Abstract
Background and Aim
Although the clinicopathological significance of the expression of programmed death ligand 1(PD‐L1) in various cancer tissues has been reported, serum PD‐L1 level has not been evaluated in patients with surgically treated gastric cancer. Therefore, we evaluated the clinicopathological characteristics and prognostic significance of preoperative serum PD‐L1 levels in patients with gastric cancer.
Patients and Methods
Serum samples were obtained before surgery from 152 patients with gastric cancer, including 75 patients with stage I, 31 with stage II, 23 with stage III, and 23 with stage IV gastric cancer. The samples were analyzed using enzyme‐linked immunosorbent assay to detect soluble PD‐L1. Using the median serum PD‐L1 level of 50 pg/mL, patients were divided into two groups, namely high serum and low serum PD‐L1 level groups. Clinicopathological characteristics and prognosis were compared between these two groups using univariate and multivariate analysis.
Results
Serum PD‐L1 level was significantly associated with older age, positive cancer antigen 19‐9 (CA19‐9), C‐reactive protein levels, and albumin levels but not with tumor stage. Patients in the high serum PD‐L1 group showed significantly worse overall survival and recurrence‐free survival than those in the low serum PD‐L1 group (P < .05). Multivariate analysis showed that high serum PD‐L1 level was an independent risk factor for poor overall survival (P = .02).
Conclusion
High serum PD‐L1 level was a prognostic factor for reduced overall survival in patients with surgically treated gastric cancer.
Keywords: C‐reactive protein, enzyme‐linked immunosorbent assay, gastric cancer, metastasis, prognosis
1. INTRODUCTION
Binding of programmed death‐1 (PD‐1) and its ligands—programmed death ligand 1 (PD‐L1) and programmed death ligand 2 (PD‐L2)—on tumor or immune cells can inhibit cytotoxic T‐cell responses, allowing tumor cells to evade immune detection.1 Clinical trials of anti‐PD‐1/PD‐L1 antibodies have reported a high response rate and significantly improved overall survival in several malignancies.2, 3, 4
PD‐L1 is constitutively expressed in several tumor tissues, including gastric cancer.5, 6, 7 Overexpression of PD‐L1 in tumor tissue has been reported to be associated with poor overall survival and good response to anti‐PD‐L1 antibody treatment.8, 9 Serum PD‐L1 level was significantly higher in patients with malignancies than in healthy individuals.10, 11, 12 Zheng et al10 reported the association of serum PD‐L1 level with prognosis in patients with advanced gastric cancer. However, their series included a small number of patients, and they did not carry out multivariate analysis to evaluate the prognostic impact of serum PD‐L1 level. Takahashi et al12 also reported the correlation of high serum PD‐L1 with shorter overall survival in patients with unresectable or recurrent advanced gastric cancer. However, they did not analyze patients with surgically treated gastric cancer.
Therefore, the present study evaluated the clinicopathological and prognostic significance of serum PD‐L1 level in patients with surgically treated gastric cancer. The main focus was to evaluate the prognostic significance of preoperative serum PD‐L1 level.
2. MATERIALS AND METHODS
2.1. Patients
A total of 152 patients (103 males and 49 females; mean age, 69.9 [range, 35‐93] years) with gastric cancer who underwent surgical resection at the Toho University Omori Hospital (Tokyo, Japan) from 2010 to 2014 without neoadjuvant chemotherapy were included. Seventy‐five patients had stage I gastric cancer, 31 had stage II, 23 had stage III, and 23 had stage IV. All patients underwent either total or subtotal gastrectomy with standard lymphadenectomy and had histologically confirmed adenocarcinoma of the stomach. Patients with stage IV disease included distant lymph node metastasis (n = 2), organ metastasis (n = 5), cancer cells on peritoneal cytology (n = 7), and peritoneal metastasis (n = 9). Three cases with liver metastases and one case with lung metastasis were treated with gastrectomy because of passage disturbance. The other 19 cases were diagnosed as stage IV at the time of laparotomy. Staging was based on the guidelines of the 14th edition (3rd English edition) of the Japanese classification of gastric carcinoma by the Japanese Gastric Cancer Association.13 All patients were regularly followed up until September 2017 or death. Thus far, none of the patients have received anti‐PD‐L1 antibody treatment. Informed consent was obtained from all patients and the study was approved by the Ethics Committee of Toho University School of Medicine (nos. 22‐112 and 22‐047).
