Abstract
Osteosarcoma, is a kind of malignant tumor derived from malignant interstitial cells. The pathogenesis of osteosarcoma remains unclear and despite use of chemotherapy drugs, resistance to these drugs affects the success of treatment. The present study was conducted to investigate the effects of icariin (ICA) on osteosarcoma cell proliferation and to investigate the role of the Wnt/β-catenin signaling pathway in the inhibition process of ICA on osteosarcoma cell proliferation. Different concentrations of ICA were selected to treat the osteosarcoma cell line 143B for 24 h, and then the onset concentration of ICA when it inhibited the growth of osteosarcoma cancer cell line 143B was detected via an MTT assay. The effect of ICA on the apoptosis of colon cancer cell line 143B under this concentration was detected using a flow cytometer. RNA in osteosarcoma cell line 143B was extracted, followed by reverse transcription. The expression levels of related and apoptotic proteins in the Wnt/β-catenin signaling pathway using ICA were detected by semi-quantitative PCR and western blot analysis, respectively. The expression quantities of vascular endothelial growth factor (VEGF) and MMP-9 were detected by ELISA. MTT assay showed that ICA inhibited the growth of 143B when its concentration was 5 µM (p<0.01). Flow cytometry showed that the number of apoptotic cells after ICA treatment was significantly higher than that in control group (p<0.01). RNA in osteosarcoma cell line 143B was extracted, followed by reverse transcription. Semi-quantitative PCR and western blot analysis revealed that the expression levels of p-GSK3β, β-catenin, c-Myc and cyclin D1 in cells after ICA treatment were significantly downregulated (p<0.01), while the expression level of caspase-3 was significantly increased (p<0.01). ELISA showed that the expression quantities of VEGF and MMP-9 were significantly decreased (p<0.01). Thus, ICA can significantly inhibit osteosarcoma cell proliferation and promote osteosarcoma cell apoptosis, which may be realized by affecting the expression of the Wnt/β-catenin signaling pathway and blocking the expression of related proteins.
Keywords: icariin, osteosarcoma, Wnt/β-catenin signaling pathway, proliferation
Introduction
Osteosarcoma, as a kind of malignant tumor derived from malignant interstitial cells, has certain osteoid characteristics, including a strong migration capacity and frequent systemic metastasis (1). The pathogenesis of osteosarcoma remains unclear and the osteosarcoma of osteoblasts is a clinically common form at present, which generally occurs in the metaphysis of long-tubular bone, but seldom in the axial skeleton (2,3). The malignant grade of osteosarcoma is high, micro-lesion metastasis is possible during diagnosis, while lung tissue is a common metastatic site (4). Prior to the application of chemotherapeutic drugs, amputation was commonly used in the treatment of osteosarcoma. The rise and development of chemotherapy drugs has been useful in the treatment of osteosarcoma. However, drug resistance of chemotherapy drugs delays the treatment of osteosarcoma (5). Icariin (ICA), also known as Epimedium brevicornu, is sweet and warm. It has the effect of nourishing kidney and strengthening yang, strengthening tendons and bones, dispelling wind and eliminating dampness, expelling furuncle and resolving carbuncle. ICA is often clinically used in the treatment of flaccid bones and muscles and rheumatic arthralgia (6–8). Recent findings have shown that ICA has the effect of promoting osteoblast proliferation and increasing ALP activity in osteoblasts, thus promoting the calcification of bone matrix and enhancing bone strength (9). Zhang et al (10) found that ICA can significantly increase osteocytes and promote osteocyte differentiation. Zhang et al (11) found that ICA can increase the apoptosis of osteoclastoma. However, to the best of our knowledge, there is no report currently available on the effect of ICA on osteosarcoma cells and its mechanism.
In this study, the effect of ICA on osteosarcoma cell proliferation and its mechanism were studied to clarify the mechanism of ICA for osteosarcoma cells, in order to provide new ideas for the efficacious development of ICA and clinical treatment of osteosarcoma.
Materials and methods
Instruments and materials
Osteosarcoma cancer cell line 143B (Cell Bank of the Chinese Academy of Sciences, Shanghai, China), methyl thiazolyl tetrazolium (MTT) and DMSO (both from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), ICA (Aladdin Shanghai Biochemical Technology Co., Ltd., Shanghai, China), TRIzol kit and reverse transcription kit (both from Invitrogen, Carlsbad, CA, USA), VEGA ELISA kit and MMP-9 ELISA kit (both from R&D Systems, Inc., Minneapolis, MN, USA), rabbit anti-DKK1, rabbit anti-caspase-3, rabbit anti-β-catenin, rabbit anti-GSK3β, rabbit anti-pGSK3β (Ser9) and GAPDH (all from Cell Signaling Technology, Inc., Danvers, MA, USA), ECI luminescent solution (Invitrogen), inversed fluorescent microscope (Thermo Fisher Scientific GmbH, Dreieich, Germany), cell culture flask (Corning Inc., Corning, NY, USA), pipettor (Eppendorf, Hamburg, Germany), PCR instrument (Applied Biosystems, Foster City, CA, USA), UV imaging system (Biometra, Goettingen, Germany), and electronic balance (BP121S; Sartorius AG, Goettingen, Germany) were used in the study. Any other equipment and reagents were previously described in the relevant section.
