Abstract
We reviewed our research concerning p53 molecules in esophageal squamous cell carcinoma by focusing on the p53 molecular diagnosis and treatment of esophageal squamous cell carcinoma. First, we developed diagnostic tools to analyze serum p53 autoantibodies to detect esophageal squamous cell carcinoma. Positive rate was around 25% to 30% in all patients and around 20% even in stage I patients. Presence of serum p53 antibodies was significantly associated with overexpression of p53 protein in tumor cells. Seropositive patients were more likely than seronegative patients to be resistant to chemotherapy. Monitoring of the titer of serum p53 autoantibodies was useful in predicting patients at high risk of recurrence and/or treatment response. Second, using Ad5CMV‐p53 for 10 patients with advanced esophageal squamous cell carcinoma, we carried out a phase I/II study of adenoviral‐mediated p53 gene therapy. Although no complete response was observed, local tumor was stabilized in nine patients. No serious adverse events related to Ad5CMV‐p53 were observed in these patients. One patient survived for over 5 years after the start of p53 gene therapy. Intratumoral injection of Ad5CMV‐p53 is therefore safe, feasible, and biologically active when given in multiple doses to patients with esophageal squamous cell carcinoma. Our observations from these clinical studies indicate that p53 is a useful molecular target both in the diagnosis and in the treatment of esophageal squamous cell carcinoma.
Keywords: autoantibody, esophageal cancer, gene therapy, p53, squamous cell carcinoma
1. INTRODUCTION
Despite improvements in surgical techniques,1, 2 perioperative care,3, 4 and the application of multimodality therapies,5 the prognosis of advanced esophageal squamous cell carcinoma (SCC) is poor, particularly in patients with T4 tumors.6 New modalities of diagnosis and therapy through molecular approaches are required to alter such circumstances. Based on the genetic alteration of esophageal SCC, p53 is reportedly one of the key molecules in the diagnosis and in the treatment of esophageal SCC.7 The most frequently mutated gene identified in esophageal SCC is the p53 gene, and its mutation and/or protein overexpression are observed in 40%‐60% of patients with esophageal cancer—even in the early phase of carcinogenesis.8, 9 Because p53 alteration was a good predictor for treatment response and survival in esophageal SCC, the 5‐year survival rate in the p53‐normal group was significantly higher than that in the p53‐alteration group.10, 11, 12 The overexpression of p53 protein usually results from genetic alterations modifying protein conformation and stability.7, 8 Such p53 protein alteration frequently induces p53 autoantibodies (s‐p53 Abs) in esophageal SCC. First, we reviewed the clinical significance of s‐p53 Abs. Second, we reviewed clinical trials of adenoviral p53 gene therapy for unresectable esophageal SCC. p53 molecular approaches signal the coming of age of esophageal cancer diagnosis and treatment.
2. S‐P53 ABS IN PATIENTS WITH ESOPHAGEAL SQUAMOUS CELL CARCINOMA
Alteration and overexpression of p53 protein is reported to induce s‐p53 Abs.13 A strong correlation between the presence of s‐p53 Abs, missense gene mutations, and protein accumulation has been observed.8 The cutoff level—based on the newly developed enzyme‐linked immunosorbent assay (ELISA)—was fixed to be 1.3 U/mL.13 The overall positive rate for s‐p53 Abs was 31%, which was significantly higher than that for carcinoembryonic antigen (CEA) and cytokeratin 19 fragment (CYFRA21‐1) (Figure 1).7 The high titer group—unlike the low titer group—showed a significant association with advanced tumor stage and poor prognosis. High s‐p53 Abs titer was an independent prognostic factor (P < .001). We found that s‐p53 Abs were useful in detecting esophageal cancer and identifying those at high risk of tumor recurrence and poor prognosis.14 The positive rate of s‐p53 Abs in stage I patients was the highest among all serum tumor markers (s‐p53 Abs at 23% vs CEA at 11.4%, SCC‐Ag at 14.3%, and CYFRA21‐1 at 5.7%) (Figure 1).15 Several superficial cancers tested s‐p53 Abs‐positive15 and were treated with endoscopic mucosal resection or endoscopic submucosal dissection (ESD). The majority of cases tested seronegative after treatment.16
Figure 1.
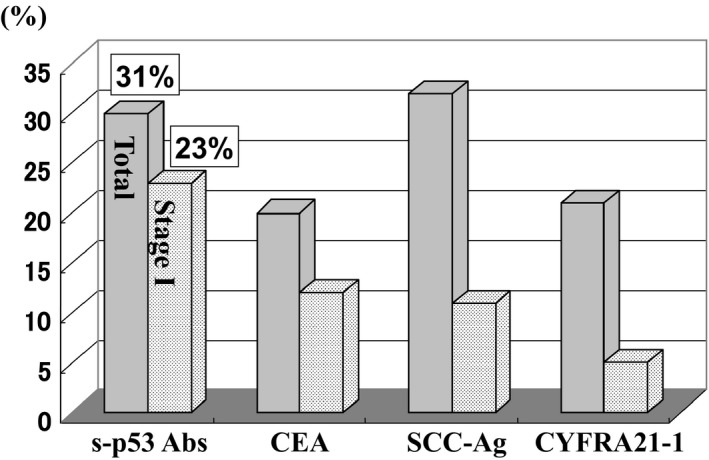
Comparison of positive rate of serum tumor markers in patients with esophageal squamous cell carcinoma. Positive rates of all patients and that of stage I patients were compared between s‐p53 Abs, carcinoembryonic antigen (CEA), squamous cell carcinoma (SCC)‐Ag and cytokeratin 19 fragment (CYFRA21‐1)
Recently, we experienced a sudden increase of s‐p53 Abs in a patient 8 years after surgical treatment for breast cancer without any recurrent signs of cancer.17 In this case, early esophageal SCC was detected by endoscopic screening. After ESD, s‐p53 Abs levels decreased to within normal range (Figure 2). Monitoring of s‐p53 Abs can be helpful not only in the early detection of the recurrence of primary‐, but also in the development of metachronous carcinoma. This case was the first in which we monitored the changing titers of s‐p53 Abs in the early phase of esophageal cancer progression into the lamina propria mucosae until the disappearance of tumor cells.
