Abstract
Effective vascular regeneration could provide therapeutic benefit for multiple pathologies, especially in chronic peripheral artery disease (PAD) and myocardial ischemia. The hypoxia inducible factors (HIFs) mediate the cellular transcriptional response to hypoxia and regulate multiple processes that are required for angiogenesis to ultimately restore perfusion and oxygen supply. In endothelial cells, both HIF1α and HIF2α are known to contribute to this role; however, the extent and individual roles of each of these HIFα remain unclear. To characterize the individual roles of HIFα, we sequenced the transcriptional outputs of stabilized forms of HIF1α and HIF2α, where they regulated 701 and 1,454 genes, respectively. HIF1α transcription primarily regulated metabolic reprogramming, whereas HIF2α exerted a larger role in regulating angiogenic extracellular signaling, guidance cues, and extracellular matrix remodeling factors. Furthermore, HIF2α almost exclusively regulated a large and diverse subset of transcription factors and coregulators that contribute to its diverse roles in hypoxia. Further understanding of how HIFs regulate cellular processes in hypoxia and angiogenesis could offer new avenues to modulate physiological angiogenesis to enhance revascularisation in ischemic conditions and other pathologies.
Keywords: HIF1a, HIF2a, EPAS1, transcription factor, transcription, hypoxia, cardiovascular disease, RNA-seq, angiogenesis
Hypoxia provokes a diverse cellular transcriptional response that is primarily mediated by the hypoxia inducible factors. Ylä-Herttuala et al. delineate the downstream genes and their associated processes regulated by the major HIF isoforms HIF1α and HIF2α, as to further understand how their non-overlapping activity could affect vascular disease and regeneration.
Introduction
Atherosclerotic stenosis and other arteriopathies can result in chronic vascular insufficiencies, which manifest as coronary, cerebrovascular, or peripheral vascular disease. In addition to being strongly associated with elevated risk for fatal cardiovascular events, peripheral vascular disease can cause chronic and debilitating complications such as critical limb ischemia, resulting in claudication, ulcers, coagulative necrosis, and distal limb loss. Reduced perfusion in these tissues impedes regeneration through oxygen and nutrient depletion. Tissue hypoxia results in the activation of the hypoxia inducible factors (HIFs) that mediate adaptive cellular processes and the secretion of angiogenic growth factors and cytokines to stimulate revascularization and restoration of functionality. Loss-of-function studies have shown that HIF1α is central to the recovery of tissue perfusion, maintenance of tissue viability, and expression of angiogenic cytokines in models of hindlimb ischemia1 and wound healing.2
The HIFs are heterodimeric transcription factors that comprise of an oxygen labile and regulated α-subunits (HIFα), encoded by three genes, HIF1A, EPAS1 (HIF2α), and HIF3A, and a stably expressed β-subunit, ARNT (HIF1β). Both HIF subunits and their respective isoforms retain a conserved domain structure and are members of the PER-ARNT-SIM (PAS) subfamily of basic helix-loop-helix (bHLH) proteins.3 To enable a rapid response to hypoxic insults without the need for de novo protein synthesis, HIFαs are continuously transcribed and translated but rapidly degraded through oxygen-dependent post-translational hydroxylation, ubiquitination, and subsequent degradation.4 In conditions of low oxygen tension, this process is inhibited and HIFαs are stabilized, enabling them to heterodimerize and translocate to the nucleus in order to undertake their transcriptional program. Beyond oxygen-dependent hydroxylation, HIFαs are extensively post-translationally regulated by various modification at different residues altering its stability and activity.5 HIF1α and HIF2α are considered to be the major HIFα isoforms that mediate the positive HIF transcriptional program. Both isoforms exhibit a highly conserved domain structure and functional similarities, made evident by their binding to the same hypoxia responsive element (HRE) characterized by a conserved RCGTG DNA motif.6
Despite these generalized regulatory, structural, and functional similarities, it is becoming increasingly evident that HIF1α and HIF2α exhibit significant differences in their transcriptional regulation in response to hypoxia and disease states.7 This has most strikingly been observed in several forms of cancer, such as renal cell carcinoma, where the regulation of HIF1α and HIF2α is perturbed resulting in HIFα-specific activities that are frequently inconsistent and occasionally contradictory between different cancer types.7 Despite utilizing the same HRE and sharing canonical hypoxia inducible target genes, HIF1α and HIF2α exhibit diverse biological activities and occupy roles outside of hypoxia and metabolic reprogramming. The mechanism accounting for target gene divergency remains incompletely understood but is known to be largely independent of DNA binding, rather differences in the C terminus of HIFs enables interaction with alternative transcription factors and coregulators.8 Furthermore, HIFs are themselves regulated in a non-equivalent manner, where HIF2α is typically stabilized at higher relative O2 concentrations compared with HIF1α.9, 10 These phenotypic differences and variation in HIF1α and HIF2α activity suggest that they exert non-equivalent regulation on divergent target genes in a context-dependent manner.
In an attempt to delineate the HIF transcriptional outputs, we expressed stabilized versions of HIF1α and HIF2α in primary human endothelial cells (ECs), a cell type central to angiogenesis and revascularization. Quantitating the resulting transcriptomes using RNA-seq, we sought to identify the biological processes preferentially associated with either of the HIFα isoforms. HIF1α was seen to specifically regulate energy metabolism compared with HIF2α’s relativity larger role in extracellular signaling, transcription, and extracellular remodeling. These results suggest that in EC HIF1α is associated with acute cellular metabolic adaptations to rapid changes in O2 concentrations, whereas HIF2α mediates a wider role in tissue revascularization and regenerative processes.
Results
Transcriptional Profiles of HIF1α and HIF2α
In order to further dissect the collective and individual roles of HIF1α and HIF2α on gene expression, primary human ECs were transduced with adenovirus vectors expressing either a stabilized form of HIF1α, HIF2α, or an empty CMV early-intermediate promoter construct (eCMV) as a control. Mutations of the proline residues within the HIF oxygen-dependent degradation domain-enabled HIF1α and HIF2α to be expressed in normoxic conditions as confirmed by western blotting (Figure S1) and qPCR for HIF targets (Figure S2). Cells from three biological replicates were harvested 48 hr post-infection, and their RNA was isolated, depleted of ribosomal RNA, and used to construct directional cDNA libraries that were sequenced on a NextSeq 500 using 75-bp single-end reads. Each replicate yielded an average of 24 million reads that were aligned using STAR11 for differential gene expression analysis in edgeR.12 After filtering out transcripts with low counts across all libraries, up to 27,648 transcripts from a total of 48,118 were identified as being expressed. Multi-dimensional scaling of the normalized log2 counts per million (CPM) for eCMV, HIF1α, and HIF2α showed strong association between replicates and the largest variation between HIF2α and eCMV in dimension 1, followed by HIF1α and eCMV in dimension 2, with both accounting for 84% of the overall variance (Figure S3).