Detection of inhibitory effect of ICA on osteosarcoma cell growth via MTT assay
After osteosarcoma cancer cell line 143B was revived (12), it was placed in the incubator, and cells in the logarithmic growth phase were inoculated onto the 96-well plate after subculture (8×103/well). [Of note, the 143B cell line used in the present study is possibly identified as osteosarcoma cell line HTK-osteosarcoma cell line (13); however, it did not affect the conclusions drawn in the study.] After inoculation for 24 h, the serum was deprived for 24 h and ICA was added at different concentrations (20, 10, 5, 2, 1 and 0.1 µM). The blank control group and the positive drug group (cisplatin) were then set up. After incubation with medication for 24 h, 1% MTT was added to incubate the cells for 4 h in the dark. The medium was discarded and 150 µl dimethyl sulfoxide was added to each well. After vibration for 10 min, the absorbance value at 570 nm was measured using the microplate reader (Thermo Fisher Scientific; Waltham, MA, USA).
Effects of ICA on osteosarcoma cell apoptosis
The 143B colon cancer cell line was cultured as follows. The cell density was adjusted to 5×106 cells/ml and added into the 6-well plate, and the cells were then divided into the blank control, ICA (5 µM) and positive drug groups. After treatment for 24 h, the cells were washed with pre-cooled PBS twice, re-suspended and centrifuged at 12,000 × g for 5 min. Fluorescent solution was added to incubate the cells at room temperature for 15 min in the dark. The cells were then detected using a flow cytometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Detection of the expression of related genes via semi-quantitative PCR
The cell proteins treated with 5 µM ICA for 24 h were extracted using a TRIzol kit, and the supernatant was discarded via centrifugation for 10 min at 6,000 × g. The RNA integrity was confirmed by agarose gel electrophoresis. The results of electrophoresis showed that 28S, 18S and 5S bands were clear, and the brightness of 28S band was approximately twice of that of 18S band, indicating that RNA was intact and can be used for subsequent experiments. cDNA was obtained via reverse transcription using the reverse transcription kit. An appropriate internal reference was selected. The expression levels of c-Myc, cyclin D1 and β-catenin in the Wnt/β-catenin signaling pathway, apoptosis-related gene caspase-3 and migration-related gene MMP-9 and VEGF were detected via semi-quantitative PCR. Reaction conditions were: denaturation, 95°C for 30 sec; annealing, 64°C for 25 sec; and extension 72°C for 30 sec, for a total of 35 cycles. Primers were produced by Tiangen Biotech Co., Ltd. (Beijing, China). Primer sequences are shown in Table I. After the reaction, agarose gel electrophoresis was performed, followed by observation using a UV imaging system.
Table I.
PCR primer.
| Gene name | Primer sequences |
|---|---|
| c-Myc | F: 5′-ATCCAGAGACAAGACATGTAC-3′ |
| R: 5′-TTCAGATGTTCTAAGCCTACGG-3′ | |
| β-catenin | F: 5′-TGGCGGTTTGCGGTGGAC-3′ |
| R: 5′-CCAGTGCAGGGTCCGAGGT-3′ | |
| Cyclin D1 | F: 5′-GATGATTGGCATGGCTTT-3′ |
| R: 5′-CACCTTCCGTTCCAGTTT-3′ | |
| Caspase-3 | F: 5′-GATGGGACTGTGGTTACCGT-3′ |
| R: 5′-GGTGAAACTCTTGCCTCGTC-3′ | |
| MMP-9 | F: 5′-GATGATTGGCATGGCTTT-3′ |
| R: 5′-GCCATACGCTGACCTTTCA-3′ | |
| VEGF | F: 5′-GGAGTCCATTGGTGCTTGA-3′ |
| R: 5′-ACACCCTTTCCAATGTGCC-3′ | |
| GAPDH | F: 5′-GATGATTGGCATGGCTTT-3′ |
| R: 5′-CACCTTCCGTTCCAGTTT-3′ |
F, forward; R, reverse; VEGF, vascular endothelial growth factor.