Figure 2.
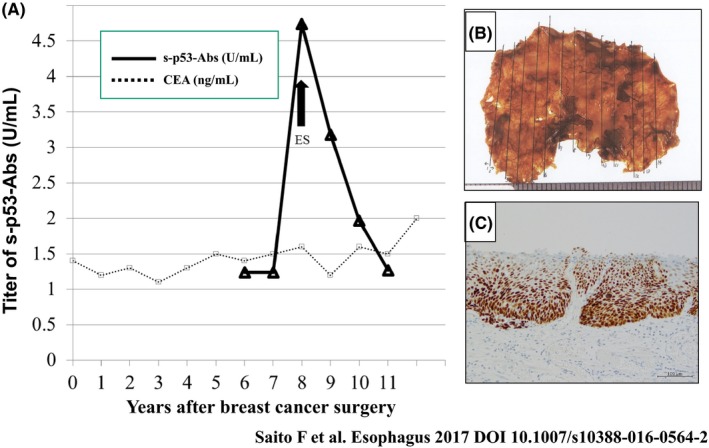
Detection of the early phase of esophageal cancer progression into lamina propria mucosae by serum p53 antibody.17 A, Changes in serum p53 antibody levels before and after endoscopic submucosal dissection (ESD) of esophageal squamous cell carcinoma. Carcinoembryonic antigen (CEA) level was continuously negative. B, Resection specimen after ESD of esophageal squamous cell carcinoma. H&E staining in the ESD specimen. C, p53 immunoreactivity in the ESD specimen
Clinical impact of s‐p53 Abs in surveillance of high‐risk patients was reported.18 Cawley et al detected s‐p53 Abs in four patients with Barrett's esophagus, including one with dysplasia that later progressed to adenocarcinoma. They also detected s‐p53 Abs in 10 cancer patients (eight squamous and two adenocarcinoma), two of whom (one squamous, one adenocarcinoma) had antibodies before cancer was diagnosed. They concluded that patients with Barrett's esophagus and esophageal cancer can develop p53 antibodies that may predate the clinical diagnosis of malignancy.
Although there was not enough evidence of s‐p53 Abs in esophageal adenocarcinoma, a few papers have shown that s‐p53 Abs seem to be useful in detecting esophageal adenocarcinoma with p53 overexpression.19, 20
3. IMPACT OF POSTOPERATIVE S‐P53 ABS TITER AND PERIOPERATIVE CHANGES OF S‐P53 ABS
We evaluated the impact of postoperative s‐p53 Abs titer and perioperative changes of s‐p53 Abs on survival in 110 patients with esophageal SCC. We generally observed a decrease in s‐p53 Abs titer after surgery.21 Among seropositive patients, those who remained seropositive after surgery (n = 28) had a worse prognosis than patients who showed seroconversion (P = .02; Figure 3). Among seropositive patients, the non‐decreased titer group showed significantly unfavorable survival. Multivariate analysis showed that postoperative s‐p53 Abs was an independent risk factor for worse overall survival (P = .03). Perioperative monitoring of s‐p53 Abs titers was useful in identifying patients with esophageal cancer at a high risk of tumor recurrence and poor prognosis. Regarding alterations in titer, continuously seropositive patients and/or the non‐decreased titer group, even after surgery, showed significantly unfavorable survival (P < .01; Figure 4).
Figure 3.
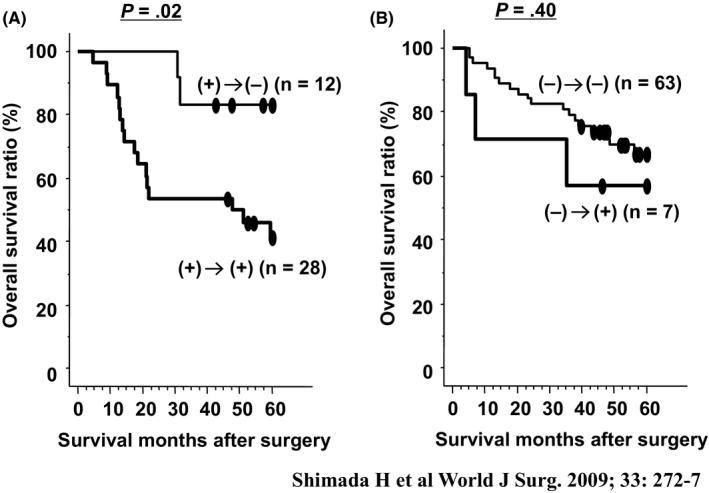
Overall survival curves according to status of serum p53 antibody.21 A, Survival according to postoperative status of serum p53 antibodies. B, Survival of patients positive for serum p53 antibody before surgery. P‐values were calculated by log‐rank test
Figure 4.
