Abstract
N-acetyl-L-cysteine (NAC) is the most abundant water-soluble component of garlic. No study to date has studied the leukemia prevention ability of NAC in mouse systemic leukemia model. The current study aimed to investigate the leukemia initiation prevention potential of NAC in a mouse model. The cytotoxic concentration of NAC was determined first in HL-60 cells, and its in vivo activity was studied in a mouse acute myelocytic leukemia model with WEHI-3 leukemia cells. The results showed that a non-toxic concentration of NAC efficiently scavenged free-radicals, lowered lipid peroxidation and reduced DNA damage induced by hydrogen peroxide in a cultured HL-60 leukemia cell line. NAC also elevated the cellular antioxidant enzyme activity significantly. Furthermore, NAC prevented mouse death induced by injection of murine WEHI-3 leukemia cells and reduced organ damage, as well as activated antioxidant mechanisms. The results of this study provided strong evidence that NAC may have potential benefits in terms of elevating antioxidant activity and preventing leukemia initiation.
Keywords: N-acetyl-L-cysteine, garlic, leukemia, antioxidant
Introduction
Garlic has traditionally been used both for culinary reasons and for the treatment of diseases in many cultures for thousands of years, as evidenced in written histories (1). Studies have shown that garlic can reduce the risk of heart disease (2), and also exerts anticarcinogenic activity against several types of cancer through similar mechanisms (3,4). Garlic is predominantly rich in organosulfur compounds, which are known to contribute to its aroma and potential biological activities (5). The organosulfur compounds derived from garlic have been under investigation for many years, and accumulated evidence has indicated that they have health benefits. However, the composition of raw garlic is variable, and garlic extracts prepared by different methods have different properties (6). Therefore, it is still unclear which components are key to its potential medical use. For example, alliin is one of the main organosulfur compounds found in whole garlic cloves, and accounts for the majority of cysteine sulfoxides in garlic; however, although alliin is known to have antioxidant properties and many bioactivities, there exists very little direct evidence to support its beneficial effect in vivo. More in-depth studies are still required in order to reveal the components of garlic that are key contributors to its medical effects.
N-acetyl-L-cysteine (NAC), a thiol-containing antioxidant, is known to prevent cell damage in disorders caused by oxidative stress (7). In our previous study, it was demonstrated that NAC is the most abundant compound in the water-soluble extract of garlic (8). The antioxidative property of NAC enables it to directly interact with reactive oxygen species (ROS) and nitrogen species. ROS play a major role in the pathogenesis of many diseases (9,10). Under normal conditions, cells express a low level of ROS to regulate intracellular signaling pathways and organismal homeostasis (11,12). ROS regulation of signaling pathways contributes beneficial results to normal cellular functions. However, excessively high levels of ROS are detected in almost all types of cancer, suggesting that ROS play a major role in cancer development (13).
Antioxidants could prevent certain reactive species in cells from damaging cellular components, including proteins, lipids and DNA (14). As DNA damage has been linked to cancer, it is reasonable to assume that reducing DNA damage might prevent or slow the progression of cancer. Decreasing the ROS level by administering an antioxidant, such as NAC, to cancer cells has been shown to cause cancer cell damage and reduce invasion and invadopodia formation (15,16), indicating that antioxidants play an important role in alleviating metastasis. However, several recent studies have demonstrated that administration of high dosages of antioxidants such as NAC or vitamin E in a mouse model or in cell culture promoted tumor growth (17–19). This evidence raises the possibility that an optimal level of antioxidant that allows maintenance of homeostasis of oxidative stress is critical for cancer treatment or prevention. Regulating cellular antioxidant activity is therefore thought to represent an important approach for cancer prevention, and is of critical clinical importance for the treatment of cancers such as acute myelocytic leukemia (AML).
AML is the most common type of leukemia in adults (20), and the second most common type of leukemia affecting children (21). Chemotherapy is still the first-line treatment for AML, though the treatment outcome is still poor. With an increasing understanding of AML biology and genetics in recent years, several emerging therapies have been proposed (22,23). Progress reports suggest that development of novel agents against AML is still necessary, especially novel agents with less toxicity.
No study has evaluated the leukemia-preventive effect of NAC. As NAC is the major compound of the water-soluble extract of garlic, we investigated whether NAC has a free-radical scavenging effect in leukemia, and whether it can prevent leukemia in vivo. The present study was therefore designed to determine the antioxidant activity of NAC in human leukemia cells and to examine its role in the cells under oxidative stress. The effect of NAC on initial occurrence of leukemiaprevention in an AML mouse model was also examined.
