Abstract
Renal cell carcinoma (RCC) is one of the most common types of cancer in adults. Previous studies have reported that the survival rate was significantly lower for renal cancer patients with diabetes than for those without diabetes. Metformin is a well-known anti-diabetic agent used for the treatment of type 2 diabetes mellitus (T2DM). It also inhibits cell proliferation and angiogenesis and is known to possess antitumor effects. However, the molecular mechanism for metformin-induced apoptosis in renal cell carcinoma is not understood. In the present study, treatment with metformin induced apoptosis in A498 cells in a dose-dependent manner. It was revealed that degradation of cellular caspase 8 (FLICE)-like inhibitory protein (c-FLIP) and activation of procaspase-8 were associated with metformin-mediated apoptosis. By contrast, treatment with metformin did not affect the mRNA level of c-FLIPL in A498 cells. Treatment with benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk, a pan-caspase inhibitor) almost completely blocked metformin-induced apoptosis and degradation of c-FLIPL protein. However, N-acetyl-L-cysteine (NAC), a reactive oxygen species (ROS) scavenger, did not inhibit metformin-mediated apoptosis in A498 cells. Taken together, the results of the present study demonstrated that metformin-induced apoptosis involved degradation of the c-FLIPL protein and activation of caspase-8 in human renal cell carcinoma A498 cells and suggested that metformin could be potentially used for the treatment of renal cancer.
Keywords: metformin, A498, apoptosis, caspase, cellular caspase 8 (FLICE)-like inhibitory protein
Introduction
Renal cell carcinoma (RCC), a neoplastic lesion of the kidney in humans, accounts for ~90% of kidney tumors (1). It is difficult to treat with conventional treatments including chemical, hormone and radiation therapy, and cannot be treated without surgery (2,3). A previous report described metformin may improve the incidence of cancer-associated diabetes (4). Thus far, RCC has been treated chemically and immunologically. However, there is an urgent requirement to identify more efficient chemo-preventive agents for treating RCC.
Metformin is the most widely used biguanide drug for treating type 2 diabetes mellitus patients (5). It has been reported that metformin has anti-diabetic and anticancer effects on colorectal and pancreatic cancer cells (6,7). It has also been revealed to exert anti-neoplastic effects in epithelial ovarian cancer (8). Furthermore, metformin has been demonstrated to reduce the risk of cancer prevalence in diabetic patients (9,10). Metformin demonstrated a marked anticancer effect in various cells of different types of human cancer, including breast cancer, renal cancer, glioblastoma, insulinoma and cholangiocarcinoma via cell growth inhibition, cell cycle arrest, apoptosis, adenosine monophosphate-activated protein kinase (AMPK) signaling and tumor growth inhibition (11–15). Although the effect of metformin on A498 cells has been reported (12), the apoptosis-mediated molecular mechanism of action of metformin remains unclear in human renal cell carcinoma A498 cells.
The cellular caspase 8 (FLICE)-like inhibitory protein (c-FLIP) gene makes three isoforms, namely c-FLIPL, c-FLIPS and c-FLIPR, via alternative splicing in humans. These proteins are well known as anti-apoptotic proteins; each exert this effect via different mechanisms (16). In previous reports, c-FLIP was demonstrated to be an independent negative prognostic factor in ovarian, endometrial and colon cancer cells (17–19). c-FLIPL is known to be involved in the inhibition of caspase-8 activation-mediated apoptosis (18,20). The activation of caspase-8 leads to death-inducing signaling complex (DISC) and augmented apoptosis via caspase-3 activation. Previous studies have demonstrated that treatment with metformin suppressed the c-FLIPL protein expression level in human lung adenocarcinoma and bladder cancer (21,22).
In the present study, the mechanism of metformin-mediated apoptosis in human renal cell carcinoma A498 cells was investigated. It was revealed that degradation of c-FLIPL protein and activation of caspase-8 were associated with metformin-induced apoptosis.
Materials and methods
Cell culture
A498 human renal carcinoma cells were procured from the American Type Culture Collection (ATCC; Manassas, VA, USA). Dulbecco's modified Eagle's medium (DMEM; catalog no. LM 001-05; Welgene, Inc., Kyungsan, Korea) containing 10% fetal bovine serum (FBS; catalog no. S001-07; Welgene, Inc.), 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; catalog no. H0887; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) buffer and 100 µg/ml gentamicin (catalog no. 15710-072; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used as the culture medium. The cells were cultured in an incubator at 37°C with humidified 5% CO2.
Cell morphology
A498 human renal carcinoma cells were treated with an inhibitor in either the absence or presence of metformin (10 mM). Following 24 h incubation, morphological changes were visualized with light microscopy (catalog no. DFC495; Leica Microsystems GmbH, Wetzlar, Germany) at ×200 magnification. The images were analyzed using the i-Solution program (IMT i-Solution, Burnaby, BC, Canada).
