SUMMARY
Phosphodiesterase-5-inhibitors, such as sildenafil, increase intracavernosal cyclic guanosine monophosphate levels, which results in corporal smooth muscle relaxation and penile erection. Here, we determined the effects of sildenafil administration on the hypo-thalamic-pituitary-gonadal axis in men with erectile dysfunction and low testosterone levels. The Testosterone and Erectile Dysfunction trial (ClinicalTrials.gov # NCT00512707) initially administered an optimized dose of sildenafil to 140 men, aged 40–70 years with erectile dysfunction, low serum total testosterone (<11.4 nmol/L; 330 ng/dL) and/or free testosterone (<173 pmol/L; 50 pg/mL) over 3–7 weeks. Sex steroids and gonadotropins were measured at baseline and after sildenafil optimization in a longitudinal study without a separate control group. Serum testosterone, dihydrotestosterone (DHT) and oestrogens were measured using liquid chromatography-tandem mass spectrometry. Administration of an optimized dose of sildenafil was associated with mean increases of 3.6 nmol/L (103 ng/dL; p < 0.001) and 110 pmol/L (31.7 pg/mL; p < 0.001) in total and free testosterone levels respectively. This was accompanied by parallel increases in serum DHT (0.17 nmol/L; 4.9 ng/dL; p < 0.001) and oestradiol (14 pmol/L; 3.7 pg/mL; p < 0.001) and significant suppression of luteinizing hormone (change −1.3 units/L; p = 0.003) levels, suggesting a direct effect at the testicular level. Androstenedione and oestrone increased by 1.3 nmol/L (38 ng/dL; p = 0.011) and 10.7 pmol/L (2.9 pg/mL; p = 0.012), respectively, supporting a possible effect of sildenafil on adrenal steroidogenesis. In conclusion, sildenafil administration was associated with increased testosterone levels likely ascribable to a direct effect on the testis.
Keywords: erectile dysfunction, selective phosphodiesterase-5 inhibitors, sildenafil, testosterone
INTRODUCTION
Phosphodiesterase-5-inhibitors (PDE5Is) have been shown to consistently and substantially improve erectile function in multiple randomized, placebo-controlled clinical trials (Rosen et al., 2002; Tsertsvadze et al., 2009). Accordingly, the American College of Physician guidelines recommend PDE5Is as the first-line therapy for erectile dysfunction (Goldstein et al., 1998; Rosen et al., 2002; Qaseem et al., 2009). Nevertheless, the effects of PDE5Is on androgen levels in men with low testosterone remain poorly understood.
PDE5Is inhibit cyclic guanosine monophosphate-specific phosphodiesterase type 5 in the human corpus cavernosum, resulting in an increase in cyclic guanosine monophosphate, activation of protein kinase G, a decrease in intracellular calcium ions, and relaxation of vascular smooth muscle (Boolell et al., 1996; Rotella, 2002). PDE5Is exert additional effects that may contribute to improved erections, including an increase in penile blood flow. In male rats, PDE5Is upregulate 17,20 lyase and steroidogenic acute regulatory protein, a rate-limiting step in the steroidogenic pathway, likely via activation of protein kinase G1 (Andric et al., 2010; Janjic et al., 2012). However, the effects of PDE5Is on serum androgen levels have not been investigated in detail (Carosa et al., 2004).
Here we describe the hormonal changes that followed the administration of sildenafil in a randomized trial (The Testosterone and Erectile Dysfunction trial, TED; ClinicalTrials.gov registration number NCT00512707) (Spitzer et al., 2012). The mean serum total and free testosterone levels increased significantly and substantially following administration of an optimized dose of sildenafil to men with erectile dysfunction and low testosterone levels. As serum luteinizing hormone (LH) levels exhibited simultaneous decreases, the observed increase in serum testosterone likely results from a direct action of sildenafil on the testis.
METHODS
Participants
Men aged 40–70 years with erectile dysfunction (International Index of Erectile Function erectile function domain score ≤25) and serum total testosterone <11.4 nmol/L (330 ng/dL) and/or free testosterone <173 pmol/L (50 pg/mL) were eligible for participation (Spitzer et al., 2012). The exclusion criteria were prostate cancer, creatinine >180 lmol/L (2 mg/dL), haemoglobin A1c ≥8.5%, prostate specific antigen >4 ng/mL, blood pressure >160/100 mmHg, myocardial infarction or stroke within 6 months or congestive heart failure. The use of androgens, antiandrogens, nitrates, high dose opiates, glucocorticoids and antiepileptics constituted additional exclusion criteria.
