Abstract
Clear cell renal cell carcinoma (ccRCC) is a common malignant kidney tumor, the pathogenesis of which remains unclear. The aim of the present study was to investigate whether caspase-10, matrix metalloproteinase-9 (MMP-9) and total laminin (LM) were involved into the pathogenesis of ccRCC. The levels of caspase-10, MMP-9 and total LM were analyzed by ELISA in tumor tissues and adjacent non-malignant tissues of 27 patients with ccRCC. The results revealed that caspase-10 levels in the tumor tissues were significantly higher than those in the adjacent non-malignant tissues (P<0.05). The MMP-9 levels in the tumor tissues were significantly lower than those in adjacent non-malignant tissues (P<0.01). The total LM levels in tumor tissues revealed no statistical difference with those in the adjacent non-malignant tissues (P=0.757). Additionally, caspase-10 levels were positively correlated with MMP-9 levels (P<0.001), but negatively correlated with total LM levels (P<0.05) in tumor tissues. Correlation analyses with clinical data of patients with ccRCC, revealed that caspase-10 levels (P<0.05) and MMP-9 levels (P<0.001) in tumor tissues were positively correlated with tumor grades of ccRCC, whereas total LM levels were positively correlated with tumor size (P<0.05). The results of the present study suggested that interactions between caspase-10, MMP-9 and LM are likely involved in the pathogenesis of ccRCC. A deeper understanding of the correlation between caspase-10, MMP-9 and LM would aid the clarification of pathogenesis of ccRCC.
Keywords: clear cell renal cell carcinoma, caspase-10, matrix metalloproteinase-9, laminin, tumor malignancy
Introduction
Renal cell carcinoma (RCC) is the most common kidney tumor, accounting for almost 3% of all human malignancies; 70–80% of RCC cases are clear cell RCC (ccRCC) (1–3). Metastasis and recurrence rates in ccRCC, as well as its poor prognosis, lead to poor survival rates in patients (4). Currently, the pathogenesis of ccRCC remains unclear.
Caspases serve an essential role in cell apoptosis. Caspases-10 and −8 act as initiators, whereas caspase-9 acts as an initiator of the intrinsic apoptosis pathway. Caspase-3 is considered to be the main effector caspase involved in extrinsic and intrinsic pathways (5). Alteration of the apoptotic pathway is essential for tumor development; thus, analysis of the expression levels of caspases in tumor tissues is necessary for a deeper understanding of tumor biology. In a previous study, immunopositivity of caspase-10 was observed in 58 (97%) of 60 advanced gastric adenocarcinomas using immunohistochemistry (IHC) using tissue microarrays (5). Tumor necrosis factor-related apoptosis-inducing ligand, which induces apoptosis in Ewing's sarcoma family tumors, requires caspase activation; furthermore, caspase-10 was revealed to be activated earlier than other caspases in the signaling pathway (6). cDNA microarrays also revealed that caspase-10 was downregulated in gastric carcinoma, compared with adjacent non-cancerous tissue (7). The antitumor effect of immunotherapeutic and chemotherapeutic agents is executed through stimulation of apoptotic programs, via the activation of members of the caspase family in susceptible cells. Resistance to drug-inducing apoptosis in RCC cell lines is considered to be correlated with downregulation of caspase-10 (8). Nevertheless, it remains unclear whether caspase-10 is involved in tumor development, particularly in RCC.
Matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, are capable of degrading components of the extracellular matrix and are implicated in tissue remodeling and tumor infiltration (9). Using various methods, a number of studies have analyzed MMP-9 expression or its activities in tumor tissues, sera, plasma or urine of patients with RCC. For example, IHC analysis revealed that MMP-9 levels in tumor tissues of RCC were significantly higher than those in non-malignant tissues, and MMP-9 overexpression was positively associated with clinical staging, pathological grade and metastasis, and the shorter survival rate of RCC patients (9–12). In addition, pro-MMP-9, but not activated MMP-9, was detected using gelatin zymography and western blot in RCC samples (13). BIOTRAK activity assay systems and in situ hybridization also established higher MMP-9 mRNA and protein levels in tumor tissues than those in adjacent non-malignant tissues (10,14). Additionally, cDNA microarray and gelatin zymography revealed that MMP-9 transcript levels were strongly correlated with MMP-9 enzymatic activity, disease-free survival rate and metastasis in RCC, suggesting that MMP-9 may be regulated at the transcript level and may be a candidate of predictors of disease-free survival rate in RCC patients (15). Other studies investigated 16 patients with ccRCC with ELISA assays, and revealed that MMP-9 levels in sera, urine and plasma samples were higher in patients with ccRCC than in healthy controls (14,16). Gelatin zymography or ELISA were also used for analysis of sera and urine of 16 patients with ccRCC, and revealed that MMP-9 activity or expression levels in sera and urine may not be useful biomarkers for kidney carcinomas, despite opposing conclusions in other publications (17,18). Therefore, the majority of these studies suggested that MMP-9 may be involved in the pathogenesis of RCC.
