Abstract
Symptomatic androgen deficiency is common in patients taking opioid analgesics, as these drugs potently suppress the hypothalamic–pituitary–gonadal axis. However, the efficacy of testosterone replacement in this setting remains unclear. The objective of this trial was to evaluate the efficacy of testosterone replacement on pain perception and other androgen-dependent outcomes in men with opioid-induced androgen deficiency. We conducted a randomized, double-blind, parallel placebo-controlled trial at an outpatient academic research center. Participants were men aged 18 to 64 years on opioid analgesics for chronic noncancer pain, and total testosterone levels were <350 ng/dL. Participants were randomly assigned to 14 weeks of daily transdermal gel that contained 5 g of testosterone or placebo. Primary outcomes were changes in self-reported clinical pain and objectively assessed pain sensitivity. Sexual function, quality of life, and body composition were also assessed. The mean age was 49 years. The median total and free testosterone levels at baseline were 243 ng/dL and 47 pg/mL and 251 ng/dL and 43 pg/mL in the testosterone and placebo arm, respectively. Of the 84 randomized participants, 65 had follow-up data on efficacy outcomes. Compared with men assigned to the placebo arm, those assigned to testosterone replacement experienced greater improvements in pressure and mechanical hyperalgesia, sexual desire, and role limitation due to emotional problems. Testosterone administration was also associated with an improvement in body composition. There were no between-group differences in changes in self-reported pain. In conclusion, in men with opioid-induced androgen deficiency, testosterone administration improved pain sensitivity, sexual desire, body composition, and aspects of quality of life.
Keywords: Opioid-induced androgen deficiency, Low testosterone, Pain perception, Sexual function, Quality of life
1. Introduction
The prescription use of opioid analgesics for the management of chronic pain grew substantially during the past 2 decades with the number of prescriptions increasing from 75.5 to 209.5 million.36 Among the many risks associated with the use of opioid analgesics, androgen deficiency due to suppression of gonadotropin-releasing hormone secretion has remained greatly underappreciated as a common side effect, with some patients experiencing severe androgen deficiency with testosterone levels in the castrate range.37 Opioid-induced androgen deficiency is often associated with distressing symptoms, such as sexual dysfunction, hot flushes, low mood, osteoporosis, and reduced quality of life.14,25
Testosterone replacement in men with androgen deficiency is associated with an improvement in sexual function, body composition, and quality of life.6 A substantial body of preclinical data also supports the notion that the opioid and gonadal systems interact to regulate sensitivity to nociceptive stimuli. In castrated male rodents, testosterone administration has been shown to improve pain tolerance to mechanical and thermal stimuli.3 These animal experiments, when taken together with findings from population studies showing that men have decreased pain perception and greater pain tolerance than women, suggest that testosterone has antinociceptive properties.10,32 It is therefore conceivable that androgen deficiency resulting from opioid use could in fact reduce their analgesic efficacy, resulting in an increase in dose requirements. Accordingly, we hypothesized that testosterone therapy of men with opioid-induced androgen deficiency would improve self-reported and experimentally induced pain. Furthermore, the correction of androgen deficiency in these men would be expected to improve sexual function and body composition, as it does in hypogonadal men not taking opioid analgesics. These improvements, along with improvements in pain sensitivity and perception, should in turn contribute to improved health-related quality of life. Surprisingly, no randomized trials have been conducted to examine the effects of testosterone replacement in men with opioid-induced androgen deficiency.
In this randomized, double-blind, placebo-controlled parallel-group trial, we determined the effects of testosterone replacement on clinical and experimental pain, sexual function, body composition, and health-related quality of life in men with opioid-induced androgen deficiency.
2. Methods
2.1. Design overview
The Testosterone and Pain (TAP) trial was a randomized, double-blind, placebo-controlled, single-center parallel-group trial involving community-dwelling men with opioid-induced androgen deficiency. The trial was funded by AbbVie Pharmaceuticals (Chicago, IL), which also provided testosterone and placebo gels for the trial. The trial was designed by the authors, and the study protocol was approved by the institutional review board of the Boston University Medical Center. The authors of the study were responsible for the design, data collection, and database maintenance of the trial and vouch for the accuracy and completeness of the data.
2.2. Setting and participants
The study participants were men, 18 to 64 years of age, who were using opioid analgesics for chronic noncancer pain and had a morning serum total testosterone concentration of <350 ng/dL measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS). Participants were also required to have been taking at least 20 mg of hydrocodone (or morphine equivalent dose of another opioid) daily for at least 4 weeks. Exclusion criteria included a previous history of hypogonadism, use of androgen therapy, any malignancy, prostate-specific antigen (PSA) level >4 ng/mL, severe lower urinary tract symptoms, myocardial infarction within 3 months before enrollment, unstable angina, uncontrolled congestive heart failure, peripheral vascular disease, uncontrolled hypertension, and oxygen-requiring chronic obstructive pulmonary disease. All participants provided written informed consent.