Detection of the expression of related proteins via western blot analysis
The cells in the logarithmic growth phase were inoculated onto the 96-well plate, and the blank control and ICA groups (5 µM) were established. After medication for 24 h, the protein was extracted and quantified, followed by separation via SDS-PAGE and the protein was transferred to PVDF membrane. The target band was cut off after sealing for 2 h. The target protein antibody [anti-caspase-3, rabbit monoclonal antibody, catalogue no. 9665; anti-β-catenin, rabbit monoclonal antibody, catalogue no. 9582; monoclonal antibody anti-GSK3β, monoclonal antibody, rabbit 12456; anti-pGSK3β (Ser9), monoclonal antibody, rabbit 5558; cyclin D1, rabbit monoclonal antibody, catalogue no. 2978; and CMYc, rabbit monoclonal antibody, catalogue no.13987 were procured from Cell Signaling Technology, Inc., Danvers, MA, USA] was used to incubate the protein at 4°C overnight. After washing with Tris-buffered saline Tween-20 (TBST) three times (5 min/time), the secondary antibody was incubated at room temperature for 2 h. After washing with TBST again three times, the appropriate amount of ECL solution (solution A and B were mixed at a ratio of 1:1) was added in the dark, followed by performing of the experiment, and the performing time was determined according to the fluorescence intensity of the protein band. Following development and fixation, the band was scanned and received gray value analysis via ImageJ software. The expression levels of related proteins in the Wnt/β-catenin signaling pathway, p-GSK3β, c-Myc, cyclin D1 and β-catenin, and apoptosis protein caspase-3 were detected.
Detection of the expression levels of VEGF and MMP-9 via ELISA
The cells in the logarithmic growth phase were inoculated onto the 24-well plate, and the blank control and ICA groups (5 µM) were set up. After medication for 24 h, the supernatant was collected and the expression levels of VEGF and MMP-9 in cells were detected using an ELISA kit.
Statistical analysis
Data were presented as mean ± standard deviation and analyzed by SPSS 19.0 (SPSS Inc., Chicago, IL, USA) software. The t-test was used for measurement data, while the Chi-square test was used for enumeration data. One-way analysis of variance (ANOVA) was used for other data. Bonferronic method was used for pairwise comparison under homogeneity of variance, while the Welch method was used under heterogeneity of variance. Dunnett's T3 method was used for multiple comparisons.
Results
Inhibitory effect of ICA on osteosarcoma cell growth
The inhibitory effect of ICA on the osteosarcoma cell 143B was detected via MTT assay. The results are shown in Fig. I. ICA concentrations were set as 20, 10, 5, 2, 1 and 0.1 µM, and the blank control and positive control groups (cisplatin) were established. The results showed that ICA significantly inhibited the 143B cell growth when its concentration reached 5 µM (p<0.01).
Figure 1.
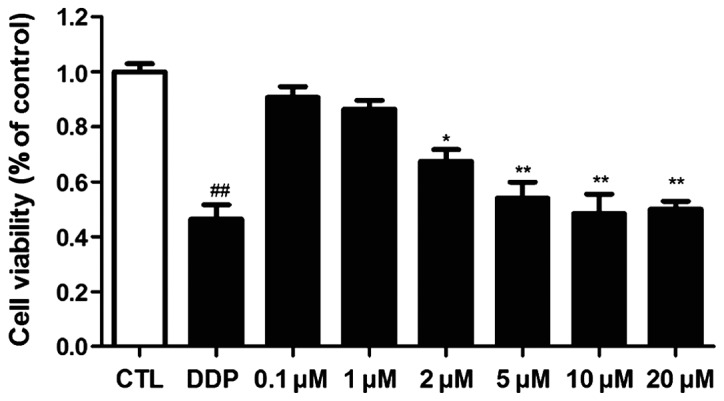
Detection of inhibitory effect of ICA on the growth of osteosarcoma cell 143B via MTT assay. When the ICA concentration reached 5 µM, the survival rate of 143B was significantly decreased (**p<0.05 and ##p<0.01), and this concentration was used as the concentration in subsequent experiments. ICA, icariin. *P>0.05.
Effects of ICA on osteosarcoma cell apoptosis
The effects of ICA on the apoptosis of osteosarcoma cell 143B were detected via a flow cytometer. The results are shown in Fig. 2. After 5 µM ICA was added to the osteosarcoma cell 143B, the number of apoptotic cells in the ICA group was significantly increased compared with that in the blank control group, and the difference was statistically significant (p<0.01).
Figure 2.