Study design
The TED trial was a randomized, double-blind, placebo-controlled, single-centre, and parallel group trial that aimed to determine the effect of adding testosterone therapy to an opti-mized dose of sildenafil citrate in men with low testosterone and erectile dysfunction (Spitzer et al., 2012). The trial consisted of a screening phase and an open-label sildenafil dose-optimization phase, followed by an intervention phase, during which participants received either testosterone or placebo gel, randomly assigned, in addition to an optimized dose of sildenafil. We have previously published comparisons of testosterone to placebo effects on sexual function (Spitzer et al., 2012). This report char-acterizes the hormonal changes among participants after initiating sildenafil monotherapy, prior to randomization.
Sildenafil dose optimization
Participants were each started and optimized on sildenafil for 3–7 weeks during the open-label phase. Men were generally started on sildenafil 50 mg p.o. daily, although sildenafil 25 mg was initiated if individuals used an α-adrenergic blocker. Those reporting prior use of sildenafil 100 mg daily were started on this dose. Men receiving 25 or 50 mg had an opportunity to increase this dose up to 100 mg p.o. as needed for sexual activity (but no more than once in any 24-h period) if they were dissatisfied with erectile response to a lower dose.
Hormone assays
Total testosterone and dihydrotestosterone (DHT) were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS), for which sensitivities are 0.07 nmol/L (2 ng/dL) and 0.2 nmol/L (5 ng/dL) respectively (Bhasin et al., 2012). Sex hormone binding globulin (SHBG) was measured using an immunofluorometric assay (PerkinElmer, Waltham, MA, USA), which has a sensitivity of 2.5 nmol/L (Basaria et al., 2010). Free testosterone was calculated from total testosterone and SHBG using the law of mass action (Vermeulen et al., 1999). Oestradiol and oestrone were measured using LC-MS/MS (sensitivity 7 pmol/L; 2 pg/mL) (Basaria et al., 2013). Interassay coefficients of variation for oestradiol were 9.2, 6.7, 6.1, 6.1 and 7.6% at 25.7 pmol/L (7.0 pg/mL), 125 pmol/L (34 pg/mL), 209 pmol/L (57 pg/mL), 433 pmol/L (118 pg/mL) and 1399 pmol/L (381 pg/mL) respectively. The interassay coefficients of variation for oestrone were 8.1, 7.3, 7.3, 9.9 and 8.7% at 23.7 pmol/L (6.4 pg/mL), 129 pmol/L (35 pg/mL), 214 pmol/L (58 pg/mL), 451 pmol/L (122 pg/mL) and 1446 pmol/L (391 pg/mL) respectively. LH and follicle-stimulating hormone (FSH) were assessed using fluoroimmunoassays (PerkinElmer), each with a sensitivity of 0.05 U/L. The interassay coefficients of variation for the LH assay were 3.1% at 3.63 U/L, 3.1% at 20.0 U/L and 4.2% at 50.8 U/L. The interassay coefficients of variation for the FSH assay were 1.8% at 2.58 U/L, 2.0% at 11.5 U/L and 1.8% at 44.8 U/L. Inhibin B was measured using an enzyme-linked immunosorbent assay (Beckman Coulter, Brea, CA, USA), whose sensitivity was 2.6 pg/mL and interassay coefficients of variation 5.4 and 5.7% at 99.9 and 363.9 pg/mL respectively (Kalra et al., 2010).
Dehydroepiandrosterone sulphate (DHEAS) and androstene-dione were measured using enzyme-linked immunosorbent assays (DRG International, Springfield, NJ, USA). Sensitivities for DHEAS and androstenedione are 4.4 lg/dL and 1.9 ng/dL respectively. Interassay coefficients of variation for androstene-dione are 9.6, 12.1 and 8.8% at 20, 230 and 440 ng/dL respectively. Interassay coefficients of variation for DHEAS are 6.5, 10.6 and 5.4% at 20, 130 and 440 lg/dL respectively. Assays were performed according to manufacturers’ recommendations and under internal quality control.