Flow cytometry analysis
Cell counting was performed using a hemocytometer. Metformin was immediately added to cell cultures at the indicated concentrations. Approximately 0.4×106 cells were resuspended in 100 µl PBS (catalog no. 17-517Q; Lonza, Walkersville, MD, USA), and 200 µl of 95% ethanol (catalog no. 1.00983.1011; Merck KGaA) was added during vortexing. The cells were incubated at 4°C for 1 h, washed in PBS, and resuspended in 250 µl 1.12% sodium citrate buffer (pH 8.4) along with 12.5 µl RNase. Incubation was continued for 30 min at 37°C. Cellular DNA was stained with 250 µl (1:1 dilution) propidium iodide (50 µg/ml; catalog no. p4170; Sigma-Aldrich; Merck KGaA) for 30 min at 37°C, and the relative DNA contents of the stained cells were analyzed using fluorescence-activated cell sorting (FACS) on the BD FACS Cato II flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
A498 whole-cell lysates were prepared by resuspending 0.4×106 cells in 50 µl lysis buffer (137 mM NaCl, 15 mM EGTA, 0.1 mM sodium orthovanadate, 15 mM MgCl2, 0.1% Triton X-100, 25 mM MOPS, 100 µM phenylmethylsulfonyl fluoride and 20 µM leupeptin, adjusted to pH 7.2). The cells were disrupted by sonication and protein extracted at 4°C for 30 min. Protein concentrations were quantified using the BCA assay kit (catalog no. 23225; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The proteins (50 µg) were separated using 10% SDS-PAGE gel and electrotransferred onto nitrocellulose membranes (catalog no. 23225; GE Healthcare, Chicago, IL, USA). The membrane was blocked with 5% skim milk in Tris-buffered saline (TBS) for 30 min at room temperature. The anti c-FLIPL (dilution, 1:700; catalog no. ALX-804-961) antibody was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). The anti-PARP (dilution, 1:1,000; catalog no. 9542) antibody was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The anti-B-cell lymphoma-2 (Bcl-2); (dilution, 1:700; catalog no. sc-783), anti-B-cell lymphoma-extra-large (Bcl-xL); (dilution, 1:1,000; catalog no. sc-634), anti-myeloid cell leukemia-1 (Mcl-1); (dilution, 1:1,000; catalog no. sc-819), anti-cellular inhibitor of apoptosis 2 (cIAP-2); (dilution, 1:1,000; catalog no. sc-7944) and anti-actin (dilution, 1:2,000; catalog no. sc-1616) antibodies were procured from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The anti-XIAP (dilution, 1:5,000; catalog no. 610762) antibody was supplied by BD Biosciences (San Jose, CA, USA). Membranes were incubated with the primary antibodies overnight at 4°C. Following six washes with TBS (each for 5 min), the membranes were incubated with the indicated secondary antibody for 1 h at room temperature and washed six times with TBS. The secondary goat anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (HRP) conjugated (dilution 1:1,000; catalog no. sc-2004) and goat anti-mouse IgG-HRP (dilution 1:1,000; catalog no. sc-2005) antibodies were procured from Santa Cruz Biotechnology, Inc. Specific proteins were detected using an ECL western blotting kit (catalog no. WBKLS0500; Merck KGaA). Proteins were detected using by ImageQuant LAS 4000 Mini Imaging System (GE Healthcare).
Transfection
A498 cells were seeded onto 6-well plates at a concentration of 0.2×106 cells/well and incubated overnight at 37°C. The pcDNA 3.1 vector and pcDNA 3.1 c-FLIPL plasmid were provided by Professor Tae-Jin Lee (Yeungnam University, South Korea). They were then transfected with control plasmid pcDNA 3.1 vector or pcDNA 3.1-c-FLIPL plasmid for 5 h using lipofectamine 2000 (catalog no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) in Opti-MEM medium (catalog no. 31985-070; Invitrogen; Thermo Fisher Scientific, Inc.). Following transfection, the cells were cultured in DMEM supplemented with 10% FBS for 12 h. Next, the cells were treated with metformin for 24 h. Finally, the cells were analyzed for c-FLIPL expression using western blotting.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
c-FLIPL mRNA expression was determined by RT-PCR. Total RNA was extracted from A498 cells using the EasyBlue reagent (catalog no. 17061; Thermo Fisher Scientific, Inc.). cDNA was prepared using M-MLV reverse transcriptase (catalog no. 18057018; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. In addition, the total cellular RNA was reverse-transcribed using a random primer and subsequently amplified using PCR. PCR primers were purchased from GenoTech (Daejeon, Korea). GAPDH was used as the internal control. The PCR cycling conditions used were as follows: For c-FLIPL: 95°C for 45 sec, 54°C for 45 sec, 72°C for 45 sec (33 cycles) and for GAPDH: 94°C for 30 sec, 58°C for 45 sec, 72°C for 42 sec (25 cycles). The following primer sequences were used to amplify c-FLIPL and GAPDH: For c-FLIPL: 5′-CGGACTATAGAGTGCTGATGG-3′ (forward) and 5′-GATTATCAGGCAGATTCCTAG-3′ (reverse); and for GAPDH: 5′-AGGTCGGAGTCAACGGATTTG-3′ (forward) and 5′-GTGATGGCATGGACTGTGGT-3′ (reverse). PCR products were analyzed by electrophoresis using 1.5% agarose gels and visualized by ethidium bromide using UV light gel (catalog no. WGD30; DAIHAN Scientific, Seoul, Korea).
Measurement of reactive oxygen species
A498 cells were plated in 6 well plates at a density of 0.4×106 cells/well and incubated for 24 h. The cells were incubated with metformin for 1 h and loaded with 10 µM 2′,7′-dichlorofluorescin diacetate (H2DCFDA; Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. Next, they were washed three times with PBS. Fluorescence was measured using flow cytometry. ROS generation was assessed by the dichlorofluorescence in fluorescence intensity (FL-1, 530 nm) of 10,000 cells using the BD FACS Cato II flow cytometer (BD Biosciences).