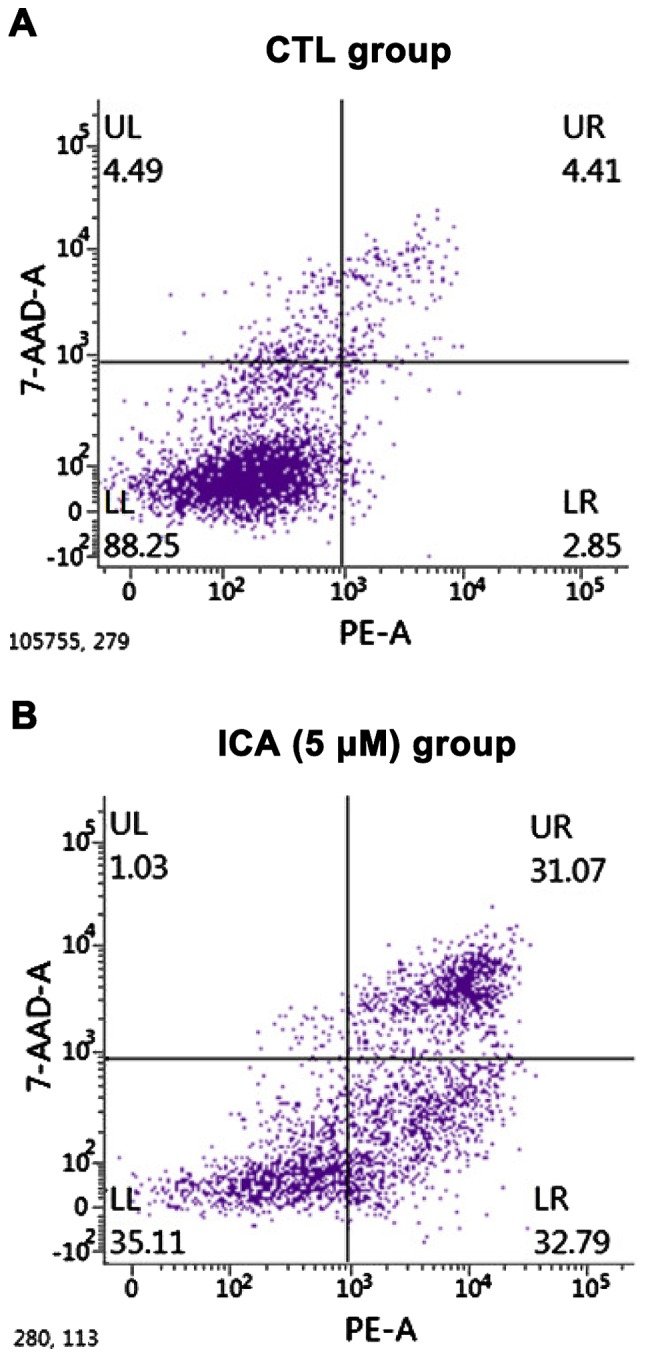
Detection of cell apoptosis via flow cytometer. (A) Blank control group, and (B) ICA (5 µM) group. Compared with the blank control group, 5 µM ICA significantly increased the apoptosis of osteosarcoma cell 143B, and the difference was statistically significant. ICA, icariin.
Detection of related gene expressions via semi-quantitative PCR
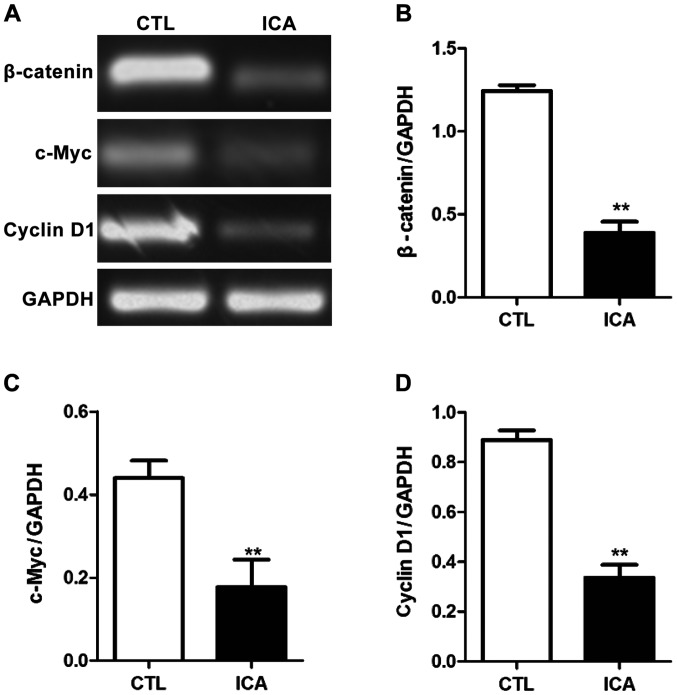
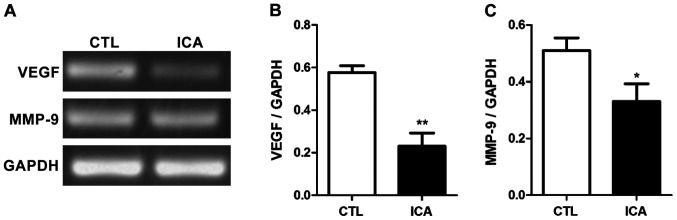
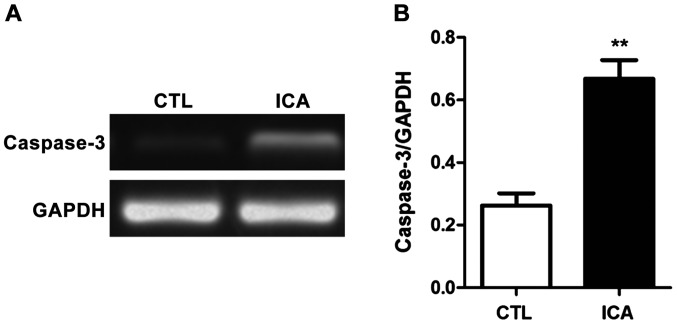
After the RNA of osteosarcoma cell 143B treated with ICA was extracted, the relative expression quantities of related genes were detected via semi-quantitative PCR with GAPDH as the internal reference. The results are shown in Figs. 3–5. Fig. 3 shows that compared with those in the blank control group, the expression levels of β-catenin were decreased after ICA treatment, and the expression levels of its downstream genes c-Myc and cyclin D1 were also significantly decreased (p<0.01). Fig. 4 shows that the expression level of caspase-3 was significantly increased after ICA treatment (p<0.01). Fig. 5 shows that the expression levels of MMP-9 and VEGF were decreased after ICA treatment (p<0.01).
Figure 3.
Detection of effect of ICA on the expression levels of related genes in the Wnt/β-catenin signaling pathway via semi-quantitative PCR. (A) Agarose gel electrophoresis. (B-D) Statistical charts of the relative expression levels of β-catenin, c-Myc and cyclin D1. mRNA expression levels in β-catenin, c-Myc and cyclin D1 in osteosarcoma cells were significantly decreased after ICA treatment (**p<0.01). ICA, icariin.