Materials and methods
Cell culture
A human acute promyelocytic leukemia HL-60 cell line was kindly provided by Dr Tzou-Chi Huang (National Pingtung University of Science and Technology, Pingtung, Taiwan). HL-60 cells were grown in Iscove's modified Dulbecco's medium (Hyclone, Logan, UT, USA) supplemented with 4 mM L-glutamine (Gibco; Invitrogen, Carlsbad, CA, USA), 15% fetal bovine serum (Hyclone), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Invitrogen). HL-60 cells from passages 20–40 were used in the experiments. A WEHI-3 mouse myelomonocytic, macrophage-like leukemia cell line purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan) was grown in Iscove's modified Dulbecco's medium with 4 mM L-glutamine, supplemented with 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) and 10% fetal bovine serum. Both cell lines were cultured in a 95% humidity atmosphere under 5% CO2 in air at 37°C.
Determination of 10% cytotoxic concentration
Cultured HL-60 cells were treated with different concentrations of H2O2 (ranging from 0–100 mM) or NAC (ranging from 0–50 mM) for 24 h. The cytotoxicities of H2O2 and NAC towards HL-60 cells were determined by trypan blue exclusion. This method is based on the principle that live cells possess intact plasma membranes that exclude the trypan blue dye. The CC10 indicated the cytotoxic concentration that kills 10% of treated cells (24,25). The 10% cytotoxic concentration (CC10) after 24 h of treatment was calculated using Microsoft Excel software.
Cellular reactive oxygen species detection assay
In order to evaluate the radical scavenging effect of NAC, the intracellular ROS level was analyzed. A DCF-DA assay was performed to detect the intracellular production of hydroxyl, peroxyl and other ROS within the cells. HL-60 cells were incubated for 30 min with 10 µM DCF-DA and washed with PBS, followed by treatment with H2O2 at the CC10 concentration alone or in combination with various concentrations of NAC for 2 h. The fluorescence intensities of DCF were quantified using a microplate reader (Turner Biosystems, Sunnyvale, CA, USA), with an excitation wavelength of 485 nm and an emission wavelength of 525 nm.
Determination of malondialdehyde (MDA), TAC, SOD and GSH/GSSG levels in cultured HL-60 cells
To further understand the antioxidant effects of NAC, the levels of MDA, TAC, SOD and GSH/GSSG in cultured HL-60 cells treated with H2O2 at the CC10 concentration were measured. Upregulation of the MDA level indicates oxidative degradation of cellular major components; however, enhancement of the total antioxidation capacity (TAC) represents a cellular protective response to oxidative stress (26). HL-60 cells were treated with H2O2 alone or in combination with various concentrations of NAC for 8 h and then subjected to MDA assay. For measurement of the TAC, SOD and GSH/GSSG levels, well-grown HL-60 cells were incubated with various concentrations of compounds for 12 h. Cells were washed twice with PBS, harvested and analyzed following the instructions of the kit manufacturers. The OD value was determined using a spectrophotometer (Bio-Rad Model 680).
Quantification of oxidative DNA damage
HL-60 cells were treated with 20 mM H2O2 alone or in combination with various concentrations of NAC for 16 h. The assay protocol followed the instruction manual provided with the kit. Briefly, cellular DNA was extracted and converted to single-stranded DNA at 95°C for 5 min, and rapidly chilled on ice, then DNA nucleosides were digested by incubating the denatured DNA with nuclease P1. The unknown sample or 8-OHdG standard was incubated with a 8-OHdG conjugate-coated plate followed by reaction with anti-8-OHdG antibody. After washing and substrate reaction, the absorbance of the microwells was determined using a spectrophotometer (Bio-Rad Model 680) at a wavelength of 450 nm.
Animal experiment
The animal test procedure examined and approved by the Institutional Animal Care and Use Committee (IACUC) of our University (NPUST-103-052). Co-author Ching-Dong Chang is a veterinary pathologists and in charge of the animal monitoring and minimize the animal distress. A leukemia mice model was established in our laboratory (27). Thirty male BALB/c mice, 6 weeks of age, were obtained from BioLASCO (Taipei, Taiwan). After accommodation for one week, the mice were randomly divided into 5 groups: Group 1 was the negative control with no treatment; group 2 received intraperitoneal (i.p.) injection with NAC 200 mg/kg for 7 days; group 3 was treated with vehicle PBS through the i.p. route for 7 days then injected with WEHI-3 cells via the tail vein; groups 4 and 5 received NAC 50 or 200 mg/kg through i.p. injection for 7 days, following which leukemia was induced by injection of WEHI-3 cells into the tail vein on day 8. The NAC administration dose was cousulted and referred to the literatures (28,29). Following death, blood and major organs were collected. All resting living mice were euthanized by carbon dioxide on day 7 post WEHI-3 injection. The mortality rate and organ weights were recorded. Blood samples were analyzed to assess liver function markers aspartate aminotransferase (AST) and alanine aminotransferase (ALT), as well as antioxidant parameters, including total antioxidant capacity (TAC) and levels of SOD, glutathione and lysozyme. Furthermore, blood samples were also subjected to total acid phosphatase (ACP) analysis, a general diagnostic marker of disease condition. Considering the animal welfare, animals inoculated with tumor cells should be euthanized if following conditions is observed and diagnosed by the co-author Ching-Dong Chang veterinarian (1) Animal is unable to present normal activities due to the tumor (2) Animal abdomen appears dark-gray/green or ascites exceed 20% of the animal weight (3). Lethargy, anorexia, dehydration, or other sign of obvious stress or pain (6). Animal is unable to feed or drink normally due to the tumor.