Statistical analysis
Data were analyzed using one-way ANOVA followed by post hoc comparisons (Student-Newman-Keuls) using the Statistical Package for Social Sciences 8.0 (SPSS, Inc., Chicago, IL, USA). At least three independent experiments were performed. The data were expressed as the mean ± standard deviation and P<0.05 was considered to indicate a statistically significant difference.
Results
Metformin induces apoptosis in human renal cell carcinoma A498 cells
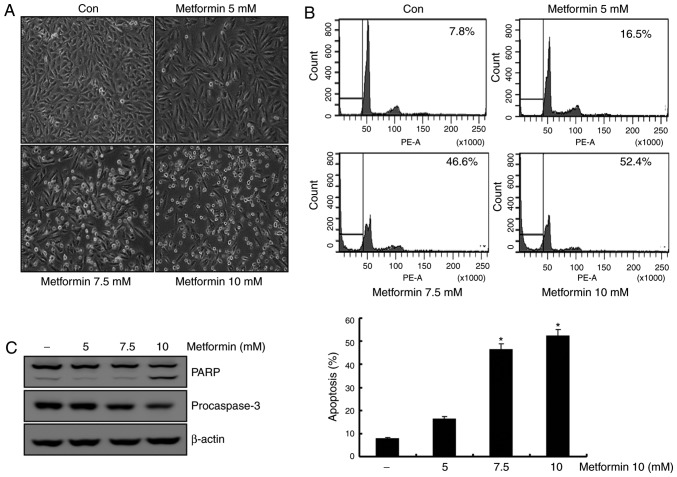
Previous studies reported that metformin induces apoptosis in renal cancer and breast cancer cell lines (12,23). To determine the apoptotic effects of metformin on A498 cells, these cells were treated with various concentrations of metformin (0, 5, 7.5 and 10 mM) for 24 h. In a dose-dependent manner, the metformin-stimulated A498 cells demonstrated features of apoptosis, including cell contraction and rounding and segregation of cells from the well (Fig. 1A). As presented in Fig. 1B, treatment of A498 cells with metformin resulted in a dose-dependent increase in sub-G1 populations. Additionally, treatment of A498 cells with metformin stimulated a reduction in the protein levels of the 32-kDa precursor (procaspase-3), along with the concomitant cleavage of PARP, a protein substrate for caspases (Fig. 1C). These results suggested that metformin-treated A498 cells demonstrated an increase in the Sub-G1 population in a dose-dependent manner.
Figure 1.
Metformin induces apoptosis in a dose-dependent manner in A498 cells. (A) A498 human renal carcinoma cells were treated with the indicated concentration of metformin. After 24 h, morphological changes were observed using a microscope at ×200 magnification. (B) A498 cells were treated with metformin (0, 5, 7.5 and 10 mM) for 24 h. Apoptosis was determined by flow cytometry. Representative FACS histograms are presented in the upper panel, cumulative data are presented in the lower panel. (C) A498 cells were treated with metformin for 24 h, and PARP, procaspase-3 and β-actin expression was analyzed using western blotting. β-actin was used as the loading control. All data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 compared with untreated cells. FACS, Fluorescence-activated cell sorting; PARP, poly (ADP-ribose) polymerase.
Metformin-induced apoptosis is modulated through degradation of the c-FLIPL protein in A498 cells
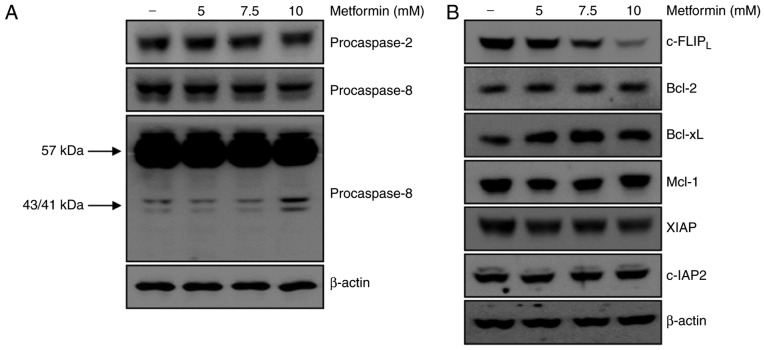
Caspases are important regulators of apoptotic cell death associated with apoptotic signaling pathways in various cancer cells (24). The present study examined whether activation of the caspase signaling pathway served a key role in metformin-mediated apoptosis. Treatment of A498 cells with metformin did not affect the activation of caspase-2. However, treatment with metformin for 24 h resulted in the appearance of p43/41-kDa fragments of caspase-8 (Fig. 2A). These results demonstrated that caspase-8 activation was involved in metformin-induced apoptosis in A498 cells. The association between metformin-induced apoptosis and regulation of other apoptotic modulators was investigated. As presented in Fig. 2B, protein expression levels of anti-apoptotic molecules including Bcl-2, Bcl-xL, Mcl-1, XIAP and c-IAP2 were not altered by metformin treatment. c-FLIP is a major regulator of the activity of caspase-8 (20). The protein level of c-FLIPL decreased following metformin treatment in A498 cells in a dose-dependent manner. Taken together, degradation of the c-FLIPL protein was involved in metformin-induced apoptosis via activation of caspase-8.
Figure 2.