Figure 5.
Detection of effect of ICA on mRNA expression levels in MMP-9 and VEGF via semi-quantitative PCR. (A) Chart of agarose gel electrophoresis. (B and C) Statistical charts of relative expression levels. After ICA treatment, mRNA expression levels in MMP-9 and VEGF in osteosarcoma cells were significantly decreased (*p<0.05, **p<0.01). ICA, icariin.
Figure 4.
Detection of the effect of ICA on the mRNA expression level in caspase-3 via semi-quantitative PCR. (A) Chart of agarose gel electrophoresis. (B) Statistical chart of relative expression level. After ICA treatment, the mRNA expression level in caspase-3 in osteosarcoma cells was significantly increased (**p<0.01). ICA, icariin
Detection of expression levels of related proteins via western blot analysis
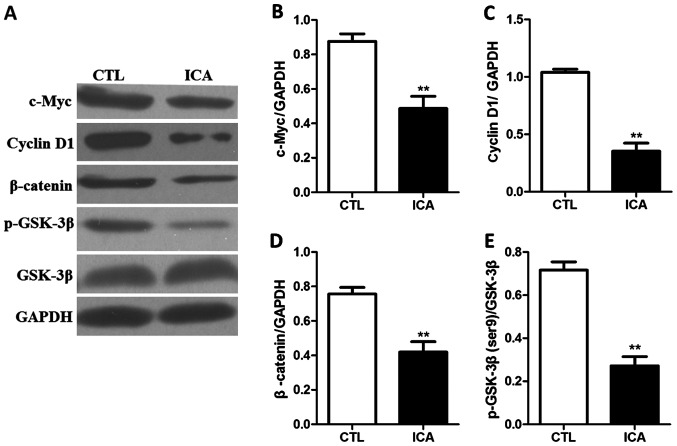
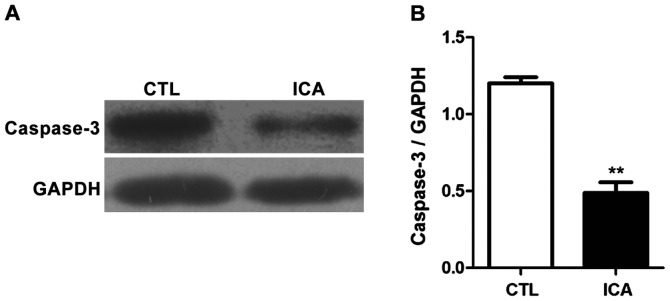
The expression levels of related proteins in osteosarcoma cell 143B treated with ICA (5 µM) were detected via western blot analysis, and the results are shown in Figs. 6 and 7. Fig. 6 shows that the protein expression levels in p-GSK3β, β-catenin and its downstream genes c-Myc and cyclin D1 were significantly decreased after ICA treatment (p<0.01). Fig. 7 shows that the protein expression level in caspase-3 was significantly increased after ICA treatment (p<0.01).