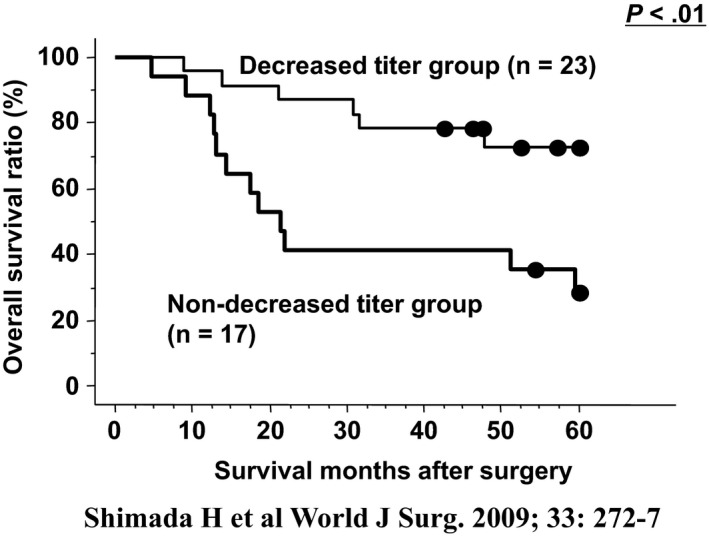
Overall survival curves according to changes in serum p53 antibody titer in 40 seropositive patients.21 A total of 23 patients showed reduction of serum titer and a total of 17 patients showed no reduction. P‐values were calculated by log‐rank test
4. PRESENCE OF S‐P53 ABS AND TREATMENT RESPONSE TO CHEMOTHERAPY
We also analyzed the clinicopathological factors of 95 patients with recurrent disease to evaluate the clinical impact of s‐p53 Abs on the prediction of treatment response.22 Of those 95 patients, 76 patients, seven patients, and 12 patients received non‐surgical treatment, operative intervention, and no treatment, respectively. Of the non‐surgical treatment group, 47 patients, 17 patients, and 12 patients received chemoradiotherapy, chemotherapy, and radiotherapy, respectively. We observed an overall clinical response in 26 of the 76 patients (34%). Multivariate analysis suggested that, at the moment of recurrence, the presence of s‐p53 Abs was an independent risk factor for poor overall survival (Table 1). These clinical impacts of s‐p53 Abs may reflect tumor chemosensitivity for cisplatin (CDDP).23 Recently, Yamashita et al24 confirmed this tendency in patients who received neoadjuvant chemotherapy followed by surgery, and seropositive patients showed poor treatment response and poor relapse‐free survival.
Table 1.
Multivariate analysis of survival of patients after recurrence of esophageal cancer22
| Variables | P‐value | Hazard ratio | 95% CI |
|---|---|---|---|
| Time of recurrencea | .089 | 2.51 | 0.87‐7.25 |
| No. of recurrent tumorsb | .262 | 1.76 | 0.66‐4.74 |
| s‐p53‐Absc | <.001 | 10.6 | 2.76‐40.00 |
| Serum albumind | .663 | 1.33 | 0.37‐4.71 |
| C‐reactive proteine | .007 | 5.05 | 1.56‐16.39 |
Within 1 y vs after 1 y.
No. of recurrent tumors: two or more vs one.
s‐p53‐Abs: positive vs negative.
Serum albumin: <3.5 mg/dL vs >3.5 mg/dL.
C‐reactive protein: <1.0 mg/dL vs >1.0 mg/dL.
Shimada et al.22
Interestingly, Hiyoshi et al25 found a significant reverse association between pathological response and the presence of serum p53 antibody before treatment in those patients who were treated with neoadjuvant chemotherapy. Hiyoshi's regimen included docetaxel combined with fluorouracil (5FU) + CDDP. There might be an unknown interaction between the presence of s‐p53 Abs and the docetaxel response. The drawing of evidence‐based conclusions about the relationship between chemosensitivities and the presence of s‐p53‐Abs requires further evaluation.
5. IMPACT OF S‐P53 ABS IN PATIENTS WITH OTHER GASTROENTEROLOGICAL CANCERS
Generally, the genetic alteration of p53 could induce s‐p53 Abs in patients with other gastroenterological cancers. We therefore evaluated the clinical significance of s‐p53 Abs in patients with various cancers—including gastric cancers, colorectal cancers, and cholangiocarcinoma (Figure 5).26, 27, 28 The positive rates were 16%, 30%, and 23% in gastric cancer, colorectal cancer, and cholangiocarcinoma, respectively. Because s‐p53 Abs tested positive independently for CEA and/or CA19‐9, s‐p53 Abs could increase the positive rates of detection in the relatively early phases of cancers. Perioperative monitoring of the titers in patients showed an association with the risk of recurrence; however, a precise evaluation should be carried out in a large series of patients in multi‐institutional studies in the future. Interestingly, some of the colorectal cancer patients with very high titers—over 200 IU/mL—showed relatively better survival compared to other patients (Figure 6).27 Such paradoxical phenomena possibly reflect the balance between immune suppression and anti‐tumoral immune reaction. Further analysis on such patients with very high titers should be carried out to evaluate the anti‐tumoral effects of the presence of s‐p53 Abs.
Figure 5.