Laminins (LMs) are large molecular-weight trimer glycoproteins consisting of α, β and γ subunits, which integrate in almost all basement membranes (BMs) (19). A previous report has suggested that LM trimers are assembled inside the cell, and the extracellular proteolytic processing of various subunits leads to their final forms (20). In addition to their function as a scaffold for BMs, LMs can also interact with cell surface receptors, including integrins, to control signaling events that regulate cell proliferation, migration and differentiation (21,22). During tumor invasion, loss of the BM barrier occurs and a discontinuous pattern of LM staining is observed (23). IHC results indicated that the loss of LM in tumor tissues was significantly correlated with symptoms, tumor size and higher tumor grades in 75 RCC cases (24). In other studies, which also used IHC, tumor BMs of RCC revealed immunoreactivity for subunits of LMα1, LMβ1, LMγ1 and LMβ2 (25,26). Additionally, in cultured RCC cells, an abundant production of LM111, but not of LM332, was observed, whereas xenografts of the same cell revealed BM-confined immunoreactivity for LM111 and LM332 (25). Assessment of microarray data of early metastatic and non-metastatic ccRCC samples confirmed LMα1, LMα2 and LMα4 as potential target genes associated with early metastatic ccRCC (27). Western blot analysis conducted in another study revealed that LM levels in urine of patients with RCC were significantly lower than those in healthy controls (28). All these results imply that abnormal expression of total LMs or certain LM subunits are possibly correlated with RCC.
The purpose of the present study was to investigate the expression levels of caspase-10, MMP-9 and total LM in tumor tissues and their adjacent non-malignant tissues of patients with ccRCC, and further elucidate the possible correlation of the three factors and ccRCC, and the possible correlation among these factors and clinical data of patients with ccRCC.
Materials and methods
Patient tissue samples
Tumor tissues and adjacent non-malignant tumor tissues were collected from 27 patients (14 male, 11 female and 2 unknown; mean age, 57.8; age range, 42–70) with ccRCC who underwent surgical procedure at the Department of Urology, Harbin Medical University Cancer Hospital (Harbin, China) from March 2015 to May 2017. Diagnosis of tumors was made by the usual clinical laboratory criteria and confirmed postoperatively by histopathological findings (16). The tumors were classified for grade and stage according to the pathological Tumor-Node-Metastasis (pTNM) classification (29). The clinical information of these 27 patients is summarized in Table I. Written informed consent was obtained from all patients. All experiments were conducted under approval from the Internal Review Board of Harbin Medical University and in compliance with the Declaration of Helsinki.
Table I.
Clinical information of patients with clear cell renal cell carcinoma.