Figure 6.
Detection of effect of ICA on expression levels of related proteins in the Wnt/β-catenin signaling pathway via western blot analysis. (A) Western blot bands. (B-E) Statistical charts of β-catenin, p-GSK3β, c-Myc and cyclin D1. The results show that the protein expression levels in β-catenin, p-GSK3β, c-Myc and cyclin D1 were significantly decreased after ICA treatment (**p<0.01). ICA, icariin.
Figure 7.
Detection of effect of ICA on the protein expression level in caspase-3 via western blot analysis. (A) Western blot bands. (B) Statistical chart. The results showed that the protein expression level in caspase-3 in osteosarcoma cells was significantly increased after ICA treatment (**p<0.01). ICA, icariin.
Detection of the expression levels of VEGF and MMP-9 via ELISA
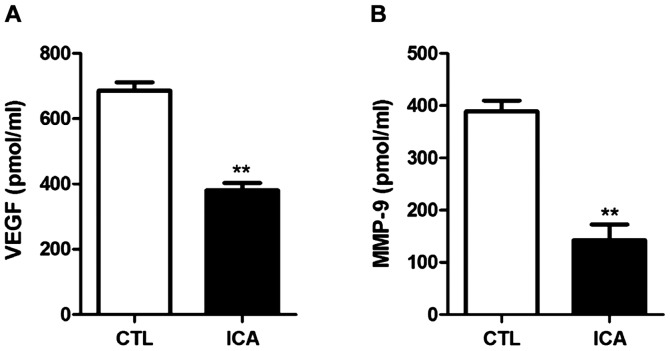
The protein expression levels in VEGF and MMP-9 in osteosarcoma cell 143B treated with ICA (5 µM) were detected via ELISA. The results are shown in Fig. 8. The protein expression levels in VEGF and MMP-9 after ICA treatment were significantly decreased compared with those in the blank control group (p<0.01).
Figure 8.
Detection of the expression levels of VEGF and MMP-9 via ELISA. Statistical chart of (A) VEGF and (B) MMP-9 expression level. The results show that the expression levels of VEGF and MMP-9 in osteosarcoma cells were significantly decreased after ICA treatment (**p<0.01). ICA, icariin.
Discussion
Osteosarcoma is a clinically common malignant bone tumor occurring in young individuals, and its main clinical manifestations are progressively aggravated bone pain and local swelling (14,15). The 5-year survival rate of osteosarcoma patients is low, placing great economic and mental burdens on the society and family (16,17). The effective constituents in natural medicine and traditional Chinese medicine have been regarded as the ideal source of antitumor drugs with high efficiency and low toxicity. Previous studies have shown that herb Epimedium has a significant development potential for antitumor drugs. ICA is one of the most studied effective constituents in the studies of herb Epimedium. Li et al (18) studied and found that ICA can cause the death of lung cancer cells in vivo, which has an inhibitory effect on lung cancer cell migration and infiltration. Zhang et al (19) found that ICA can promote the proliferation and differentiation of osteoblasts, which may be realized via the BMP2/Smads/Runx2/Osterix signaling pathways. Hartemayer et al (20) studied and proved that the Wnt/β-catenin signaling pathway in chondrocytes can lead to hypertrophy and matrix mineralization of chondrocytes, induce the expressions of MMP-9 and VEGF and promote cell proliferation and migration. In addition, non-steroidal anti-inflammatory drugs can inhibit the proliferation of tumor cells by reducing β-catenin/TCF transcriptional activity and disturbing the signal transduction of the Wnt pathway (21).