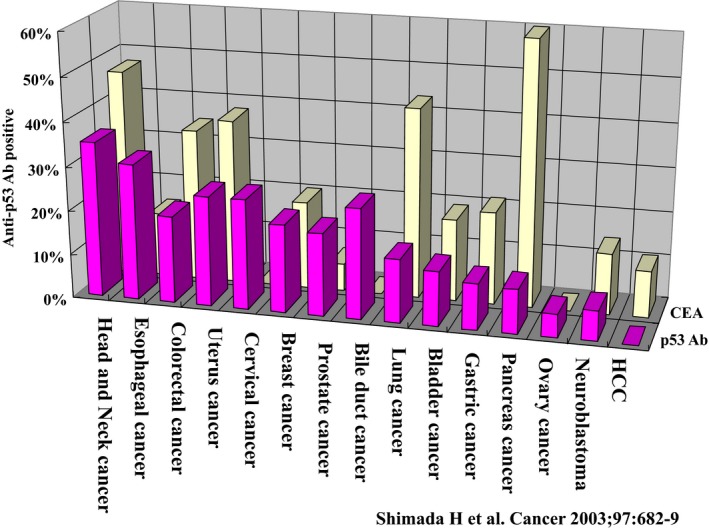
Comparisons of positive rates of s‐p53 Abs and carcinoembryonic antigen (CEA) in various cancers13
Figure 6.
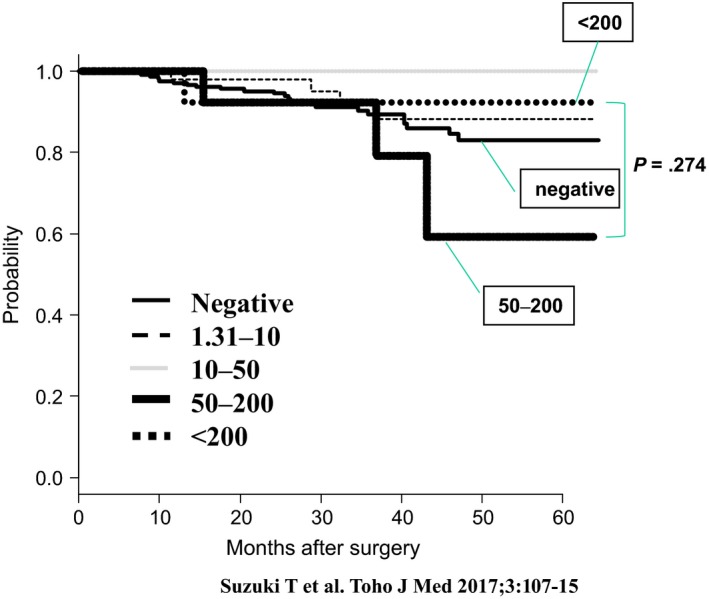
Survival curves of stage II and stage III patients with colorectal cancer according to various ranges of s‐p53 Abs titers27
6. PHASE I/II STUDY OF P53 GENE THERAPY FOR ESOPHAGEAL SQUAMOUS CELL CARCINOMA
Based on a preclinical study using p53 recombinant adenovirus (Ad5CMV‐p53) on human esophageal SCC cell lines (Figure 7),29 we conducted a phase I/II study of p53 gene therapy for treatment‐resistant esophageal SCC.30 The primary objective was to determine feasibility and safety of this adenoviral‐mediated p53 gene therapy. The secondary objective was to observe biological responses and anti‐tumor effects. Individual doses were 10 × 1011 to 25 × 1011 viral particles, depending on tumor size. Ten patients were enrolled in this trial.28 Before gene therapy, five patients had tumor invasion into adjacent organs (patients 3, 4, 7, 9, and 10) and four had high‐risk factors for surgery (patients 1, 2, 5, and 8). The remaining patient had a T3 tumor with multiple lymph node metastases (patient 6). Eight patients had tumors with p53 mutations among exons 5 to 8. Nine of the 10 patients had completed at least two cycles of treatment or four injections, according to the protocol. Treatment cycles ranged from one cycle to five cycles, with two cycles as the median number of cycles. Patient 6 did not undergo a second cycle of treatment because, after the first cycle, distant metastases rapidly progressed. Injected lesions were large and ranged from 25 mm to 100 mm at their longest axes (median = 43 mm). Adverse events attributed to treatment with Ad.5CMV‐p53 were mild to moderate. The most common adverse events were slight fever and local pain. Fever was observed in all patients, and pain in 30%. Overall, the drug administration was feasible and well tolerated.
Figure 7.