| Patient number | Sex | Age | Tumor stage | Tumor grade | Maximal tumor diameter, cm |
|---|---|---|---|---|---|
| 1 | Female | 55 | T1aN0M0 | 1 | 3.5 |
| 2 | Male | 55 | T1aN0M0 | 1 | 3.5 |
| 3 | Female | 70 | T3aN0M0 | 3 | 7 |
| 4 | Male | 52 | T2aN0M0 | 2 | 8 |
| 5 | Female | 64 | T1bN0M0 | 1 | 4.7 |
| 6 | Male | 59 | T1aN0M0 | 1 | 3.8 |
| 7 | Male | 69 | UC | UC | 4.7 |
| 8 | Male | 42 | T4N1M1 | 4 | 9 |
| 9 | Female | 49 | T1bN0M0 | 1 | 5.5 |
| 10 | Female | 66 | T1bN0M0 | 1 | 7.6 |
| 11 | Female | 66 | T1aN0M0 | 1 | 3 |
| 12 | Female | 46 | UC | UC | UC |
| 13 | Female | 62 | T2aN0M0 | 2 | 8 |
| 14 | Female | 61 | T1aN0M0 | 1 | 4 |
| 15 | Male | 59 | T3b | 3 | 5 |
| 16 | Male | 46 | T1bN0M0 | 1 | 6.5 |
| 17 | Female | 67 | T3bN0M0 | 3 | 3.5 |
| 18 | UC | UC | UC | UC | UC |
| 19 | Male | 56 | T1bN0M0 | 1 | 4.5 |
| 20 | Female | 67 | T1aN0M0 | 1 | 4 |
| 21 | Male | 52 | T1aN0M0 | 1 | 3.5 |
| 22 | Male | 55 | T1aN0M0 | 1 | 4 |
| 23 | Male | 57 | T1aN0M0 | 1 | 3.5 |
| 24 | Male | 58 | T1bN0M0 | 1 | 6 |
| 25 | UC | UC | UC | UC | UC |
| 26 | Male | 44 | UC | UC | UC |
| 27 | Male | 69 | T2bN0M0 | 2 | 2.5 |
T, tumor; N, node; M, metastasis; UC, unclear.
Tissue protein lysate preparation and protein concentration determination
Tissue samples were lysed with cold radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime Institute of Biotechnology, Shanghai, China) at 4°C overnight. Following centrifugation at 14,000 × g at 4°C for 20 min, the supernatant was harvested as tissue protein lysate, which was stored at −80°C until use. The protein concentration of all tissue protein lysates was measured using a bicinchoninic acid assay kit (cat. no. P0011; Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's protocols. Tissue protein lysates were diluted to a concentration of 1 µg/ml prior to the any subsequent experiments.
ELISA
Capsase-10, MMP-9 and total LM levels in tumor tissues and adjacent non-malignant tissues of patients with ccRCC were determined by a capase-10 ELISA kit (cat. no. JL45340; Jianglaibio, Shanghai, China), MMP-9 ELISA kit (cat. no. CSB-E08006h; Cusabio, Wuhan, China) and total LM ELISA kit (cat. no. E-EL-H0128c; Elabscience, Houston, TX, USA), respectively. The antibodies used in the LM ELISA kit were polyclonal antibodies against all types of LMα, LMβ and LMγ subunits. All experiments were performed according to the manufacturer's protocols for the respective ELISA kits. All sample measurements were repeated twice.
Statistical analysis
All sample measurements were repeated twice and the data were presented as mean ± standard deviation. Statistical analysis was performed using SigmaPlot 12.0 (Hulinks, Inc., Tokyo, Japan). Paired Student's t-tests and Wilcoxon signed rank tests were used to compare the difference between caspase-10, MMP-9 and total LM levels in tumor tissues and their adjacent non-malignant tissues in patients with ccRCC. Pearson's correlation was used to analyze the correlation between ccRCC clinical data and caspase-10, MMP-9 and total LM. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of caspase-10, MMP-9 and total LM in tumor and adjacent non-malignant tissues of patients with ccRCC by ELISA
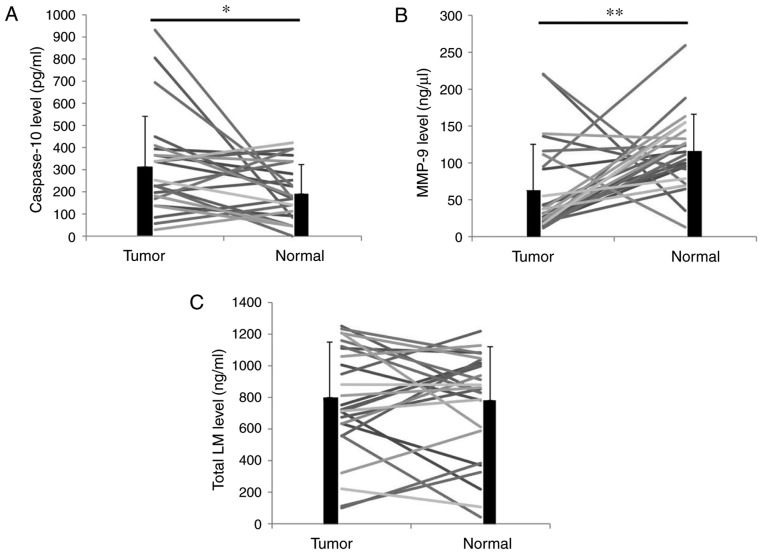
Tumor tissues and adjacent non-malignant tissues were obtained from 27 patients with ccRCC. The protein concentration of all tissue protein lysates was analyzed and resuspended at 1 µg/ml. ELISA kits were then used to detect caspase-10, MMP-9 and total LM levels in these protein lysates. The caspase-10 levels in the tumor tissues were significantly higher than those in the adjacent non-malignant tissues (P<0.05; Fig. 1A). However, the MMP-9 levels in the tumor tissues were significantly lower than those in the adjacent non-malignant tissues (P<0.01; Fig. 1B). No statistical difference was observed between the total LM levels in the tumor tissues and those in the adjacent non-malignant tissues (P=0.757; Fig. 1C).