In the present study, the effect of ICA on the proliferation of osteosarcoma cell 143B was investigated and it was found that the proliferation of 143B was significantly inhibited when the concentration of ICA reached 5 µM. At the same time, the flow cytometry showed that the cell apoptosis after ICA treatment was significantly increased. The aforementioned results indicated that ICA has a good inhibitory effect on the proliferation of osteosarcoma cells with a low onset concentration and high activity. The expression levels of related genes were detected via semi-quantitative PCR, and the results showed that the expression level of β-catenin was significantly decreased after ICA treatment, while the expression levels of its downstream genes c-Myc and cyclin D1 were also significantly decreased. Previous findings have shown that multiple ligands and receptors in the Wnt/β-catenin signaling pathway are highly expressed in osteosarcoma cells, suggesting that ICA may inhibit the proliferation of osteosarcoma cells by inhibiting the transcriptional level of β-catenin (22). Additionally, the protein expression levels in p-GSK3β and β-catenin were significantly decreased after ICA treatment. During typical Wnt/β-catenin signal transduction, the intracellular accumulation of β-catenin and its transfer to the nucleus were dominated in regulating the signal activity. GSK3β is an important negative regulator in the signal transduction process. Activation of GSK3β can promote the phosphorylation of β-catenin and eventually lead to the degradation of β-catenin and inactivation of the Wnt/β-catenin signal (23). ICA can increase the catalytic activity of GSK3β by inhibiting the phosphorylation of 9-serine of GSK3β, thus promoting β-catenin degradation and downregulating the protein level of β-catenin in cells. It was also found that the expression levels of downstream proteins of β-catenin, c-Myc and cyclin D1, were also inhibited. Therefore, it was inferred that ICA inhibits the Wnt/β-catenin signal and the expression of related target proteins in the pathway through inhibition of the phosphorylation of p-GSK3β, thus regulating the proliferation and invasion of osteosarcoma cells. At the same time, the expression level of caspase-3 in osteosarcoma cells after ICA treatment was increased, suggesting that ICA can also inhibit the proliferation of osteosarcoma cells by inhibiting their apoptosis.
In conclusion, ICA has a significant effect of inhibiting the proliferation and inducing the apoptosis of osteosarcoma cell 143B, which may be realized by activating GSK3β and inhibiting the activity of the Wnt/β-catenin signaling pathway. However, there were still some shortcomings in this experiment. The inhibitory effect of ICA on osteosarcoma cell proliferation and its mechanism were investigated only through in vitro experiments, and the abovementioned results were not verified in in vivo experiments. In the follow-up studies, the author aims to verify the efficacy of ICA and the role of Wnt/β-catenin signaling pathway from in vivo experiments again in the hope to provide new ideas for the efficacious development of ICA and the clinical treatment of osteosarcoma.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shimizu T, Kido A, Honoki K, Murata K, Fujii H, Higuchi B, Ishihara T, Takeshita Y, Shima M, Yajima H, et al. A successful reconstruction using a frozen autograft and a pedicled latissimus dorsi flap after a S12345B shoulder girdle resection in a patient with osteosarcoma. J Reconstr Microsurg. 2012;28:155–159. doi: 10.1055/s-0031-1296031. [DOI] [PubMed] [Google Scholar]
- 2.Qiu Q, Jiang J, Lin L, Cheng S, Xin D, Jiang W, Shen J, Hu Z. Downregulation of RSK2 influences the biological activities of human osteosarcoma cells through inactivating AKT/mTOR signaling pathways. Int J Oncol. 2016;48:2508–2520. doi: 10.3892/ijo.2016.3481. [DOI] [PubMed] [Google Scholar]
- 3.Hurley C, McCarville MB, Shulkin BL, Mao S, Wu J, Navid F, Daw NC, Pappo AS, Bishop MW. Comparison of (18) F-FDG-PET-CT and bone scintigraphy for evaluation of osseous metastases in newly diagnosed and recurrent osteosarcoma. Pediatr Blood Cancer. 2016;63:1381–1386. doi: 10.1002/pbc.26014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waresijiang N, Sun J, Abuduaini R, Jiang T, Zhou W, Yuan H. The downregulation of miR 125a 5p functions as a tumor suppressor by directly targeting MMP 11 in osteosarcoma. Mol Med Rep. 2016;13:4859–4864. doi: 10.3892/mmr.2016.5141. [DOI] [PubMed] [Google Scholar]
