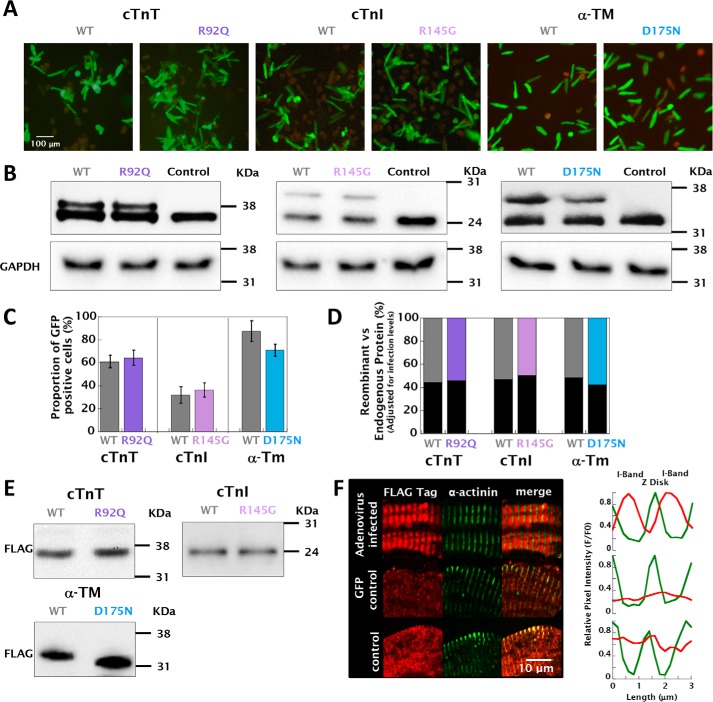
Isolated guinea pig left ventricular cardiomyocytes were infected with ∼1000 MOI of recombinant adenovirus. The level of infection was between 32.1 ± 7.2 (WT TnI) and 87.6 ± 9.1% (WT TM), calculated from the coexpression of human recombinant GFP (Fig. 1, A and C). The relative expression of thin filament regulatory protein subunits was determined by Western blotting and subsequently adjusted by the infection levels for each cell preparation tested. Conjugation of an N-terminal (cTnT and cTnI) or C-terminal (α-TM) FLAG tag increased the molecular weight of the recombinant proteins sufficiently to allow differentiation from the endogenous subunit (Fig. 1E). The relative expression level of recombinant protein in purified cardiomyocytes coexpressing human recombinant GFP was found to be 54.3 ± 9.5% (cTnT R92Q), 49.3 ± 8.5% (cTnI R145G), and 57.6 ± 7.5% (TM D175N) (Fig. 1, B and D), thus likely to closely reflect the levels of mutant protein found in patients with autosomal dominant cardiomyopathy. The recombinant FLAG-tagged thin filament proteins were localized to the I-band of the myofilaments of infected cardiomyocytes (Fig. 1F and Fig. S2). To assess the impact of culture time on cardiomyocyte function, we compared the contractility and Ca2+ transients of cardiomyocytes cultured for 1, 24, and 48 h along with the extent of t-tubular dedifferentiation (Fig. S3). We observed a small but significant reduction in the number of t-tubules and a prolongation in the relaxation and Ca2+ reuptake after 48 h (Fig. S4 and Table S1).
Figure 1.
Recombinant adenoviral infection, protein expression, and localization in guinea pig left ventricular cardiomyocytes. A shows the representative GFP expression in cardiomyocytes infected with recombinant adenovirus at ∼1000 MOI 48 h after transfection. B shows the relative protein expression of FLAG-tagged human recombinant WT/R92Q cTnT, WT/R145G cTnI, and WT/D175N α-TM compared with endogenous guinea pig subunits probed using anti-cTnT, anti-cTnI, and anti-α-TM primary antibodies, respectively. The third lane of each gel in B shows that endogenous protein in uninfected control cardiomyocytes is higher than endogenous levels from infected cardiomyocytes, therefore suggesting that recombinant protein replaces the endogenous at the myofilament. The bar graph in C shows the relative infection ratio of each virus as a percentage of green cells, whereas D shows the relative expression levels of endogenous (dark gray bars) versus recombinant (colored bars) protein, calculated from densitometery measurements of doublet bands in B multiplied by the relative expression levels of each virus calculated in C. E shows Western blots for cell lysates expressing each different recombinant protein using anti-FLAG tag primary antibody. F shows a magnified image of adenovirally expressed FLAG-tagged protein localized to the I-band in cardiomyocytes. Colocalization used an anti-FLAG tag primary antibody (conjugated to Alexa 568, red), with counterstain provided using an α-actinin antibody (conjugated to Alexa 633, false colored green) to stain the z disks. Colocalization was confirmed in the adjacent intensity profile plots. I-band staining usually presents as a doublet; however, because of the contracted nature of isolated cells, the signal from the I-band appeared as a single band with the limited resolution provided by the confocal microscope. (A full breakdown of the localization of each recombinant protein used can be found in Fig. S2.)
The presence of the Ca2+-sensitizing mutations increases myofilament Ca2+ buffering and drives altered Ca2+ homeostasis
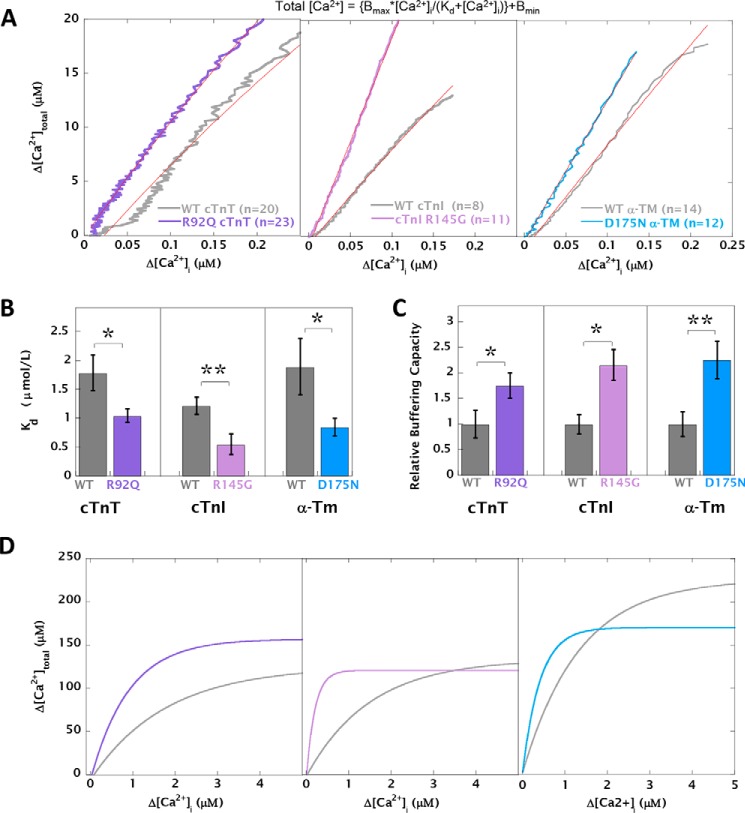
[Ca2+]total and [Ca2+]i were measured upon the application of 10 mm caffeine using simultaneous measurement of fura2 fluorescence ratio and whole-cell voltage-clamp current in a method adapted from Trafford et al. (22) (Fig. S6). We postulated that increasing the Ca2+ affinity of myofilaments by introducing a Ca2+ sensitizing HCM mutation will in turn increase total Ca2+ buffering in the intact cardiomyocytes. We fitted the calibrated total versus free [Ca2+]i data to a Michaelis–Menten type equation (Fig. 2A). The Kd of Ca2+ buffering was significantly decreased in the presence of each HCM-causing mutation (Fig. 2B and Table S2). However, the total Ca2+ occupancy of the myofilaments (Bmax) was not significantly altered between groups (Table S2). The relative buffering at low [Ca2+] is described by Bmax/Kd, and this ratio was increased for all three mutants compared with WT (Fig. 2C). The calculated relationship between [Ca2+]total and [Ca2+]i over the full range of [Ca2+] is shown in Fig. 2D. We have used these fits to convert observed [Ca2+]free to [Ca2+]total for each cell and calculate changes in SR load and SERCA activity. This incorporates the effect of altered Ca2+ buffering when considering these key Ca2+-handling parameters.