Figure 1.
Detection of caspase-10, MMP-9 and total LM by ELISA in tumor tissues and adjacent non-malignant tissues of patients with clear cell renal cell carcinoma. (A) Caspase-10 ELISA; (B) MMP-9 ELISA; and (C) total LM ELISA. Tumor, tumor tissues; normal, adjacent non-malignant tissues. *P<0.05, **P<0.01. MMP, matrix metalloproteinase; LM, laminin.
Correlation of caspase-10, MMP-9 and total LM protein levels in tumor and adjacent non-malignant tissues of patients with ccRCC
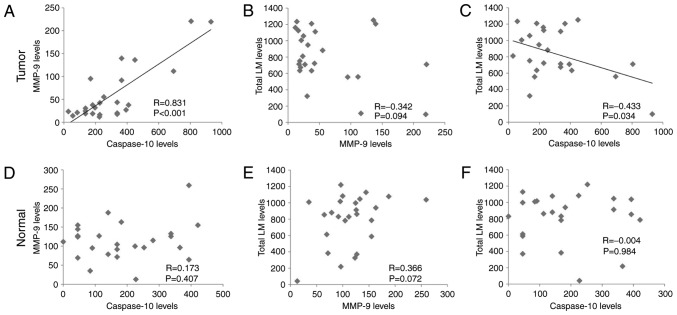
The protein levels of caspase-10, MMP-9 and total LM in tumor and adjacent non-malignant tissues were subjected to correlation analyses. In the tumor tissues, caspase-10 levels were positively correlated with MMP-9 levels (P<0.001; Fig. 2A), but MMP-9 levels revealed no correlation with total LM levels (P=0.094; Fig. 2B) and total LM levels were negatively correlated with caspase-10 levels (P<0.05; Fig. 2C). In adjacent non-malignant tissues, the levels of caspase-10, MMP-9 and total LM exhibited no correlation among each other (Fig. 2D-F).
Figure 2.
Correlation of caspase-10, MMP-9 and total LM in tumor tissues and adjacent non-malignant tissues of patients with clear cell renal cell carcinoma. (A) Correlation of MMP-9 and caspase-10 in tumor tissues. (B) Correlation of MMP-9 and total LM in tumor tissues. (C) Correlation of caspase-10 and total LM in tumor tissues. (D) Correlation of MMP-9 and caspase-10 in adjacent non-malignant tissues. (E) Correlation of MMP-9 and total LM in adjacent non-malignant tissues. (F) Correlation between caspase-10 and total LM in adjacent non-malignant tissues. Tumor, tumor tissues; Normal, adjacent non-malignant tissues; MMP, matrix metalloproteinase; LM, laminin.