Sexual activity
The Male Sexual Health Questionnaire provided estimates of self-reported sexual activity levels before and after sildenafil therapy (Rosen et al., 2004).
Statistical methods
Statistical analyses were performed in R version 2.15.1 (R Foundation, Vienna, Austria). Graphical displays were generated using the ggplot2 library (Wickham, 2009). Paired Student’s t-tests were used to assess evidence in favour of changes in mean hormonal levels during the period that subjects were exposed to sildenafil monotherapy. Hormonal changes on silde-nafil 100 and 50 mg were contrasted using simple linear regression. Changes in serum total testosterone at different levels of sexual activity were compared using adjusted means in a multivariate linear model involving baseline and post-sildenafil sexual activity reports. Results were considered significant if null hypotheses could be rejected at the 0.05 level.
RESULTS
Baseline characteristics
A total of 140 participants were included in this analysis. The mean age of these men was 55 years, and at baseline their average total testosterone was 8.7 nmol/L (251 ng/dL; Table 1).
Table 1.
Baseline characteristics
| Age (years) | 55 (8; 137) |
| Race – n (%) | |
| Black | 61 (44) |
| White | 68 (49) |
| Other | 9 (7) |
| BMI (kg/m2) | 32 (6; 117) |
| Diabetes mellitus – n (%) | 27 (21) |
| Hypertension – n (%) | 56 (42) |
| Total testosterone (ng/dL) | 251 (65; 140) |
| Free testosterone (pg/mL) | 46 (15; 138) |
| Dihydrotestosterone (ng/dL) | 24 (11; 132) |
| Oestradiol (pg/mL) | 28 (10; 132) |
| Androstenedione (ng/dL) | 247 (102; 133) |
| Oestrone (pg/mL) | 41 (16; 132) |
| Luteinizing hormone (U/L) | 5.6 (3.7; 133) |
| Follicle-stimulating hormone (U/L) | 6.4 (5.2; 133) |
| DHEAS (lg/dL) | 115 (70; 133) |
| SHBG (nmol/L) | 31 (16; 119) |
| Inhibin B (pg/mL) | 141 (51; 133) |
BMI, body mass index; DHEAS, dehydroepiandrosterone sulphate; SHBG, sex hormone binding globulin. Continuous variables are described as mean (SD; n). Numbers of participants are less than 140 ascribable to missing data.
Sildenafil dose and frequency
Overall, 106 (76%) were optimized to 100 mg, 33 (24%) to 50 mg and 1 (1%) to 25 mg dose as needed for sexual activity respectively (Spitzer et al., 2012). Participants used sildenafil on average 2.6 (SD 1.2) times weekly (Spitzer et al., 2012).
Hormone levels
Mean total and free testosterone increased significantly with the addition of sildenafil by 3.58 nmol/L (103 ng/dL; p < 0.001) and 110 pmol/L (31.7 pg/mL; p < 0.001; Fig. 1) respectively. Serum concentrations of DHT and oestradiol, metabolites of testosterone, also increased by an average 0.17 nmol/L (4.9 ng/dL; p < 0.001) and 14 pmol/L (3.7 pg/mL; p < 0.001) respectively (Fig. 1).
Figure 1.