HIF1α and HIF2α overexpression resulted in 1,485 and 3,067 differentially expressed transcripts representing 701 and 1,454 genes, respectively, using a fold change (FC) of 2 and FDR < 0.01 (Table S1). Of the differentially expressed genes (DEGs), for both HIF1α and HIF2α the majority, 85.5% and 73.6%, respectively were upregulated as seen in Figures 1 and 2, in agreement with previous reported findings.13 Hierarchical clustering of the variance of log2 FC revealed four primary gene clusters (HIF1α upregulated, HIF2α upregulated, upregulated by both, and downregulated by both) that exhibited distinct forms of regulation under HIF1α and HIF2α (Figure 2, clusters). HIF1α and HIF2α exhibited either parallel or non-overlapping regulation of their target genes; only nine genes were inversely regulated between the HIF isoforms, suggesting that their forms of regulation are not antagonistic.
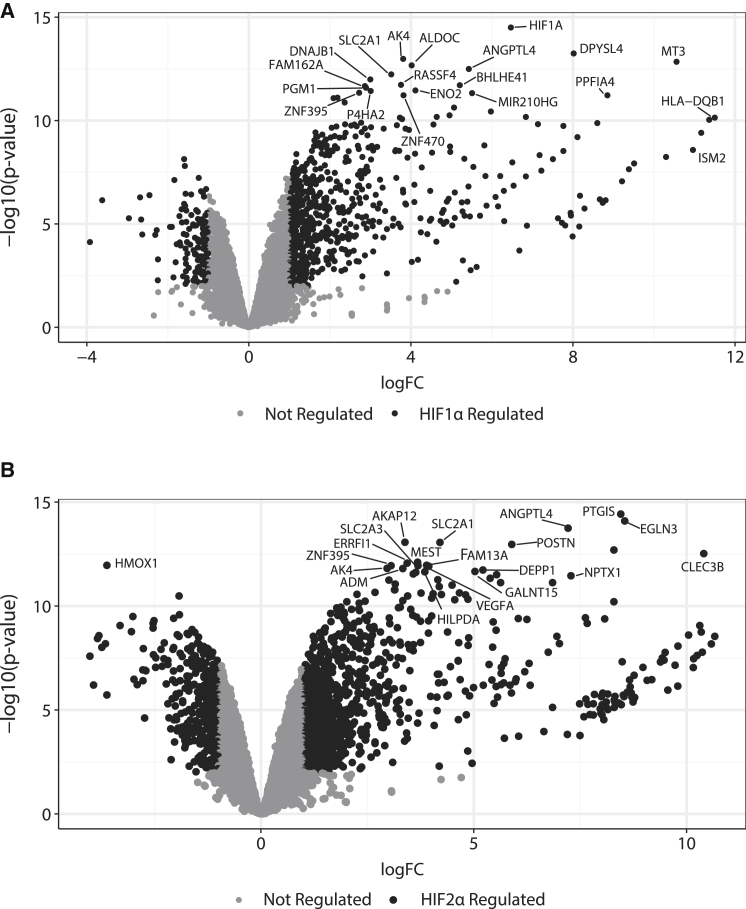
Figure 1.
HIF1α and HIF2α Expression Profiles
Volcano plot of log2 fold change and −log10 (p value) of gene expression under HIF1α (A) and HIF2α (B) overexpression. Differentially regulated genes (fold change >2, FDR < 0.01) are highlighted with the top 20 genes annotated.
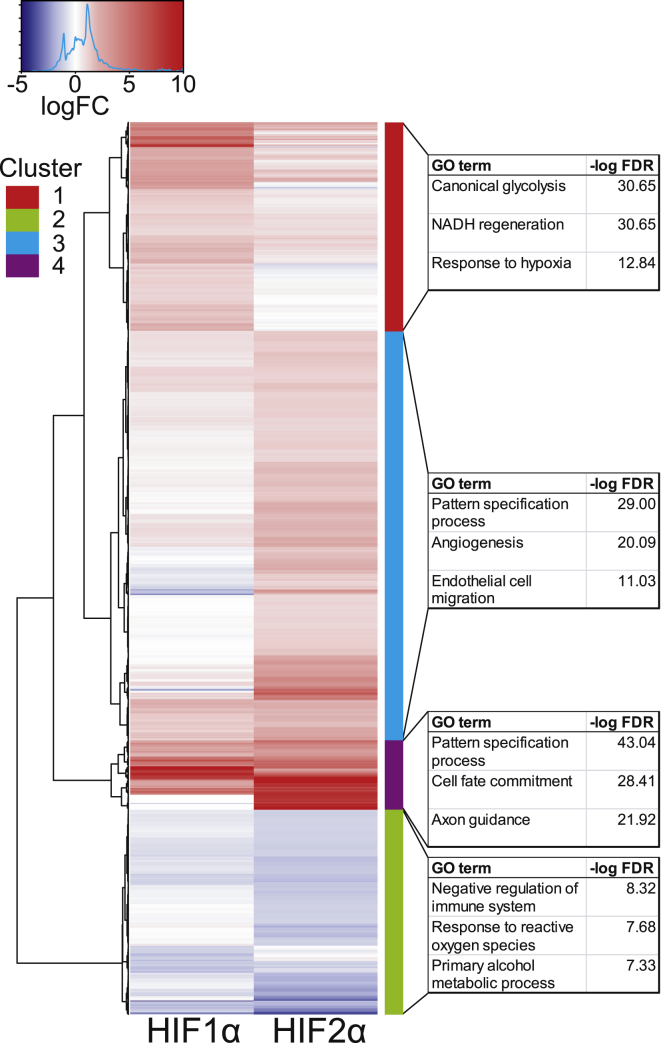
Figure 2.
Heatmap of HIF1α and HIF2α Differentially Regulated Genes
Expression values are shown as log2 fold-change ratios. Rows are hieratically clustered (k = 4) using Ward’s least absolute error with Manhattan distance. The highest enriched GO terms associated with genes in each cluster are shown on the right.
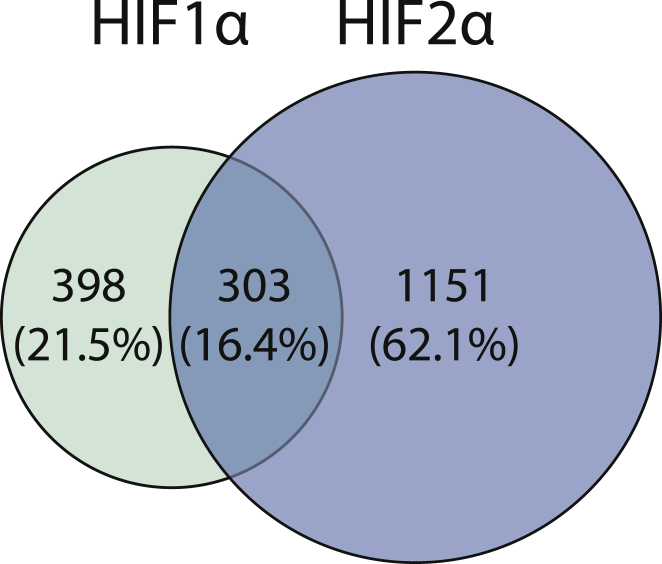
When comparing the genes regulated by HIF1α and HIF2α, less than 20% were regulated by both HIF isoforms; however, these overlapping genes constitute 48% of the HIF1α-regulated genes, as shown in Figure 3. Comparing the regulated RNA classes, HIF1α and HIF2α exerted a significantly different proportion of non-coding to coding RNA species (7.67% and 12.7%, respectively, p value < 0.01, chi-square). The proportion of up- and downregulated genes between HIFs remained approximately equivalent in both RNA classes (data not shown).