- 5.Ma Q, Zhang Y, Liu T, Jiang K, Wen Y, Fan Q, Qiu X. Hypoxia promotes chemotherapy resistance by down-regulating SKA1 gene expression in human osteosarcoma. Cancer Biol Ther. 2017;18:177–185. doi: 10.1080/15384047.2017.1294285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Yuan Y, Zhang Y, Zhang X, Gao L, Xu R. Icariin inhibits AMPK-dependent autophagy and adipogenesis in adipocytes in vitro and in a model of Graves' orbitopathy. Front Physiol. 2017;8:45. doi: 10.3389/fphys.2017.00045. doi: 10.3389/fphys.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su YS, Fan ZX, Xiao SE, Lin BJ, Miao Y, Hu ZQ, Liu H. Icariin promotes mouse hair follicle growth by increasing insulin-like growth factor 1 expression in dermal papillary cells. Clin Exp Dermatol. 2017;42:287–294. doi: 10.1111/ced.13043. [DOI] [PubMed] [Google Scholar]
- 8.Xiao HB, Sui GG, Lu XY. Icariin improves eNOS/NO-pathway to prohibit the atherogenesis of apolipoprotein E null mice. Can J Physiol Pharmacol. 2017;95:625–633. doi: 10.1139/cjpp-2016-0367. [DOI] [PubMed] [Google Scholar]
- 9.Qian ZQ, Wang YW, Li YL, Ling-Zhu Li YQ, Yang DL. Icariin prevents hypertension-induced cardiomyocyte apoptosis through the mitochondrial apoptotic pathway. Biomed Pharmacother. 2017;88:823–831. doi: 10.1016/j.biopha.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Yin L, Zheng N, Zhang L, Liu J, Liang W, Wang Q. Icariin enhances remyelination process after acute demyelination induced by cuprizone exposure. Brain Res Bull. 2016;34:713–725. doi: 10.1016/j.brainresbull.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Feng P, Mo G, Li D, Li Y, Mo L, Yang Z, Liang D. Icariin influences adipogenic differentiation of stem cells affected by osteoblast-osteoclast co-culture and clinical research adipogenic. Biomed Pharmacother. 2017;88:436–442. doi: 10.1016/j.biopha.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Brennecke P1, Arlt MJ, Muff R, Campanile C, Gvozdenovic A, Husmann K, Holzwarth N, Cameroni E, Ehrensperger F, Thelen M, et al. Expression of the chemokine receptor CXCR7 in CXCR4-expressing human 143B osteosarcoma cells enhances lung metastasis of intratibial xenografts in SCID mice. PLoS One. 2013;8:e74045. doi: 10.1371/journal.pone.0074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamie Yu, Selvaraj Suresh K., Liang-Chu MM, Aghajani S, Busse M, Yuan J, Lee G, Peale F, Klijn C, et al. A resource for cell line authentication, annotation and quality control. Nature. 2015;520:307–311. doi: 10.1038/nature14397. [DOI] [PubMed] [Google Scholar]
- 14.Dang H, Wu W, Wang B, Cui C, Niu J, Chen J, Chen Z, Liu Y. CXCL5 plays a promoting role in osteosarcoma cell migration and invasion in autocrine- and paracrine-dependent manners. Oncol Res. 2017;25:177–186. doi: 10.3727/096504016X14732772150343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Liu L, Lv Z, Li Q, Gong W, Wu H. MicroRNA-342-3p inhibits the proliferation, migration, and invasion of osteosarcoma cells by targeting astrocyte-elevated gene-1 (AEG-1) Oncol Res. 2017;8:435–447. doi: 10.3727/096504017X14886485417426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M, Zhang YY, Wang HF, Yang GS. The expression and function of miRNA-106 in pediatric osteosarcoma. Eur Rev Med Pharmacol Sci. 2017;21:715–722. [PubMed] [Google Scholar]
- 17.Rushing CJ, Rogers DE, Spinner SM, Gajzer DC. A case report of heel pain mimicking plantar fasciitis and osteosarcoma: A unique presentation of a Nora's lesion. J Foot Ankle Surg. 2017;56:670–673. doi: 10.1053/j.jfas.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Wang L, Chu X, Cui H, Bian Y. Icariin combined with human umbilical cord mesenchymal stem cells significantly improve the impaired kidney function in chronic renal failure. Mol Cell Biochem. 2017;428:203–212. doi: 10.1007/s11010-016-2930-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Wang XZ, Li YS, Zhang L, Hao LR. Icariin ameliorates IgA nephropathy by inhibition of nuclear factor kappa b/Nlrp3 pathway. FEBS Open Bio. 2016;7:54–63. doi: 10.1002/2211-5463.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartemayer R, Kuo C, Kent P. Osteosarcoma metastases with direct cardiac invasion: A case report and review of the pediatric literature. J Pediatr Hematol Oncol. 2017;39:188–193. doi: 10.1097/MPH.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 21.Sareddy GR, Kesanakurti D, Kirti PB, Babu PP. Nonsteroidalanti-inflammatory drugs diclofenac and celecoxib attenuatesWnt/β-catenin/Tcf signaling pathway in human glioblastomacells. Neurochem Res. 2013;38:2313–2322. doi: 10.1007/s11064-013-1142-9. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Wang C, Wang J, Yin S, Gao H, Xiang LU, Liu H, Xiong Y, Wang P, Zhu X, Yang LI, Zhang R. Antiosteoporoticeffect of icariin in ovariectomized rats is mediated via the Wnt/β-catenin pathway. Exp Ther Med. 2016;12:279–287. doi: 10.3892/etm.2016.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Dong T, Hu C, Lu J, Dai J, Liu P. Salinomycinrepressed the epithelial-mesenchymal transition of epithelialovarian cancer cells via downregulating Wnt/β-cateninpathway. Onco Targets Ther. 2017;28:328–335. [Google Scholar]