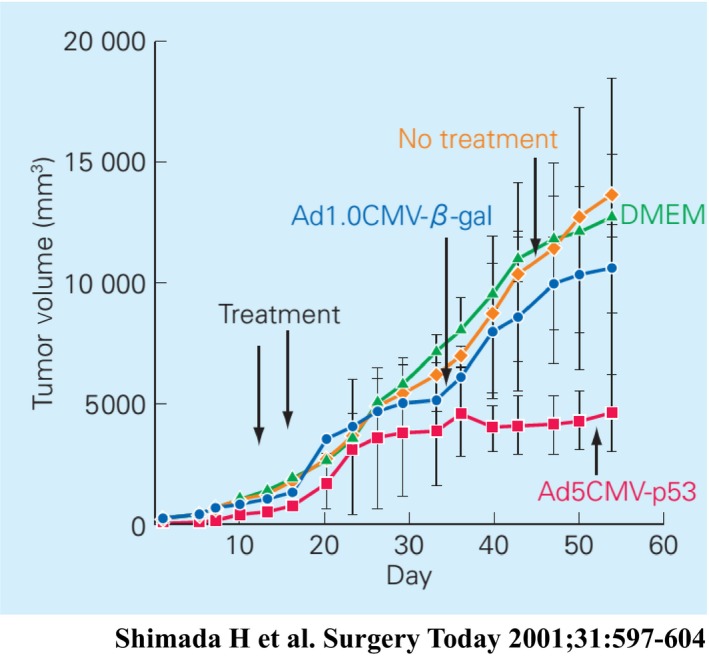
Effect of Ad5CMV‐p53 infection on in vivo tumor growth of human esophageal squamous cell carcinoma cell line (T.Tn).29 Established tumors were treated after reaching a size of 200 mm3 with 2 injections delivered on days 14 and 16 of DMEM/F‐12 medium or the virus (total dose 2× 1010 pfu). Mean tumor volumes per 5 mice following injection are plotted against the number of days since s.c. transplantation. Bars, SD; ●, Ad1.0CMV‐β‐gal; ■, Ad5CMV‐p53; ♦, no treatment;▲, DMEM
Nine of the 10 eligible patients have died of their disease. During the initial 56 days, one patient had local tumor progression and four had systemic progression. Six of the 10 patients survived for over 1 year (Table 2). In three patients, there was no evidence of tumor on multiple biopsies after treatment (patients 2, 3, and 5). Patient 2 had a tumor with a deep ulceration before treatment. After three or four cycles, the ulcer became shallower, and no viable cancer cells were observed in the biopsy specimens. We found a stable local tumor and no residual SCC on esophageal biopsies during the five cycles of treatment. Patient 3 had a complete obstruction just before gene therapy (Figure 8A). Analysis of the biopsies after the first cycles of treatment showed mild dysplasia and necrotic tissue without any sign of cancer cells. He was able to swallow liquid and solid foods after two injections (Figure 8B). Although we could not pass the endoscope through the stenotic portion of the lesion before treatment (Figure 8C), we succeeded in doing so after treatment (Figure 8D). Although the local tumor had been controlled during treatment, distant metastases occurred and the patient died 3 months after the start of gene therapy. Patient 5 showed no tumor progression over the 24 months after the start of gene therapy. He remains progression‐free and alive 63 months after the completion of treatment.
Table 2.
Characteristics of patients with esophageal squamous cell carcinoma treated with Ad.CMV‐p5330
| Patient no. | Age & gender | Time to tumor progressiona | Site of p53 mutation | Viral particles | Treatment cycles | Tumor size (mm)c | Local & overall responsed | Prognosis after gene therapye |
|---|---|---|---|---|---|---|---|---|
| 1 | 64M | 7M | Exon 5 | 15 × 1011 | 2 | 43 | SD & SD | 19M dead |
| 2 | 71M | 7M | Exon 7 | 10 × 1011 | 5 | 25 | SD & SD | 15M dead |
| 3 | 62M | 5M | Negativeb | 15 × 1011 | 3 | 50 | SD & PD | 3M dead |
| 4 | 78M | 2M | Exon 8 | 10 × 1011 | 2 | 30 | PD & PD | 6M dead |
| 5 | 66M | 2M | Exon 7 | 10 × 1011 | 3 | 40 | SD & SD | 63M alive |
| 6 | 60M | 1M | Exon 7 | 20 × 1011 | 1 | 70 | SD & PD | 2M dead |
| 7 | 67F | 4F | Exon 5 | 10 × 1011 | 2 | 38 | SD & SD | 13M dead |
| 8 | 58M | 8M | Exon 6 | 10 × 1011 | 2 | 40 | SD & SD | 15M dead |
| 9 | 48M | 2M | Exon 7 | 25 × 1011 | 4 | 100 | SD & SD | 12M dead |
| 10 | 77M | 12M | Negativeb | 20 × 1011 | 2 | 68 | SD & PD | 2M dead |
Time to tumor progression after completion of chemoradiation therapy (M, months).
No mutation among exons 5, 6, 7 and 8.
Tumor size is sum of esophageal tumor and lymph node.
Treatment response was determined 4 wks after completion of therapy by external review board.
Survival months after first injection of Ad.CMV‐p53 (M, months).
Shimada et al.30
PD, progressive disease; SD, stable disease.
Figure 8.
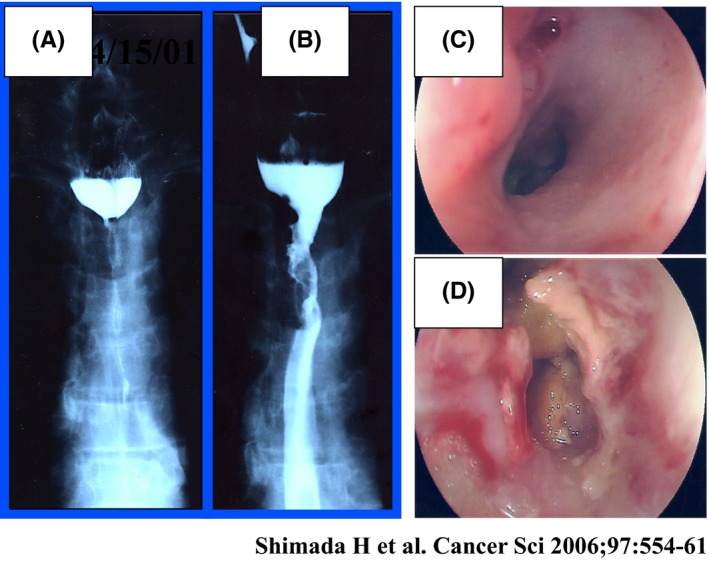
Upper thoracic esophageal cancer inoperable because of tracheal invasion. A and C, Complete obstruction is seen before p53 gene transfer. B and D, Esophageal obstruction was reduced after treatment30
Overall survival rate with an intent‐to‐treat analysis was 60% at 1 year (Figure 9). Four patients developed clinically diagnosed distant metastases (two bone metastases, one liver and lymph node metastasis, and one lung metastasis). Three of the four patients with development of distant metastases died of their disease within 3 months of the initial viral treatment. Median time to both local and distant progression was 6 months.