Figure 3.
Venn Diagram of the Proportion of Unique and Shared Differentially Regulated Genes of HIF1α and HIF2α
Ontology and Major Functional Categories of HIF-Regulated Genes
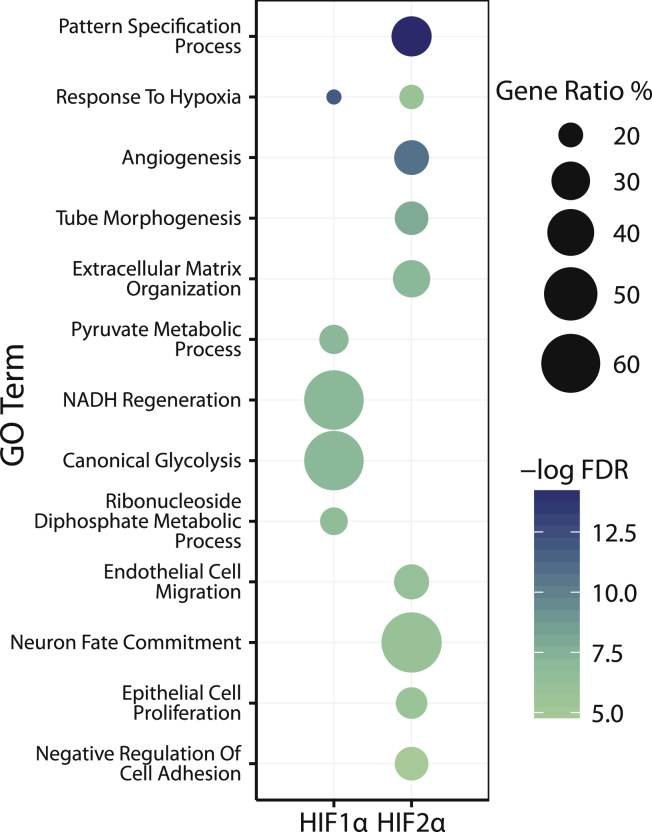
In order to comprehend the complex biological functions, processes, and phenotypes that occur downstream of HIF transcriptional regulation, we sought to cluster the DEGs based on semantic similarities, expression patterns, and interaction networks which could reveal system level differences in how HIF1α and HIF2α regulate their target genes. In order to identify predominant biological themes associated with the regulated genes of HIF1α and HIF2α, overrepresented Gene Ontology (GO) terms for each set of HIF DEGs was analyzed using the clusterProfiler package.14 Redundant GO terms were removed, and the most informative GO terms were selected using minimum FDR and Rel similarity.15 The top enriched biological processes unique and shared between HIF1α and HIF2α are shown in Figure 4. Overlap between top enriched biological processes (FDR < 0.05) was limited to response to hypoxia. HIF1α-specific enriched processes centered on glycolysis, NADH regeneration and other metabolic processes. In contrast, HIF2α-enriched processes included angiogenesis, EC migration, and extracellular matrix reorganization.
Figure 4.
Gene Ontology Enrichment for Biological Processes Regulated by HIF1α and HIF2α
Enriched biological processes are shown in a descending order according to their −log2FDR values. The size of the dots is representative of the percentage of genes regulated for the given GO term.
To further identify biological processes positively or negatively regulated by HIF1α and HIF2α, we performed GO analysis on the genes categorized by the unsupervised gene clustering determined previously in Figure 2. Consistent with the terms previously identified, the HIF1α upregulated cluster 1 was associated with glycolysis and NADH regeneration, whereas the HIF2α upregulated cluster 3 and 4 was associated with EC migration, pattern specification process, and activation of GTPase signaling. Processes negatively regulated by both HIF1α and HIF2α in cluster 2 included responses to reactive oxygen species, and negative regulation of immune system processes. Furthermore, CAMERA16 was used to identify inter-gene correlation enrichment against the MSigDB (Broad Institute) hallmark collection to represent well-defied and non-redundant biological processes. As anticipated, hypoxia was the highest enriched hallmark for both HIF1α and HIF2α, but interestingly, both showed decreased activation for c-Myc-regulated genes (Figure S4A). In addition to the general negative trend, HIF2α overexpression in particular resulted in the significant downregulation of 15 c-Myc regulated genes (Figure S4B). While HIF2α overexpression only resulted in a modest downregulation of c-Myc expression (FC −0.7; FDR < 7e-04), both HIF1α and HIF2α induced robust expression of the c-Myc suppressor MXI1.
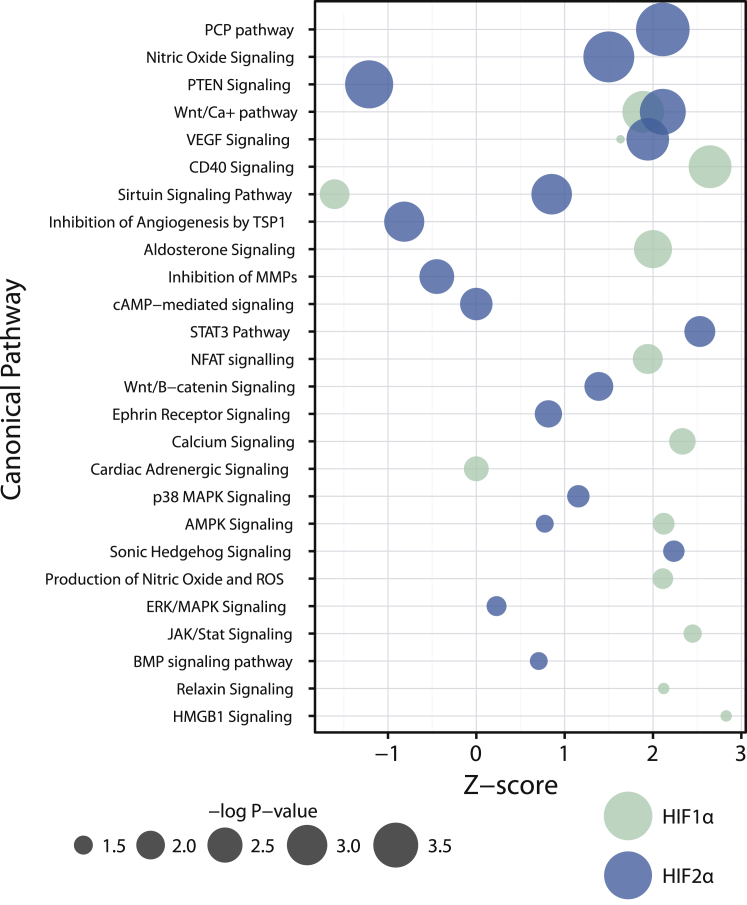
Since several known HIF target genes mediate their effects through a variety of signaling pathways,17 DEGs were analyzed using Ingenuity Pathway Analysis (IPA; QIAGEN Redwood City) to identify activation or repression of cellular signaling and metabolic processes downstream of HIF1α and HIF2α. Thirteen and seventeen canonical pathways with an activation Z score were seen to be enriched (p value < 0.05) for HIF1α and HIF2α, respectively, as shown in Figure 5. The majority of the enriched pathways were seen to be activated with only four pathways between HIF1α and HIF2α and had an activation Z score < 0. Consistently with the GO analysis, there was minimal overlap between the significantly enriched pathways where only four were shared by HIF1α and HIF2α.