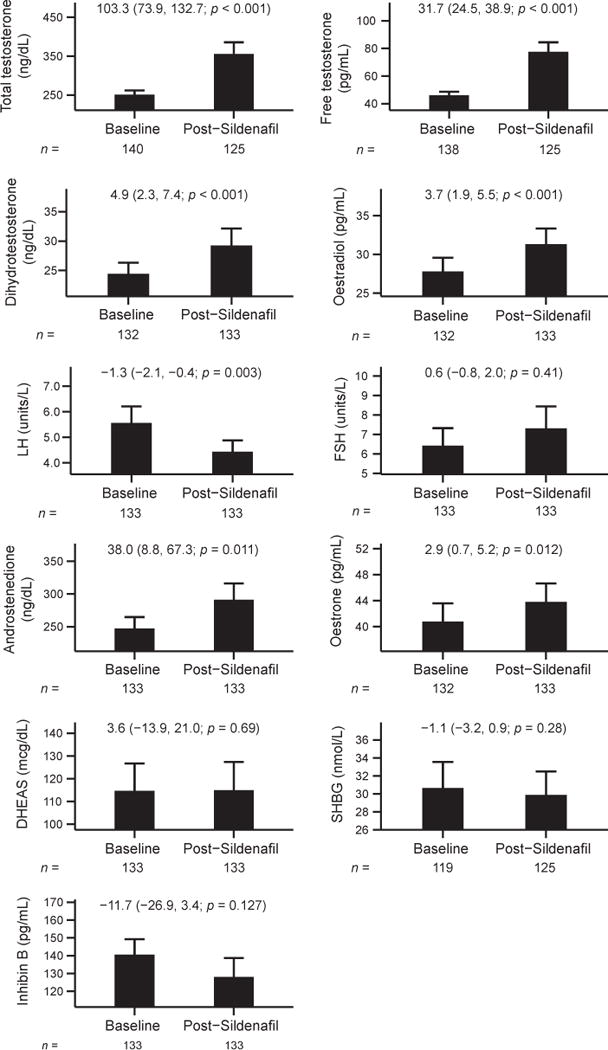
Hormonal changes with sildenafil therapy in men with low testosterone and erectile dysfunction. Mean hormone levels with 95% confidence intervals (CI) are displayed pre-and post-sildenafil therapy. Mean changes (95% CI; p-value), calculated using paired t-tests, are printed in each graph. The numbers of participants contributing to data at each time point are shown below the graphs.
To determine whether sildenafil increased testosterone levels by a direct effect on the testis, we measured serum LH concentrations. Serum LH decreased significantly from baseline by an average 1.3 U/L (p = 0.003), suggesting that sildenafil likely directly stimulates Leydig cell steroidogenesis. Serum FSH, SHBG and inhibin B levels remained unchanged.
Androstenedione, oestrone and DHEAS were assessed to evaluate the effects of sildenafil on adrenal androgens. Serum androstenedione levels increased by 1.3 nmol/L (38 ng/dL; p = 0.011), which was paralleled by an increase in serum oestrone (its metabolite) of 11 pmol/L (2.9 pg/mL; p = 0.012). There was no change in serum DHEAS levels.
Sildenafil dose and hormone levels
The increases in total and free testosterone levels with sildena-fil therapy were not different between the 50 and 100 mg groups (Table 2).
Table 2.
Hormonal changes on sildenafil 100 and 50 mg
| Hormone | Change with sildenafil 100 mg | Change with sildenafil 50 mg | Difference (95% confidence interval) | p-value | n |
|---|---|---|---|---|---|
| Total testosterone (ng/dL) | 112.8 | 74.6 | 38.3 (−30.2, 106.7) | 0.27 | 124 |
| Free testosterone (pg/mL) | 33.5 | 26.5 | 7.0 (− 9.8, 23.7) | 0.41 | 122 |
| Dihydrotestosterone (ng/dL) | 5.1 | 4.5 | 0.6 (− 5.3, 6.5) | 0.84 | 125 |
| Oestradiol (pg/mL) | 3.8 | 3.8 | −0.1 (− 4.2, 4.1) | 0.98 | 125 |
| Luteinizing hormone (U/L) | −1.4 | −0.8 | −0.6 (−2.5, 1.3) | 0.52 | 125 |
| Androstenedione (ng/dL) | 51.0 | 7.6 | 43.4 (− 23.4, 110.2) | 0.2 | 125 |
| Oestrone (pg/mL) | 1.9 | 6.0 | −4.1 (− 9.2, 1.1) | 0.12 | 125 |
Hormonal changes on sildenafil 100 and 50 mg were compared using simple linear regression, in which sildenafil dose was the independent variable and change in hormone was the dependent variable.
Effect of sexual activity on serum testosterone levels
Three men reporting no sexual activity while on sildenafil were excluded from the analysis of sexual activity and serum testosterone levels attributable to insufficient sample size. Among men who reported sexual activity post-sildenafil, changes in serum testosterone levels were not associated with post-sildenafil sexual activity, even after adjusting for baseline sexual activity (Table 3).
Table 3.