Reagents and kits
2′,7′-Dichlorofluorescin diacetate (DCF-DA), N-acetyl-L-cysteine (NAC) and H2O2 were purchased from Sigma-Aldrich. A Lipid Peroxidation (MDA) Colorimetric Assay Kit, Total Antioxidant Capacity (TAC) Colorimetric Assay Kit, Superoxide Dismutase (SOD) Activity Assay Kit, Glutathione (GSH/GSSG/Total) Fluorometric Assay Kit, Aspartate aminotransferase (AST) Assay Kit, Alanine aminotransferase (ALT) Assay Kit, Total Acid Phosphatase (ACP) and Lysozyme Activity Assay Kit were purchased from Biovision (Mountain View, CA, USA). An 8-OHdG DNA Damage ELISA kit was obtained from Cell Biolabs, Inc. (San Diego, CA, USA).
Statistical analysis
All cell-based experiments were performed at least 3 times, and data are presented as mean ± standard deviation (SD). Statistical significance was determined between groups using Student's t-test. In the animal experiment, the data were analyzed by one-way analysis of variance (ANOVA), with the post-hoc t-test. A value of P<0.05 was considered to be significant.
Results
Cytotoxicity response
First of all, the non-toxic maximum concentration based on our experimental treatment duration needed to be determined. In order to determine the cytotoxicity response, the initial testing concentration range of NAC from 0–50 mM. As the duration of compound treatment in all cell culture assays did not exceed 16 h, we needed to identify the concentration that did not cause the death of more than 10% of cells after 24 h of incubation. Our data indicated that the CC10 values of H2O2 and NAC were 11.16 and 12.34 mM, respectively. The growth curve showed the higher NAC concentration, the cell viability decreased gradually (data not shown). The working concentration of the next experiments will not over the CC10 dose. Thus, we can then investigate the benefit effects under the enough safety prerequisites.
NAC alleviatesthe cellular ROS, lipid peroxidation and oxidative DNA damage induced by H2O2
Next, we analyzed the oxidative response induced by H2O2 in our system, and characterized the protective dose-response in the presence of NAC. Several experiments were performed. A fluorescent probe, DCF-DA, has been widely-used to measure intracellular oxidant levels. As shown in Fig. 1A, H2O2 produced significantly higher levels of ROS. Critically, at the CC10, NAC efficiently blocked the H2O2-generated ROS; lower dosages of NAC also exerted significant activity in terms of reducing the ROS level in a dose-response manner. Additionally, MDA is one of the most critical byproducts of lipid peroxidation during oxidative stress, and therefore analysis of lipid peroxidation is essential in the study of pathophysiological processes. The results for ROS and MDA showed an identical trend with very similar response patterns. Furthermore, in order to demonstrate that H2O2-elicited ROS affected DNA integrity, we measured the concentration of 8-hydroxydeoxyguanosine (8-OHdG), a critical oxidative DNA damage byproduct. As shown in Fig. 1B, NAC exerted an antioxidant protective activity against H2O2-mediated oxidative stress in HL-60 cells.
Figure 1.
The antioxidant activity of NAC protects HL-60 cells from oxidative stress. Cells were incubated with various treatments as indicated for 2 h (A) ROS (left y-axis) and lipid peroxidation (right y-axis) levels were analyzed by measuring the DCF-DA and MDA contents, respectively. (B) DNA damage was detected by measuring the amount of 8-hydroxydeoxyguanosine (8-OHdG). ‘*’ indicates P<0.001.