2.2. Sample collection and enzyme‐linked immunosorbent assay
Blood samples were collected before surgery. Each sample was centrifuged at 3000 g for 5 minutes, aliquoted, and frozen at −80°C until analysis. Repeated freezing and thawing of samples was avoided. Serum PD‐L1 levels were measured using a commercially available enzyme‐linked immunosorbent assay (ELISA) for PD‐L1 (R&D Systems, Inc., Minneapolis, MN, USA). This assay uses the quantitative sandwich enzyme immunoassay technique using a monoclonal antibody specific for human B7‐H1 precoated onto a microplate. The assay procedure was as follows: (i) A 96‐well plate, all reagents, working standards, and samples were prepared according to the manufacturer's instructions. (ii) 100 μL of the standard and samples were added to each well and incubated for 2 hours at room temperature on a horizontal orbital microplate shaker. (iii) After aspirating and washing four times, 100 μL of the horseradish peroxidase conjugate was added and incubated for 2 hours at room temperature on the shaker. (iv) The aspiration and wash were repeated 4 times. (v) The substrate solution was added to each well and incubated for 30 minutes at room temperature on the benchtop and protected from light. (vi) Stop solution was added to each well, which changed the colors in the wells from blue to yellow. (vii) Using a microplate reader set to 450 nm, optical density of each well was determined, and wavelength correction was set to 540 nm.
2.3. Serum biomarker analysis
The inflammatory marker, C‐reactive protein (CRP), albumin, and hemoglobin were also analyzed before surgery to evaluate their association with serum PD‐L1 level. White blood cell, neutrophil, lymphocyte, and eosinophil counts were also examined. Cutoff values were determined in accordance with the institutional standards for white blood cell count (9000 cells/mm3) and CRP levels (0.3 mg/mL).
2.4. Statistical analysis
Continuous data from patients were expressed as mean ± SD. Comparisons between unpaired groups for these variables were conducted using the Mann‐Whitney U test. Differences in the distribution of two variables were evaluated using Fisher's exact test or χ2 test. Clinicopathological data were analyzed using logistic regression analysis to evaluate the association with serum PD‐L1 level. Survival curves were calculated using the Kaplan‐Meier method and compared using log‐rank test. Significant predictors identified using univariate analysis were assessed using multivariate analysis with the Cox proportional hazards model. All statistical analyses were carried out using EZR statistical software.14 Statistical significance was defined as P < .05.
3. RESULTS
3.1. Correlation of clinical characteristics, biomarkers, TNM stage, and pathological characteristics with serum PD‐L1 level
In terms of clinical variables, serum PD‐L1 level was positively correlated with age (P = .01) and lymph node metastases (P = .04) but was not associated with depth of tumor, tumor size, and histological differentiation (Table 1). Such a tendency was very similar among stage I/II/III patients (Table S1). There were no significant differences between serum PD‐L1 levels in relation to tumor stage (Figure 1).
Table 1.
Comparison of serum programmed death ligand 1 (PD‐L1) levels according to clinicopathological characteristics of patients
| Variable | No. patients | Serum PD‐L1 (pg/mL), median (min‐max) | P‐valuea |
|---|---|---|---|
| Gender | |||
| Male | 103 | 50.3 (24.4‐163.6) | .73 |
| Female | 49 | 50.4 (28.3‐109.2) | |
| Age (y) | |||
| <65 | 48 | 47.3 (28.3‐107.0) | .01 |
| ≥65 | 104 | 54.0 (24.4‐163.6) | |
| Macroscopic type | |||
| 0, I, II | 100 | 50.7 (28.3‐151.0) | .31 |
| III, IV | 52 | 47.8 (24.4‐163.6) | |
| Tumor depth | |||
| T1 | 61 | 48.8 (28.3‐151.0) | .50 |
| T2‐T4 | 91 | 51.6 (24.4‐163.6) | |
| Lymph node metastasis | |||
| N0 | 82 | 50.3 (28.3‐163.6) | .94 |
| N1 | 70 | 50.9 (24.4‐121.6) | |
| Distant metastasis | |||
| M0 | 130 | 50.4 (24.4‐163.6) | .53 |
| M1 | 22 | 49.2 (30.4‐118.0) | |
| Size (mm)b | |||
| <50 | 93 | 50.3 (24.4‐151.0) | .89 |
| ≥50 | 58 | 52.3 (28.3‐163.6) | |
| Differentiation | |||
| Differentiated | 79 | 51.6 (28.3‐163.6) | .33a |
| Poorly differentiated | 73 | 48.4 (24.4‐121.6) | |
Mann‐Whitney U test.