Changes in serum total testosterone at different levels of sexual activity
| Post-sildenafil sexual activity | Change in serum total testosterone (95% CI; ng/dL) | n |
|---|---|---|
| 1–3 times/month | 102.7 (25.4, 180.1) | 22 |
| 4–6 times/month | 149.1 (89.1, 209.1) | 47 |
| >6 times/month | 102.0 (40.0, 164.0) | 39 |
| Daily or almost daily | 144.5 (52.6, 236.4) | 14 |
Least-squares mean changes in serum total testosterone at various levels of post-sildenafil sexual activity are displayed, after adjusting for baseline sexual activity (n = 122). Change in serum total testosterone was the dependent variable, and pre- and post-sildenafil sexual activities were independent variables in a multivariate linear regression model. Pairwise comparisons of serum total testosterone levels reveal no differences across levels of sexual activity (p ≥ 0.22). Three men reporting no sexual activity post-sildenafil were excluded from this analysis due to small sample size. Sexual activity was ascertained using the Male Sexual Health Questionnaire.
DISCUSSION
The increases in serum total and free testosterone in conjunction with a significant decrease in serum LH levels with sildenafil therapy in men with erectile dysfunction and low testosterone levels suggest a direct effect of sildenafil on the testis. Circulating concentrations of DHT and oestradiol, which are derived mostly from peripheral conversion of testosterone, also increased. Taken together, these hormonal changes are most consistent with a direct stimulatory effect of sildenafil on Leydig cell steroidogenesis, which likely resulted in the expected feedback inhibition of LH secretion. Androstenedione and its metabolite oestrone rose from baseline to post-sildenafil. Although DHEAS levels did not change significantly after sildenafil administration, we cannot exclude the possibility that sildenafil may also stimulate adrenal androgen synthesis.
Preclinical studies support a direct effect of sildenafil on Leydig cell steroidogenesis. PDE5 is abundantly expressed in the rat Leydig and peritubular cells (Scipioni et al., 2005) and administration of sildenafil to rats stimulates the expression of steroidogenic acute regulatory protein and protein kinase G1, possible mechanisms behind this increase in serum testosterone levels (Andric et al., 2007, 2010; Janjic et al., 2012). An increase in sexual activity associated with sildenafil administration may contribute to the increase in testosterone levels, as has been reported previously following successful treatment of erectile dysfunction (Jannini et al., 1999; Aversa et al., 2013); however, in this study, greater sexual activity did not predict greater change in serum total testosterone (Table 3). Sexual arousal has been associated with acute increases in LH and testosterone, suggesting central neurohormonal stimulation (LaFerla et al., 1978; Rowland et al., 1987); however, we observed a significant suppression of serum LH. Other studies also have reported an inconsistent and complex relationship between sexual activity and testosterone levels (Anonymous, 1970). The observed pattern of hormonal changes in our trial – increased testosterone and decreased LH levels – is more suggestive of a direct testicular effect of sildenafil. Further investigation of the relationship between sildenafil and sexual activity in other contexts would be informative.
Few clinical investigations have systematically investigated the link between PDE5I use and testosterone levels. An open-label, retrospective study of men with erectile dysfunction who reported improvement in erectile function with PDE5I therapy found similar increases in testosterone levels (Carosa et al., 2004). However, in another study, neither sildenafil 100 mg by mouth daily nor vardenafil 20 mg daily for 6 months significantly changed testosterone, LH, or FSH levels in eugonadal men (Jarvi et al., 2008). The lower baseline testosterone levels in participants of our trial may have contributed to the more robust increase in testosterone levels with sildenafil.
The adrenal glands are the major site for production of androstenedione, which is converted to oestrone by aromatase (Payne & Hales, 2004; de Ronde et al., 2005). Enzyme 17,20 lyase, which converts 17α-hydroxyprogesterone to androstenedione, is upregulated with sildenafil treatment in rats (Payne & Hales, 2004; Andric et al., 2010). This could explain the increase in serum androstenedione levels in our participants. However, an increase in 17,20-lyase activity should also result in an increase in DHEAS levels, which was not observed. Further investigation is needed to study the effect of sildenafil on adrenal androgens.