NAC possesses antioxidant activity
To further confirm the comprehensive antioxidant function of NAC in a HL-60 leukemia cell line, we examined whether NAC elevated cellular endogenous antioxidant mechanisms. There are three different types of antioxidant species, including enzyme systems, small molecules and proteins. The total antioxidant capacity (TAC) assay kit used in this study provided a method by which to measure the combined nonenzymatic antioxidant capacity from culture medium. Thus, both small-molecule antioxidants and protein antioxidants were determined in the NAC-treated HL-60 cell system. Another antioxidant enzyme, superoxide dismutase (SOD), which is considered one of the body's most powerful enzymes, was also evaluated. As shown in Fig. 2A, NAC upregulated the endogenous cellular antioxidant activity, and increased TAC and SOD in a dose-response manner. Furthermore, glutathione (GSH) is critical in terms of protecting cells against free-radical damage, and an increased ratio of GSSG to GSH indicates oxidative stress. As clearly demonstrated in Fig. 2B, H2O2 reduced the GSH/GSSG ratio, and NAC upregulated the GSH/GSSG ratio by itself; furthermore, NAC reversed the H2O2-mediated oxidative effects in HL-60 cells dramatically. Taken together, these results demonstrated that NAC is a good antioxidant for use in this leukemia cell line, not only upregulating the endogenous cellular antioxidant machinery, but also scavenging free-radicals in cells facing oxidative stress.
Figure 2.
NAC elevated the cellular endogenous antioxidant machinery. Cultured HL-60 cells were treated with NAC at various concentrations as indicated. (A) SOD activity assay is shown on the left y-axis; cellular total antioxidant capacity is shown on the right y-axis. (B) The ratio of GSH/GAAG is shown. ‘*’ indicates P<0.001.
Leukemia chemoprevention activity of NAC in BALB/c mice
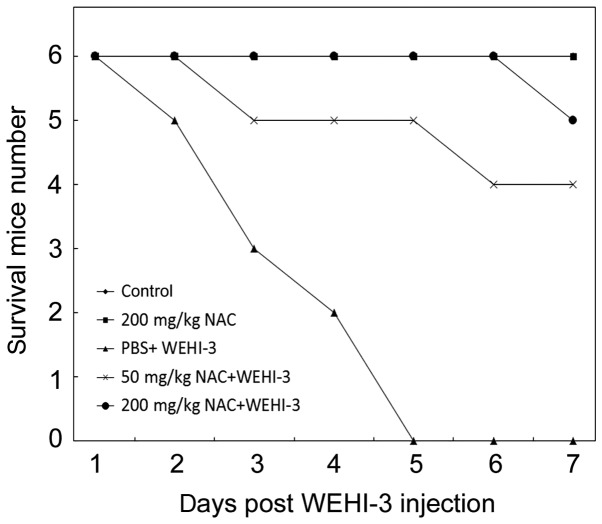
To compare and confirm the antioxidant protective activity of NAC against leukemia in vivo, a circulated leukemia mouse model was utilized, created by WEHI-3 injection via the tail vein and resulting in mouse death within one week (30,31). Based on our previous publication, the non-treated-mice died of leukemia by histopathological examination (27). The mortality rates of the individual mouse groups are shown in Fig. 3. Additionally, the spleen size of each group was showed in Fig. 4. The result clearly illustrated the larger spleen in mice received WEHI-3 cells. All mice had died by day 5 after WEHI-3 cell injection; however, in mice that received 50 mg/kg NAC, survival was significantly increased, and in mice receiving 200 mg/kg NAC, all mice were alive on day 7 after WEHI-3 administration (Fig. 3). There are nine mice died in our total thirty mice. These nine died mice occurs during the daytime and we isolate the blood and organs immediately.
Figure 3.
NAC protected cells from leukemia-induced death. Five groups of mice received various treatments, and mouse deaths were recorded each day after administration of WEHI-3 leukemia cells. The mouse survival response was as shown.
Figure 4.
Spleen size of mice received various treatment. Spleen isolated from mice with each group were shown as indicated. The precise size of spleen were measured by ruler and shown.
NAC does not cause organ damage, increases serum antioxidant marker activity and protects mouse organs
Mouse sera and organs were collected and analyzed. First, we evaluated the safety of the dosage of NAC we used. Based on organ weights/10 g body weight (Table I) and serum levels of AST, ALT and ACP markers (Table II), a comparison of group 2 with corn oil-treated healthy mice was performed, and no significant difference was observed in any of these parameters. Thus, 200 mg/kg NAC was sufficiently safe for use in our mouse model. In the group 3 mice, WEHI-3 cells were used to establish leukemia, and the liver, spleen and lung weights were significantly higher than those of the group 1 mice. The food consumption of the WEHI-3-injected mice was significantly lower than that of the control mice. The leukemia mice in group 3 also exhibited higher levels of AST, ALT and ACP, as well as lower antioxidant serum parameters of TAC, SOD, and GSH/GSSG. In the 50 mg/kg NAC administration group, some parameter reversed as compared with group 3, indicated the in vivo protective effects of NAC. In the 200 mg/kg NAC treatment groups, the levels of AST, ALT and ACP were not significantly different to those of the control mice. The levels of TAC, SOD and GSH/GSSG indicated that NAC promoted good health. Finally, administration of both 50 and 200 mg/kg NAC reduced mouse death (Fig. 3).