Figure 2.
HCM mutations acutely decrease the apparent Kd of cytosolic Ca2+ buffering. Cardiomyocytes expressing WT or mutant cTnT/cTnI/α-TM were simultaneously assessed to measure [Ca2+]i by fura2 fluorescence and NCX current (to calculate [Ca2+]total) by voltage clamping upon the rapid application of 10 mm caffeine. A, plots of Δ[Ca2+]i versus Δ[Ca2+]total for cardiomyocytes containing WT cTnT (n = 20) versus R92Q cTnT (n = 23), WT cTnI (n = 9) versus R145G cTnI (n = 11), and WT α-TM (n = 12) versus D175N α-TM (n = 14) reveal increased buffering capacity in cells containing HCM mutants. The red lines show best fits to the following: [Ca2+]total = {Bmax*[Ca2+]i/(Kd + [Ca2+]i)} + Bmin. B, bar graph comparing mean Kd values for the fits calculated in A. C, bar graph showing the relative estimated changes in buffering capacity (calculated from relative Kd and Bmax values for individual cells undergoing the buffing protocol), where mutant calls have been compared with WT-infected cells set to 1. D, cytosolic buffering curves over a wide range of Ca2+ concentration drawn using the Kd and Bmax values calculated in A. Comparison of WT with HCM mutant: **, p < 0.01; *, p < 0.05.
Cardiomyocytes expressing Ca2+-buffering HCM-causing mutations exhibit altered Ca2+ transients and contractility
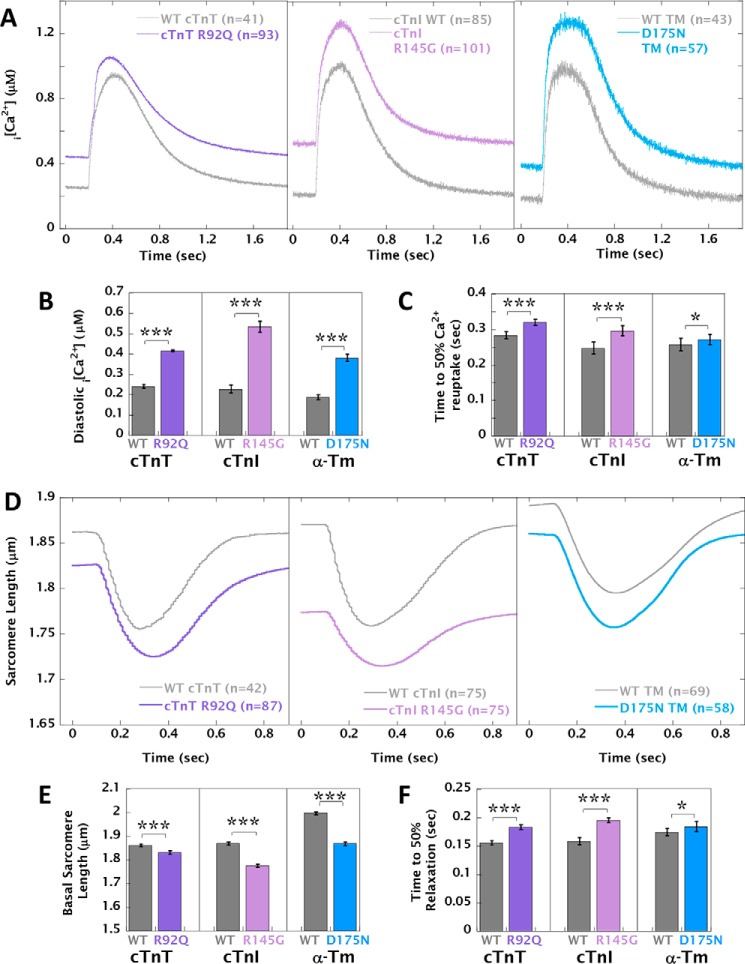
In analyses of cytosolic [Ca2+]i transients at 0.5-Hz pacing (Fig. 3A), cells expressing HCM-causing mutations had significantly higher diastolic [Ca2+]i (Δ[Ca2+]i = 0.175 ± 0.007 to 0.307 ± 0.011 μm) (Fig. 3B and Table S3A) and significantly prolonged rates of Ca2+ reuptake (Δτ = 0.027 ± 0.003 to 0.041 ± 0.002 s−1) (Fig. 3C and Table S3A) compared with cardiomyocytes expressing the corresponding WT protein. The time to 50% Ca2+ release from the SR and the Ca2+ transient amplitude were not significantly different between groups. In measurements of sarcomere shortening at 1-Hz pacing, all three HCM mutations were found to decrease basal sarcomere length compared with WT (ΔSL = −0.030 ± 0.007 to −0.094 ± 0.007 μm) in line with the higher levels of diastolic [Ca2+]i. Cardiomyocytes expressing HCM mutations also had significantly prolonged relaxation (ΔT50 = 0.011 ± 0.002 to 0.037 ± 0.003 s) (Fig. 3, D–F, and Table S3B). Cardiomyocytes expressing the R145G mutation in cTnI displayed reduced fractional shortening compared with WT; these cells also had the shortest diastolic sarcomere length (1.776 ± 0.007 versus 1.865 ± 0.009 μm in WT), and this may impose some limit on the extent of shortening compared with the other two mutations, because of fura2 accumulation (23) and resultant Ca2+ chelation in our loaded cardiomyocytes. The phenotype conferred by HCM mutations in fura2-loaded cardiomyocytes at 0.5 Hz was qualitatively the same, despite reduced fractional shortening caused by the presence of the chemical dye (Fig. S7 and Table S3C).
Figure 3.
Unloaded sarcomere shortening and Ca2+ transient measurements in adenovirally infected guinea pig left ventricular cardiomyocytes. A, intracellular Ca2+ transients of cardiomyocytes loaded with 1 mm fura2 at 0.5 Hz pacing, expressing HCM-causing mutations cTnT R92Q, cTnI R145G, and α-TM D175N compared with cardiomyocytes infected with the equivalent WT protein. B and C, the average diastolic [Ca2+]i and time to 50% Ca2+ reuptake, respectively. D, the corresponding unloaded sarcomere shortening measurements at 1-Hz pacing. E and F, the average basal sarcomere length and time to 50% relaxation, respectively. The pairwise sarcomere shortening of fura2-loaded cardiomyocytes acquired at 0.5 Hz is shown in Fig. S7. Each curve was averaged from multiple cells (n) taken from at least four separate cell preparations; total n numbers are given in the legends of each plot. Comparison of WT with HCM mutant: ***, p < 0.001; *, p < 0.05.
Increased myofilament Ca2+ buffering results in higher peak NCX current, SR load, and RyR receptor leak/load relationship along with activation of CaMKII signaling
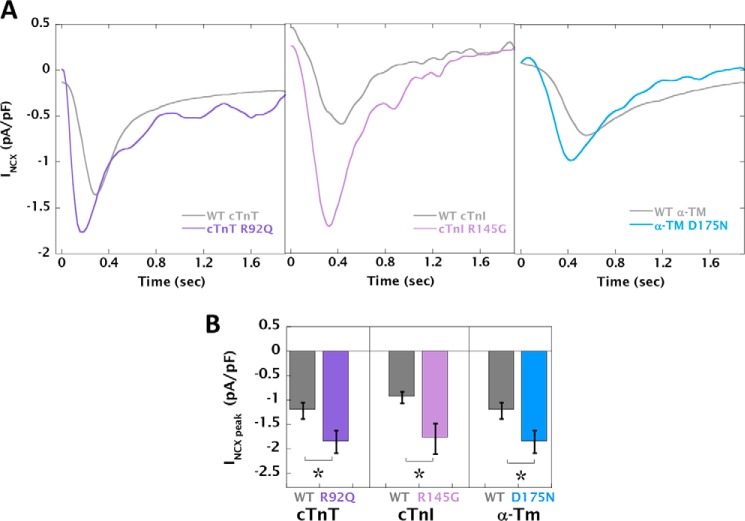
The NCX current was measured during the direct application of 10 mm caffeine by whole-cell voltage-clamp recordings (Fig. S6). The peak INCX current was significantly increased in cardiomyocytes expressing the HCM mutants. (Fig. 4, A and B, and Table S4). Simultaneous measurement of L-type Ca2+ current showed no difference between WT and HCM mutant cardiomyocytes (Fig. S8 and Table S4).