2.3. Randomization and interventions
Eligible participants were randomly assigned to receive either 5 g of a transdermal testosterone gel (AndroGel 1%; Abbvie Pharmaceuticals) or placebo gel, applied once daily for 3 months. The target range for serum total testosterone level was between 500 and 1000 ng/dL. Two weeks after randomization, the dose was adjusted by an unblinded study physician if the serum total testosterone level was <500 ng/dL, in which case the dose was increased to 7.5 g daily. For any subject in the testosterone group requiring a dose increase, a placebo subject was chosen at random by the unblinded physician and was assigned a (simulated) dose increase, thus maintaining blinding.
2.4. Outcomes and assessments
Efficacy outcomes were assessed at baseline and at week 14.
2.4.1. Pain outcomes
The primary outcome was the change in self-reported pain, determined by the Brief Pain Inventory (BPI) questionnaire, and experimentally assessed pain sensitivity, determined by quantitative sensory testing (QST). The BPI is a validated measure of pain severity and pain interference for patients with chronic pain.34 The QST was performed in the pain laboratory to assess sensitivity to calibrated mechanical and thermal (cold) noxious stimuli while the subjects sat comfortably in a reclining chair.4,19,21 Pressure pain thresholds were assessed using a digital pressure algometer (Somedic, Hörby, Sweden) at the trapezius muscle and the metacarpophalangeal joint of the thumb. At each site, mechanical force was applied using a 0.5-cm2 probe; pressure was increased at a steady rate of 30 kPa/s until the subject indicated that pressure was “first perceived as painful.” Reaction to prolonged pressure pain was ascertained through cuff pressure algometry. Tonic, deep-tissue mechanical stimulation was applied over the gastrocnemius muscle using a pneumatic tourniquet cuff, which was inflated to and maintained at a particular pressure.21 A computer-controlled air compressor maintained the pressure at a level that was individually tailored for each subject to produce a pain intensity rating of 40/100. Participants then underwent an assessment of mechanical pain by temporal summation, an analog of central sensitization, using weighted pinprick stimulators.19 The lowest-force stimulator that produced a sensation of discomfort was used to apply a train of 10 stimuli to the skin on the dorsum of the hand at the rate of 1 per second. Participants rated the painfulness of the first and 10th stimuli. Cold-induced pain was evaluated using a repeated cold-pressor task involving immersion of the right hand in a circulating cold water bath maintained at 4°C. The first 2 tasks involved serial immersions of the right hand for 30 seconds, with 2 minutes between immersions. The final task involved immersion of the hand until a participant reached pain tolerance (or 3 minutes maximum). Participants rated the intensity of the cold pain on a 0 to 100 scale. Cold pain intensity ratings (0-100) were also obtained at 30-second intervals after cold-pressor tests to assess painful after-sensations.18
2.4.2. Sexual function, quality of life, and body composition
Sexual function was assessed using the International Index of Erectile Function,31 and the Medical Outcomes Study Short Form-36 was used to assess health-related quality of life.38 Body composition was measured using dual-energy X-ray absorpti-ometry scan (QDR 4500A; Hologic, Waltham, MA), calibrated before each scan using a soft tissue phantom.8
2.5. Hormone assays
Total testosterone was measured in a CDC-certified laboratory using an LC-MS/MS method with a sensitivity of 2 ng/dL.8 Sex hormone binding globulin and luteinizing hormone were measured using immunofluorometric assays (PerkinElmer, Waltham, MA), with limits of quantification of 2.5 nmol/L and 0.05 U/L, respectively.8 Free testosterone was calculated using a law of mass action equation.35 Because proinflammatory cytokines have been implicated in the pathophysiology of pain, we measured interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) using high-sensitivity enzyme-linked immunospecific assay (Millipore, Billerica, MA) with sensitivities of 0.039 pg/mL and 0.1 pg/mL, respectively.5
2.6. Safety monitoring
Safety monitoring included measurements of hemoglobin, hematocrit, and PSA, a prostate examination and assessment of adverse events. An independent Data and Safety Monitor Board reviewed all serious adverse events as they occurred and cumulative adverse events every 6 months.