Table I.
Major organ weight and food and water consumption of the BALB/c mice under various treatment protocols.
| Groups | |||||
|---|---|---|---|---|---|
| Parameters | Control | 200 mg/kg NAC | PBS + WEHI-3 | 50 mg/kg NAC + WEHI-3 | 200 mg/kg NAC + WEHI-3 |
| Heart (g/10g bw) | 0.090±0.007 | 0.093±0.010 | 0.103±0.023 | 0.086±0.013 | 0.093±0.006 |
| Liver (g/10g bw) | 0.687±0.074 | 0.647±0.136 | 1.608±0.154a | 1.005±0.079a | 0.755±0.132 |
| Spleen (g/10g bw) | 0.051±0.006 | 0.078±0.023 | 0.178±0.032a | 0.097±0.027b | 0.064±0.013 |
| Lungs (g/10g bw) | 0.101±0.025 | 0.129±0.030 | 0.252±0.019 | 0.168±0.035 | 0.129±0.027 |
| Kidneys (g/10g bw) | 0.381±0.062 | 0.450±0.085 | 0.488±0.154 | 0.408050 | 0.379±0.060 |
| Food (g/days) | 3.098±0.166 | 3.085±0.149 | 2.222±0.165a | 2.750±0.130b | 2.909±0.193 |
| Water (ml/days) | 4.508±0.353 | 4.366±0.542 | 4.445±0.464 | 4.656±0.560 | 4.550±0.383 |
All treatment groups were compared with the control group.
P<0.001
P<0.01
P<0.05. NAC, N-Acetyl-L-cysteine.
Table II.
Serum marker analysis of the BALB/c mice under various treatment protocols.
| Parameters | Control | 200 mg/kg NAC | PBS + WEHI-3 | 50 mg/kg NAC + WEHI-3 | 200 mg/kg NAC + WEHI-3 |
|---|---|---|---|---|---|
| AST (mU/ml) | 63.83±22.06 | 61.69±16.59 | 585.4±113.25a | 210.23±76.61a | 77.52±26.35 |
| ALT (mU/ml) | 70.12±27.52 | 69.41±28.21 | 989.50±165.12a | 378.73±106.69a | 80.37±21.36 |
| ACP (U/ml) | 0.40±0.20 | 0.37±0.15 | 0.85±0.23b | 0.62±0.11c | 0.41±0.14 |
| TAC (Trolox mM) | 14.48±3.18 | 19.67±2.98a | 7.34±0.63a | 16.53±3.35b | 21.47±4.04a |
| SOD (U/ml) | 40.95±8.73 | 43.09±9.6 | 19.03±5.42a | 33.95±4.68 | 47.58±7.40 |
| GSH/GSSG | 7.09±1.88 | 7.36±1.99 | 3.17±1.22b | 3.90±1.07b | 8.82±2.04 |
| Lysozyme (µg/ml) | 1.78±0.91 | 1.66±0.62 | 1.65±0.31 | 2.37±0.55 | 2.19±0.58 |
All treatment groups were compared with the control group.
P<0.001
P<0.01
P<0.05. AST, aspartate aminotransferase; ALT, alanine aminotransferase; ACP, acid phosphatase; TAC, total antioxidant capacity; SOD, superoxide dismutase; GSH/GSSG, glutathione/glutathione disulfide; NAC, N-Acetyl-L-cysteine.
Discussion
Several organosulfur compounds of garlic have been shown to exert multiple pharmacological activities (32–38), and studies have demonstrated that aqueous garlic extract has antioxidant properties (39,40). It is well-known and well-published that garlic extract possessed the strong antioxidant activities in vitro and in vivo (41–43). However, there have been no further studies of the active components of garlic extract in terms of the antioxidant effect. In comparison with garlic oil, garlic water-soluble extract has a higher antioxidant activity, and among the identified constituents of garlic water-soluble extract, NAC has been found to be the most abundant (8). In this study, we explored whether NAC has the potential to prevent damage caused by leukemia cells owing to its antioxidant capacity. Further efforts will trying to establish the standard extraction method of garlic containing high amount of NAC and evaluate its possible broad-spectrum cancer-preventive ability.