Figure 4.
HCM mutant-infected cardiomyocytes have increased NCX current. A, representative traces from voltage-clamp recordings during the application of 10 mm caffeine. B, the average peak amplitude for WT cTnT (n = 24), cTnT R92Q (n = 23), WT cTnI (n = 8), cTnI R145G (n = 8), WT α-TM (n = 15), and α-TM D175N (n = 19). Comparison of WT with HCM mutant: *, p < 0.05.
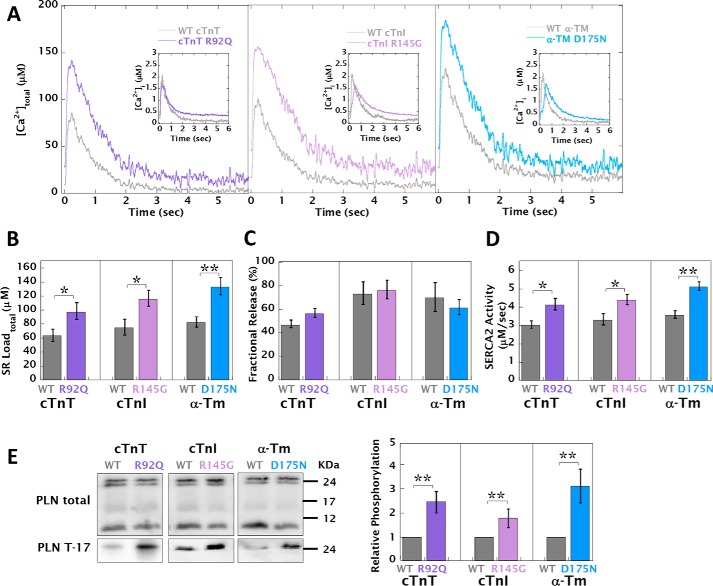
Using a standard method (24) that utilizes the fura2-based measurement of caffeine [Ca2+]i transients, SR load, fractional SR Ca2+ release, and SERCA activity were calculated and found to be decreased, increased, and unchanged, respectively (Fig. 5A, insets, and Table S5A). Furthermore, we found that these alterations were independent of pause duration prior to caffeine spritz (Table S6). However, we also calculated caffeine and Ca2+ transients expressed as [Ca2+]total derived from the NCX current integral, thus incorporating any alterations to Ca2+ buffering, and used this approach to recalculate SR load, fractional SR Ca2+ release and SERCA activity (Fig. 5A and Table S5B). HCM mutant cells consistently displayed a higher SR load (Δ[Ca2+]total = 34.1 ± 3.2 to 51.3 ± 8.2 μm) (Fig. 5B). The preceding [Ca2+]i transient amplitude (Table S5A) was also converted to [Ca2+]total (Table S5B) to calculate the fractional SR Ca2+ release and SERCA2 activity. HCM cells had unchanged SR fractional release compared with WT (Fig. 5C) and had increased SERCA2 activity (Δrate = 1.53 ± 0.30 to 3.87 ± 0.42 s−1) (Fig. 5D).
Figure 5.
HCM mutant-infected cardiomyocytes have increased SR load, fractional SR Ca2+ release, and SERCA2 activity via CaMKII-dependent PLN phosphorylation. A, representative caffeine-induced [Ca2+]total transients generated by the direct perfusion of 10 mm caffeine after a 5-s pause of pacing comparing WT and HCM mutant-transfected cardiomyocytes, where each transient amplitude has been adjusted from [Ca2+]i (inset) to [Ca2+]total using the buffering curves in Fig. 1A. Bar graphs show the SR load of [Ca2+]total (B), fractional release of Ca2+ (calculated from the [Ca2+]total caffeine transient amplitudes in B and Table S5B subtracted from the preceding [Ca2+]total transient amplitude shown in Table S5B) (C), and the SERCA2 activity (calculated by the subtraction of [Ca2+]total τ-decay constants (total) from the [Ca2+]total caffeine transient (NCX) τ-decay rates Table S5B) (D). The extracted parameters from [Ca2+]i baseline and caffeine transients are also tabulated in Table S5A. E, representative Western blots of total and phosphothreonine 17 PLN. The bar graph shows the densitometric quantification of phosphorylation increase (n = 5). All preparations were paced for 8 h at 0.5 Hz. **, p < 0.01; *, p < 0.05; ns, p > 0.05.
The increases in SR load and SERCA2 activity appear to be driven by increased CaMKII-dependent phosphorylation of phospholamban (PLN). Relative phosphothreonine 17 PLN levels were increased in HCM cells (between 87 ± 27 and 220 ± 72% more than WT) (Fig. 5E), whereas the PKA site at serine 16 of PLN and absolute levels of NCX and SERCA2 remained unaltered (Fig. S9). This result also confirms the need to consider the [Ca2+]total transient amplitudes when investigating SERCA2 activity and SR load in cardiomyocytes with altered Ca2+ buffering.
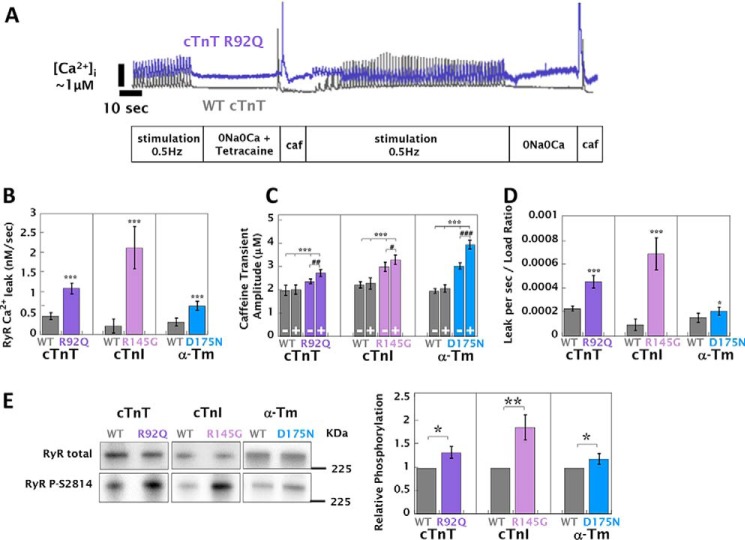
Increased SR load and diastolic [Ca2+]i in HCM cardiomyocytes suggest there may be Ca2+ leak from the RyR. To test this, we used a method adapted from Shannon et al. (25) (Fig. 6A) and found that RyR leak is profoundly increased in cells expressing HCM mutations (Fig. 6B). It was also observed that RyR blockade with tetracaine increased the [Ca2+]i caffeine transient amplitude by 12–25% in HCM cells (Fig. 6C) but had no effect on WT-infected or uninfected control myocytes. Despite the combination of increased leak (Fig. 6B) and [Ca2+]i SR load (adjusted for myofilament buffering calculated in Fig. 2C) (Fig. 6C), the relative leak load relationship was still higher in HCM cardiomyocytes (Δμm/s/μm = 5.24 × 10−5 ± 1.65 × 10−5 to 2.22 × 10−4 ± 3.75 × 10−5) (Fig. 6D), suggesting that other factors may be driving RyR channel open probability. We measured the phosphorylation status of each of the known regulatory sites on the RyR (serine 2030, 2808, and 2814) and found that (consistent with changes in PLN phosphorylation), there was higher phosphorylation at the RyR CaMKII site serine 2814 (20 ± 11 to 87 ± 27% greater than WT) (Fig. 6E); phosphorylation at the PKA/PKG sites (serine 2030 and 2808) did not differ between WT and HCM mutant cardiomyocytes (Fig. S9).