Loss value.
Figure 1.
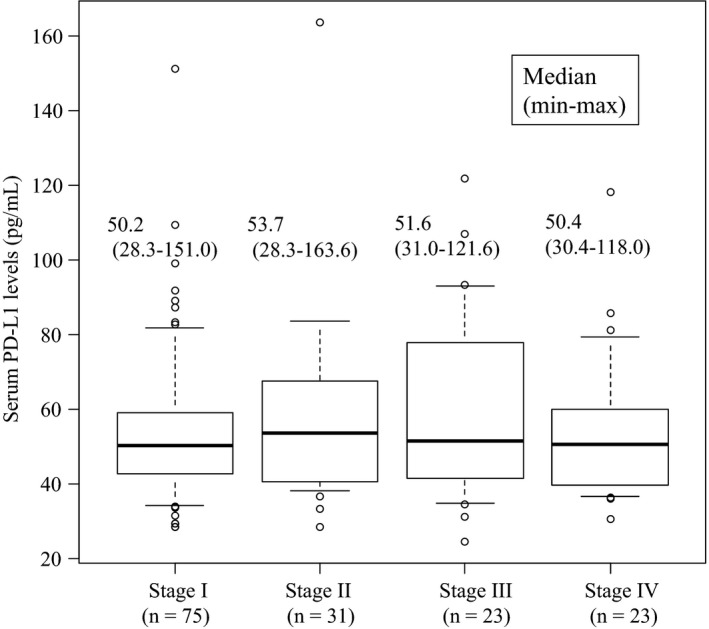
Comparison of serum programmed death ligand 1 (PD‐L1) level between each TNM stage. TNM, tumor, node, and metastases
3.2. Association of serum PD‐L1 level with other serum biomarkers
Serum PD‐L1 level positively correlated with high CRP (P < .01), low albumin (P < .01) levels and CA19‐9 levels (P < .01; Table 2). Such a tendency was very similar among stage I/II/III patients (Table S2). Median value of serum PD‐L1 level in patients with gastric cancer was 50.4 ± 22.5 pg/mL; therefore, we fixed the cutoff value for serum PD‐L1 level at 50 pg/mL for further analyses.
Table 2.
Comparison of serum programmed death ligand 1 (PD‐L1) levels according to various laboratory data
| Variable | No. patients | Median (min‐max) | P‐valuea |
|---|---|---|---|
| WBC (/μL) | |||
| <8000 | 135 | 50.2 (24.4‐163.6) | .79 |
| ≥8000 | 17 | 54.1 (33.9‐98.9) | |
| Neutrophils (%) | |||
| <70 | 113 | 50.2 (24.4‐163.6) | .64 |
| ≥70 | 39 | 52.3 (33.5‐151.0) | |
| Lymphocytes (%) | |||
| 35 | 123 | 50.6 (28.3‐163.6) | .30 |
| ≥35 | 29 | 48.3 (24.4‐107.0) | |
| Eosinophils (%) | |||
| <5 | 136 | 49.5 (24.4‐163.6) | .09 |
| ≥5 | 16 | 59.2 (33.2‐118.0) | |
| CRP (mg/dL) | |||
| <0.3 | 109 | 48.2 (24.4‐121.6) | <.01 |
| ≥0.3 | 43 | 59.6 (31.5‐163.6) | |
| Albumin (g/dL) | |||
| <3.5 | 20 | 63.7 (38.4‐151.0) | <.01 |
| ≥3.5 | 132 | 48.6 (24.4‐163.6) | |
| CEAb | |||
| Negative | 126 | 49.5 (24.4‐151.0) | .13 |
| Positive | 25 | 56.5 (30.4‐163.6) | |
| CA19‐9b | |||
| Negative | 133 | 48.4 (24.4‐163.6) | .01 |
| Positive | 16 | 57.2 (38.4‐107.0) | |
| s‐p53‐Absb | |||
| Negative | 84 | 47.5 (28.3‐163.6) | .18 |
| Positive | 25 | 51.8 (28.3‐121.6) | |
Mann‐Whitney U test.
Loss value.