Figure 5.
Ingenuity Canonical Pathway Analysis of HIF1α and HIF2α Differentially Expressed Gene
Enriched pathways are shown descending according to their −log2 p values. The size of the dots is proportional to the −log2 p values. The activation Z score on the x axis is used to predict the regulation direction based on the observed differentially expressed genes.
Differentially Regulated HIF Target Genes and Processes
Energy Metabolism
HIF1α DEGs were most significantly related to anaerobic metabolic reprogramming (Figure 6A), where HIF1α regulated almost 80% of the glycolytic-related genes expressed in EC (GO:0061621), including the regulatory rate-limiting enzyme phosphofructokinase (PFK).18 Both HIFs upregulated several glucose transporters also listed in Figure 6A. HIF1α further regulated the switch to glycolysis by upregulating pyruvate dehydrogenase kinase (PDK), which redirects pyruvate metabolism away from the Krebs cycle to instead be anaerobically reduced to lactate for NAD+ regeneration, a process catalyzed by the HIF1α-induced lactate dehydrogenase A (LDHA). Furthermore, coordinate HIF1α regulation of the lactate and proton symporter SLC16A3 and carbonic anhydrase 9 (CA9) prevents acidification from intracellular accumulation of lactate. Hydroxyacylglutathione hydrolase (HAGH), encoding an enzyme used to remove the cytotoxic glycolysis byproduct methylglyoxal, was also seen to be induced under HIF1α overexpression.
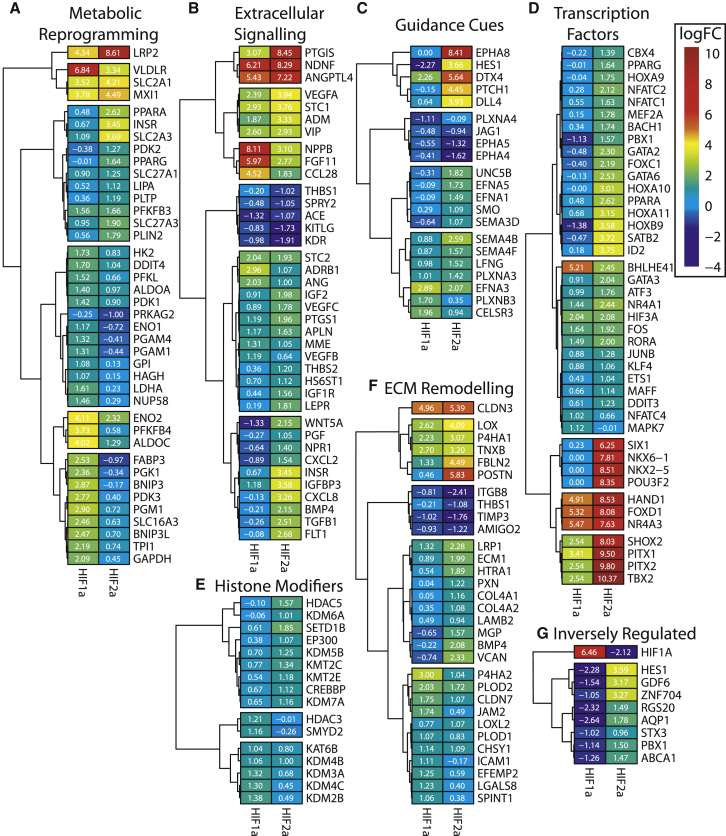
Figure 6.
Heatmaps of Selected HIF1α and HIF2α Regulated Genes Central to HIF-Regulated Biological Processes
(A) Metabolic reprogramming constitutes genes involved in glycolysis, NADH regeneration, glucose transport, fatty acid transport, and autophagy. (B) Genes involved in extracellular signaling including ligands and receptors of growth factors, cytokines, and vasodilatory molecules. (C) Diverse transcription factors from ETS, GATA, AP-1/2, FOX, NFATC, HOX, and other families. (D) Guidance cues and receptors related to axon guidance, including semaphorins, ephrins, and plexins. (E) Genes encoding matricellular basement membrane proteins, collagen fibril assembly, adhesion, and junction molecules. (F) Histone modifiers including lysine demethylases, methyltransferases, deacetylases, and acetyltransferases. (G) Genes that are inversely regulated by HIF1α and HIF2α.
HIF1α also regulates the autophagy-related genes BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) and BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 like (BNIP3L), which have also been shown to increase mitochondrial autophagy in order to reduce mitochondrial mass and as an adaptive survival mechanism to maintain ATP levels.19 Conversely, HIF2α induced the expression of PPARα, PPARγ, and other genes related to fatty acid and lipoprotein uptake, including the very low-density lipoprotein receptor (VLDLR), LDL receptor-related protein 2 (LRP2), lipase A (LIPA), phospholipid transfer protein (PLTP), perilipin 2 (PLIN2), and the fatty acid transporter SLC27A3.
Extracellular Signaling and Guidance Cues
The HIFs primary means of regulating angiogenesis is through the induction of multiple extracellular signaling ligands and receptors with diverse functional activities on multiple vascular, mural, and immune cell types. HIF1α and HIF2α regulated multiple growth factors of the vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), Wnt, and the transforming growth factor (TGF) families (Figure 6B). VEGF-A, the principle pro-angiogenic growth factor, was ranked as one of the highest induced HIF2α target genes (Figure 1B) but was also regulated by HIF1α to a lesser degree. The other VEGF members were exclusively regulated such that VEGFC was a HIF2α-specific target, while VEGFB was regulated by HIF1α. Angiopoietin-like 4 (ANGPTL4) ranked as the highest upregulated growth factor genes for both HIF1α and HIF2α. HIF2α regulated several other signaling cytokines related to angiogenesis, including interleukin 33 (IL-33), C-X-C motif chemokine ligand 8 (CXCL8), C-C motif chemokine ligand 28 (CCL28), and bone morphogenetic protein 4 (BMP4). Furthermore, HIF2α was also seen to regulate several corresponding receptors, including receptor tyrosine kinases VEGF receptor 1 (FLT1), VEGF receptor 2 (KDR), FGF receptor 3 (FGFR3), insulin receptor (INSR), and the cytokine receptors C-X-C chemokine receptor type 4 (CXCR4), leptin receptor (LEPR), and tumor necrosis factor receptor superfamily member 10B (TNFRSF10B). Multiple genes encoding vasodilator peptide hormones and receptors including adrenomedullin (ADM), natriuretic peptide receptor (NPR1/3), natriuretic peptide B (NPPB), vasoactive intestinal peptide (VIP), and prostaglandin synthases (PTGS1, PTGIS) were regulated by HIF1α and HIF2α. HIF2α, unlike HIF1α, regulated juxtacrine signaling pathways, including the Notch-related genes Delta-like 4 (Dll4), hairy and enhancer of split-1 (HES1), Deltex E3 Ubiquitin Ligase 4 (DTX4), and Lunatic Fringe Homolog (LFNG) as shown in Figure 6C. Other guidance pathways regulated by HIF2α included the Sonic Hedgehog pathway-related genes Patched 1 (PTCH) and Smoothened, Frizzled Class Receptor (SMO), in addition to axon-guidance-related signaling molecules and receptors (class 3 and 4 semaphorins, ephrins, and plexins) also shown in Figure 6C. Overall, both HIF1α and HIF2α regulated a variety of extracellular signaling processes but HIF2α appeared to regulate a larger and more diverse subset of target genes.