2.7. Statistical analysis
The trial was designed to detect a between-group treatment difference in change in pain perception and pain sensitivity with 80% power, which required the enrollment of 35 evaluable subjects per group. In anticipation of approximately 15% cumulative attrition, the planned enrollment was 84 subjects. Of the 84 subjects randomized, 65 had follow-up data on efficacy outcomes, a total close to that assumed in original computations. Descriptive statistics were computed for each outcome and randomization arm, and graphical depictions of change were obtained. For analytes that typically exhibit some level of right skew (eg, total testosterone, luteinizing hormone), central tendency was summarized using medians and quartiles. The prespecified primary estimate of treatment effects computed the mean change in outcomes for each treatment arm along with 95% confidence intervals. Quantification of statistical significance of mean differences between arms was generated using Student’s t test for unpaired samples. Nonparametric assessments of distributional differences on opioid dose were obtained using Wilcoxon’s rank sum test. Control for covariate interference used multiple linear regression. Measures of association between change in circulating hormone concentrations and outcomes were obtained using Spearman’s rank correlation statistic supported by scatter plot smoothing through a generalized additive model and smoothing splines. Safety analyses computed the number of subjects with 1 or more adverse events overall and by physiologic system; consistency with the null hypothesis (no difference between arms) was evaluated using Fisher’s exact test. Hypothesis tests allowed type I error of 0.05 (α). Confidence intervals were estimated at the 95% level. As hypotheses were prespecified, no adjustment of significance threshold was made for multiple comparisons.
3. Results
3.1. Study participants and baseline characteristics
Enrollment took place between 2008 and November 2012. Of 84 enrolled men, 43 were randomized to the testosterone group and 41 to the placebo group (Fig. 1). Among these men, 36 in the testosterone arm and 29 in the placebo group completed the 14-week intervention, yielding an “analytic sample” of 65 participants.
Figure 1.
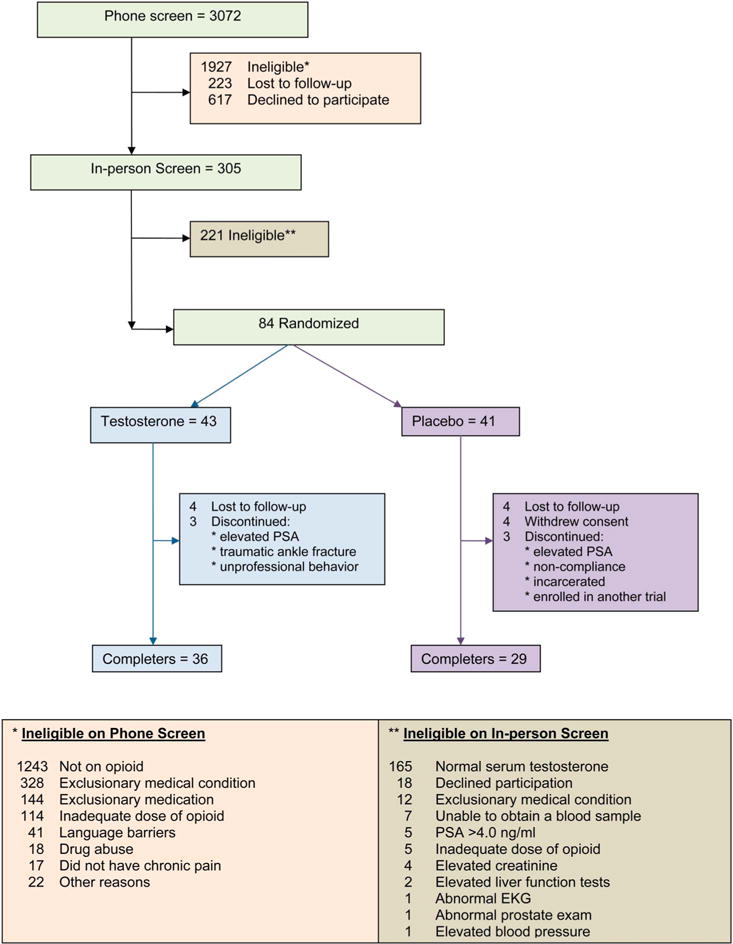
CONSORT diagram showing the flow of participants through the trial.
The mean age of the participants was 49 years, and the majority were obese (Table 1). The median (first quartile, third quartile) total and free testosterone levels in the testosterone and placebo arms were 243 (183, 286) ng/dL and 47 (27, 63) pg/mL and 251 (195, 313) ng/dL and 43 (38, 60) pg/mL, respectively. The most common indication for prescription of opioid analgesics was musculoskeletal pain (>95%). The mean ± SD morphine equivalent opioid dose at baseline was 114 ± 176 mg in the testosterone arm and 76 ± 139 mg in the placebo arm. The average time on opioids at the time of enrollment was 54 ± 48 months in the testosterone arm and 40 ± 54 months in the placebo arm. The baseline characteristics of the overall sample of 84 randomized participants were also similar between the 2 groups.
Table 1.