H2O2 is an extremely strong reagent that causes oxidizing damage in cells, as treatment with H2O2 leads to high levels of ROS in cells (44). To evaluate the protective effect of NAC against H2O2-induced HL-60 cell damage, we used 11.16 µM of H2O2 in subsequent assays, as this concentration only caused a ~10% reduction in cell viability after 24 h of incubation; this treatment also caused an increase in the ROS content of ~12-fold and in the MDA content of 3.5-fold.
In the past few decades, many bioactive natural products with potential therapeutic applications have been widely-studied as sources for new drug development (45). Although numerous compounds derived from plants have been consumed as food for centuries, cytotoxicity is still an important issue in relation to their medical use. Our results showed that at a concentration of 12.34 mM, NAC only caused a decrease in cell viability of 10%, while at this concentration NAC efficiently diminished the number of free-radicals, lowered lipid peroxidation, and reduced the DNA oxidative damage induced by H2O2 in an HL-60 leukemia cell line. The results showed that even at low, non-toxic concentrations, NAC significantly reduced ROS and MDA, signs of H2O2-induced oxidative stress (Fig. 1).
Increased oxidative stress and ROS levels have been found to be associated with many aspects of tumor development and progression (46). The GSH precursor, NAC, can increase the GSH/GSSG ratio. NAC is an antioxidant that act as ROS-scavenger during stressful conditions (47). To confirm that NAC induced antioxidant activity in HL-60 cells, we investigated whether NAC induced cellular endogenous antioxidant mechanisms. We found that administration of NAC resulted in a significant increase in SOD activity in HL-60 cells, and reversed the reduction in the GSH/GSSG ratio caused by H2O2 treatment. The dose response to NAC showed the effects of NAC treatment on the TAC and SOD levels in the cells, indicating upregulation of endogenous cellular antioxidant activity. In addition, incubation of cells with H2O2 reduced the GSH/GSSG ratio, while NAC treatment reversed the reduction in the GSH/GSSG level in HL-60 cells. These results also indicated that NAC has a good antioxidant activity in leukemia cells, and also acts as a scavenger of free-radicals when cells face oxidative stress. In order to evaluate whether the antioxidant capacity of NAC has a protective effect in vivo, we investigated whether administration of NAC inhibited WEHI-3 leukemia cells in a BALB/c mouse model. The current study did not perform antioxidant experiments on WEHI-3 cells, there is still some procedures points are not totally covered. Peritoneal inoculation of BALB/c mice with WEHI-3 leukemia cells has been reported (48) and widely-used as an in vivo mouse leukemia model in many studies examining the anti-leukemia activities of several agents (30,49). In our laboratory, we developed an acute promyelocytic leukemia mouse model by intravenous injection of BALB/c mice with WEHI-3 leukemia cells, and employed this model to study the anti-proliferation and preventive effects of NAC on leukemia development in mice (27). We first tested the safety of NAC by i.p. injection with 50 or 200 mg/kg body weight for 7 days, and no organ abnormalities resulted at either dosage. We then used these doses to study the effect of NAC on WEHI-3-induced ALM mice. Based on the results of the present study, we demonstrated that NAC significantly reduced WEHI-3-induced organ damage in the liver, spleen and lungs (Table I). In addition, mice treated with WEHI-3 alone exhibited increased AST, ALT and ACP serum levels, while in mice pre-treated with 200 mg/kg NCA, these increases were prevented, and in addition, the levels of SOD and GSH/GSSG were returned to the same ranges as those of the control animals (Table II). Although WEHI-3 inoculated mice showed reduced average food intake ~28% as compared to control group mice and reduced body weight ~14% (27), not yet reached the euthanasia criteria. Additionally, WEHI-3 inoculated mice died very soon when symptoms appear, there was no detectable and noticeable suffering which diagnosis by a veterinarian. Taken together, the results indicated that NAC provided a good health promotion function that protected the mice from damage caused by WEHI-3 leukemia cell injection. Lysozyme is present in secretions and body fluid, and is associated with immunity; additionally, it exerts antioxidant activity in terms of clearing free-radicals and hydroxyl molecules. In our mouse model, the role of lysozyme was not evident. On the other hand, as the majority of cancer clinic drugs are oxidative agents, NAC might be a potential cancer treatment drug candidate, however, NAC might also prompt chemoresistance.
Taken together, the results of the in vivo mouse model revealed NAC to be a good antioxidant agent for use in the initial occurrence prevention of leukemia. The longer observation and complete pathological examination togerther with the involved molecular mechanisms regarding the NAC-mediated effects will be investigated in our next coming study.