Metformin-induced apoptosis is associated with activation of procaspase-8 and degradation of c-FLIPL. (A) A498 cells were treated with metformin for 24 h, and procaspase-2 and procaspase-8 expression was analyzed using western blotting. β-actin was used as the loading control. (B) A498 cells were treated with various concentrations of metformin for 24 h. c-FLIPL, Bcl-2, Bcl-xL, Mcl-1, XIAP and c-IAP2 expression was determined using western blot analysis. β-actin served as the loading control. c-FLIP, cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein; Bcl-2, B-cell lymphoma 2; Bcl-xL, B-cell lymphoma-extra-large; Mcl-1, myeloid cell leukemia-1, XIAP, X-linked inhibitor of apoptosis protein; c-IAP, cellular inhibitor of apoptosis protein, baculoviral IAP repeat containing 3.
Metformin-mediated apoptosis is associated with activation of the caspase signaling pathway
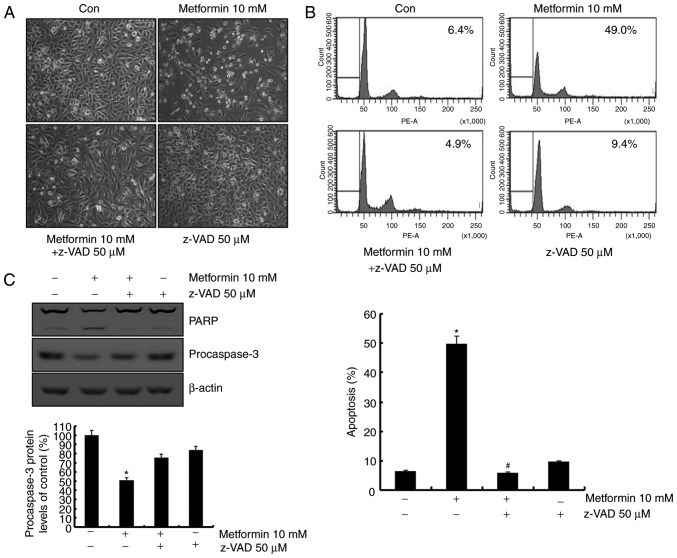
The role of the caspase signaling pathway in metformin-mediated apoptosis was investigated. As presented in Fig. 3A, metformin-induced apoptosis was blocked following pretreatment with a general caspase inhibitor, z-VAD-fmk. Sub-G1 population was markedly decreased by treatment with z-VAD-fmk in the presence of metformin (Fig. 3B). In addition, treatment with z-VAD-fmk prevented the cleavage of PARP and caspase-3 activation (Fig. 3B). These data indicated that metformin-induced apoptosis was mediated by caspase-dependent apoptosis in the presence of z-VAD-fmk.
Figure 3.
Metformin induces apoptosis by a caspase-dependent signaling pathway in A498 cells. (A) A498 cells were pretreated with 50 µM z-VAD-fmk or a solvent for 30 min and incubated with 10 mM metformin for 24 h. Morphological features were analyzed using light microscopy at ×200 magnification. (B) A498 cells were cultured with 50 µM z-VAD-fmk or the vehicle for 30 min prior to treatment with metformin (10 mM) for 24 h. The sub-G1 fraction was determined using flow cytometry. Representative FACS histograms are presented in the upper panel, cumulative data are presented in the lower panel. (C) A498 cells were incubated with 10 mM metformin for 24 h in the presence or absence of 50 µM z-VAD-fmk. PARP, procaspase-3 and β-actin expression was analyzed using western blotting. β-actin was used as the loading control. Band density of procaspase-3 was analyzed using ImageJ software. All data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 compared with untreated cells, #P<0.05 compared with metformin-treated cells. z-VAD-fmk, benzyloxycarbonyl-Val-Ala-Asp-fluorometyhlketone; PARP, poly(ADP-ribose) polymerase.
Metformin-mediated apoptosis is dependent on the degradation of c-FLIPLprotein in A498 cells
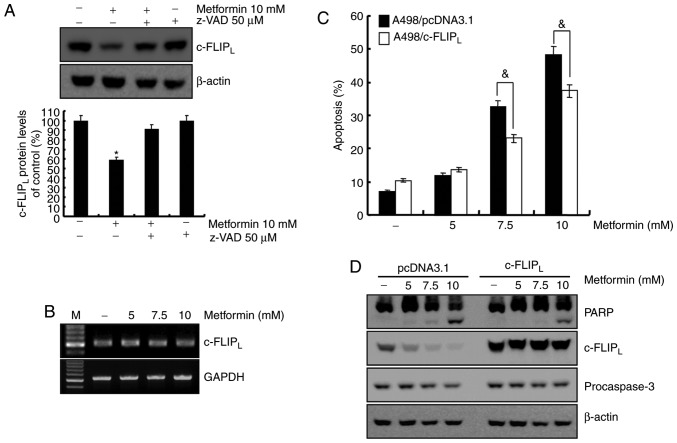
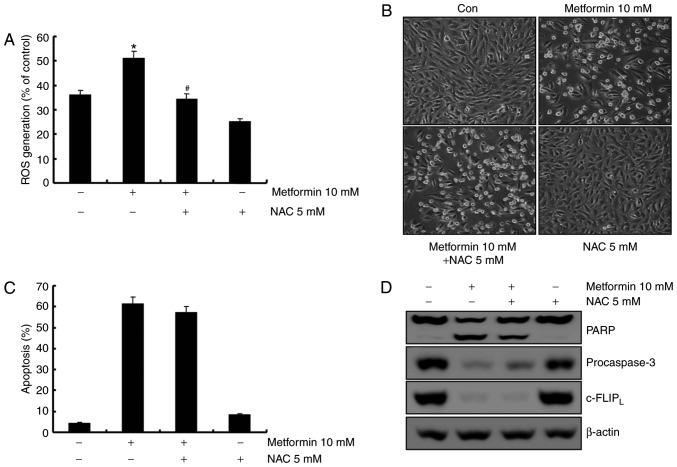
The association between caspase-8 activation and metformin-mediated apoptosis was investigated. Treatment with metformin led to c-FLIPL degradation, which was recovered by z-VAD-fmk (Fig. 4A). To determine whether the mRNA level of c-FLIPL was associated with protein level in A498 human renal cell carcinoma cells, the c-FLIPL mRNA level was examined using RT-RCR. As demonstrated in Fig. 4B, c-FLIPL mRNA level remained constant following metformin treatment in A498 cells at indicated concentrations. A previous study reported that c-FLIP functions as an anti-apoptotic regulator and is overexpressed in various cancer cell lines (25). To determine whether the reduced level of c-FLIPL was involved in the induction of apoptosis in metformin-treated A498 cells, c-FLIPL overexpressing cells were established. Overexpression of c-FLIPL attenuated apoptosis (Fig. 4C) and PARP cleavage (Fig. 4D) induced by metformin. Taken together, metformin-induced apoptosis was associated with the degradation of c-FLIPL through activation of caspase-8. ROS is not involved in metformin-mediated apoptosis in A498 cells. ROS is an important regulator of apoptosis (26). A previous report demonstrated that metformin induces ROS production in breast cancer cells (23). Therefore, the capacity of metformin to induce ROS production in A498 human renal carcinoma cells was