CEA, carcinoembryonic protein; CRP, C‐reactive protein; WBC, white blood cell count.
3.3. Multivariate analysis of the association of high serum PD‐L1 level with age and CA19‐9, CRP, and albumin levels
Based on the univariate analyses shown in Tables 1 and 2, contribution of the three serum biomarkers (CRP, CA19‐9, and albumin) to high serum PD‐L1 level (>50 pg/mL) was evaluated. Multivariate analysis showed that CA19‐9 and CRP, or CA19‐9 and albumin, were associated with high serum PD‐L1 level (P < .05, P = .05; Table 3). Such a tendency was very similar among stage I/II/III patients (Table S3).
Table 3.
Multivariate analysis of various parameters to increase serum programmed death ligand 1 levels
| Model A | Model B | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐valuea | Odds ratio | 95% CI | P‐valuea | |
| Age (y) | ||||||
| ≥65/<65 | 2.15 | 1.03‐4.51 | <.05 | 1.83 | 0.87‐3.85 | <.05 |
| CA19‐9 | ||||||
| Positive/Negative | 6.45 | 1.36‐30.5 | <.05 | 6.15 | 1.31‐28.8 | <.05 |
| CRP (mg/dL) | ||||||
| ≥0.3/<0.3 | 2.66 | 1.21‐5.85 | <.05 | |||
| Albumin (g/dL) | ||||||
| <3.5/≥3.5 | 3.24 | 0.98‐10.7 | .05 | |||
Logistic regression analysis.
CRP, C‐reactive protein.
3.4. Overall survival and progression‐free survival curves according to serum PD‐L1 level
Patients in the high serum PD‐L1 group showed significantly poorer survival than those in the low serum PD‐L1 group (P < .05, Figure 2). Although this tendency was not confirmed in patients with stage I or II gastric cancer, a similar tendency was confirmed in patients with stage III or IV gastric cancer (Figure 3). In the multivariate analysis, gender, serum PD‐L1 level, and tumor stage were independent risk factors for poor overall survival (Table 4). In the multivariate analysis, such a tendency was very similar focusing on stage I/II/III patients only (Table S4).
Figure 2.
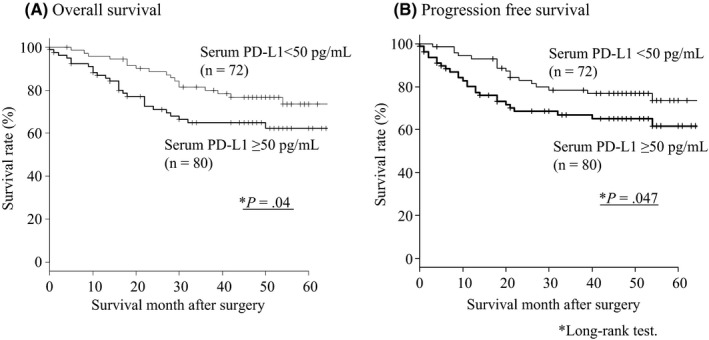
(A) Overall survival and (B) disease‐free survival curves according to the status of serum programmed death ligand 1 (PDL1) level
Figure 3.
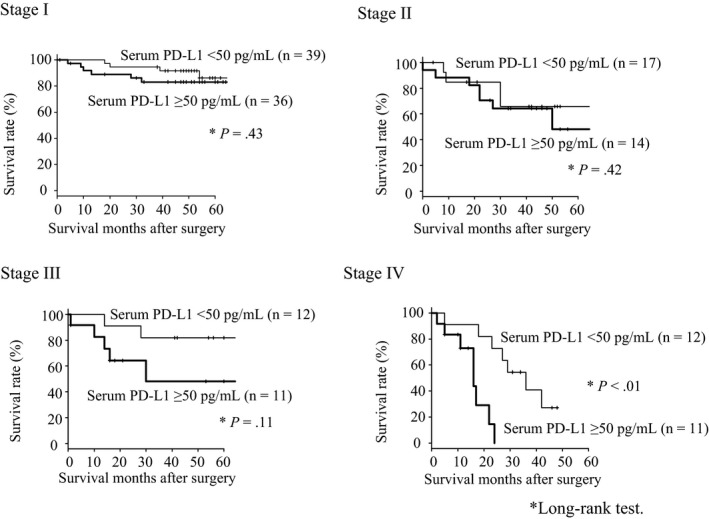
Comparison of overall survival between patients in the high serum programmed death ligand 1 (PD‐L1) group and those in the low PD‐L1 group at each TNM stage. TNM, tumor, node, and metastases
Table 4.