Baseline characteristics of the study participants.
| Testosterone (N = 36) | Placebo (N = 29) | |
|---|---|---|
| Age, y | 48 ± 9 | 50 ± 6 |
| Body mass index, kg/m2 | 32.0 ± 7.0 | 33.4 ± 8.0 |
| Opioid dose (morphine equivalent), mg | 114 ± 176 | 76 ± 139 |
| Time on opioids, mo | 54 ± 48 | 40 ± 54 |
| Total testosterone, ng/dL | 243 (183, 286) | 251 (195, 313) |
| Free testosterone, pg/mL | 46.7 (27.4, 63.3) | 43.0 (37.6, 59.6) |
| Sex hormone binding globulin, nmol/L | 29.7 (22.3, 42.2) | 33.5 (26.5, 41.9) |
| Luteinizing hormone, U/L | 2.6 (1.4, 4.4) | 3.3 (2.2, 5.1) |
| TNF-a, pg/mL | 1.7 (1.4, 2.1) | 1.6 (1.3, 2.0) |
| IL-6, pg/mL | 1.5 (1.1, 3.1) | 1.9 (1.2, 2.7) |
| Hemoglobin, g/dL | 15 (13, 16) | 14 (13, 15) |
| Hematocrit, % | 44 (40, 46) | 43 (39, 45) |
| Pain scores and parameters | ||
| BPI: total | 11.5 ± 3.2 | 10.4 ± 3.1 |
| BPI: severity | 5.7 ± 1.5 | 5.5 ± 1.7 |
| BPI: interference | 5.8 ± 2.2 | 4.9 ± 1.8 |
| Pressure pain threshold, trapezius | 341 ± 116 | 353 ± 197 |
| Pressure pain threshold, thumb | 369 ± 125 | 383 ± 185 |
| Mechanical probe pain ratings, first stimulus | 12.0 ± 13.0 | 8.0 ± 7.4 |
| Mechanical probe pain ratings, 10th stimulus | 27.0 ± 20.6 | 18.6 ± 19.5 |
| Cold pain tolerance | 40.1 ± 33.4 | 53.3 ± 51.3 |
| Cold pain after sensation: 30 s | 16.7 ± 19.2 | 13.8 ± 18.0 |
| Sexual functioning: IIEF | ||
| Total score | 44.6 ± 19.0 | 41.1 ± 21.7 |
| Erectile function | 18.8 ± 9.2 | 16.8 ± 10.6 |
| Orgasmic function | 6.5 ± 3.5 | 6.1 ± 3.6 |
| Sexual desire | 5.5 ± 2.5 | 6.2 ± 2.1 |
| Intercourse satisfaction | 8.2 ± 4.6 | 7.1 ± 5.6 |
| Overall satisfaction | 4.9 ± 2.5 | 5.0 ± 2.9 |
| Quality of life: Short Form-36 (SF-36) | ||
| Physical functioning | 41 ± 22 | 38 ± 25 |
| Bodily pain | 56 ± 15 | 55 ± 17 |
| Role limitations due to physical health problems | 10 ± 24 | 18 ± 31 |
| Role limitations due to personal or emotional problems | 39 ± 45 | 40 ± 45 |
| Emotional well-being | 37 ± 18 | 37 ± 18 |
| Social functioning | 60 ± 16 | 59 ± 17 |
| Energy/fatigue | 53 ± 13 | 54 ± 15 |
| General health perceptions | 54 ± 14 | 51 ± 14 |
| Body composition: DEXA | ||
| Lean mass, kg | 57.4 ± 7.8 | 60.1 ± 6.9 |
| Fat mass, kg | 27.0 ± 9.7 | 26.8 ± 9.5 |
| Total mass, kg | 84.4 ± 16.1 | 86.8 ± 13.8 |
| Appendicular lean mass, kg | 27.4 ± 4.0 | 28.6 ± 4.2 |
| Appendicular fat mass, kg | 11.7 ± 4.9 | 11.9 ± 5.1 |
| Appendicular total mass, kg | 38.3 ± 7.8 | 39.4 ± 7.0 |
Values are expressed in mean ± SD or median (25th percentile, 75th percentile).
BPI, Brief Pain Inventory; DEXA, dual-energy X-ray absorptiometry; IIEF, International Index of Erectile Function; IL-6, interleukin-6; TNF-α, tumor necrosis factor α.
3.2. Testosterone dose and adherence
Twenty-four men in the testosterone group required an increase in the testosterone dose to 7.5 mg/d. Adherence, as assessed by counts of used and unused gel packets, exceeded 90% in both groups.
3.3. Hormone levels
The changes in hormone levels are depicted in Figure 2. After adjustment of the testosterone dose, the mean (±SD) on-treatment total testosterone levels were 790 ± 544 ng/dL in the testosterone group and 328 ± 185 ng/dL in the placebo group. Similarly, on-treatment mean free testosterone levels were 189 ± 152 pg/mL in the testosterone group and 65 ± 36 pg/mL in the placebo group. The median (Q1, Q3) on-treatment total testosterone levels were 633 (404, 957) ng/dL and 303 (201, 458) ng/dL, whereas free testosterone levels were 123 (84, 246) pg/mL and 55 (39, 88) pg/mL in the testosterone and placebo groups, respectively.