investigated using flow cytometry. As demonstrated in Fig. 5A, metformin treatment increased ROS production 1 h post-treatment, and pretreatment with the anti-oxidant N-acetyl-L-cysteine (NAC, a ROS scavenger) inhibited metformin-induced ROS production. To confirm whether ROS generation served a key role in metformin-mediated apoptosis, A498 cells were pretreated with NAC for 30 min. Pretreatment with NAC did not inhibit metformin-induced morphological changes and apoptosis in A498 cells (Fig. 5B and C). Furthermore, NAC did not prevent PARP cleavage, caspase activation and degradation of c-FLIPL protein in metformin-treated cells (Fig. 5D). These results suggested that ROS generation was not critical for the induction of apoptosis by metformin.
Figure 4.
Degradation of c-FLIPL protein is regulated via a caspase-dependent signaling pathway. (A) A498 cells were treated with 10 mM metformin for 24 h in the presence or absence of 50 mM z-VAD-fmk. c-FLIPL and β-actin expression was analyzed using western blotting. β-actin was used as the loading control. Band density of the c-FLIPL protein was determined using the ImageJ program. (B) A498 cells were incubated with the indicated concentrations of metformin for 24 h. c-FLIPL mRNA level was determined using reverse transcription-polymerase chain reaction. (C) A498 cells transfected with pcDNA3.1 or c-FLIPL plasmid were treated for 24 h with different concentrations of metformin. The sub-G1 cell population was analyzed using flow cytometry. (D) The pcDNA3.1 and c-FLIPL transfected cells were treated with varying concentrations of metformin. PARP, procaspase-3 and c-FLIPL expression levels were determined using western blotting. β-actin was used as the loading control. All data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 compared with untreated cells, &P<0.05 compared with metformin-treated pcDNA3.1 cells. z-VAD-fmk, benzyloxycarbonyl-Val-Ala-Asp-fluoro-methyl ketone; c-FLIP, cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein; PARP, poly(ADP-ribose) polymerase.
Figure 5.
Metformin-mediated apoptosis in A498 cells was not affected by reactive oxygen species generation. (A) Cells were pretreated with 5 mM NAC for 30 min and incubated with 10 mM metformin for 1 h. H2DCFDA fluorescence was quantified with flow cytometry. (B) A498 cells were incubated with 5 mM NAC or the vehicle for 30 min prior to treatment with 10 mM metformin for 24 h. Morphological changes were analyzed using a light microscope at ×200 magnification. (C) A498 cells were pretreated with 5 mM NAC or the solvent for 30 min and incubated in the presence or absence of 10 mM metformin for 24 h. The sub-G1 cell fraction was analyzed using flow cytometry. (D) A498 cells were pretreated with 5 mM NAC or the solvent for 30 min and incubated with 10 mM metformin for 24 h. PARP, procaspase-3, c-FLIPL and β-actin expression levels were analyzed using western blotting. β-actin was used as the loading control. All data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 compared with untreated cells, #P<0.05 compared with metformin-treated cells. NAC, N-acetyl-L-cysteine; PARP, poly(ADP-ribose) polymerase; c-FLIP, cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein; ROS, reactive oxygen species.
Discussion
Metformin has been used as a therapeutic agent for diabetic patients (27). It was reported to possess various effects, including anti-proliferation, anti-inflammation, anti-angiogenesis and anti-invasion (12,28–30). Metformin also has anti-apoptotic effects on prostate, hepatocellular carcinoma, gall bladder and breast cancer cell lines (31–34). In addition, the insulin receptor may serve a major role in facilitating cancer development via increases in insulin levels (35,36). However, the specific apoptotic mechanism of action of metformin in human renal cancer cells has not been reported. The present study investigated whether metformin had an anti-cancer effect on human renal cell carcinoma A498 cells. It was revealed that metformin-mediated apoptosis led to the activation of caspase-8 through the downregulation of c-FLIPL protein expression level in A498 cells.