Transcription
Analysis of the molecular function GO terms associated with HIF1α and HIF2α showed the largest difference was for multiple transcription factor activity-associated terms, where HIF2α regulated 66 genes classified under the term GO:0000982 relative to 20 regulated by HIF1α, several of which are shown in Figure 6D. Both HIF1α and HIF2α regulated multiple immediate-early response genes, such as AP-1 subunits (FOS, JUNB, ATF3), ETS, and MAPK7, which can act as downstream terminal effector nodes for extracellular signaling pathways.20 Interestingly, HIF2α virtually exclusively upregulated a significant fraction (n = 24) of the class I HOX genes from the HOXA, HOXB and HOXD clusters. Furthermore, HIF2α regulated another 36 homeobox related genes from various subclasses including PRD, NKL, LIM, POU, SINE, TALE, CUT, and ZF. Forty-one of these genes were associated with the pattern specification GO term (GO:0007389), which represents the highest enriched HIF2α biological process (Figure 4). Other regulated transcription factor families include GATA, KLF, NFATC, and FOX. In addition to transcription factors, HIF1α and HIF2α both modestly regulated various histone modifiers involved in chromatin remodeling and epigenetic regulation (Figure 6E). HIF1α and HIF2α regulated a number of repressive histone deacetylases (HDACs) known to act on H3K27ac of promotors and enhancers. Alternatively, they both regulated several Jumonji domain containing lysine-specific demethylases (KDMs) responsible for demethylating multiple histones, including H3K9, H3K27, and H3K36, which require O2 as a cofactor.21 HIF1α upregulated the histone acetyltransferase KAT6B, whereas HIF2α upregulated the classical HIF histone acetyltransferase cofactors EP300 and CREB binding protein (CREBBP), which mediates activating H3K27 acetylation.22
Extracellular Remodeling
HIF2α, and to a lesser degree HIF1α, appear to regulate extracellular matrix (ECM) remodeling through induction of the basement membrane components laminin and collagen IV, in addition to enzymes required for collagen post-translational modification and fibril assembly (Figure 6F). HIF1α and HIF2α both induce gene expression of enzymes responsible for mediating the hydroxylation of the procollagen proline residues by the collagen prolyl 4-hydroxylases P4HA1 and P4HA2, along with procollagen lysine residues by the lysyl hydroxylases, procollagen-lysine,2-oxoglutarate 5-dioxygenase (PLOD) 1 and 2. Furthermore, the lysyl oxidases LOX and LOXL2 genes that mediate the lysine-derived inter-polypeptide chain crosslinks required for the stabilization of collagen fibrils, are also HIF regulated. Likewise, both HIFs regulated a number of matricellular proteins associated with wound healing and tissue repair including fibulin 2 and 7 (FBLN), periostin (POSTN), versican (VCAN), and tenascin X (TNXB), which contribute to vessel formation and vessel wall integrity.23 Additionally, the Claudin 3 (CLDN3), component of endothelial tight junctions as part of the paracellular barrier between ECs was highly induced by both HIF1α and HIF2α. Conversely, the anti-angiogenic matricellular protein thrombospondin 1 (THBS1) was downregulated by HIF2α.
Interaction Networks of HIF1α and HIF2α Target Genes
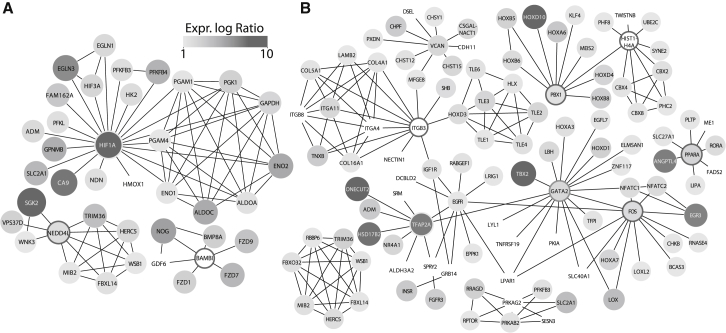
To identify functional interactions between HIF DEGs, we used Cytoscape with the Reactome-FI plugin to create modules of known functional complexes. In Figure 7A, HIF1α DEGs formed three modules where HIF1α was seen to act as a central hub, forming 21 interactions with its regulated genes with the majority associated with glycolysis (n = 9, FDR 4e-15). In Figure 7B, HIF2α DEGs formed four modules, the largest consisting of 69 connected nodes with transcription activity as the most overrepresented function (FDR 1.7e-4). The transcription factors GATA2 and FOS formed the central hubs with 17 and 13 first neighbor nodes, collectively. Pathway analysis of the GATA2 and FOS hubs were associated with the AP-1 transcription factor network (GATA2 FDR < 0.01; FOS FDR < 3e-5) and nuclear factor of activated T cells (NFAT) transcription (GATA2 FDR < 0.01; FOS FDR < 6e-5). A HIF2α DEG module with PPARα as a hub was associated to the already identified fatty acid uptake-related proteins SLC27A1, PLTP, and LIPA.
Figure 7.
Functional Interaction Networks of HIF-Regulated Genes
Cytoscape with the Reactome-FI plugin was used to identify genes regulated by HIF1α (A) or HIF2α (B) that formed functional interaction networks. Gene nodes are colored according to their log2 fold change. Genes that act as central hubs are highlighted with borders, whereas edge genes are shown without borders.