Figure 9.
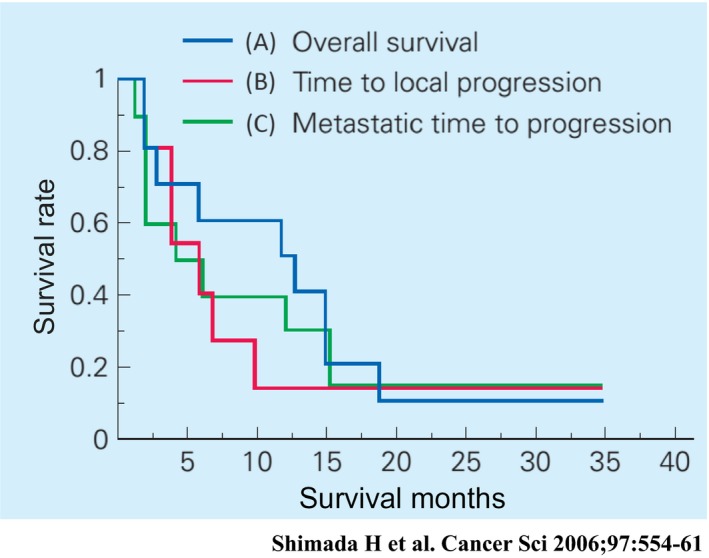
A, Overall survival; B, time to local progression; and C, time to metastatic progression of all patients30
7. VIRAL SHEDDING AFTER P53 ADENOVIRAL TRANSFER
We analyzed the presence of adenoviral DNA fragments by DNA‐PCR after Ad5CMV‐p53 injection. We assessed a total of 220 samples consisting of feces, gargling saliva, urine, and blood plasma. A total of 29.7% of feces samples and 13.2% of gargling saliva samples were positive for adenoviral DNA fragments. After follow up, 89.7% of the positive feces samples and 100% of the positive gargling saliva samples tested negative on day 12 after tumor injection (Figure 10).31 Although adenoviral DNA fragments may be pathogen‐free for 12 days after injection, patients’ feces and gargling saliva still contain adenoviral DNA fragments. Patients treated with adenoviral gene therapy need not be separated because of concerns relating to viral shedding, but it should be considered that viral DNA fragments are contained in patients’ excreta for up to 12 days after injection.
Figure 10.
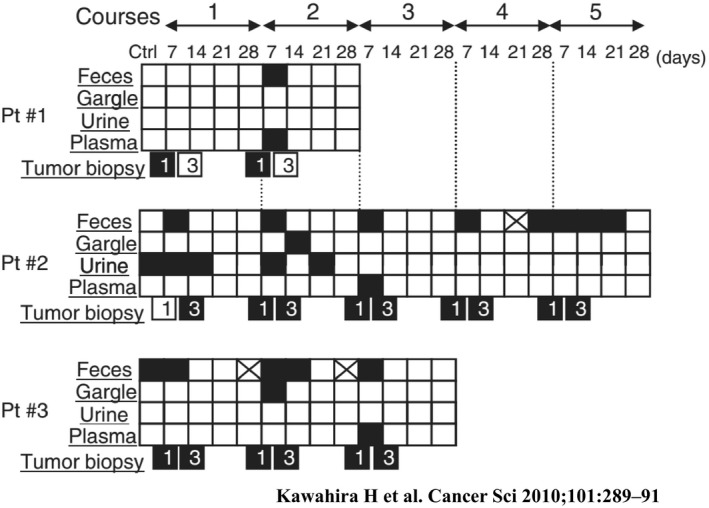
Summary of Ad‐CMV vector biodistribution in three patients with esophageal cancer after Ad5CMV‐p53 tumoral injection.31 Black box, Ad5CMV‐p53 positive; crossed box, samples not available; white box, Ad5CMV‐p53 negative. Pt, patient
8. NEXT CLINICAL TRIAL OF P53 GENE THERAPY COMBINED WITH CHEMORADIOTHERAPY
According to recent reports on p53 gene therapy for cancer, Ad5CMV‐p53 is frequently used, together with chemotherapy32, 33 or ionizing radiation.34, 35 We have also found that Ad5CMV‐p53 treatment combined with CDDP or radiation was efficacious for human esophageal SCC, both in vitro and in vivo.36 Further experiments are required to develop new combination therapy with low‐dose CDDP and/or 5‐FU and Ad5CMV‐p53 at a low multiplicity of infection and a new combination therapy with concurrent chemoradiotherapy. Because long‐term survival is strongly associated with treatment response, combination treatment with chemoradiation and Ad5CMV‐p53 may be an attractive modality in the forthcoming clinical trial.