Figure 2.
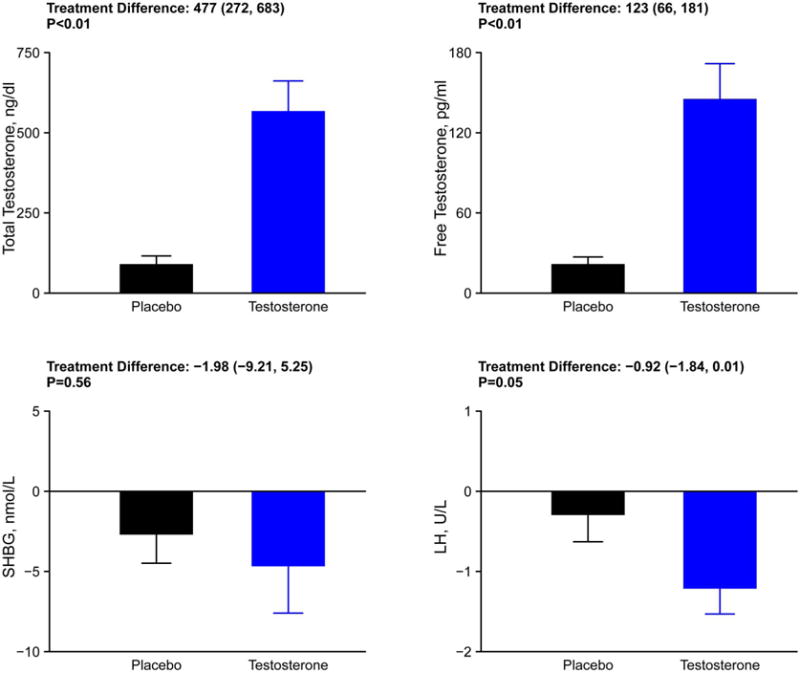
Postintervention changes in serum concentrations of total and free testosterone, luteinizing hormone, and sex hormone binding globulin levels in the 2 study arms.
3.4. Effects on experimentally induced and self-reported pain
Comparisons of testosterone and placebo response as quantified by QST are presented in Figure 3. Men randomized to testosterone arm exhibited a significantly greater improvement in pressure pain threshold at the thumb (P = 0.03; treatment difference, 90 points; 95% confidence interval, 9-171) than those randomized to the placebo arm, with a statistically nonsignificant though similar improvement at the trapezius (75 points; 28 to 158) (Fig. 3). Similarly, testosterone administration was associated with significantly lower punctate mechanical pain ratings (−5.9 points; −12.4 to 0.6) and lower temporal summation scores for mechanical pain (P = 0.05; −10 points; −19.9 to −0.04). Nonsignificant trends toward improvements in cold pain after-sensations were also seen in the testosterone group. Men randomized to testosterone reported a decrease in pain interference score on the BPI, but this trend was not statistically significant (Fig. 3). The changes in the dose of opioids between the 2 groups did not differ appreciably (mean dose increase of 4.8 mg and 2.4 mg in the testosterone and placebo groups, respectively; P = 0.79 by Wilcoxon’s rank sum test). Changes in the mean levels of inflammatory cytokines, IL-6 or TNF-α, did not differ between the 2 groups (Fig. 4).
Figure 3.
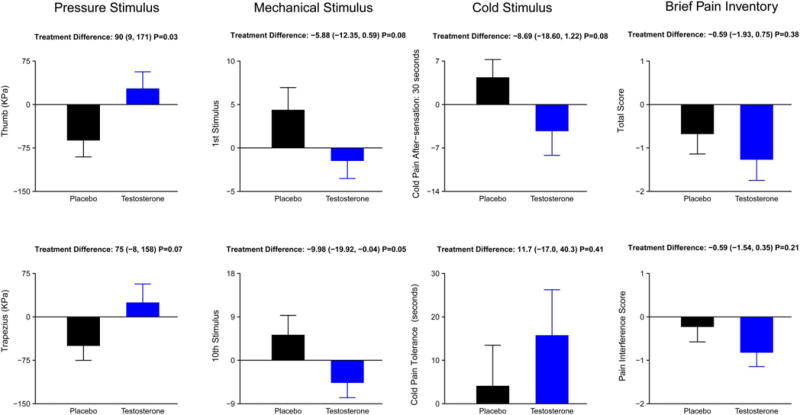
Postintervention changes in pain perception and tolerance with testosterone or placebo. Men in the testosterone arm exhibited greater tolerance to (1) algometer-induced pressure pain, (2) weighted pinprick stimulator–induced mechanical pain, and (3) ice water–induced cold pain and its after-sensations.