HRE Motif Occurrence in Promoters of Target Genes
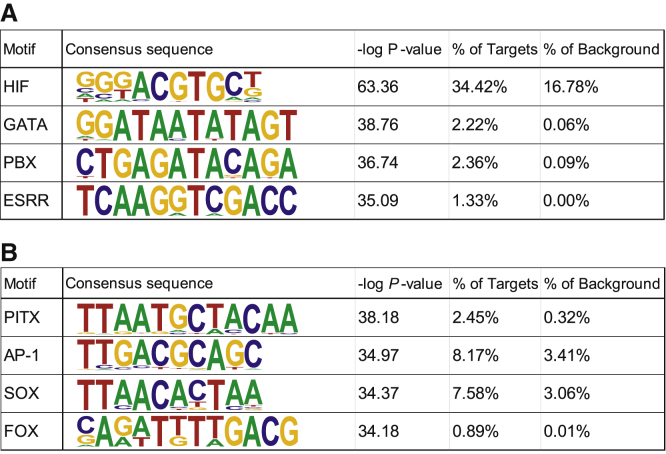
To further explore the role of HIF transcriptional regulation, the enrichment of the consensus HRE motif and other motifs was analyzed in regions proximal to HIF1α and HIF2α target gene transcriptional start sites (TSSs). Transcription factor motifs enriched in HIF1α and HIF2α target gene TSSs is shown in Figures 8A and 8B, respectively. As anticipated, the HRE motif was identified as the most significantly enriched motif present in 34.42% of HIF1α target genes (p value < 1e-27); unexpectedly however, the HRE motif was not significantly enriched in HIF2α DEG TSSs over the GC% matched background. Using public ChIP-seq data for H3K4me2 H3K27ac with co-occupancy as a mark of regulatory regions, we performed motif enrichment analysis for those intergenic regions occurring within 100 kb of HIF DEGs. Motif analysis revealed a similar trend in that the HRE was only significantly identified in HIF1α DEG regulatory regions, with the top motifs shown in Figure S5. These findings suggest that HIF2α may either regulate a significant subset of its DEGs either directly through mechanisms independent of its DNA binding or through indirect regulation of other transcription factors.
Figure 8.
Motif Analysis of the Proximal Regulatory Regions of HIF Target Genes
Enriched motifs identified in the proximal regions flanking (±750 bp) the transcription start site of HIF1α (A) and HIF2α (B) target genes calculated relative to a GC% matched background sequence.
Discussion
Critical limb ischemia resulting from advanced peripheral artery disease (PAD) requires extensive intervention to maximize limb salvage, vessel patency, and wound healing. The capacity for physiological angiogenesis is frequently impaired in patients with PAD due to the presence of multiple comorbidities, such as aging and diabetes mellitus, which contribute to endothelial dysfunction24, 25 and reduced HIF1α activity.1, 2 Current revascularization strategies to preserve limb viability include surgical vascular bypass and endovascular angioplasty, with or without stenting.26 Nevertheless, surgical revascularization carries periprocedural complications and remains of limited use to patients exhibiting diffuse and distal forms of the disease that is frequently associated with diabetes mellitus.27
Therapeutic vascular regeneration through the use of minimally invasive gene- or cell-based therapies is anticipated to circumvent the disease constraints placed on endogenous angiogenesis and to stimulate robust collateral growth as to alleviate ischemia and restore limb functionality. Angiogenesis, however, represents a complex process that consists of multiple spatially and temporally regulated processes such as migration, sprout/tube formation, proliferation, and vascular and stromal remodeling. As the master systemic and cellular transcriptional regulators of the hypoxia response, the HIFs remain an attractive, if complex and incompletely understood, candidate for vascular regeneration. While several studies have attributed a critical role to HIF1α in angiogenesis,28 the full extent of its transcriptional activity in comparison with HIF2α has yet to be defined.
Our present findings indicate that in ECs, HIF2α transcriptionally regulates a larger and more functionally diverse set of target genes compared with those of HIF1α. These findings contrast results from studies in other cell types, where HIF1α was the predominantly active isoform and HIF2α was seen to be largely redundant.29, 30 We hypothesize that these differences could result from the more limited specific expression profile of HIF2α31 or a prerequisite for cell-specific transcriptional cofactors required for productive HIF2α transcriptional elongation.8 Not only did HIF1α and HIF2α exhibit substantially different degrees of gene regulation, but there was also limited overlap in their target genes, which was surprising given their close structural, functional, and regulatory similarities.
HIF1α was seen to primarily regulate genes affecting metabolic reprogramming, suggesting it mediates a shift in energy production away from O2-dependent oxidative phosphorylation to anoxic glycolysis. As part of a comprehensive metabolic transcriptional program, HIF1α concurrently regulated genes associated with NADH redox balance, lactate efflux, methylglyoxal detoxification, and cellular autophagy, likely to enable ECs to adapt and survive in an acute, low O2 environment. Conversely, the predominantly HIF2α-regulated fatty acid and lipoprotein uptake may appear counterintuitive but may represent a mechanism to acquire energy reserves for proliferation, membrane biosynthesis, and lipid signaling.32
HIF2α, and to a lesser degree HIF1α, transcriptionally regulate biological processes involved in angiogenesis through the regulation of numerous extracellular signaling ligands and receptors, comprised of growth factors, cytokines, vasoactive compounds, and guidance cues. The growth factors VEGF, FGF, and TGFβ have well-characterized roles in vascular biology, where they can stimulate EC proliferation, sprouting, migration, and survival.33 Within angiogenesis, vascular patterning is critical for optimal vascular network density requiring precisely regulated spatiotemporal signaling between EC and their environment. This process is primarily controlled through chemoattractant gradients, EC tip/stalk phenotype selection and other attraction and repulsion cues. Migratory sprout “tip” ECs closest to the chemoattractant gradient express high levels of the Notch ligand Dll4, which is regulated by MEF2 and HIF2α.34, 35 Subsequent juxtacrine signaling between tip cell Dll4 and the trailing stalk cell Notch receptors laterally supresses the migratory tip cell phenotype through reducing Dll4 and KDR expression to prevent excessive branching.36 In addition to Notch signaling, HIF2α also appeared to specifically induce a number of axonal guidance-associated molecules, including semaphorins, ephrins, plexins, and their receptors, which are known to act as attraction or repulsion cues in vascular morphology and branching but also arterial/venous vascular patterning.37
This additional layer of regulation of angiogenesis through Notch signaling and vascular guidance cues may enhance the formation of organized, well-perfused, and functional vasculature. A similar observation has been reported in conditional EC knockout mice, where EPAS1 deletion resulted in poor perfusion caused by disordered vasculature in a Dll4-dependent manner.35 In addition to effects on EC, HIF2α also contributes to vessel normalization by inducing the expression of multiple collagens, collagen-modifying enzymes, and other secreted ECM proteins. Collagen IV, periostin, fibulin, and laminins are used to synthesize the basement membrane of the immature vascular structure with parallel recruitment of pericytes.38 Since edema and poor perfusion caused by leaky, immature, and irregular collateral vessels is a major clinical limitation when using gene transfer with single growth factor like VEGFA39, 40, HIF2α may alternatively support normalized collateral growth due to its capacity to regulate multiple balanced pro-angiogenic pathways.
Strikingly, the largest difference between HIF1α and HIF2α transcriptional profiles was the degree of regulation of other transcription factors. HIF2α overexpression resulted in over 100 genes characterized as transcription factors or transcriptional regulators being induced. Motif analysis of HIF2α DEG promotors or distal regulatory regions failed to identify enrichment for the canonical HRE, suggesting that HIF2α regulation is either indirectly mediated through other induced transcription factors or through independent DNA-binding mechanisms. HIF2α has been shown to preferentially bind to intergenic regions rather than proximal promoters relative to HIF1α.6 Several HIF2α-regulated transcription factors have well-defined roles in EC angiogenic processes, such as GATA2 and FOS, both of which are known to interact with a network of other HIF2α DEGs, including NFATC1/2 and TFAP2A. The role of other transcription factors, such as the large magnitude of Hox and non-Hox homeobox genes, remains less well characterized in the context of HIF activation. Various individual Hox genes are known to be differentially expressed in conditions of vascular quiescence, sprouting angiogenesis and vessel normalization in addition to wound healing.41 However, how HIF2α induced Hox cluster expression and subsequent spatiotemporal gene regulation would influence revascularization is currently poorly defined. It would be interesting to delineate the role of HIF-induced Hox activity in the spatiotemporal regulation of gene expression in development and disease. Induction of various transcriptional cofactors and histone modifiers likely represents an additional layer of intricate transcriptional regulation.