In China, Gendicine (recombinant human p53 adenovirus) developed by Shenzhen SiBiono GeneTech Co. Ltd (Shenzen, China), was approved in 2003 by the China Food and Drug Administration (CFDA) and entered the commercial market. Based on 12 years of commercial use in >30 000 patients and >30 published clinical studies, Gendicine seems to be safe and effective. When combined with chemotherapy and radiotherapy, Gendicine showed significantly higher response rates than standard therapies alone.37
So far, four types of p53 transfer system have been explored as follows: (i) cationic liposome‐DNA plasmid complexes; (ii) a replication‐deficient adenovirus vector; (iii) a replication‐competent adenovirus vector; and (iv) a protein transduction system. Moreover, technical advancements were observed in therapeutic methods for enhancing tumor cell death and induction of bystander effects within tumor tissues in p53 replacement therapy.38
Here we reviewed the findings from clinical studies of molecular diagnosis and treatment with p53 for esophageal SCC. Further multi‐institutional studies are required to evaluate clinical significance of p53 targeting therapies.
DISCLOSURE
Hideaki Shimada received research grants and technical lecture fees from the Medical & Biological Laboratories Co., Ltd, Nagoya, Japan.
ACKNOWLEDGEMENT
This work was supported, in part, by the 21st Century COE (Center of Excellence) Programs Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The majority of this review was presented at the award ceremony of 2016 JSGS Science of the Year.
Shimada H. p53 molecular approach to diagnosis and treatment of esophageal squamous cell carcinoma. Ann Gastroenterol Surg. 2018;2:266–273. https://doi.org/10.1002/ags3.12179
REFERENCES
- 1. Koyanagi K, Ozawa S, Tachimori Y. Minimally invasive esophagectomy in the prone position improves postoperative outcomes: role of C‐reactive protein as an indicator of surgical invasiveness. Esophagus. 2018;15:95–102. [DOI] [PubMed] [Google Scholar]
- 2. Shimada H, Okazumi S, Matsubara H, et al. Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three‐field lymph node dissection. World J Surg. 2006;30:1441–9. [DOI] [PubMed] [Google Scholar]
- 3. Watanabe M, Mine S, Nishida K, et al. Improvement in short‐term outcomes after esophagectomy with a multidisciplinary perioperative care team. Esophagus. 2016;13:337–42. [Google Scholar]
- 4. Shimada H, Fukagawa T, Haga Y, Oba K. Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann Gastroenterol. 2017;1:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsukada Y, Higashi T, Shimada H, Kikuchi Y, Terahara A. The use of neoadjuvant therapy for resectable locally advanced thoracic esophageal squamous cell carcinoma in an analysis of 5016 patients from 305 designated cancer care hospitals in Japan. Int J Clin Oncol. 2018;23:81–91. [DOI] [PubMed] [Google Scholar]
- 6. Hamamoto Y, Sakakibara N, Nagashima F, Kitagawa Y, Higashi T. Treatment selection for esophageal cancer: evaluation from a nationwide database. Esophagus. 2018;15:109–14. [DOI] [PubMed] [Google Scholar]
- 7. Shimada H, Ochiai T. Molecular diagnosis and treatment for esophageal carcinoma with p53. Esophagus. 2007;4:165–8. [Google Scholar]
- 8. Bennett WP, Hollstein MC, Metcalf RA, et al. p53 mutation and protein accumulation during multistage human esophageal carcinogenesis. Cancer Res. 1992;52:6092–7. [PubMed] [Google Scholar]
- 9. Liu X, Zhang M, Ying S, et al. Genetic alterations in esophageal tissues from squamous dysplasia to carcinoma. Gastroenterology. 2017;153:166–77. [DOI] [PubMed] [Google Scholar]
- 10. Salnikova LE, Kolobkov DS. Germline and somatic genetic predictors of pathological response in neoadjuvant settings of rectal and esophageal cancers: systematic review and meta‐analysis. Pharmacogenomics J. 2016;16:249–65. [DOI] [PubMed] [Google Scholar]
- 11. Kandioler D, Schoppmann SF, Zwrtek R, et al. The biomarker TP53 divides patients with neoadjuvantly treated esophageal cancer into 2 subgroups with markedly different outcomes. A p53 Research Group study. J Thorac Cardiovasc Surg. 2014;148:2280–6. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Z, Wang P, Gao Y, He J. The high expression instead of mutation of p53 is predictive of overall survival in patients with esophageal squamous‐cell carcinoma: a meta‐analysis. Cancer Med. 2017;6:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimada H, Ochiai T, Nomura F. Japan p53 Antibody Research Group. Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: a multi‐institutional analysis by the Japan p53 Antibody Research Group. Cancer. 2003;97:682–9. [DOI] [PubMed] [Google Scholar]
- 14. Shimada H, Nabeya Y, Okazumi S, et al. Prognostic significance of serum p53 antibody in patients with esophageal squamous cell carcinoma. Surgery. 2002;132:41–7. [DOI] [PubMed] [Google Scholar]
- 15. Shimada H, Takeda A, Arima M, et al. Serum p53 antibody is a useful tumor marker in superficial esophageal squamous cell carcinoma. Cancer. 2000;89:1677–83. [PubMed] [Google Scholar]