Figure 4.

Change in serum concentrations of inflammatory cytokines with testosterone or placebo.
3.5. Effects on sexual function and quality of life
Men randomized to testosterone experienced a significantly greater increase in sexual desire as compared with men in the placebo arm (P = 0.05; 1.11 units [0.002-2.17]) (Fig. 5) that was positively related to on-treatment serum testosterone concentration (Spearman’s correlation, 0.39; P = 0.002). The changes in orgasmic domain and erectile function domain did not differ between groups.
Figure 5.

Change in sexual function, quality of life and body composition with testosterone or placebo.
Compared with men in the placebo arm, the men in the testosterone group also experienced greater improvements in some domains of quality of life such as role limitations secondary to emotional problems (20.1 points; 2.2-42.4) (Fig. 5).
3.6. Effects on body composition
Men in the testosterone group experienced a significantly greater reduction of 0.8 kg in fat mass compared with those in the placebo (P = 0.01) (Fig. 5). The change in fat mass in men assigned to testosterone arm was negatively associated with on-treatment serum testosterone concentration (Spearman’s correlation, −0.45; P < 0.01). Similarly, the increase in lean body mass of 1.0 kg observed in the testosterone group was greater than the corresponding mean change (0.2 kg) observed in the placebo group (Fig. 5).
3.7. Adverse events
The frequency of adverse events did not differ between the 2 study arms (Table 2). There were 3 serious adverse events, 1 in the testosterone group (pancreatitis) and 2 in the placebo arm (elective total knee replacement and elective wrist arthrodesis). These events were not considered to be related to the study intervention. Elevation in PSA >4 ng/mL was seen in 2 subjects, 1 in each group, while erythrocytosis did not occur in either group.
Table 2.
Incidence of on-treatment adverse events by physiologic system.
| System | Testosterone (N = 43) | Placebo (N = 41) | P* |
|---|---|---|---|
| Cardiovascular | 2 (5) | 2 (5) | >0.99 |
| Respiratory | 1 (2) | 1 (2) | >0.99 |
| Gastrointestinal | 2 (5) | 2 (5) | >0.99 |
| Hepatic/Biliary | 0 (0) | 1 (2) | 0.49 |
| Genital/Urinary | 0 (0) | 3 (7) | 0.11 |
| Musculoskeletal | 7 (16) | 6 (15) | >0.99 |
| Hematologic/Lymphatic | 1 (2) | 0 (0) | >0.99 |
| Neurologic | 1 (2) | 2 (5) | 0.61 |
| Dermatologic | 1 (2) | 1 (2) | >0.99 |
| Psychiatric | 0 (0) | 1 (2) | 0.49 |
| Infectious disease | 6 (14) | 7 (17) | 0.77 |
| No. of subjects having other AEs | 6 (14) | 2 (5) | 0.27 |
| One or more AEs† | 18 (42) | 21 (51) | 0.51 |
| No. of subjects having serious AE‡ | 1 (2) | 2 (5) | 0.61 |
The P values reported are of Fisher’s exact test.
Fifty-six AEs were reported by 39 subjects.
Three serious AEs were reported in 3 subjects. The subject in the testosterone arm suffered from pancreatitis. Elective right total knee replacement and arthrodesis in the left wrist were reported in the subjects in the placebo arm. AE, adverse event.
4. Discussion
Opioid analgesics are widely prescribed for chronic pain. Testosterone deficiency is a common consequence of opioid analgesics in men who often present with sexual dysfunction, depressive symptoms, low bone mass, and decreased quality of life. Although small uncontrolled studies of testosterone replacement have been conducted in this patient population,2,13 no randomized controlled trials had been performed. In this first double-blind, randomized placebo-controlled trial of testosterone replacement in men with opioid-induced androgen deficiency, testosterone administration was associated with reduced sensitivity to multiple modalities of experimentally induced noxious stimuli, including pressure algometry and repetitive noxious punctate stimuli. The men randomized to testosterone did not show significant difference from placebo toward improvement in self-reported clinical pain. Studies have reported that improvements in QST-assessed pain responses precede improvements in self-reported clinical pain.20,23,39 For example, patients with carpal tunnel syndrome treated with manual therapy showed an immediate improvement in pain thresholds on QST, followed weeks later by a decrease in self-reported clinical pain.9 These findings suggest that QST responses serve as an early and sensitive “biomarker” of clinical response. Because changes in self-reported pain often lag behind changes in QST-assessed pain, it is possible that had the treatment duration been longer, significant differences in self-reported pain might have emerged.