Figure 6.
HCM mutant-infected cardiomyocytes have increased RyR leak/load relationships and CAMKII activation of RyR. SR Ca2+ load and Ca2+ leak via the RyR were assessed by the sequential perfusion of 1.8 mm CaCl2 (A), Na+- and Ca2+-free solution containing 1 mm tetracaine for 50 s (B), the direct application of 10 mm caffeine (C), 1.8 mm CaCl2 for 100 s (D), Na+- and Ca2+-free solution for 50 s (E), and a direct application of 10 mm caffeine (F). A, representative experimental traces for WT cTnT (gray) and cTnT R92Q (purple) cardiomyocytes. All mutants tested were analogous to cTnT R92Q, whereas all control and WT-infected cells tested resembled WT cTnT. B, the observed RyR-dependent leak rate. C, the buffering-adjusted caffeine transient amplitude taken after perfusion with either 0Ca0Na (−) or with 0Na0Ca solution containing 1 μm tetracaine (+). D, the leak/load ratio, calculated as the leak rate divided by the caffeine transient amplitude at the end of each experiment. Each bar graph is an average of 30 cells, 15 of which were acquired as shown in A or B, whereas a further 15 were acquired by reversing the sequence of 0Na0Ca solution with or without 1 μm tetracaine to prevent errors from fura2 signal degradation and cell fatigue. A full breakdown of Δ[Ca2+]i for each perfusion switch can be found in Fig. S10, and the extracted parameters are also tabulated in Table S7. Representative Western blots of total and phosphoserine 2814 RyR are given in E, with the adjacent bar graph showing the average change in phosphorylation from densitometry measurements, all preparations were paced for 8 h at 0.5 Hz. Unpaced preparations showed no significant changes at the same site (data not shown). ***, p < 0.001; **, p < 0.01; *, p < 0.05 for comparing WT to HCM mutant. ###, p < 0.001; ##, p < 0.01; #, p < 0.05 for comparing the presence or absence of 1 mm tetracaine.
Altered Ca2+ homeostasis in HCM mutant cardiomyocytes activates NFAT and ERK signaling
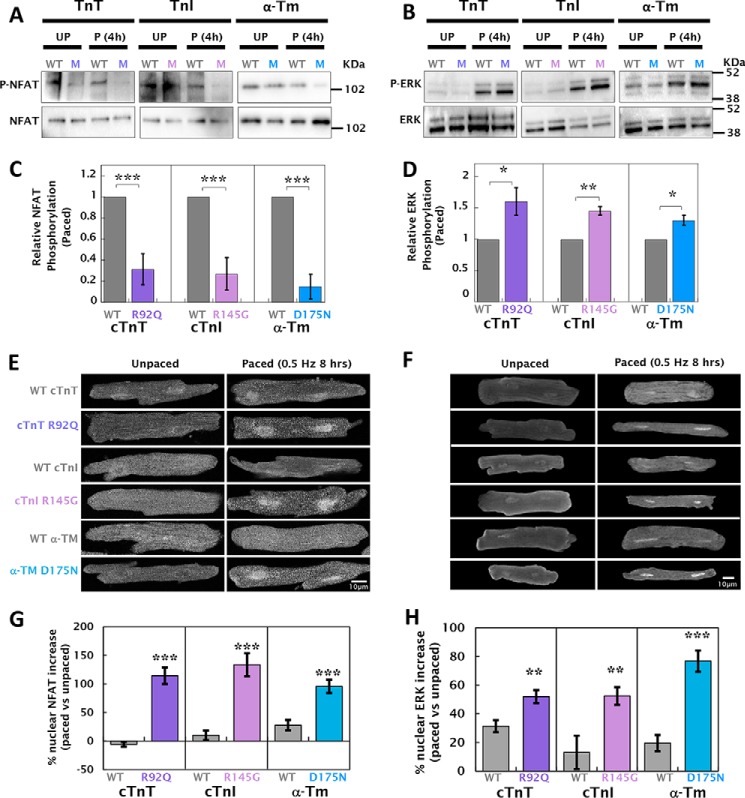
Ca2+-dependent signaling was assessed by the phosphorylation status and immunolocalization of the NFAT and ERK, two key mediators of HCM pathogenesis (26). Fig. 7 (A and B) show that both NFAT and ERK phosphorylation (NFAT at serine 165 and ERK at threonine 202/tyrosine 204) are altered in paced cardiomyocytes containing HCM mutations. NFAT-c3 phosphorylation is unchanged in unpaced cells but is substantially dephosphorylated in paced (0.5 Hz for 8 h) HCM mutant cardiomyocytes (phosphorylation reduced by 68 ± 15 to 85 ± 12% compared with WT) (Fig. 7C). ERK phosphorylation is increased in WT cells upon pacing but significantly more in the presence of HCM mutations (30 ± 8 to 60 ± 22% greater than WT) (Fig. 7D). Concordant nuclear translocation of NFAT-c3 and ERK is shown in Fig. 7 (E and F). In the absence of pacing, the distribution of NFAT-c3 was equal between the nucleus and cytosol in both mutant and WT cells (Table S8). However, the presence of a Ca2+-sensitizing HCM mutation in cells that had been paced for 8 h prior to fixation caused a 95.6 ± 11.6 to 133.6 ± 20.2% increase in the nuclear localization of NFAT-c3 (Fig. 7G). Pacing of WT cardiomyocytes resulted in a 13.1 ± 11.6 to 31.4 ± 4.2% increase in nuclear ERK; however, HCM cardiomyocytes had significantly higher nuclear translocation (52.1 ± 4.5 to 76.8 ± 7.4%) (Fig. 7H and Table S8).
Figure 7.
HCM mutant-infected cardiomyocytes have altered NFAT and ERK phosphorylation resulting in increased nuclear localization. A, total NFAT and phosphoserine 165 NFAT were measured by Western blotting of transfected cardiomyocytes either paced at 0.5 Hz for 8 h (P) or unpaced (UP). B, densitometric quantification of blots from paced cells indicates that expression of HCM mutations results in a significant decrease in phospho-NFAT compared with WT (n = 6). Similarly, C, ERK phosphorylated at threonine 202/tyrosine 204 and total ERK were measured by Western blotting of transfected cardiomyocytes either paced or unpaced. D, densitometric quantification of blots from paced cells show that expression of HCM mutations results in a significant increase in phospho-ERK compared with WT (n = 6). Relative changes in phosphorylation of NFAT and ERK in unpaced cardiomyocytes and the percentages of change in phosphorylation caused by pacing for each group are tabulated in Table S8. E and F show representative immunofluorescence images using anti-NFAT and anti-ERK antibodies, respectively. Individual panels compare HCM mutant to WT-infected cardiomyocytes either unpaced or paced at 0.5 Hz for 8 h. G and H, percentage of change in NFAT or ERK nuclear localization between paced and unpaced cardiomyocytes for each mutant and WT-infected group (n = 30–40 cells in each group). ***, p < 0.001; **, p < 0.01; *, p < 0.05; ns, p > 0.05. Normalized relative localization versus background cytosolic NFAT and ERK comparing WT versus mutant measurements are tabulated in Table S8.