Univariate and multivariate analysis of risk factor of survival
| Univariate | Multivariate P‐valuec | |||
|---|---|---|---|---|
| P‐valuea | Hazard ratio 95% CIb | P‐value | ||
| Gender | ||||
| Male/Female | .04 | 2.63 | 1.20‐5.88 | .02 |
| Age (y) | ||||
| ≥65/<65 | .28 | |||
| PD‐L1 (pg/mL) | ||||
| ≥50/<50 | .04 | 2.12 | 1.12‐3.99 | .02 |
| CEA | ||||
| Positive/Negative | .56 | |||
| CA19‐9 | ||||
| Positive/Negative | .25 | |||
| s‐p53‐Abs | ||||
| Positive/Negative | .94 | |||
| CRP (mg/dL) | ||||
| ≥0.3/<0.3 | .31 | |||
| Albumin (g/dL) | ||||
| <3.5/≥3.5 | .02 | 1.28 | 0.63‐2.63 | .50 |
| Stage | ||||
| II III IV/I | <.01 | 4.05 | 1.89‐8.71 | <.01 |
| Size (mm) | ||||
| ≥50/<50 | <.01 | 1.58 | 0.83‐3.01 | .16 |
| Differentiation | ||||
| poorly diff./diff. | .94 | |||
Log‐rank test.
Adjusted 95% confidence interval.
Cox proportional hazard model.
CEA, carcinoembryonic antigen; CRP, C‐reactive protein; diff., differentiated; PD‐L1, programmed death ligand 1.
A variety of recurrent tumors was observed among 129 patients with stage I/II/III cancer. The recurrent patterns included seven for liver metastases, three for lung metastases, six for peritoneal metastases, three for lymph node metastases, and one for local recurrence. Although serum PD‐L1 levels of the patients were higher in the recurrent group than in the non‐recurrent group, the difference was not statistically significant (P = .09, Figure S1). Moreover, no significant differences were observed among recurrent patterns.
4. DISCUSSION
In the present study, preoperative serum PD‐L1 level was significantly associated with age and CA19‐9, CRP, and albumin levels but not with tumor stage. With a cutoff value of 50 pg/mL, preoperative high serum PD‐L1 level was an independent risk factor for the poor overall survival of patients with gastric cancer.
Among the serum biomarkers, CRP, albumin, and CA19‐9 were independent risk factors for increased serum PD‐L1 level. Some inflammatory cytokines, such as interleukin‐6, may affect such association of CRP level with serum PD‐L1 level. Although Xu et al have reported that high interleukin‐6 (IL‐6) level in patients with prostate cancer was associated with the expression of PD‐L1 in prostate cancer tissues,15 the mechanism of high serum PD‐L1 levels is unclear.
Average serum PD‐L1 level has been reported to be much higher in healthy volunteers aged >70 years than in those aged <70 years.16 Our present data confirmed this tendency. Because human immunological potency decreases with age,16 serum PD‐L1 level may be negatively correlated with immunological status. Based on these findings, age and IL‐6 and serum PD‐L1 levels may be correlated with the poor overall survival of patients with cancer.
Clinicopathological and prognostic impact of serum PD‐L1 level could be affected by the population of patients with or without stage IV tumors. We first evaluated patients with all stages including stage IV. Then, we re‐evaluated the patients without stage IV tumors (Tables S1‐S4). Interestingly, both clinicopathological and prognostic impacts of serum PD‐L1 level were consistent among the patients with and without stage IV tumors. We speculated that soluble PD‐L1 may inhibit anti‐tumoral immune response among the patients with stage III or IV tumors. Residual cancer volume might also be associated with high serum PD‐L1 level. Therefore, serum PD‐L1 level may be helpful for selecting high‐risk patients among stage III who need adjuvant chemotherapy.