In this trial, we show that restoration of serum testosterone levels improved pain sensitivity. These data are consistent with preclinical studies that show that gonadectomy in male rats predisposes to hyperalgesia to noxious stimuli, which is improved by testosterone replacement.3 Population studies find that use of analgesics is generally higher among women compared with men and that women complain of increased prevalence of pain on any given day compared with men.28 These data suggest that testosterone has antinociceptive properties. Hence, androgen deficiency resulting from opioid use could, in fact, reduce their pain-relieving efficacy. Many men on opioid analgesics also develop “opioid-induced hyper-algesia,” a condition in which pain sensitivity is increased despite the use of opioid analgesics.11 The pathophysiology behind this phenomenon remains unclear, however. It is conceivable that suppression of testosterone by opioids may also be a contributing factor in the pathogenesis of this condition.
Proinflammatory cytokines have been implicated in the pathophysiology of pain as they promote amplification of pain transmission, and administration of proinflammatory cytokines such as IL-6 and TNF-α induces muscle, joint, and visceral pain.16,17,27,33 As testosterone replacement has been shown to reduce circulating levels of these cytokines,26 we evaluated whether changes in these markers could represent 1 potential mechanism of testosterone’s antinociceptive action. Interestingly, testosterone administration was not associated with reduction in serum concentration of these cytokines, suggesting that testosterone improved pain by another mechanism.
Men with opioid-induced androgen deficiency experience deterioration in libido and erectile function.12,37 In this trial, men in the testosterone group showed significant improvement in sexual desire compared with those in placebo. Opioid-induced androgen deficiency is also associated with reduced quality of life.2 Previous uncontrolled reports have shown improvement in some aspects of health-related quality of life in men receiving testosterone.2,13 In this trial, men treated with testosterone demonstrated a nonsignificant trend toward improvement in role limitations due to emotional problems.
Testosterone deficiency is associated with a decrease in lean mass and an increase in fat mass, a phenotype associated with reduced muscle strength and function.6,24 Although effects of testosterone therapy on body composition are well established in other patient populations, it had not been studied in men with opioid-induced androgen deficiency. In this trial, men in testosterone group experienced a significant reduction in fat mass and an improvement in lean body mass compared with those in placebo. The improvement in body composition has importance because men on opioids are susceptible to developing metabolic abnormalities and cardiovascular disease.15,22,29 Loss of lean mass, particularly in older men on opioids, could also predispose to falls.25,36
This trial has many attributes of good trial design such as the inclusion of a placebo control, blinding, concealed randomization, and parallel-group design. Randomization effectively generated 2 intervention groups that were similar in baseline characteristics. Screening and on-treatment testosterone levels were measured using LC-MS/MS, which is widely considered the reference method. At baseline, mean total and free testosterone levels were well below the lower limits of normal established in community-based samples and validated against outcomes in epidemiologic studies7 and consistent with previous observational data in men with opioid-induced androgen deficiency.1,30 Testosterone dose was adjusted and was effective in increasing testosterone levels into the target range. Finally, validated tools were used to evaluate both self-reported and experimentally induced pain measures. The trial also had some limitations. The 14-week intervention duration was not long enough to observe changes in self-reported pain; a longer duration may have allowed detection of meaningful changes in this outcome. This was a single-site trial that limits the generalizability of results.
In summary, we conducted the first randomized, double-blind placebo-controlled trial to determine the efficacy of testosterone replacement in men with opioid-induced androgen deficiency and showed that testosterone therapy improved pain sensitivity to a number of noxious painful stimuli, confirming its antinociceptive role. Testosterone therapy also improved sexual desire and body composition. There is need for larger randomized trials of longer duration to further evaluate the efficacy of testosterone in chronic pain syndromes.
Acknowledgments
The authors thank Dr Alexander Walley, Lauren Collins, RNP, Maithili Davda, and Ulises Alvarez for their efforts on the trial. They also thank Dr Andre Guay, who was the Safety Monitor for this study, and the staff of the Boston University Clinical and Translational Science Institute.
Dr S. Basaria has received research grant on an investigator-initiated study from AbbVie Pharmaceuticals. He also consults for Eli Lilly. Dr S. Bhasin has previously received research grant from AbbVie Pharmaceuticals.
This research was supported by an investigator-initiated grant from AbbVie Pharmaceuticals, which also provided testosterone and placebo gels for this trial. AbbVie Pharmaceuticals did not have any role in the trial design, data analysis, or article preparation.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Conflict of interest statement
The remaining authors have no conflicts of interest to declare.
Author Contributions: S. Basaria and R. R. Edwards designed this trial, implemented the protocol, and collected data from participants. H. Stroh conducted hormonal assays. Statistical analyses were conducted by T. G. Travison. S. Basaria and R. R. Edwards wrote the first draft of the article. All authors reviewed, edited, and approved the final article.
Clinical Trials Registration Number: NCT00351819.