Correlation of ccRCC clinical data with the levels of caspase-10, MMP-9 and total LM
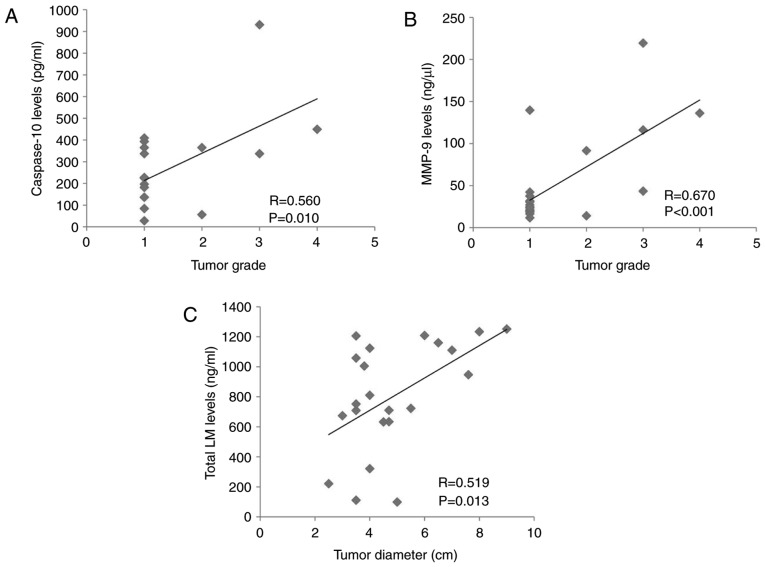
The clinical data of the patients with ccRCC involved in the present study are presented in Table I. Positive correlation was revealed between tumor grades and maximal tumor diameters (P<0.05), but no other correlation was revealed among the parameters of the clinical data of patients with ccRCC (data not shown). Analyses were next performed between the levels of caspase-10, MMP-9 and total LM and the clinical data of patients with ccRCC, including age, sex, tumor grade and maximal tumor diameters. The statistically significant data are included in Fig. 3. The tumor grade of patients with ccRCC was positively correlated with caspase-10 levels (P<0.05; Fig. 3A) and MMP-9 levels (P<0.001; Fig. 3B) in the tumor tissue. In addition, the maximal tumor diameter was positively correlated with the level of total LM in the tumor tissue (P<0.05; Fig. 3C).
Figure 3.
Analyses of the correlation between clear cell renal cell carcinoma clinical data and the levels of caspase-10, MMP-9 and total LM. (A) Correlation between caspase-10 levels in tumor tissues and tumor grade. (B) Correlation between MMP-9 levels in tumor tissues and tumor grade. (C) Correlation between total LM levels in tumor tissues and tumor diameters. MMP, matrix metalloproteinase; LM, laminin.
Discussion
In the present study, caspase-10, MMP-9 and total LM levels in tumor and adjacent non-malignant tissue from 27 patients with ccRCC were detected by ELISA. The tumor tissues revealed significantly higher levels of caspase-10 and lower levels of MMP-9 than the adjacent non-malignant tissues, but similar total LM levels. Statistical analysis indicated that caspase-10 levels were positively correlated with MMP-9 levels (P<0.001), but negatively correlated with total LM levels (P<0.05) in the tumor tissues. Correlation analyses confirmed that levels of caspase-10 (P<0.05) and MMP-9 (P<0.001) in tumor tissues were positively correlated with ccRCC tumor grades, and total LM levels in the tumor tissues were positively correlated with the tumor size in patients with ccRCC.
Caspase-10 has been investigated in tumor-related studies mainly due to its involvement in cell apoptosis. To the best of our knowledge, the current study is the first report on caspase-10 detection in tumor tissues from patients with ccRCC. Using ELISA, it was confirmed that caspase-10 levels in tumor tissues from patients with ccRCC were significantly higher than those in the adjacent non-malignant tissues. Furthermore, caspase-10 levels in tumor tissues were positively correlated with the tumor grades of patients with ccRCC. These results indicated that caspase-10 may promote tumor development and be a potential pathological marker for ccRCC. Furthermore, it was revealed that caspase-10 levels were positively correlated with MMP-9 levels (P<0.001) and negatively correlated with total LM levels (P<0.05) in the tumor tissues. These results imply that caspase-10 may promote tumor development of ccRCC via upregulation of MMP-9 and downregulation of LMs, which should be clarified in future studies.