Discussion
This study set out to investigate the effects of Ca2+-sensitizing HCM mutations in thin filament regulatory proteins on myofilament Ca2+ buffering and the resultant consequences to intracellular Ca2+ handling and hypertrophic signaling. We examined mutants in three different regulatory proteins in adult guinea pig cardiomyocytes transfected with recombinant adenovirus, which resulted in ∼50% incorporation of the mutant protein. Mutations in troponin and tropomyosin only comprise ∼10% of all mutations found in HCM patients (27); however, they affect contractile regulation in a similar way to the more common mutations in βMyHC and MyBPC (3). The selected mutations cTnT R92Q, cTnI R145G, and α-TM D175N are also among the most prevalent in each gene and have similar HCM phenotypes (28, 29). Characterization of the infected cardiomyocytes showed that the presence of a Ca2+-sensitizing thin filament mutant doubled the Ca2+ buffering of the myofilament and had a profound effect on Ca2+ handling. These changes were consistent among the disease genes with the cTnI R145G mutation having the largest effect in many of the assays. These functional alterations are likely to be maintained or even accentuated by the increased CaMKII-dependent phosphorylation of Ca2+ handling proteins (PLN and RyR). We also show the direct link between altered intracellular Ca2+ handling and activation of key regulators of cardiac hypertrophy. Both NFAT and ERK are translocated to the nucleus as a result of Ca2+ dysregulation in cardiomyocytes containing HCM mutations. These data emphasize the intimate link between myofilament Ca2+ buffering, Ca2+ handling, and the initiation of Ca2+-mediated hypertrophic signaling.
Our study builds on and extends earlier work, much of it using cardiomyocytes isolated from mouse models of HCM. Our experimental design provides the strongest evidence to date that altered Ca2+ buffering can be directly attributed to the primary effect of a mutation on myofilament Ca2+ affinity, because of the short-term transfection model used. Previous detailed electrophysiological characterization of HCM cardiomyocytes used transgenic mice in which the disease-causing mutation is expressed from birth or earlier, and thus the observed functional changes reflect a mixture of primary effects caused by the mutation and secondary compensatory alterations caused by molecular and (in longer-term studies) physiological remodeling. Furthermore, we opted to use adult guinea pig cardiomyocytes in which the myosin isoform and Ca2+ handling more closely resemble that found in human. Murine cardiomyocytes contain principally α-MyHC that has faster enzyme kinetics than the β isoform, which predominates in both guinea pig and human cardiomyocytes (19), and has been shown to be an important determinant when assessing myocyte or cardiac function (30). Also, in mice, Ca2+ reuptake during diastole is almost entirely dependent on SERCA2, whereas in humans and guinea pigs, the sarcolemmal NCX makes a substantial contribution (20). This and the shorter action potential lacking an appreciable plateau (21) may make the guinea pig a more accurate model for determining the effects of altered myofilament Ca2+ affinity on Ca2+ handling in HCM patients.
Some recent work analyzing Ca2+ handling in transgenic mice containing Ca2+-sensitizing mutations has shown profound increases in basal [Ca2+]i in the presence of increased Ca2+ buffering (13, 14), whereas others have found the opposite effect depending on the age of the mice (15, 16). One of the former experiments also shows a pause-dependent increase in SR load when HCM variants are present (13); however, using guinea pig cardiomyocytes, which have slower Ca2+ cycling, alterations to SR Ca2+ are seen independently of pausing prior to caffeine application. In the latter cases there are profound alterations in the levels of Ca2+-handling proteins, such as SERCA and PLN, and age-dependent alterations to CAMKII phosphorylation levels. These findings are in agreement with our shorter-term study where Ca2+ buffering appears to directly affect SERCA2 activity via CaMKII activation and subsequent phosphorylation of PLN threonine 17. Adenoviral-mediated transfection of HCM mutations has previously been carried out using rat primary cardiomyocytes. The restrictive cardiomyopathy R193H TnI mutant increased myofilament Ca2+ sensitivity and decreased sarcomere length, as well as increasing both relaxation time and Ca2+ transient decay (31). Of note, cardiomyocytes isolated from a transgenic mouse model of the same mutant showed prolonged relaxation with no change in Ca2+ transient decay, suggesting secondary changes at the level of Ca2+ handling (32).
The observed increase in diastolic Ca2+ is likely to be caused by the slower release of Ca2+ from the myofilament during Ca2+ reuptake by SERCA and maintained by increased RyR leak. Isolated cardiomyocytes from myectomy samples taken from HCM patients with different genetic mutations showed increases in diastolic Ca2+ (33) analogous to those in our study. This may indicate that the primary defects in Ca2+ handling may again begin to predominate as disease pathogenesis progresses to end stage and the chronically remodeled myocardium begins to fail. More recent work from human samples shows that CaMKII signaling and functional effects on SERCA/PLN are preserved in end stage HCM; interestingly the study also shows compensatory changes of absolute SERCA levels (34). In contrast with our findings, RyR phosphorylation was unchanged in cardiomyocytes from 10 patients. This suggests that some aspects of Ca2+ buffering in HCM are preserved throughout the natural history of the disease in nonmurine HCM, whereas others are compensated for to preserve long-term myocyte function during disease progression. It has also been suggested that CaMKII plays a nodal role in intracellular signaling (35); our study confirms that CaMKII mediates phosphorylation of both PLN and RyR, and its activity is increased in HCM cardiomyocytes. Longer-term studies suggest secondary changes, potentially under the control of mechanochemotransduction pathways such as nitric oxide, which may shut off some of these nodes (35, 36), because the myocardium attempts to achieve a homeostatic equilibrium of the Ca2+ pool to preserve contractile function.
Work on both human tissue and transgenic animals shows conflicting results when assessing SR Ca2+, NCX, and SERCA2 activity (13–15). This is the first study to directly account for the myofilament buffering when calculating these parameters. Increased myofilament Ca2+ occupancy will mask recordings that rely on cytoplasmic fura2 fluorescence, leading to under representations of the [Ca2+] used to estimate NCX and SERCA2 activity. If the necessary adjustments for buffering are made (as in this study), a paradigm of Ca2+ overload with altered Ca2+ compartmentalization begins to develop. There is a need for greater mechanistic insight of subdomain Ca2+ in HCM. The emerging next generation of genetically encoded Ca2+ sensors (GECOs) are now sensitive and fast enough kinetically to be used in the cardiomyocyte (37). They can be cross-linked to a range of proteins across the contracting cardiomyocyte and dynamically signal changes in Ca2+ microdomains (38).
We show that NFAT and ERK are activated in HCM infected cardiomyocytes when paced in culture, in agreement with the mechanisms proposed by Davis et al. (39). Here we show that Ca2+ handling directly facilitates NFAT dephosphorylation, leading to nuclear translocation in addition to CaMKII activation, which differs from findings in neonatal rat cardiomyocytes, where the opposite effect is seen (40). However, we also describe acute ERK phosphorylation and nuclear translocation in HCM in the absence of pacing. This suggests a primary mechanism distinct from that of NFAT driving these changes. Microdomain Ca2+ or indirect HCM mutation effects caused by altered contractility or energetics may underlie these observations.
Our work and that of others help to establish a paradigm of Ca2+-dependent myocardial remodeling in HCM: the underlying genetic defect causes increased myofilament Ca2+ buffering and altered Ca2+ handling. Ca2+-dependent downstream signaling cascades may then drive deleterious cellular remodeling. Other proposed mechanisms include energetic compromise from inefficient ATP utilization affecting the metabolic milieu of the cardiomyocyte and impairing energy-requiring ionic homeostasis, especially Ca2+ reuptake by SERCA2 (3, 41). Thus, both direct and indirect alterations in Ca2+ handling may work in concert to generate the macroscopic HCM disease phenotype. Furthermore previous studies have implicated myocyte disarray and interstitial fibrosis as the main propagators of ventricular arrhythmias in HCM pathophysiology (42). Our findings re-enforces the view that altered intracellular Ca2+ homeostasis in the diseased cardiomyocyte, brought about as a direct consequence by the primary gene mutation, may be important in arrhythmic events observed in HCM patients.
These data raise the possibility that correction of Ca2+ dysregulation and signaling could be disease-modifying in HCM and improve outcomes. Direct targeting of myofilament Ca2+ sensitivity provides the most attractive potential therapeutic approach. Small molecules such as the green tea polyphenol epigallocatechin-3-gallate have been shown to bind to cTnC (43) and desensitize the myofilament (44), although it should be noted that epigallocatechin-3-gallate currently lacks specificity and potency to be useful in itself (45). Such approaches using derivative compounds may provide a tractable method for drug treatment to prevent or even regress HCM disease pathology by targeting its primary cause.