This study has some limitations. Because low testosterone levels were a condition of enrolment, some portion of the observed increases in testosterone occurring during sildenafil administration is almost certainly attributable to regression to the mean. Given the lack of a control regime during this phase, its contribution to the observed increase in testosterone levels cannot be estimated, although the significant suppression of LH after silde-nafil administration suggests a direct effect on testosterone production. The magnitude of the observed increase in testosterone of ~3.5 nmol/L (100 ng/dL) greatly exceeds the changes reported for placebo groups from other clinical trials, which imposed similar ‘low testosterone’ criteria as a condition of enrolment (Sih et al., 1997; Snyder et al., 1999; Steidle et al., 2003; Basaria et al., 2010; Srinivas-Shankar et al., 2010). Consequently, it seems unlikely that the change in serum total testosterone observed during the TED run-in phase was entirely ascribable to placebo effect or regression to the mean.
Hormonal changes in men receiving 100 mg sildenafil did not differ from those in men receiving 50 mg sildenafil (Table 2). The failure to demonstrate a robust dose–response relationship between the sildenafil dose and increase in testosterone levels may either be attributable to the small number of men receiving the lower doses of sildenafil (e.g. only 33 men received the 50 mg dose and one man received the 25 mg dose) or because the maximum effect of sildenafil on testicular steroidogenesis occurs at the 50 mg dose.
This investigation also has notable strengths. We measured total testosterone, DHT, oestradiol and oestrone levels using LC-MS/MS, widely considered the reference method with the highest sensitivity and specificity. Second, the dose of sildenafil was based on a standardized regimen. Lastly, we included men with erectile dysfunction and testosterone levels that were well below the lower limit of the reference range (Bhasin et al., 2011).
The unexpected increase in serum testosterone with sildenafil has potential clinical applications, especially among men with ED and low serum total testosterone levels. As PDE5I therapy, in addition to improving erectile function, also raises testosterone levels, it seems reasonable to first administer PDE5I monothera-py to men with ED and borderline low testosterone levels and measure testosterone levels post-PDE5I treatment to assess the need for additional testosterone therapy.
CONCLUSIONS
Administration of an optimized dose of sildenafil to men with erectile dysfunction and low baseline serum testosterone increases serum testosterone levels likely by a direct action on the testes.
Acknowledgments
This research was supported by a grant from the National Institute of Child Health and Human Development (5R01HD047722). The Boston University Clinical and Translational Science Institute (1UL1RR025771) and Boston Claude D. Pepper Older Americans Independence Center (grant 5P30AG031679 from the National Institute of Aging) also contributed support. Pfizer, Inc. provided sildenafil for this trial, and Auxilium Pharmaceuticals provided testosterone gel for this trial. Funding agencies, Pfizer, and Auxilium Pharmaceuticals did not have any role in the trial design, data analysis or manuscript preparation. The authors thank the members of the Data and Safety Monitoring Board [Abraham Morgentaler, MD (Chair); Leonard Marks, MD; Andre Guay, MD; and Ridwan Shabsigh, MD] and the staff of the Boston University Clinical and Translational Science Institute.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Shalender Bhasin designed this trial. Shalender Bhasin and Shehzad Basaria implemented the protocol and collected data from participants. Helene Stroh conducted hormonal assays. Statistical analyses were conducted by Matthew Spitzer, Maithili Davda, and Thomas Travison. Matthew Spitzer wrote the first draft of the manuscript. All authors reviewed, edited, and approved the final manuscript.