MMP-9 protein expression levels in patients with RCC have been reported in a number of studies, the majority of which analyzed MMP-9 levels in tumor tissue samples by IHC. The findings of these investigations revealed that MMP-9 levels in tumor tissues were higher than those in the adjacent non-malignant tissues, and MMP-9 overexpression was positively correlated with tumor grades of patients with RCC (9–12). In the present study, MMP-9 levels were analyzed by ELISA; it was revealed that MMP-9 levels were significantly lower in tumor tissues than in the adjacent non-malignant tissues of ccRCC, which is contradictory to previous studies (9–12). The different tissue samples and methods used for detection of MMP-9 levels may have led to these discrepancies in results. The contradictory results may also imply the complexities of the role of MMP-9 in the pathogenesis of RCC. In the present study, it was confirmed that MMP-9 levels in tumor tissues were positively correlated with tumor grades of ccRCC (P<0.001), which is consistent with the results of previous studies (9,11). The finding from the present study indicated that MMP-9 may promote tumor development in ccRCC. However, this point also seems to be contradictory to the relatively lower MMP-9 levels in the tumor tissues, compared with those in the adjacent non-malignant tissues. Therefore, it is hypothesized that MMP-9 levels in the tumor and the adjacent non-malignant tissues may be independent, or be two different isoforms of MMP-9 with different functions. Further analyses of the various molecular weights of MMP-9 in the tumor and adjacent non-malignant tissues of RCC cases and their functions using experimental models may be of use. MMP-9 is known to enhance tumor migration and angiogenesis via degradation of extracellular matrix molecules including LMs (30). In the present study, MMP-9 levels in the tumor tissues of ccRCC tended to be negatively correlated with the total LM levels (P=0.094). This result suggested that MMP-9 may contribute to the pathogenesis of ccRCC via degradation of LMs. The lack of statistical significance may be caused by the limited numbers of samples analyzed or targeting of only certain LM subunit(s)/trimer(s) by MMP-9. Therefore, it is necessary to clarify what LM subunits or LM trimers are influenced by MMP-9 in ccRCC.
During tumor invasion, the BM barrier is removed, and a discontinuous pattern of LM staining is observed (23). In RCC, LM loss in the tumor tissues, as established by IHC, was suggested to be correlated with symptoms, tumor size and a higher tumor grade (24). However, in the present study, no differences in total LM levels in the tumor and adjacent non-malignant tissues of ccRCC were revealed by ELISA. These discrepancies in results may be due to the different detection methods used. ELISAs which used coating and primary antibodies against LMs in the present study may have a relatively higher specificity than IHC, in which only the primary antibody is used. In the present study, it was also demonstrated that total LM levels in tumor tissues were positively correlated with tumor size in patients with ccRCC, which is similar to the findings of a previous study (24). This result suggested that LMs in tumor tissues may promote the tumor development in ccRCC. Therefore, considering the results obtained, it can be concluded that certain LM subunit(s) or trimer(s) likely contribute to tumor development. LM has 5 α, 3 β and 3 γ subunits and their distribution is tissue-specific (20). LM exists extensively in malignant cells, non-malignant cells, BM and endothelial cells of blood vessels (20). Therefore, in tumor tissues of patients with RCC, the total LM levels reflect the levels of various LM subunits in all cells and tissues involved. It has been suggested that RCC tumor tissues may have LMα1-4, LMβ1-3 LMγ1-2 subunits (25–27). In future studies, it is necessary to define what LM subunit(s) contributed to the total LM changes in RCC tumor tissues and to elucidate the possible mechanisms of pathogenesis.
It is well accepted that decreased expression levels of caspase-3 and LMs and increased MMP-9 in tumor tissue were linked to the pathogenesis of tumor development in various malignant types of cancer (23,31,32). By contrast, increased caspase-10, decreased MMP-9 and unchanged total LMs were revealed in the tumor tissues of ccRCC in the present study. These apparently discrepant results provide new and important insights into the pathogenesis of ccRCC. The positive correlation between caspase-10 and MMP-9, the negative correlation between caspase-10 and total LMs imply that caspase-10, MMP-9 and LMs may regulate each other in vivo and contribute to the pathogenesis of ccRCC. In the present study, tumor grades were positively correlated with maximal tumor diameters in patients with ccRCC. Caspase-10 and MMP-9 were positively correlated with tumor grades but not tumor diameters, whereas total LMs were positively correlated with tumor diameters but not tumor grades; these results appear to be contradictory. It is hypothesized that caspase-10 and MMP-9 may contribute directly to tumor invasion (one factor of tumor grade) and indirectly to tumor diameters (another factor of tumor grade) by regulation of LMs.