In summary, NAC efficiently scavenged free-radicals, lowered lipid peroxidation and reduced DNA damage induced by HL-60 leukemia cells under oxidative stress. NAC prevented death from WEHI-3 leukemia cell-induced AML, and was associated with reduced organ damage, and may be mediated by its activation of antioxidant mechanisms. The results of the present study suggested that NAC is a potential agent for development as a new drug for the prevention of leukemia initiation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data are available from the corresponding author on reasonable request.
Authors' contributions
CDC, HTC and KKF were involved in data collection. WLS, CDC, HTC and KKF performed analysis and interpretation of data. WLS was involved in study conception and design, drafting of the manuscript, and was the project leader. WLS, CDC, HTC and KKF critically revised the manuscript.
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee (IACUC) of National Pingtung University of Science and Technology approved the animal experiments, which was conducted in accordance with the highest standards of animal welfare and care (NPUST-103-052). All contributed authors were fully informed of the procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rivlin RS. Historical perspective on the use of garlic. J Nutr. 2001;131(Suppl 3):951S–954S. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 2.Orekhov AN, Grünwald J. Effects of garlic on atherosclerosis. Nutrition. 1997;13:656–663. doi: 10.1016/S0899-9007(97)83010-9. [DOI] [PubMed] [Google Scholar]
- 3.Milner JA. A historical perspective on garlic and cancer. J Nutr. 2001;131(Suppl 3):1027S–1031S. doi: 10.1093/jn/131.3.1027S. [DOI] [PubMed] [Google Scholar]
- 4.El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr. 2006;136(Suppl 3):864S–869S. doi: 10.1093/jn/136.3.864S. [DOI] [PubMed] [Google Scholar]
- 5.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136(3 Suppl):716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 6.Rahman K, Lowe GM. Garlic and cardiovascular disease: A critical review. J Nutr. 2006;136(Suppl 3):736S–740S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 7.Cotgreave IA. N-acetylcysteine: Pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. doi: 10.1016/S1054-3589(08)60985-0. [DOI] [PubMed] [Google Scholar]
- 8.Dewi ADR, Kusnad J, Shih WL. Comparison of the main bioactive compounds and antioxidant activity from garlic water-soluble and garlic oil. NRLS Conference Proceedings, International Conference on Natural Resources and Life Sciences (2016). KnE Life Sciences. 2017. pp. 20–34. In:
- 9.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Görlach A, Dimova EY, Petry A, Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP, Palmeira CM, Kietzmann T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015;6:372–385. doi: 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Autréaux B, Toledano MB. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 12.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105. doi: 10.1016/j.canlet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 16.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 18.Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P3, Nilsson J, Bergo MO. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7:308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 19.Diao QX, Zhang JZ, Zhao T, Xue F, Gao F, Ma SM, Wang Y. Vitamin E promotes breast cancer cell proliferation by reducing ROS production and p53 expression. Eur Rev Med Pharmacol Sci. 2016;20:2710–2717. [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia S, Neglia JP. Epidemiology of childhood acute myelogenous leukemia. J Pediatr Hematol Oncol. 1995;17:94–100. doi: 10.1097/00043426-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kavanagh S, Murphy T, Law A, Yehudai D, Ho JM, Chan S, Schimmer AD. Emerging therapies for acute myeloid leukemia: translating biology into the clinic. JCI Insight. 2017;2(pii):95679. doi: 10.1172/jci.insight.95679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saygin C, Carraway HE. Emerging therapies for acute myeloid leukemia. J Hematol Oncol. 2017;10:93. doi: 10.1186/s13045-017-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Hough AM, Lawrence TS. The role of p53 in gemcitabine-mediated cytotoxicity and radiosensitization. Cancer Chemother Pharmacol. 2000;45:369–374. doi: 10.1007/s002800051004. [DOI] [PubMed] [Google Scholar]
- 25.Monge-Fuentes V, Muehlmann LA, Longo JP, Silva JR, Fascineli ML, de Souza P, Faria F, Degterev IA, Rodriguez A, Carneiro FP, et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J Photochem Photobiol B. 2017;166:301–310. doi: 10.1016/j.jphotobiol.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 27.Rawendra RD, Lin PY, Chang CD, Hsu JL, Huang TC, Shih WL. Potentiation of acute promyelocytic leukemia cell differentiation and prevention of leukemia development in mice by oleanolic acid. Anticancer Res. 2015;35:6583–6590. [PubMed] [Google Scholar]