It is becoming increasingly evident that HIF1α and HIF2α fulfil non-overlapping niche roles in regulating the cellular and systemic hypoxia response and their related biological processes. Further understanding of their activities and downstream effects will hopefully enable appropriate clinical use of either coordinate or individual HIFs in vascular regeneration.
Materials and Methods
Cell Culture
Human umbilical vein ECs (HUVECs) were isolated from cords obtained from the Kuopio University Hospital with approval from the Kuopio University Hospital Ethics Committee. HUVECs were maintained in endothelial growth medium (EGM; basal medium with SingleQuots supplements CC-4133; Lonza) on T-75 cell culture flasks coated with 10 g/mL fibronectin and 0.05% gelatin (Sigma, St. Louis, MO) and maintained in humidified 5% CO2.
Adenovirus Transductions
Sub-confluent HUVECs (70%) at passage 5 were infected 24 hr post-seeding with E1/E3-deleted replication-incompetent adenovirus serotype 5 overexpressing a stabilized HIF1α (P402→A, P563→A), HIF-2α (P405→A, P530→A), or an empty construct containing the CMV minimal promoter (eCMV) as a control.42 Prior to transduction, the media was exchanged for endothelial basal media without supplements, and diluted adenovirus at an MOI of 20 was added and incubated for 4 hr. Subsequently, media was exchanged for complete EGM with supplements. Cells were collected for RNA and protein isolation 48 hr post-infection.
Protein Extraction and Western Blotting
Cells were lysed using RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and protease inhibitor cocktail [Roche]), and the total protein content was determined with the BCA protein assay (Pierce, Thermo Fisher Scientific). Nine micrograms of protein was separated on Mini-PROTEAN TGX Stain-Free gels (Bio-Rad) and transferred to a 0.2-μm nitrocellulose membrane (Bio-Rad). The membrane was blocked with Tris-buffered saline-Tween 20 (TBST) containing 5% nonfat dry milk overnight at 4°C and incubated with primary antibodies diluted 1:1,000 in TBST containing 5% BSA for 1 hr at room temperature. Antibodies used were as follows: anti-HIF1α (Thermo Fisher Scientific, MA1-16504), anti-HIF2α (Thermo Fisher Scientific, MA1-16519), anti-PECAM1 (Dako, M0823). HRP-conjugated goat anti-IgG (Thermo Fisher Scientific, 32430) and was diluted 1:5,000 with TBST containing 5% nonfat dry milk and the membranes incubated for 1 hr at room temperature before ECL detection (ChemiDox XRS).
Library Preparation, Sequencing, and qPCR
Total RNA from cells was isolated using RNeasy Kit (QIAGEN) followed by DNase treatment using the Turbo DNase kit (Ambion). Two hundred fifty nanograms RNA was reverse transcribed using SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific). qPCR was performed using a StepOne Plus (Applied Biosystems) with TaqMan universal master mix (Applied Biosystems) to confirm induction of HIF target genes using TaqMan assays (Applied Biosystems) for VEGFA (Hs00903129) and GAPDH (Hs99999905_m1) with RPLP0 (Hs00420895_gH) as an endogenous control. Gene expression was estimated using the 2−ΔΔCT method. RNA-seq libraries were prepared from 250 ng of total RNA using the TruSeq Stranded Total RNA Library Prep Kit with Ribo-Zero Gold (Illumina). Sequencing was performed with the Nextseq 500 using 75 cycles in single end high output mode.
Data Analysis
RNA-seq was mapped using STAR11 allowing up to two mismatches and reporting only one alignment for each read. Poor-quality reads were filtered out (minimum 97% of bp over quality cutoff 10) and tag per base value was set to 3. Transcripts with low counts of CPM < 1 not present in at least two libraries above this threshold were considered not expressed and removed from the analysis. Differential gene expression was estimated using edgeR,12 using TMM library size normalization and quasi-likelihood F-testing. Transcripts with a log2 FC of ≥ 1 or ≤ −1 and FDR < 0.01 were considered differentially expressed. Plots were produced and visualized in R using ggplot2. Multi-dimensional scaling (MDS) was performed using cmdscale. Heatmaps were produced using Heatmap.2 and Pheatmap. Hierarchal clustering was performed using Ward’s least absolute error with Manhattan distance.43 Gene ontology was performed using clusterProfiler14 with FDR cutoff of 0.05, and the most informative GO terms were selected with “Simplify” using minimum FDR with the Rel method. Interaction analysis of DEGs was performed using the Cytoscape software with the Reactome functional interaction (FI) plugin.44 Pathway analysis of the DEGs was performed using IPA (QIAGEN Redwood City). Promoter and proximal regulatory element motif analysis of differentially regulated genes was performed using the “findmotifs.pl” command in HOMER using a ±750-bp region of the TSS with a motif length of 8, 10, and 12 bases. A random set of genomic positions with matched GC% composition was used as background for enrichment. Co-bound HUVEC H3K4me2 and H3K27ac intergenic regions were determined using annotation from the “annotatePeaks.pl” command. Motif analysis was performed on those regions within 100 kb of HIF target genes using “findMotifsGenome.pl” command with default settings with the total co-bound H3K4me2 and H3K27ac peaks used as background.
Data Access
The experiments performed in this study are available in GEO: GSE98060. The public ChIP-seq data for H3K4me2 and H3K27ac are available in GEO: GSE29611 and GSE38555.
Author Contributions
N.L.D. conducted the experiments, analyzed the data, and wrote the manuscript. S.Y.-H., M.U.K., and N.L.-K. conceived and supervised the project.
Acknowledgments
We thank the EMBL GeneCore Sequencing Service (https://www.genecore.embl.de) for RNA-seq library preparation and sequencing. Viral vectors were obtained from the National Virus Vector Laboratory (Kuopio), which is supported by Biocentre Finland and EU EATRIS infrastructure networks. We would also like to thank Annakaisa Tirronen for assistance with the western blots and Henri Niskanen for assistance with the RNA-seq data. N.L.D. and S.Y.-H. were supported by the Finnish Academy Centre of Excellence and ERC Advanced Grant. M.U.K. was supported by grants from the Academy of Finland (287478 and 294073), the Finnish Foundation for Cardiovascular Research, the Jane and Aatos Erkko Foundation, the Sigrid Juselius Foundation, and the Finnish Diabetes Research Foundation.
Footnotes
Supplemental Information includes six figures and one table and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.05.004.