- 16. Shimada H, Arima M, Nakajima K, et al. Detection of serum p53 antibodies in mucosal esophageal cancer and negative conversion after treatment. Am J Gastroenterol. 1998;93:1388–9. [DOI] [PubMed] [Google Scholar]
- 17. Saito F, Shimada H, Ogata H, et al. Detection of the early phase of esophageal cancer progression into lamina propria mucosae by the serum p53 antibody. Esophagus. 2017;14:366–9. [Google Scholar]
- 18. Cawley HM, Meltzer SJ, De Benedetti VM, et al. Anti‐p53 antibodies in patients with Barrett's esophagus or esophageal carcinoma can predate cancer diagnosis. Gastroenterology. 1998;115:19–27. [DOI] [PubMed] [Google Scholar]
- 19. Shimada H, Nagata M, Cho A, et al. Long‐term monitoring of serum p53 antibody after neoadjuvant chemotherapy and surgery for esophageal adenocarcinoma: report of a case. Surg Today. 2014;44:1957–61. [DOI] [PubMed] [Google Scholar]
- 20. Blanchard P, Quero L, Pacault V, et al. Prognostic significance of anti‐p53 and anti‐KRas circulating antibodies in esophageal cancer patients treated with chemoradiotherapy. BMC Cancer. 2012;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimada H, Shiratori T, Takeda A, et al. Perioperative changes of serum p53 antibody titer is a predictor for survival in patients with esophageal squamous cell carcinoma. World J Surg. 2009;33:272–7. [DOI] [PubMed] [Google Scholar]
- 22. Shimada H, Kitabayashi H, Nabeya Y, et al. Treatment response and prognosis of patients after recurrence of esophageal cancer. Surgery. 2003;133:24–31. [DOI] [PubMed] [Google Scholar]
- 23. Shimada H, Okazumi S, Takeda A, et al. Presence of serum p53 antibodies is associated with decreased in vitro chemo‐sensitivity in patients with esophageal cancer. Surg Today. 2001;31:591–6. [DOI] [PubMed] [Google Scholar]
- 24. Yamashita K, Makino T, Tanaka K, et al. Peritherapeutic serum p53 antibody titers are predictors of survival in patients with esophageal squamous cell carcinoma undergoing neoadjuvant chemotherapy and surgery. World J Surg. 2017;41:1566–74. [DOI] [PubMed] [Google Scholar]
- 25. Hiyoshi Y, Yoshida N, Watanabe M, et al. The Presence of serum p53 antibody predicts the pathological tumor response to neoadjuvant chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) in esophageal squamous cell carcinoma. World J Surg. 2017;41:480–6. [DOI] [PubMed] [Google Scholar]
- 26. Nakajima K, Suzuki T, Shimada H, Hayashi H, Takeda A, Ochiai T. Detection of preoperative serum anti‐p53 antibodies in gastric cancer. Tumour Biol. 1999;20:147–52. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki T, Funahashi K, Shimada H, et al. Diagnostic and prognostic impact of serum p53 antibody titration in colorectal cancer. Toho J Med. 2017;3:107–15. [Google Scholar]
- 28. Okada R, Shimada H, Otsuka Y, et al. Serum p53 antibody as a potential tumor marker in extrahepatic cholangiocarcinoma. Surg Today. 2017;47:1492–9. [DOI] [PubMed] [Google Scholar]
- 29. Shimada H, Shimizu T, Ochiai T, et al. Preclinical study of adenoviral p53 gene therapy for esophageal cancer. Surg Today. 2001;31:597–604. [DOI] [PubMed] [Google Scholar]
- 30. Shimada H, Matsubara H, Shiratori T, et al. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci. 2006;97:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawahira H, Matsushita K, Shiratori T, et al. Viral shedding after p53 adenoviral gene therapy in 10 cases of esophageal cancer. Cancer Sci. 2010;101:289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Li LJ, Wang LJ, et al. Selective intra‐arterial infusion of rAd‐p53 with chemotherapy for advanced oral cancer: a randomized clinical trial. BMC Med. 2014;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guan YS, Liu Y, He Q, et al. p53 gene therapy in combination with transcatheter arterial chemoembolization for HCC: one‐year follow‐up. World J Gastroenterol. 2011;17:2143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su X, Chen WJ, Xiao SW, et al. Effect and safety of recombinant adenovirus‐p53 transfer combined with radiotherapy on long‐term survival of locally advanced cervical cancer. Hum Gene Ther. 2016;27:1008–14. [DOI] [PubMed] [Google Scholar]
- 35. Liu S, Chen P, Hu M, et al. Randomized, controlled phase II study of post‐surgery radiotherapy combined with recombinant adenoviral human p53 gene therapy in treatment of oral cancer. Cancer Gene Ther. 2013;20:375–8. [DOI] [PubMed] [Google Scholar]
- 36. Oohira G, Yamada S, Ochiai T, et al. Growth suppression of esophageal squamous cell carcinoma induced by heavy carbon‐ion beams combined with p53 gene transfer. Int J Oncol. 2004;25:563–9. [PubMed] [Google Scholar]
- 37. Zhang WW, Li L, Li D, et al. The first approved gene therapy product for cancer Ad‐p53 (Gendicine): 12 Years in the Clinic. Hum Gene Ther. 2018;29:160–79. [DOI] [PubMed] [Google Scholar]
- 38. Tazawa H, Kagawa S, Fujiwara T. p53 replacement therapy for cancer. Recent Results Cancer Res. 2016;209:1–15. [DOI] [PubMed] [Google Scholar]