- 28.Rocksén D, Lilliehöök B, Larsson R, Johansson T, Bucht A. Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000;122:249–256. doi: 10.1046/j.1365-2249.2000.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL., Jr Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res. 2006;173:211–216. doi: 10.1016/j.bbr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Su CC, Yang JS, Lin SY, Lu HF, Lin SS, Chang YH, Huang WW, Li YC, Chang SJ, Chung JG. Curcumin inhibits WEHI-3 leukemia cells in BALB/c mice in vivo. In Vivo. 2008;22:63–68. [PubMed] [Google Scholar]
- 31.Chang YH, Yang JS, Yang JL, Wu CL, Chang SJ, Lu KW, Lin JJ, Hsia TC, Lin YT, Ho CC, et al. Ganoderma lucidum extracts inhibited leukemia WEHI-3 cells in BALB/c mice and promoted an immune response in vivo. Biosci Biotechnol Biochem. 2009;73:2589–2594. doi: 10.1271/bbb.90357. [DOI] [PubMed] [Google Scholar]
- 32.Yang CS, Wang ZY, Hong JY. Inhibition of tumorigenesis by chemicals from garlic and tea. Adv Exp Med Biol. 1994;354:113–122. doi: 10.1007/978-1-4899-0939-8_8. [DOI] [PubMed] [Google Scholar]
- 33.Salman H, Bergman M, Bessler H, Punsky I, Djaldetti M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int J Immunopharmacol. 1999;21:589–597. doi: 10.1016/S0192-0561(99)00038-7. [DOI] [PubMed] [Google Scholar]
- 34.Bordia A, Verma SK, Srivastava KC. Effect of garlic on platelet aggregation in humans: A study in healthy subjects and patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1996;55:201–205. doi: 10.1016/S0952-3278(96)90099-X. [DOI] [PubMed] [Google Scholar]
- 35.Hall A, Troupin A, Londono-Renteria B, Colpitts TM. Garlic organosulfur compounds reduce inflammation and oxidative stress during dengue virus infection. Viruses. 2017;9(pii):E159. doi: 10.3390/v9070159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho SC, Su MS. Evaluating the anti-neuroinflammatory capacity of raw and steamed garlic as well as five organosulfur compounds. Molecules. 2014;19:17697–17714. doi: 10.3390/molecules191117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trio PZ, You S, He X, He J, Sakao K, Hou DX. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014;5:833–844. doi: 10.1039/c3fo60479a. [DOI] [PubMed] [Google Scholar]
- 38.You S, Nakanishi E, Kuwata H, Chen J, Nakasone Y, He X, He J, Liu X, Zhang S, Zhang B, Hou DX. Inhibitory effects and molecular mechanisms of garlic organosulfur compounds on the production of inflammatory mediators. Mol Nutr Food Res. 2013;57:2049–2060. doi: 10.1002/mnfr.201200843. [DOI] [PubMed] [Google Scholar]
- 39.Boonpeng S, Siripongvutikorn S, Sae-Wong C, Sutthirak P. The antioxidant and anti-cadmium toxicity properties of garlic extracts. Food Sci Nutr. 2014;2:792–801. doi: 10.1002/fsn3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasul Suleria HA, Sadiq Butt M, Muhammad Anjum F, Saeed F, Batool R, Nisar Ahmad A. Aqueous garlic extract and its phytochemical profile; special reference to antioxidant status. Int J Food Sci Nutr. 2012;63:431–439. doi: 10.3109/09637486.2011.634786. [DOI] [PubMed] [Google Scholar]
- 41.Bravi E, Marconi O, Sileoni V, Rollo MR, Perretti G. Antioxidant effects of supercritical fluid garlic extracts in canned artichokes. J Food Sci Technol. 2016;53:3744–3751. doi: 10.1007/s13197-016-2352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naji KM, Al-Shaibani ES, Alhadi FA, Al-Soudi SA, D'Souza MR. Hepatoprotective and antioxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits. BMC Complement Altern Med. 2017;17:411. doi: 10.1186/s12906-017-1916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petropoulos S, Fernandes Â, Barros L, Ciric A, Sokovic M, Ferreira ICFR. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018;245:7–12. doi: 10.1016/j.foodchem.2017.10.078. [DOI] [PubMed] [Google Scholar]
- 44.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 45.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 46.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun SY. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther. 2010;9:109–110. doi: 10.4161/cbt.9.2.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Q, Na X. The effects and mechanisms of a novel 2-aminosteroid on murine WEHI-3B leukemia cells in vitro and in vivo. Leuk Res. 2001;25:455–461. doi: 10.1016/S0145-2126(00)00153-3. [DOI] [PubMed] [Google Scholar]
- 49.Lin JP, Yang JS, Lu CC, Chiang JH, Wu CL, Lin JJ, Lin HL, Yang MD, Liu KC, Chiu TH, Chung JG. Rutin inhibits the proliferation of murine leukemia WEHI-3 cells in vivo and promotes immune response in vivo. Leuk Res. 2009;33:823–828. doi: 10.1016/j.leukres.2008.09.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data are available from the corresponding author on reasonable request.