Apoptosis is the process of programmed cell death that is closely associated with caspase activation. Caspases can be divided into two main groups, namely initiator caspases (caspase-2, −8, −9 and −10) and executioner caspases (caspase-3, −6 and −7) (37). Caspase-8 enhances apoptosis through caspase-3 activation. Caspase-3 is a primary caspase because it is associated with the intrinsic and extrinsic signaling pathways of apoptosis (38). In the present study, the involvement of caspases in metformin-mediated apoptosis was examined. Caspase-8 was activated by metformin, resulting in the emergence of p43/41 kDa fragments (Fig. 2A). Activation of the executioner caspase, caspase-3, was increased via metformin-induced apoptosis (Fig. 1C). In addition, z-VAD-fmk (a pan-caspase inhibitor) completely blocked metformin-induced apoptosis (Fig. 3). These results suggested that metformin-induced apoptosis was associated with caspase activation. Activation of the caspase cascade is modulated via upregulation of pro-apoptotic proteins and/or downregulation of anti-apoptotic proteins (39). In addition, activation of caspase-8 at DISC is blocked via c-FLIP, which inhibits the death receptor-mediated apoptotic pathways (40). In the present study, metformin also decreased c-FLIPL protein level in A498 cells in a dose-dependent manner (Fig. 2B). However, treatment with metformin did not affect c-FLIPL mRNA levels (Fig. 4B) and proteasomal degradation (data not shown). Previous reports also indicated that metformin had no effect on c-FLIPL mRNA level and the proteasomal pathway in non-muscle invasive bladder cancer (NMIBC) (22). It was also revealed that c-FLIPL-overexpressing cells partly prevented metformin-induced apoptosis in A498 cells (Fig. 4). These results suggested that metformin-induced apoptosis occurred via degradation of c-FLIPL protein. Metformin has also been reported to suppress the expression levels of anti-apoptotic proteins, including Bcl-2, Bcl-XL, Mcl-1, c-IAP2 and XIAP in primary ovarian cell lines, p53-deficient cells, colorectal and breast cancer cell lines, respectively (41–44). It was demonstrated that Bcl-2, Bcl-XL, Mcl-1, c-IAP2 and XIAP did not affect metformin-treated A498 cells (Fig. 2B). These results demonstrated that suppression of the anti-apoptotic protein, c-FLIPL, was associated with metformin-induced apoptosis in A498 cells.
Reactive oxygen species (ROS) are important mediators of apoptosis in several cancer cell lines (45–48). Previous reports demonstrated that metformin modulated apoptosis through ROS production in hepatoma, ovary and breast cancer cell lines (49,50). Therefore, the association between metformin-mediated apoptosis and ROS generation was investigated. In the present study, metformin was observed to stimulate ROS generation in A498 cells. Pretreatment with N-acetylcysteine did not prevent metformin-induced apoptosis (Fig. 5). These results suggested that metformin-induced ROS generation was not associated with metformin-mediated apoptosis in A498 cells.
Taken together, these results demonstrated that metformin-induced apoptosis was mediated by the degradation of c-FLIPL protein via activation of caspase-8 in A498 human renal cell carcinoma cells. This suggested that metformin can serve the role of a chemotherapeutic agent for diabetes, as well as an anti-cancer agent.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant no. 2017R1D1A1B3030961).
Availability of data and materials
All data generated or analyzed during present study are included within the article.
Authors' contributions
JJ and JK designed the study, collected and analyzed the data. TL, IS and ES advised on the morphological images and performed the data analysis. JJ and JK drafted and wrote the manuscript. TL and JK revised the manuscript critically for intellectual content. All authors gave intellectual input to the study and approved the final version of the manuscript.