The lower number of tissue samples of patients with ccRCC and the application of only one detection method are limitations of the present study. Therefore, further studies with a greater number of tissue samples and detection methods would be of benefit. In addition, the correlation among caspase-10, MMP-9 and LM in in vitro and in vivo experimental models of ccRCC should be investigated in future studies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- RCC
renal cell carcinoma
- ccRCC
clear cell RCC
- IHC
immunohistochemistry
- MMP-9
matrix metalloproteinase-9
- LM
laminin
- BMs
basement membranes
Funding
This study was supported by the Scientific Research Fund of Heilongjiang Provincial Education Department (grant nos. 12521262 and 12531415), the Scientific Research Fund of Heilongjiang Provincial Health Bureau (grant nos. 2011-031 and 2012-566), the Natural Science Foundation of Heilongjiang Province of China (grant no. QC2016129) and the Postdoctoral Grant of Heilongjiang Province (grant no. LBH-Z14154).
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HQ, XL, CL and YC designed the study. YSC, LT, WW, DL, YX and YC gathered samples and data. WZ and XL provided project guidance. HQ, XL, WZ, LM, XT, JS, YSC, LT, WW, DL and YX performed the experiments and data analysis. XL and YC revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments were performed with the approval of the Internal Review Board of Harbin Medical University and in compliance with the Declaration of Helsinki. Informed consent was obtained from all patients prior to mortality or from their families following mortality for inclusion of autopsy data.
Consent for publication
The patients involved in this study provided written informed consent for the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Diaz JI, Mora LB, Hakam A. The mainz classification of renal cell tumors. Cancer Control. 1999;6:571–579. doi: 10.1177/107327489900600603. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Rosner I, Bratslavsky G, Pinto PA, Linehan WM. The clinical implications of the genetics of renal cell carcinoma. Urol Oncol. 2009;27:131–136. doi: 10.1016/j.urolonc.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novara G, Ficarra V, Antonelli A, Artibani W, Bertini R, Carini M, Cosciani Cunico S, Imbimbo C, Longo N, Martignoni G, et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: Are further improvements needed? Eur Urol. 2010;58:588–595. doi: 10.1016/j.eururo.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Yoo NJ, Kim HS, Kim SY, Park WS, Kim SH, Lee JY, Lee SH. Stomach cancer highly expresses both initiator and effector caspases: An immunohistochemical study. APMIS. 2002;110:825–832. doi: 10.1034/j.1600-0463.2002.1101109.x. [DOI] [PubMed] [Google Scholar]
- 6.Mitsiades N, Poulaki V, Mitsiades C, Tsokos M. Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 2001;61:2704–2712. [PubMed] [Google Scholar]
- 7.Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M. Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol. 2002;8:580–585. doi: 10.3748/wjg.v8.i4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolenko V, Uzzo RG, Bukowski R, Bander NH, Novick AC, His ED, Finke JH. Dead or dying: Necrosis versus apoptosis in caspase-deficient human renal cell carcinoma. Cancer Res. 1999;59:2838–2842. [PubMed] [Google Scholar]
- 9.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–3119. [PubMed] [Google Scholar]
- 10.Bhuvarahamurthy V, Kristiansen GO, Johannsen M, Johannsen M, Loening SA, Schnorr D, Jung K, Staack A. In situ gene expression and localization of metalloproteinases MMP1, MMP2, MMP3, MMP9, and their inhibitors TIMP1 and TIMP2 in human renal cell carcinoma. Oncol Rep. 2006;15:1379–1384. [PubMed] [Google Scholar]
- 11.Lin H, Pan JC, Zhang FM, Huang B, Chen X, Zhuang JT, Wang H, Mo CQ, Wang DH, Qiu SP. Matrix metalloproteinase-9 is required for vasculogenic mimicry by clear cell renal carcinoma cells. Urol Oncol. 2015;33:168.e9–16. doi: 10.1016/j.urolonc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Kawata N, Nagane Y, Igarashi T, Hirakata H, Ichinose T, Hachiya T, Takimoto Y, Takahashi S. Strong significant correlation between MMP-9 and systemic symptoms in patients with localized renal cell carcinoma. Urology. 2006;68:523–527. doi: 10.1016/j.urology.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya N, Kishimoto T, Suzuki H, Sekita N, Nagai Y, Oosumi N, Kito H, Tochigi N, Shinbo M, Nemori R, et al. Increased in situ gelatinolytic activity in renal cell tumor tissues correlates with tumor size, grade and vessel invasion. Int J Cancer. 2003;106:480–485. doi: 10.1002/ijc.11272. [DOI] [PubMed] [Google Scholar]