References
- Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Protein kinase G-mediated stimulation of basal Leydig cell steroidogenesis. Am J Physiol Endocrinol Metab. 2007;293:E1399–E1408. doi: 10.1152/ajpendo.00482.2007. [DOI] [PubMed] [Google Scholar]
- Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Sildenafil treatment in vivo stimulates Leydig cell steroidogenesis via the cAMP/cGMP signaling pathway. Am J Physiol Endocrinol Metab. 2010;299:E544–E550. doi: 10.1152/ajpendo.00337.2010. [DOI] [PubMed] [Google Scholar]
- Anonymous. Effects of sexual activity on beard growth in man. Nature. 1970;226:869–870. doi: 10.1038/226869a0. [DOI] [PubMed] [Google Scholar]
- Aversa A, Jannini EA, Maggi M, Lenzi A. Effects of testosterone replacement on response to sildenafil citrate. Ann Intern Med. 2013;158:569–570. doi: 10.7326/0003-4819-158-7-201304020-00018. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68:153–160. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Travison TG, Storer TW, Lakshman K, Kaushik M, Mazer NA, et al. Effect of testosterone supplementation with and without a dual 5alpha-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012;307:931–939. doi: 10.1001/jama.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- Carosa E, Martini P, Brandetti F, Di Stasi SM, Lombardo F, Lenzi A, et al. Type V phosphodiesterase inhibitor treatments for erectile dysfunction increase testosterone levels. Clin Endocrinol (Oxf) 2004;61:382–386. doi: 10.1111/j.1365-2265.2004.02108.x. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- Janjic MM, Stojkov NJ, Bjelic MM, Mihajlovic AI, Andric SA, Kostic TS. Transient rise of serum testosterone level after single sildenafil treatment of adult male rats. J Sex Med. 2012;9:2534–2543. doi: 10.1111/j.1743-6109.2012.02674.x. [DOI] [PubMed] [Google Scholar]
- Jannini EA, Screponi E, Carosa E, Pepe M, Lo Giudice F, Trimarchi F, et al. Lack of sexual activity from erectile dysfunction is associated with a reversible reduction in serum testosterone. Int J Androl. 1999;22:385–392. doi: 10.1046/j.1365-2605.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- Jarvi K, Dula E, Drehobl M, Pryor J, Shapiro J, Seger M. Daily vardenafil for 6 months has no detrimental effects on semen characteristics or reproductive hormones in men with normal baseline levels. J Urol. 2008;179:1060–1065. doi: 10.1016/j.juro.2007.10.077. [DOI] [PubMed] [Google Scholar]
- Kalra B, Kumar A, Patel K, Patel A, Khosravi MJ. Development of a second generation Inhibin B ELISA. J Immunol Methods. 2010;362:22–31. doi: 10.1016/j.jim.2010.08.002. [DOI] [PubMed] [Google Scholar]
- LaFerla JJ, Anderson DL, Schalch DS. Psychoendocrine response to sexual arousal in human males. Psychosom Med. 1978;40:166–172. doi: 10.1097/00006842-197803000-00007. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Snow V, Denberg TD, Casey DE, Jr, Forciea MA, Owens DK, et al. Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2009;151:639–649. doi: 10.7326/0003-4819-151-9-200911030-00151. [DOI] [PubMed] [Google Scholar]
- de Ronde W, Hofman A, Pols HA, de Jong FH. A direct approach to the estimation of the origin of oestrogens and androgens in elderly men by comparison with hormone levels in postmenopausal women. Eur J Endocrinol. 2005;152:261–268. doi: 10.1530/eje.1.01830. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Cappelleri JC, Gendrano N., 3rd The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226–244. doi: 10.1038/sj.ijir.3900857. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Catania J, Pollack L, Althof S, O’Leary M, Seftel AD. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology. 2004;64:777–782. doi: 10.1016/j.urology.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Rotella DP. Phosphodiesterase 5 inhibitors: current status and potential applications. Nat Rev Drug Discov. 2002;1:674–682. doi: 10.1038/nrd893. [DOI] [PubMed] [Google Scholar]
- Rowland DL, Heiman JR, Gladue BA, Hatch JP, Doering CH, Weiler SJ. Endocrine, psychological and genital response to sexual arousal in men. Psychoneuroendocrinology. 1987;12:149–158. doi: 10.1016/0306-4530(87)90045-x. [DOI] [PubMed] [Google Scholar]
- Scipioni A, Stefanini S, Santone R, Giorgi M. Immunohistochemical localisation of PDE5 in Leydig and myoid cells of prepuberal and adult rat testis. Histochem Cell Biol. 2005;124:401–407. doi: 10.1007/s00418-005-0057-1. [DOI] [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Basaria S, Travison TG, Davda MN, Paley A, Cohen B, et al. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med. 2012;157:681–691. doi: 10.7326/0003-4819-157-10-201211200-00004. [DOI] [PubMed] [Google Scholar]
- Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- Tsertsvadze A, Fink HA, Yazdi F, MacDonald R, Bella AJ, Ansari MT, et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med. 2009;151:650–661. doi: 10.7326/0003-4819-151-9-200911030-00150. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; New York, NY: 2009. [Google Scholar]