Ethics approval and consent to participate
This research was approved by the Ethics Committee of Yeungnam University (Daegu, South Korea). All procedures were performed according to the ethical standards of Ethics Committee of Yeungnam University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016;8:484–500. doi: 10.4329/wjr.v8.i5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atzpodien J, Kirchner H, Poliwoda H. Interleukin 2 based ambulatory therapy of metastatic renal cell carcinoma. Med Klin (Munich) 1996;91(3):S38–S43. (Suppl. (In German) [PubMed] [Google Scholar]
- 3.Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: Implications for adjuvant local and systemic therapy. J Clin Oncol. 1994;12:206–212. doi: 10.1200/JCO.1994.12.1.206. [DOI] [PubMed] [Google Scholar]
- 4.Malek M, Aghili R, Emami Z, Khamseh ME. Risk of cancer in diabetes: The effect of metformin. ISRN Endocrinol. 2013;2013:636927. doi: 10.1155/2013/636927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: A meta-analysis. Diabetes Care. 2011;34:2323–2328. doi: 10.2337/dc11-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair V, Pathi S, Jutooru I, Sreevalsan S, Basha R, Abdelrahim M, Samudio I, Safe S. Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors. Carcinogenesis. 2013;34:2870–2879. doi: 10.1093/carcin/bgt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: From the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013;34:126–135. doi: 10.1016/j.tips.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 11.Rice S, Pellat L, Ahmetaga A, Bano G, Mason HD, Whitehead SA. Dual effect of metformin on growth inhibition and oestradiol production in breast cancer cells. Int J Mol Med. 2015;35:1088–1094. doi: 10.3892/ijmm.2015.2108. [DOI] [PubMed] [Google Scholar]
- 12.Fang Z, Xu X, Zhou Z, Xu Z, Liu Z. Effect of metformin on apoptosis, cell cycle arrest migration and invasion of A498 cells. Mol Med Rep. 2014;9:2251–2256. doi: 10.3892/mmr.2014.2097. [DOI] [PubMed] [Google Scholar]
- 13.Ucbek A, Ozunal ZG, Uzun O, Gepdiremen A. Effect of metformin on the human T98G glioblastoma multiforme cell line. Exp Ther Med. 2014;7:1285–1290. doi: 10.3892/etm.2014.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung TW, Lee MW, Lee YJ, Kim SM. Metformin prevents endoplasmic reticulum stress-induced apoptosis through AMPK-PI3K-c-Jun NH2 pathway. Biochem Biophys Res Commun. 2012;417:147–152. doi: 10.1016/j.bbrc.2011.11.073. [DOI] [PubMed] [Google Scholar]
- 15.Fujimori T, Kato K, Fujihara S, Iwama H, Yamashita T, Kobayashi K, Kamada H, Morishita A, Kobara H, Mori H, et al. Antitumor effect of metformin on cholangiocarcinoma: In vitro and in vivo studies. Oncol Rep. 2015;34:2987–2996. doi: 10.3892/or.2015.4284. [DOI] [PubMed] [Google Scholar]
- 16.He MX, He YW. A role for c-FLIP(L) in the regulation of apoptosis, autophagy, and necroptosis in T lymphocytes. Cell Death Differ. 2013;20:188–197. doi: 10.1038/cdd.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagnoli M, Ambrogi F, Pilotti S, Alberti P, Ditto A, Barbareschi M, Galligioni E, Biganzoli E, Canevari S, Mezzanzanica D. c-FLIPL expression defines two ovarian cancer patient subsets and is a prognostic factor of adverse outcome. Endocr Relat Cancer. 2009;16:443–453. doi: 10.1677/ERC-08-0218. [DOI] [PubMed] [Google Scholar]
- 18.Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): A novel target for cancer therapy. Current Cancer Drug Targets. 2008;8:37–46. doi: 10.2174/156800908783497087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: A key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42:210–213. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Koenig A, Buskiewicz IA, Fortner KA, Russell JQ, Asaoka T, He YW, Hakem R, Eriksson JE, Budd RC. The c-FLIPL cleavage product p43FLIP promotes activation of extracellular signal-regulated kinase (ERK), nuclear factor kappaB (NF-κB), and caspase-8 and T cell survival. J Biol Chem. 2014;289:1183–1191. doi: 10.1074/jbc.M113.506428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nazim UM, Moon JH, Lee JH, Lee YJ, Seol JW, Eo SK, Lee JH, Park SY. Activation of autophagy flux by metformin downregulates cellular FLICE-like inhibitory protein and enhances TRAIL-induced apoptosis. Oncotarget. 2016;7:23468–23481. doi: 10.18632/oncotarget.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, Wang X, He D, Jin X, Guo P. Metformin sensitizes human bladder cancer cells to TRAIL-induced apoptosis through mTOR/S6K1-mediated downregulation of c-FLIP. Anticancer Drugs. 2014;25:887–897. doi: 10.1097/CAD.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 23.Gao ZY, Liu Z, Bi MH, Zhang JJ, Han ZQ, Han X, Wang HY, Sun GP, Liu H. Metformin induces apoptosis via a mitochondria-mediated pathway in human breast cancer cells in vitro. Exp Ther Med. 2016;11:1700–1706. doi: 10.3892/etm.2016.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 25.Ili CG, Brebi P, Tapia O, Sandoval A, Lopez J, Garcia P, Leal P, Sidransky D, Guerrero-Preston R, Roa JC. Cellular FLICE-like inhibitory protein long form (c-FLIPL) overexpression is related to cervical cancer progression. Int J Gynecol Pathol. 2013;32:316–322. doi: 10.1097/PGP.0b013e31825d8064. [DOI] [PubMed] [Google Scholar]
- 26.Ding H, Han C, Guo D, Chin YW, Ding Y, Kinghorn AD, D'Ambrosio SM. Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr Cancer. 2009;61:348–356. doi: 10.1080/01635580802567158. [DOI] [PubMed] [Google Scholar]
- 27.Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and Meta-analysis. Ann Intern Med. 2016;164:740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 28.Xu HY, Fang W, Huang ZW, Lu JC, Wang YQ, Tang QL, Song GH, Kang Y, Zhu XJ, Zou CY, et al. Metformin reduces SATB2-mediated osteosarcoma stem cell-like phenotype and tumor growth via inhibition of N-cadherin/NF-κB signaling. Eur Rev Med Pharmacol Sci. 2017;21:4516–4528. [PubMed] [Google Scholar]