Supplemental Information
References
- 1.Bosch-Marce M., Okuyama H., Wesley J.B., Sarkar K., Kimura H., Liu Y.V., Zhang H., Strazza M., Rey S., Savino L. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 2.Botusan I.R., Sunkari V.G., Savu O., Catrina A.I., Grünler J., Lindberg S., Pereira T., Ylä-Herttuala S., Poellinger L., Brismar K., Catrina S.B. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Dengler V.L., Galbraith M., Espinosa J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014;49:1–15. doi: 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schödel J., Oikonomopoulos S., Ragoussis J., Pugh C.W., Ratcliffe P.J., Mole D.R. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith B., Johnson R.S., Simon M.C. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu C.-J., Sataur A., Wang L., Chen H., Simon M.C. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol. Biol. Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamidian A., von Stedingk K., Munksgaard Thorén M., Mohlin S., Påhlman S. Differential regulation of HIF-1α and HIF-2α in neuroblastoma: estrogen-related receptor alpha (ERRα) regulates HIF2A transcription and correlates to poor outcome. Biochem. Biophys. Res. Commun. 2015;461:560–567. doi: 10.1016/j.bbrc.2015.04.083. [DOI] [PubMed] [Google Scholar]
- 10.Uchida T., Rossignol F., Matthay M.A., Mounier R., Couette S., Clottes E., Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J. Biol. Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 11.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy D.J., Chen Y., Smyth G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhry H., Schödel J., Oikonomopoulos S., Camps C., Grampp S., Harris A.L., Ratcliffe P.J., Ragoussis J., Mole D.R. Extensive regulation of the non-coding transcriptome by hypoxia: role of HIF in releasing paused RNApol2. EMBO Rep. 2014;15:70–76. doi: 10.1002/embr.201337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlicker A., Domingues F.S., Rahnenführer J., Lengauer T. A new measure for functional similarity of gene products based on Gene Ontology. BMC Bioinformatics. 2006;7:302. doi: 10.1186/1471-2105-7-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D., Smyth G.K. Camera: a competitive gene set test accounting for inter-gene correlation. Nucleic Acids Res. 2012;40:e133. doi: 10.1093/nar/gks461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramakrishnan S., Anand V., Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014;9:142–160. doi: 10.1007/s11481-014-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie J., Dai C., Hu X. Evidence that does not support pyruvate kinase M2 (PKM2)-catalyzed reaction as a rate-limiting step in cancer cell glycolysis. J. Biol. Chem. 2016;291:8987–8999. doi: 10.1074/jbc.M115.704825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellot G., Garcia-Medina R., Gounon P., Chiche J., Roux D., Pouysségur J., Mazure N.M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah A.V., Birdsey G.M., Randi A.M. Regulation of endothelial homeostasis, vascular development and angiogenesis by the transcription factor ERG. Vascul. Pharmacol. 2016;86:3–13. doi: 10.1016/j.vph.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukada Y., Fang J., Erdjument-Bromage H., Warren M.E., Borchers C.H., Tempst P., Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 22.Tie F., Banerjee R., Stratton C.A., Prasad-Sinha J., Stepanik V., Zlobin A., Diaz M.O., Scacheri P.C., Harte P.J. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136:3131–3141. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubmacher D., Apte S.S. The biology of the extracellular matrix: novel insights. Curr. Opin. Rheumatol. 2013;25:65–70. doi: 10.1097/BOR.0b013e32835b137b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momose M., Abletshauser C., Neverve J., Nekolla S.G., Schnell O., Standl E., Schwaiger M., Bengel F.M. Dysregulation of coronary microvascular reactivity in asymptomatic patients with type 2 diabetes mellitus. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1675–1679. doi: 10.1007/s00259-002-0977-0. [DOI] [PubMed] [Google Scholar]
- 25.Taddei S., Virdis A., Ghiadoni L., Salvetti G., Bernini G., Magagna A., Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 26.Menard M.T., Farber A., Assmann S.F., Choudhry N.K., Conte M.S., Creager M.A., Dake M.D., Jaff M.R., Kaufman J.A., Powell R.J. Design and rationale of the best endovascular versus best surgical therapy for patients with critical limb ischemia (BEST-CLI) trial. J. Am. Heart Assoc. 2016;5:e003219. doi: 10.1161/JAHA.116.003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawarada O., Fujihara M., Higashimori A., Yokoi Y., Honda Y., Fitzgerald P.J. Predictors of adverse clinical outcomes after successful infrapopliteal intervention. Catheter. Cardiovasc. Interv. 2012;80:861–871. doi: 10.1002/ccd.24370. [DOI] [PubMed] [Google Scholar]
- 28.Manalo D.J., Rowan A., Lavoie T., Natarajan L., Kelly B.D., Ye S.Q., Garcia J.G., Semenza G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 29.Mole D.R., Blancher C., Copley R.R., Pollard P.J., Gleadle J.M., Ragoussis J., Ratcliffe P.J. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu C.-J., Iyer S., Sataur A., Covello K.L., Chodosh L.A., Simon M.C. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol. Cell. Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiesener M.S., Jürgensen J.S., Rosenberger C., Scholze C.K., Hörstrup J.H., Warnecke C., Mandriota S., Bechmann I., Frei U.A., Pugh C.W. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 32.Rankin E.B., Rha J., Selak M.A., Unger T.L., Keith B., Liu Q., Haase V.H. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol. Cell. Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behm B., Babilas P., Landthaler M., Schreml S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012;26:812–820. doi: 10.1111/j.1468-3083.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- 34.Sacilotto N., Chouliaras K.M., Nikitenko L.L., Lu Y.W., Fritzsche M., Wallace M.D., Nornes S., García-Moreno F., Payne S., Bridges E. MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev. 2016;30:2297–2309. doi: 10.1101/gad.290619.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skuli N., Majmundar A.J., Krock B.L., Mesquita R.C., Mathew L.K., Quinn Z.L., Runge A., Liu L., Kim M.N., Liang J. Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes. J. Clin. Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams C.K., Li J.L., Murga M., Harris A.L., Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams R.H., Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stratman A.N., Malotte K.M., Mahan R.D., Davis M.J., Davis G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ylä-Herttuala S. The pharmacology of gene therapy. Mol. Ther. 2017;25:1731–1732. doi: 10.1016/j.ymthe.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ylä-Herttuala S., Bridges C., Katz M.G., Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur. Heart J. 2017;38:1365–1371. doi: 10.1093/eurheartj/ehw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kachgal S., Mace K.A., Boudreau N.J. The dual roles of homeobox genes in vascularization and wound healing. Cell Adhes. Migr. 2012;6:457–470. doi: 10.4161/cam.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niemi H., Honkonen K., Korpisalo P., Huusko J., Kansanen E., Merentie M., Rissanen T.T., André H., Pereira T., Poellinger L. HIF-1α and HIF-2α induce angiogenesis and improve muscle energy recovery. Eur. J. Clin. Invest. 2014;44:989–999. doi: 10.1111/eci.12333. [DOI] [PubMed] [Google Scholar]
- 43.Strauss T., von Maltitz M.J. Generalising Ward’s method for use with Manhattan distances. PLoS ONE. 2017;12:e0168288. doi: 10.1371/journal.pone.0168288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Croft D., O’Kelly G., Wu G., Haw R., Gillespie M., Matthews L., Caudy M., Garapati P., Gopinath G., Jassal B. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39:D691–D697. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.