One of the limitations of the present study was that we did not compare the expression of PD‐L1 in cancer tissue and serum PD‐L1 level using immunohistochemical analysis. Yamagiwa et al17 reported the association of serum PD‐L1 level with the expression of PD‐L1, which was induced by inflammatory cytokines including interferon gamma, on hepatocytes. Therefore, serum PD‐L1 level in patients with gastric cancer in the present study may also be associated with the expression of PD‐L1 on the gastric cancer cell surface. The other limitation was that there were no age‐matched healthy controls to compare their serum PD‐L1 level with those of patients with gastric cancer. Using the same ELISA kit as that used in the present study, Chen et al18 reported that the median serum PD‐L1 level in healthy controls was 48.15 pg/mL, which is very similar to the median serum PD‐L1 level reported in our series of patients with gastric cancer. Therefore, we suspect that there is no statistically significant difference in serum PD‐L1 level between healthy controls and patients with gastric cancer. Another limitation was that there were no paired serum samples of these patients after surgery. Therefore, we could not compare serum PD‐L1 level between before and after surgery. Further comparison study may assess the cause of high serum PD‐L1 levels.
Thus far, association of the expression of PD‐L1 in the treatment response of anti‐PD‐L1 antibody in cancer tissues has not been evident in previous clinical trials. One possible explanation for such confusing results is that circulating PD‐L1, rather than tissue PD‐L1 protein, may have some interaction with the anti‐PD‐L1 antibody. Further prospective observation study is required to monitor fluctuations in serum PD‐L1 level during anti‐PD‐L1 antibody treatment, which will further clarify the clinical significance of serum PD‐L1 level in patients with gastric cancer.
In conclusion, high serum PD‐L1 level was found to be an independent prognostic factor in patients with surgically treated gastric cancer. Because serum PD‐L1 may reflect the systemic immune checkpoint pathway status, serum PD‐L1 level seems to be a convenient prognostic serum biomarker.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGEMENTS
This research was supported by the Project for Cancer Research and Therapeutic Evolution (P‐CREATE) from the Japan Agency for Medical Research and Development, AMED.
Ito M, Oshima Y, Yajima S, et al. Is high serum programmed death ligand 1 level a risk factor for poor survival in patients with gastric cancer? Ann Gastroenterol Surg. 2018;2:313–318. https://doi.org/10.1002/ags3.12175
REFERENCES
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gong J, Chehrazi‐Raffle A, Reddi S, Salgia R. Development of PD‐1 and PD‐L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdin SM, Zaher DM, Arafa EA, Omar HA. Tackling cancer resistance by immunotherapy: updated clinical impact and safety of PD‐1/PD‐L1 Inhibitors. Cancers (Basel) 2018;10:pii: E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amatatsu M, Arigami T, Uenosono Y, et al. PD‐L1 is a promising blood marker for predicting tumor progression and prognosis in patients with gastric cancer. Cancer Sci. 2018;109:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xing X, Guo J, Wen X, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2017;7:e1356144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42–52. [DOI] [PubMed] [Google Scholar]
- 7. Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD‐L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandini S, Massi D, Mandalà M. PD‐L1 expression in cancer patients receiving anti PD‐1/PD‐L1 antibodies: a systematic review and meta‐analysis. Crit Rev Oncol Hematol. 2016;100:88–98. [DOI] [PubMed] [Google Scholar]
- 9. Gu L, Chen M, Guo D, et al. PD‐L1 and gastric cancer prognosis: a systematic review and meta‐analysis. PLoS ONE. 2017;12:e0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Z, Bu Z, Liu X, et al. Level of circulating PD‐L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Wang H, Chen H, et al. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget. 2015;6:41228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi N, Iwasa S, Sasaki Y, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first‐line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol. 2016;142:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Japanese Gastric Cancer Association : Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- 14. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu L, Chen X, Shen M, et al. Inhibition of IL‐6‐JAK/Stat3 signaling in castration‐resistant prostate cancer cells enhances the NK cell mediated cytotoxicity via alteration of PD‐L1/NKG2D ligand levels. Mol Oncol. 2018; 12:269–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y, Wang Q, Shi B, et al. Development of a sandwich ELISA for evaluating soluble PD‐L1 (CD274) in human sera of different ages as well as supernatants of PD‐L1 + cell lines. Cytokine. 2011;56:231–8. [DOI] [PubMed] [Google Scholar]
- 17. Yamagiwa S, Ishikawa T, Waguri N, et al. Increase of soluble programmed cell death ligand 1 in patients with chronic hepatitis C. Int J Med Sci. 2017;14:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Li M, Liu J, et al. sPD‐L1 expression is associated with immunosuppression and infectious complications in patients with acute pancreatitis. Scand J Immunol. 2017;86:100–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