- 14.Lein M, Jung K, Laube C, Hübner T, Winkelmann B, Stephan C, Hauptmann S, Rudolph B, Schnorr D, Loening SA. Matrix-metalloproteinases and their inhibitors in plasma and tumor tissue of patients with renal cell carcinoma. Int J Cancer. 2000;85:801–804. doi: 10.1002/(SICI)1097-0215(20000315)85:6<801::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Cho NH, Shim HS, Rha SY, Kang SH, Hong SH, Choi YD, Hong SJ, Cho SH. Increased expression of matrix metalloproteinase 9 correlates with poor prognostic variables in renal cell carcinoma. Eur Urol. 2003;44:560–566. doi: 10.1016/S0302-2838(03)00362-2. [DOI] [PubMed] [Google Scholar]
- 16.DI Carlo A. Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol Lett. 2013;5:1677–1681. doi: 10.3892/ol.2013.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Carlo A. Matrix metalloproteinase-2 and −9 in the sera and in the urine of human oncocytoma and renal cell carcinoma. Oncol Rep. 2012;28:1051–1056. doi: 10.3892/or.2012.1864. [DOI] [PubMed] [Google Scholar]
- 18.DI Carlo A. Matrix metalloproteinase-2 and −9 and tissue inhibitor of metalloproteinase-1 and −2 in sera and urine of patients with renal carcinoma. Oncol Lett. 2014;7:621–626. doi: 10.3892/ol.2013.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schéele S, Nyström A, Durbeej M, Talts JF, Ekblom M, Ekblom P. Laminin isoforms in development and disease. J Mol Med (Berl) 2007;85:825–836. doi: 10.1007/s00109-007-0182-5. [DOI] [PubMed] [Google Scholar]
- 20.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 21.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Res Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 23.Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 24.Morell-Quadreny L, Rubio J, Lopez-Guerrero JA, Casanova J, Ramos D, Iborra I, Solsona E, Llombart-Bosch A. Disruption of basement membrane, extracellular matrix metalloproteinases and E-cadherin in renal-cell carcinoma. Anticancer Res. 2003;23:5005–5010. [PubMed] [Google Scholar]
- 25.Lohi J, Tani T, Leivo I, Linnala A, Kangas L, Burgeson RE, Lehto VP, Virtanen I. Expression of laminin in renal-cell carcinomas, renal-cell carcinoma cell lines and xenografts in nude mice. Int J Cancer. 1996;68:364–371. doi: 10.1002/(SICI)1097-0215(19961104)68:3<364::AID-IJC15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Rissanen J, Korhonen M, Lehto VP, Virtanen I. Laminin alpha1 chain in human renal cell carcinomas and integrin-mediated adhesion of renal cell carcinoma cells to human laminin isoforms. J Pathol. 2003;200:157–167. doi: 10.1002/path.1347. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Huo P, Hu G, Wei B, Kong D, Li H. Identification of gene markers associated with metastasis in clear cell renal cell carcinoma. Oncol Lett. 2017;13:4755–4761. doi: 10.3892/ol.2017.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherief MH, Low SH, Miura M, Kudo N, Novick AC, Weimbs T. Matrix metalloproteinase activity in urine of patients with renal cell carcinoma leads to degradation of extracellular matrix proteins: Possible use as a screening assay. J Urol. 2003;169:1530–1534. doi: 10.1097/01.ju.0000049201.91150.9d. [DOI] [PubMed] [Google Scholar]
- 29.Sobin LH, Wittekind CH, editors. International Union Against Cancer (UICC) TNM classification of malignant tumors. 6th edition. Wiley-Liss; New York; 2002. pp. 193–195. [Google Scholar]
- 30.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 31.Tian HY, Li ZX, Li HY, Wang HJ, Zhu XW, Dou ZH. Effects of 14 single herbs on the induction of caspase-3 in tumor cells: A brief review. Chin J Integr Med. 2013;19:636–640. doi: 10.1007/s11655-013-1539-y. [DOI] [PubMed] [Google Scholar]
- 32.Himelstein BP, Canete-Soler R, Bernhard EJ, Dilks DW, Muschel RJ. Metalloproteinases in tumor progression: The contribution of MMP-9. Invasion Metastasis. 1994;14:246–258. -1995. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.