Experimental procedures
Adenoviral design and production
Adenoviruses were engineered to contain either WT or HCM mutant FLAG-tagged thin filament proteins (cTnT R92Q, cTnI R145G, and α-TM D175N) using the AdEasy XL viral production system (Agilent Technologies). Viral particles were purified by CsCl gradient centrifugation and desalted by dialysis, and the number of plaque-forming units per ml was determined using the manufacturer's standard protocols.
Isolation of guinea pig left ventricular cardiomyocytes
This investigation was approved by the Animal Welfare and Ethical Review Board at the University of Oxford and conforms to the UK Animals (Scientific Procedures) Act, 1986, incorporating Directive 2010/63/EU of the European Parliament. Left ventricular cardiomyocytes were isolated from guinea pig heart, by standard collagenase perfusion and mechanical agitation (46). Cardiomyocytes were incubated in ACCITT3 culture medium (47) at 37 °C and 5% CO2 in the presence of ∼1000 MOI of adenovirus for 48 h. All subsequent functional experiments were carefully controlled for culture time, whereas viral MOI ratios were assessed throughout the duration of the study to ensure that the validity of the model was maintained. All experiments detailed herein compare HCM mutant-infected cardiomyocytes with similarly infected cardiomyocytes expressing human FLAG-tagged recombinant WT protein. Uninfected control cardiomyocytes broadly resembled the WT-infected controls; any exception to this is detailed in Tables S2–S7.
Determination of optimum fura2-AM-ester loading concentration
Optimum fura2-AM-ester loading in isolated cardiomyocytes was determined by signal-to-noise analysis using Ionwizard software (IonOptix). 100,000–150,000 viable guinea pig left ventricular cardiomyocytes were incubated with 5, 1, 0.5, or 0.1 μm fura2-AM ester (Life Technologies) in buffer containing 150 mm NaCl, 10 mm HEPES, 7 mm glucose, 1 mm MgCl, 1 mm KCl, 0.3 mm NaH2PO3, and 250 μm CaCl2, pH 7.4, with NaOH. F365/380 was determined using IonOptix μstep under electrical pacing at 40 V and 0.5 Hz to establish basal noise and peak stimulated fluorescence conditions. It was determined that 1 μm was the minimum concentration/time required to give a dynamic Ca2+ signal (with lower concentrations giving signal to noise ratios of 1 or lower) and was therefore used in all subsequent experiments (Fig. S5).
Measurement of Ca2+ buffering, NCX current, SR content, and SERCA activity
Cardiomyocytes were loaded with fura2, attached to a whole-cell voltage-clamp pipette, and spritzed with 10 mm caffeine. Intracellular Ca2+ buffering was calculated from the simultaneous measurement of total and free Ca2+ ([Ca2+]total and [Ca2+]i, respectively) using a previously reported technique (22). Specifically, caffeine caused the release of Ca2+ from SR and induced a brief Ca2+ transient measured using fura2 (“caffeine transient”), as well as Ca2+ efflux via NCX (NCX current), allowing [Ca2+]i and [Ca2+]total to be calculated. To exclude the other flux mechanism's contribution to the extrusion of Ca, the measured NCX currents were integrated and corrected as previously described (48). After this correction, we now assume that extrusion of Ca2+ released from SR by caffeine is only via NCX. Based on this foundation, we then determined (at two time points) the change of integral of NCX current. This gave the Δ[Ca2+]total, whereas the change of intracellular Ca2+ transient measured by fura2 gave Δ[Ca2+]i during caffeine application. Thus, the relationship between Δ[Ca2+]i and Δ[Ca2+]total represents the Ca2+ buffering regardless of differential bound myofilament Ca2+ in control and mutant cells at baseline. Plots of [Ca2+]i versus [Ca2+]total were fitted to a Michaelis–Menten equation to give estimates of buffering Kd and Bmax. NCX amplitudes were taken from currents obtained during the buffering measurement protocol. Relative changes in SR load were calculated using [Ca2+]total transients upon caffeine spritz to account for the Ca2+ buffering of each cell. SERCA2 activity was determined from the decay constants from the caffeine transient and the preceding Ca2+ transient using standard methods (49).
Measurement of sarcomere shortening and cytoplasmic Ca2+ transients
fura2-loaded guinea pig left ventricular cardiomyocytes were then allowed to settle to the bottom of a perfusion chamber with a 0 thickness coverslip base, which was mounted on an inverted fluorescence microscope. The cells were perfused with buffer containing 150 mm NaCl, 10 mm HEPES, 7 mm glucose, 1 mm MgCl, 1 mm KCl, 0.3 mm NaH2PO3, and 1.8 mm CaCl2, pH 7.4, and electrically paced at 40 V. Pacing frequency was set at 1 Hz for cells not loaded with Ca2+ indicator or 0.5 Hz for fura2-loaded cells to accurately measure resting diastolic [Ca2+]i. The effects of HCM mutations on contractility were found to be qualitatively unaltered compared with their effect in cells not loaded with fura2 at 1 Hz (Fig. S7 and Table S3). Sarcomere shortening was captured by Fourier transform of the cardiomyocyte striations under phase contrast microscopy using a switching rate of 100 Hz. Ca2+ transients were captured simultaneously, using the ratio of fura2 fluorescence emission at 365/380 nm at a switching rate of 1000 Hz. All contracting cardiomyocytes were measured for contractility and fura2 Ca2+; any cells displaying asynchronous contractility or excessive blebbing/dysmorphology were ignored for acquisition. fura2 fluorescence ratio was subsequently calibrated to [Ca2+]i by selective membrane permeabilization using ionomycin (Fig S1).
Measurement of RyR leak
RyR leak was calculated using the RyR channel blocker tetracaine as previously reported (25). Briefly, contracting cardiomyocytes were identified under field stimulation at 0.5 Hz in the presence of 1.8 mm CaCl2. RyR leak was measured for 50 s in the absence of field stimulation in a Na+- and Ca2+-free solution. Field stimulation and perfusion of 1.8 mm CaCl2 were restarted to allow verification that basal functional conditions were as at the start of the experiment. Perfusion was again switched to Na+- and Ca2+-free solution containing 1 μm of tetracaine, and field stimulation was stopped to make a baseline reading that was subtracted from the preceding RyR leak measurement. Leak/load relationships were measured by 10 mm caffeine spritz following each perfusion switch.
Western blotting and immunolocalization
Western blots were performed to detect cTnT, cTnI α-TM and FLAG tag, PLN, phosphoserine 16 PLN, phosphothreonine 17 PLN, RyR, phosphoserine 2030 RyR, phosphoserine 2808 RyR, phosphoserine 2814 RyR, phosphoserine 22/24 cTnI, NCX, SERCA2A, NFAT, phosphoserine 165 NFAT, ERK, or phosphothreonine 202/tyrosine 204 ERK in WT and mutant-infected cardiomyocytes. In immunolocalization experiments, recombinant FLAG-tagged protein was detected versus α-actinin. NFAT and ERK nuclear localization was also measured versus nucleoplasmic marker 4′,6′-diamino-2-phenylindole.
Statistics
The data are expressed as the average of n experiments ± S.E. throughout. Statistically significance was determined using unpaired Student–Newman–Keuls analysis for nonnormally distributed data (InStat, GraphPad Software), with significance values defined as p < 0.05.
Author contributions
P. R., Y.-H. Z., B. C., H. W., and C. R. conceptualization; P. R., X. L., A. S., S. P., and C. R. data curation; P. R., X. L., and S. P. formal analysis; P. R., B. C., H. W., and C. R. supervision; P. R., X. L., A. S., S. P., and Y.-H. Z. investigation; P. R. and X. L. methodology; P. R. and C. R. writing-original draft; P. R. project administration; P. R., X. L., Y.-H. Z., B. C., H. W., and C. R. writing-review and editing.
Supplementary Material
The work is supported by British Heart Foundation Programme Grant RG/12/16/29939 and the British Heart Foundation Centre of Research Excellence (Oxford, UK). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains text, Tables S1–S8, and Figs. S1–S10.