References
- 1.Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, Verlooy J, Van Havenbergh T, Smet M, Van Acker K. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215–22. doi: 10.1210/jcem.85.6.6615. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi AM, Ceccarelli I, Carlucci M, Suman A, Sindaco G, Mameli S, Paci V, Ravaioli L, Passavanti G, Bachiocco V, Pari G. Hormone replacement therapy in morphine-induced hypogonadic male chronic pain patients. Reprod Biol Endocrinol. 2011;9:9–26. doi: 10.1186/1477-7827-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloisi AM, Ceccarelli I, Fiorenzani P. Gonadectomy affects hormonal and behavioral responses to repetitive nociceptive stimulation in male rats. Ann N Y Acad Sci. 2003;1007:232–7. doi: 10.1196/annals.1286.022. [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–72. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68:153–60. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basaria S. Male hypogonadism. Lancet. 2013;13:61126–5. [Google Scholar]
- 7.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–9. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhasin S, Travison TG, Storer TW, Lakshman K, Kaushik M, Mazer NA, Ngyuen AH, Davda MN, Jara H, Aakil A, Anderson S, Knapp PE, Hanka S, Mohammed N, Daou P, Miciek R, Ulloor J, Zhang A, Brooks B, Orwoll K, Hede-Brierley L, Eder R, Elmi A, Bhasin G, Collins L, Singh R, Basaria S. Effect of testosterone supplementation with and without a dual 5a-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012;307:931–9. doi: 10.1001/jama.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bialosky JE, Bishop MD, Robinson ME, Price DD, George SZ. Heightened pain sensitivity in individuals with signs and symptoms of carpal tunnel syndrome and the relationship to clinical outcomes following a manual therapy intervention. Man Ther. 2011;16:602–8. doi: 10.1016/j.math.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Català E, Reig E, Artés M, Aliaga L, López JS, Segú JL. Prevalence of pain in the Spanish population: telephone survey in 5000 homes. Eur J Pain. 2002;6:133–40. doi: 10.1053/eujp.2001.0310. [DOI] [PubMed] [Google Scholar]
- 11.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 12.Cicero TJ, Bell RD, Wiest WG, Allison JH, Polakoski K, Robins E. Function of the male sex organs in heroin and methadone users. N Engl J Med. 1975;292:882–7. doi: 10.1056/NEJM197504242921703. [DOI] [PubMed] [Google Scholar]
- 13.Daniell HW, Lentz R, Mazer NA. Open-label pilot study of testosterone patch therapy in men with opioid-induced androgen deficiency. J Pain. 2006;7:200–10. doi: 10.1016/j.jpain.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3:377–84. doi: 10.1054/jpai.2002.126790. [DOI] [PubMed] [Google Scholar]
- 15.Darrouj J, Karma L, Arora R. Cardiovascular manifestations of sedatives and analgesics in the critical care unit. Am J Ther. 2009;16:339–53. doi: 10.1097/01.pap.0000249925.76324.47. [DOI] [PubMed] [Google Scholar]
- 16.De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96:1096–103. doi: 10.1213/01.ANE.0000055362.56604.78. [DOI] [PubMed] [Google Scholar]
- 17.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–5. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, Schreiber KL, Campbell C, Wasan AD, Jamison RN. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage. 2013;46:30–42. doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain. 2011;12:953–63. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer EZ. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–6. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6:599–606. doi: 10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 22.Guido M, Romualdi D, Lanzone A. Role of opioid antagonists in the treatment of women with glucoregulation abnormalities. Curr Pharm Des. 2006;12:1001–12. doi: 10.2174/138161206776055895. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YW, Somma J, Hung YC, Tsai PS, Yang CH, Chen CC. Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology. 2005;103:613–8. doi: 10.1097/00000542-200509000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 25.Kim TW, Alford DP, Malabanan A, Holick MF, Samet JH. Low bone density in patients receiving methadone maintenance treatment. Drug Alcohol Depend. 2006;85:258–62. doi: 10.1016/j.drugalcdep.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 27.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–32. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 28.Paller CJ, Campbell CM, Edwards RR, Dobs AS. Sex-based differences in pain perception and treatment. Pain Med. 2009;10:289–99. doi: 10.1111/j.1526-4637.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereska Z, Bozinovska C, Dimitrovski C, Cakalarovski K, Chibishev A, Zdravkovska M, Babulovska A, Janicevic D. Heroin dependence duration influences the metabolic parameters: mechanisms and consequences of impaired insulin sensitivity in hepatitis C virus seronegative heroin dependents. J Addict Med. 2012;6:304–10. doi: 10.1097/ADM.0b013e31826bd76c. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100:851–8. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 31.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 32.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain. 2004;112:248–53. doi: 10.1016/j.pain.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 36.Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–8. doi: 10.1059/0003-4819-155-5-201109060-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 39.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. PAIN. 2008;138:22–8. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]