- 29.Figueroa-González G, Garcia-Castillo V, Coronel-Hernández J, López-Urrutia E, León-Cabrera S, Arias-Romero LE, Terrazas LI, Rodríguez-Sosa M, Campos-Parra AD, Zúñiga-Calzada E, et al. Anti-inflammatory and antitumor activity of a triple therapy for a colitis-related colorectal cancer. J Cancer. 2016;7:1632–1644. doi: 10.7150/jca.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Jin G, Liu H, Liu K, Zhao J, Chen X, Wang D, Bai R, Li X, Jang Y, et al. Metformin inhibits esophageal squamous cell carcinoma-induced angiogenesis by suppressing JAK/STAT3 signaling pathway. Oncotarget. 2017;8:74673–74687. doi: 10.18632/oncotarget.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran LNK, Kichenadasse G, Butler LM, Centenera MM, Morel KL, Ormsby RJ, Michael MZ, Lower KM, Sykes PJ. The combination of metformin and valproic acid induces synergistic apoptosis in the presence of p53 and androgen signaling in prostate cancer. Mol Cancer Ther. 2017;16:2689–2700. doi: 10.1158/1535-7163.MCT-17-0074. [DOI] [PubMed] [Google Scholar]
- 32.Tsai HH, Lai HY, Chen YC, Li CF, Huang HS, Liu HS, Tsai YS, Wang JM. Metformin promotes apoptosis in hepatocellular carcinoma through the CEBPD-induced autophagy pathway. Oncotarget. 2017;8:13832–13845. doi: 10.18632/oncotarget.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi T, Zhu A, Yang X, Qiao H, Tang J, Liu Y, Lv R. Metformin synergistically enhances antitumor activity of cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway. Cytotechnology. 2018;70:439–448. doi: 10.1007/s10616-017-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Zaidan L, El Ruz RA, Malki AM. Screening novel molecular targets of metformin in breast cancer by proteomic approach. Front Public Health. 2017;5:277. doi: 10.3389/fpubh.2017.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 36.Rizos CV, Elisaf MS. Metformin and cancer. Eur J Pharmacol. 2013;705:96–108. doi: 10.1016/j.ejphar.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity. 2016;44:221–231. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Walters J, Pop C, Scott FL, Drag M, Swartz P, Mattos C, Salvesen GS, Clark AC. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem J. 2009;424:335–345. doi: 10.1042/BJ20090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spagnuolo C, Cerella C, Russo M, Chateauvieux S, Diederich M, Russo GL. Quercetin downregulates Mcl-1 by acting on mRNA stability and protein degradation. Br J Cancer. 2011;105:221–230. doi: 10.1038/bjc.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruidering M, Evan GI. Caspase-8 in apoptosis: The beginning of ‘the end’? IUBMB Life. 2000;50:85–90. doi: 10.1080/15216540050212088. [DOI] [PubMed] [Google Scholar]
- 41.Patel S, Singh N, Kumar L. Evaluation of effects of metformin in primary ovarian cancer cells. Asian Pac J Cancer Prev. 2015;16:6973–6979. doi: 10.7314/APJCP.2015.16.16.6973. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Li B, Ni Z, Zhou P, Wang B, He J, Xiong H, Yang F, Wu Y, Lyu X, et al. Metformin synergizes with BCL-XL/BCL-2 inhibitor ABT-263 to induce apoptosis specifically in p53-defective cancer cells. Mol Cancer Ther. 2017;16:1806–1818. doi: 10.1158/1535-7163.MCT-16-0763. [DOI] [PubMed] [Google Scholar]
- 43.Park SH, Lee DH, Kim JL, Kim BR, Na YJ, Jo MJ, Jeong YA, Lee SY, Lee SI, Lee YY, Oh SC. Metformin enhances TRAIL-induced apoptosis by Mcl-1 degradation via Mule in colorectal cancer cells. Oncotarget. 2016;7:59503–59518. doi: 10.18632/oncotarget.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strekalova E, Malin D, Rajanala H, Cryns VL. Metformin sensitizes triple-negative breast cancer to proapoptotic TRAIL receptor agonists by suppressing XIAP expression. Breast Cancer Res Treat. 2017;163:435–447. doi: 10.1007/s10549-017-4201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jang JH, Iqbal T, Min KJ, Kim S, Park JW, Son EI, Lee TJ, Kwon TK. Helenalin-induced apoptosis is dependent on production of reactive oxygen species and independent of induction of endoplasmic reticulum stress in renal cell carcinoma. Toxicol In Vitro. 2013;27:588–596. doi: 10.1016/j.tiv.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Woo SM, Min KJ, Kwon TK. Calyculin A causes sensitization to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by ROS-mediated down-regulation of cellular FLICE-inhibiting protein (c-FLIP) and by enhancing death receptor 4 mRNA stabilization. Apoptosis. 2012;17:1223–1234. doi: 10.1007/s10495-012-0753-y. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Yu X, Song H, Feng D, Jiang Y, Wu S, Geng J. The STAT-ROS cycle extends IFN-induced cancer cell apoptosis. Int J Oncol. 2018;52:305–313. doi: 10.3892/ijo.2017.4196. [DOI] [PubMed] [Google Scholar]
- 48.Sheikh BY, Sarker MMR, Kamarudin MNA, Mohan G. Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomed Pharmacother. 2017;96:834–846. doi: 10.1016/j.biopha.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 49.Rogalska A, Bukowska B, Marczak A. Metformin and epothilone A treatment up regulate pro-apoptotic PARP-1, Casp-3 and H2AX genes and decrease of AKT kinase level to control cell death of human hepatocellular carcinoma and ovary adenocarcinoma cells. Toxicol In Vitro. 2018;47:48–62. doi: 10.1016/j.tiv.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Haugrud AB, Zhuang Y, Coppock JD, Miskimins WK. Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast Cancer Res Treat. 2014;147:539–550. doi: 10.1007/s10549-014-3128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during present study are included within the article.