- HCM
- hypertrophic cardiomyopathy
- α-TM
- α-tropomyosin
- [Ca2+]total
- total intracellular calcium
- CaMKII
- Ca2+/calmodulin-dependent protein kinase
- cTnI
- cardiac troponin I
- cTnT
- cardiac troponin T
- ERK
- extracellular signal-regulated kinase
- MOI
- multiplicity of infection
- MyBPC
- myosin-binding protein C
- MyHC
- myosin heavy chain
- NCX
- sodium/calcium exchanger
- NFAT
- nuclear factor of activated T-cells
- PLN
- phospholamban
- RyR
- ryanodine receptor
- SERCA2
- sarcoendoplasmic reticulum calcium ATPase 2
- SR
- sarcoplasmic reticulum.
References
- 1. Maron B. J., Gardin J. M., Flack J. M., Gidding S. S., and Bild D. E. (1996) HCM in the general population. Circulation 94, 588–589 [PubMed] [Google Scholar]
- 2. Maron B. J. (2003) Sudden death in young athletes. N. Engl. J. Med. 349, 1064–1075 10.1056/NEJMra022783 [DOI] [PubMed] [Google Scholar]
- 3. Watkins H., Ashrafian H., and Redwood C. (2011) Inherited cardiomyopathies. N. Engl. J. Med. 364, 1643–1656 10.1056/NEJMra0902923 [DOI] [PubMed] [Google Scholar]
- 4. Bing W., Redwood C. S., Purcell I. F., Esposito G., Watkins H., and Marston S. B. (1997) Effects of two hypertrophic cardiomyopathy mutations in α-tropomyosin, Asp175Asn and Glu180Gly, on Ca2+ regulation of thin filament motility. Biochem. Biophys. Res. Commun. 236, 760–764 10.1006/bbrc.1997.7045 [DOI] [PubMed] [Google Scholar]
- 5. Robinson P., Mirza M., Knott A., Abdulrazzak H., Willott R., Marston S., Watkins H., and Redwood C. (2002) Alterations in thin filament regulation induced by a human cardiac troponin T mutant that causes dilated cardiomyopathy are distinct from those induced by troponin T mutants that cause hypertrophic cardiomyopathy. J. Biol. Chem. 277, 40710–40716 10.1074/jbc.M203446200 [DOI] [PubMed] [Google Scholar]
- 6. Burton D., Abdulrazzak H., Knott A., Elliott K., Redwood C., Watkins H., Marston S., and Ashley C. (2002) Two mutations in troponin I that cause hypertrophic cardiomyopathy have contrasting effects on cardiac muscle contractility. Biochem. J. 362, 443–451 10.1042/bj3620443,10.1042/0264-6021:3620443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elliott K., Watkins H., and Redwood C. S. (2000) Altered regulatory properties of human cardiac troponin I mutants that cause hypertrophic cardiomyopathy. J. Biol. Chem. 275, 22069–22074 10.1074/jbc.M002502200 [DOI] [PubMed] [Google Scholar]
- 8. Lakdawala N. K., Dellefave L., Redwood C. S., Sparks E., Cirino A. L., Depalma S., Colan S. D., Funke B., Zimmerman R. S., Robinson P., Watkins H., Seidman C. E., Seidman J. G., McNally E. M., and Ho C. Y. (2010) Familial dilated cardiomyopathy caused by an α-tropomyosin mutation: the distinctive natural history of sarcomeric dilated cardiomyopathy. J. Am. Coll. Cardiol. 55, 320–329 10.1016/j.jacc.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson P., Griffiths P. J., Watkins H., and Redwood C. S. (2007) Dilated and hypertrophic cardiomyopathy mutations in troponin and α-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ. Res. 101, 1266–1273 10.1161/CIRCRESAHA.107.156380 [DOI] [PubMed] [Google Scholar]
- 10. Lowey S. (2002) Functional consequences of mutations in the myosin heavy chain at sites implicated in familial hypertrophic cardiomyopathy. Trends Cardiovasc. Med. 12, 348–354 10.1016/S1050-1738(02)00181-0 [DOI] [PubMed] [Google Scholar]
- 11. Witt C. C., Gerull B., Davies M. J., Centner T., Linke W. A., and Thierfelder L. (2001) Hypercontractile properties of cardiac muscle fibers in a knock-in mouse model of cardiac myosin-binding protein-C. J. Biol. Chem. 276, 5353–5359 10.1074/jbc.M008691200 [DOI] [PubMed] [Google Scholar]
- 12. Smith G. A., Dixon H. B., Kirschenlohr H. L., Grace A. A., Metcalfe J. C., and Vandenberg J. I. (2000) Ca2+ buffering in the heart: Ca2+ binding to and activation of cardiac myofibrils. Biochem. J. 346, 393–402 10.1042/0264-6021:3460393,10.1042/bj3460393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schober T., Huke S., Venkataraman R., Gryshchenko O., Kryshtal D., Hwang H. S., Baudenbacher F. J., and Knollmann B. C. (2012) Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ. Res. 111, 170–179 10.1161/CIRCRESAHA.112.270041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coppini R., Mazzoni L., Ferrantini C., Gentile F., Pioner J. M., Laurino A., Santini L., Bargelli V., Rotellini M., Bartolucci G., Crocini C., Sacconi L., Tesi C., Belardinelli L., Tardiff J., et al. (2017) Ranolazine prevents phenotype development in a mouse model of hypertrophic cardiomyopathy. Circ. Heart Fail. 10, e003565 10.1161/CIRCHEARTFAILURE.116.003565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haim T. E., Dowell C., Diamanti T., Scheuer J., and Tardiff J. C. (2007) Independent FHC-related cardiac troponin T mutations exhibit specific alterations in myocellular contractility and calcium kinetics. J. Mol. Cell Cardiol. 42, 1098–1110 10.1016/j.yjmcc.2007.03.906 [DOI] [PubMed] [Google Scholar]
- 16. Martins A. S., Parvatiyar M. S., Feng H. Z., Bos J. M., Gonzalez-Martinez D., Vukmirovic M., Turna R. S., Sanchez-Gonzalez M. A., Badger C. D., Zorio D. A. R., Singh R. K., Wang Y., Jin J. P., Ackerman M. J., and Pinto J. R. (2015) In vivo analysis of troponin C knock-in (A8V) mice: evidence that TNNC1 is a hypertrophic cardiomyopathy susceptibility gene. Circ. Cardiovasc. Genet. 8, 653–664 10.1161/CIRCGENETICS.114.000957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Periasamy M., and Huke S. (2001) SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J. Mol. Cell Cardiol. 33, 1053–1063 10.1006/jmcc.2001.1366 [DOI] [PubMed] [Google Scholar]
- 18. Hamdani N., Bishu K. G., von Frieling-Salewsky M., Redfield M. M., and Linke W. A. (2013) Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc. Res. 97, 464–471 10.1093/cvr/cvs353 [DOI] [PubMed] [Google Scholar]
- 19. Deacon J. C., Bloemink M. J., Rezavandi H., Geeves M. A., and Leinwand L. A. (2012) Identification of functional differences between recombinant human α and β cardiac myosin motors. Cell Mol. Life Sci. 69, 2261–2277 10.1007/s00018-012-0927-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bers D. M. (2002) Cardiac excitation-contraction coupling. Nature 415, 198–205 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- 21. Luo C. H., and Rudy Y. (1994) A dynamic model of the cardiac ventricular action potential: I. simulations of ionic currents and concentration changes. Circ. Res. 74, 1071–1096 10.1161/01.RES.74.6.1071 [DOI] [PubMed] [Google Scholar]
- 22. Trafford A. W., Díaz M. E., and Eisner D. A. (1999) A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflugers Arch. 437, 501–503 10.1007/s004240050808 [DOI] [PubMed] [Google Scholar]
- 23. Williams D. A., Fogarty K. E., Tsien R. Y., and Fay F. S. (1985) Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature 318, 558–561 10.1038/318558a0 [DOI] [PubMed] [Google Scholar]
- 24. O'Neill S. C., and Eisner D. A. (1990) A mechanism for the effects of caffeine on Ca2+ release during diastole and systole in isolated rat ventricular myocytes. J. Physiol. 430, 519–536 10.1113/jphysiol.1990.sp018305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shannon T. R., Ginsburg K. S., and Bers D. M. (2002) Quantitative assessment of the SR Ca2+ leak-load relationship. Circ. Res. 91, 594–600 10.1161/01.RES.0000036914.12686.28 [DOI] [PubMed] [Google Scholar]
- 26. Molkentin J. D. (2004) Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 63, 467–475 10.1016/j.cardiores.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 27. Walsh R., Thomson K. L., Ware J. S., Funke B. H., Woodley J., McGuire K. J., Mazzarotto F., Blair E., Seller A., Taylor J. C., Minikel E. V., Exome Aggregation, Consortium, MacArthur D. G., Farrall M., Cook S. A., et al. (2017) Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet. Med. 19, 192–203 10.1038/gim.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thierfelder L., Watkins H., MacRae C., Lamas R., McKenna W., Vosberg H. P., Seidman J. G., and Seidman C. E. (1994) α-Tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 77, 701–712 10.1016/0092-8674(94)90054-X [DOI] [PubMed] [Google Scholar]
- 29. Kimura A., Harada H., Park J. E., Nishi H., Satoh M., Takahashi M., Hiroi S., Sasaoka T., Ohbuchi N., Nakamura T., Koyanagi T., Hwang T. H., Choo J. A., Chung K. S., Hasegawa A., et al. (1997) Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat. Genet. 16, 379–382 10.1038/ng0897-379 [DOI] [PubMed] [Google Scholar]
- 30. Rice R., Guinto P., Dowell-Martino C., He H., Hoyer K., Krenz M., Robbins J., Ingwall J. S., and Tardiff J. C. (2010) Cardiac myosin heavy chain isoform exchange alters the phenotype of cTnT-related cardiomyopathies in mouse hearts. J. Mol. Cell Cardiol. 48, 979–988 10.1016/j.yjmcc.2009.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davis J., Wen H., Edwards T., and Metzger J. M. (2007) Thin filament disinhibition by restrictive cardiomyopathy mutant R193H troponin I induces Ca2+-independent mechanical tone and acute myocyte remodeling. Circ. Res. 100, 1494–1502 10.1161/01.RES.0000268412.34364.50 [DOI] [PubMed] [Google Scholar]
- 32. Davis J., Yasuda S., Palpant N. J., Martindale J., Stevenson T., Converso K., and Metzger J. M. (2012) Diastolic dysfunction and thin filament dysregulation resulting from excitation-contraction uncoupling in a mouse model of restrictive cardiomyopathy. J. Mol. Cell Cardiol. 53, 446–457 10.1016/j.yjmcc.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coppini R., Ferrantini C., Yao L., Fan P., Del Lungo M., Stillitano F., Sartiani L., Tosi B., Suffredini S., Tesi C., Yacoub M., Olivotto I., Belardinelli L., Poggesi C., Cerbai E., et al. (2013) Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127, 575–584 10.1161/CIRCULATIONAHA.112.134932 [DOI] [PubMed] [Google Scholar]
- 34. Helms A. S., Alvarado F. J., Yob J., Tang V. T., Pagani F., Russell M. W., Valdivia H. H., and Day S. M. (2016) Genotype-dependent and -independent calcium signaling dysregulation in human hypertrophic cardiomyopathy. Circulation 134, 1738–1748 10.1161/CIRCULATIONAHA.115.020086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tardiff J. C. (2016) The role of calcium/calmodulin-dependent protein kinase II activation in hypertrophic cardiomyopathy. Circulation 134, 1749–1751 10.1161/CIRCULATIONAHA.116.025455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jian Z., Han H., Zhang T., Puglisi J., Izu L. T., Shaw J. A., Onofiok E., Erickson J. R., Chen Y. J., Horvath B., Shimkunas R., Xiao W., Li Y., Pan T., Chan J., et al. (2014) Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci. Signal. 7, ra27 10.1126/scisignal.2005046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Helassa N., Podor B., Fine A., and Török K. (2016) Design and mechanistic insight into ultrafast calcium indicators for monitoring intracellular calcium dynamics. Sci. Rep. 6, 38276 10.1038/srep38276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Y., Araki S., Wu J., Teramoto T., Chang Y. F., Nakano M., Abdelfattah A. S., Fujiwara M., Ishihara T., Nagai T., and Campbell R. E. (2011) An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 10.1126/science.1208592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davis J., Davis L. C., Correll R. N., Makarewich C. A., Schwanekamp J. A., Moussavi-Harami F., Wang D., York A. J., Wu H., Houser S. R., Seidman C. E., Seidman J. G., Regnier M., Metzger J. M., Wu J. C., et al. (2016) A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 165, 1147–1159 10.1016/j.cell.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacDonnell S. M., Weisser-Thomas J., Kubo H., Hanscome M., Liu Q., Jaleel N., Berretta R., Chen X., Brown J. H., Sabri A. K., Molkentin J. D., and Houser S. R. (2009) CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ. Res. 105, 316–325 10.1161/CIRCRESAHA.109.194035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ashrafian H., Redwood C., Blair E., and Watkins H. (2003) Hypertrophic cardiomyopathy:a paradigm for myocardial energy depletion. Trends Genet. 19, 263–268 10.1016/S0168-9525(03)00081-7 [DOI] [PubMed] [Google Scholar]
- 42. O'Hanlon R., Grasso A., Roughton M., Moon J. C., Clark S., Wage R., Webb J., Kulkarni M., Dawson D., Sulaibeekh L., Chandrasekaran B., Bucciarelli-Ducci C., Pasquale F., Cowie M. R., McKenna W. J., et al. (2010) Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 56, 867–874 10.1016/j.jacc.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 43. Robertson I. M., Li M. X., and Sykes B. D. (2009) Solution structure of human cardiac troponin C in complex with the green tea polyphenol, (−)-epigallocatechin 3-gallate. J. Biol. Chem. 284, 23012–23023 10.1074/jbc.M109.021352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tadano N., Du C. K., Yumoto F., Morimoto S., Ohta M., Xie M. F., Nagata K., Zhan D. Y., Lu Q. W., Miwa Y., Takahashi-Yanaga F., Tanokura M., Ohtsuki I., and Sasaguri T. (2010) Biological actions of green tea catechins on cardiac troponin C. Br. J. Pharmacol. 161, 1034–1043 10.1111/j.1476-5381.2010.00942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng W., Hwang H. S., Kryshtal D. O., Yang T., Padilla I. T., Tiwary A. K., Puschner B., Pessah I. N., and Knollmann B. C. (2012) Coordinated regulation of murine cardiomyocyte contractility by nanomolar (−)-epigallocatechin-3-gallate, the major green tea catechin. Mol. Pharmacol. 82, 993–1000 10.1124/mol.112.079707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stemmer P., Akera T., Brody T. M., Rardon D. P., and Watanabe A. M. (1989) Isolation and enrichment of Ca2+-tolerant myocytes for biochemical experiments from guinea-pig heart. Life Sci. 44, 1231–1237 10.1016/0024-3205(89)90319-6 [DOI] [PubMed] [Google Scholar]
- 47. Ellingsen O., Davidoff A. J., Prasad S. K., Berger H. J., Springhorn J. P., Marsh J. D., Kelly R. A., and Smith T. W. (1993) Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am. J. Physiol. 265, H747–H754 [DOI] [PubMed] [Google Scholar]
- 48. Sears C. E., Bryant S. M., Ashley E. A., Lygate C. A., Rakovic S., Wallis H. L., Neubauer S., Terrar D. A., and Casadei B. (2003) Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ. Res. 92, e52–e59 10.1161/01.RES.0000064585.95749.6D [DOI] [PubMed] [Google Scholar]
- 49. Bode E. F., Briston S. J., Overend C. L., O'Neill S. C., Trafford A. W., and Eisner D. A. (2011) Changes of SERCA activity have only modest effects on sarcoplasmic reticulum Ca2+ content in rat ventricular myocytes. J. Physiol. 589, 4723–4729 10.1113/jphysiol.2011.211052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.