Abstract
Breast fibromatosis is a benign fibroblastic proliferation accounting for less than 0.2% of breast tumors. It presents sporadically or as a manifestation of familial adenomatous polyposis (FAP). Fibromatosis in FAP may develop in patients with adenomatous polyposis coli (APC) gene mutations at any location through the gene. Notably, there is an increased risk if mutation is downstream codon 1400. The present case report described a 33-year-old woman with recurrent bilateral breast fibromatosis after breast implants in a context of classic FAP. APC mutation (codon-935) was detected at the age of 16. In the same year, a thyroidectomy for a cribriform-morular papillary thyroid carcinoma (pT1) was performed. Seven years later, a prophylactic total colectomy with >100 adenomas without invasive carcinoma was performed and the patient was kept under surveillance. At the age of 30 years old, she underwent breast silicone implantation for cosmetic reasons. One year later, bilateral breast tumors were diagnosed in core biopsy as fibromatosis (nuclear β-catenin+, estrogen receptors-). After no success with medical treatment with tamoxifen, bilateral mastectomy was performed. The patient relapsed one year later and a fibromatosis lesion in the right thoracic wall was excised again. The patient demonstrated no signs of relapse 24 months after the surgery. This rare case illustrates that the increased risk of developing fibromatosis in patients with FAP, even in the classic form, should be considered before deciding to place breast implants.
Keywords: fibromatosis, breast implants, familial adenomatous polyposis, cribriform-morular papillary thyroid carcinoma, β-catenin, APC gene
Introduction
In familial adenomatous polyposis (FAP) context, extra-colonic features may include osteomas and dental abnormalities, congenital hypertrophy or hyperplasia of the retinal pigment epithelium, upper gastrointestinal tumors, epidermoid cysts and lipomas, adrenal tumors, hepatoblastoma, brain tumors and pancreatic cancer. Also, papillary thyroid cancer and fibromatosis can occur (1,2).
The cribriform-morular papillary thyroid carcinoma is the most common morphologic variant found in FAP-associated thyroid carcinoma, exhibits nuclear and cytoplasmic accumulation of β-catenin and is associate with germline or somatic mutation of adenomatous polyposis coli (APC) and 3-catenin (CTNNB1) genes (3).
Fibromatosis is a benign neoplasm with infiltrative growth and consequent high potential to recur after surgical excision (2,4,5). It occurs predominantly in adults, sporadically or in association with FAP syndrome (2). In FAP setting, fibromatosis usually follows a surgical trauma, occurs in 15 to 20% of the patients, is more frequent in women (ratio 1:3) (2,6), and has a significantly increased rate of occurrence. Studies on APC genotype-phenotype correlation identified that mutations in the 3′end and downstream of codon 1400 in the APC have an increased risk of fibromatosis development (1). Although breast fibromatosis is very rare (less than 0.2% of all breast tumors), it occurs mainly as a sporadic form. Trauma and previous surgery are the most important contributing factors (6,7) namely after the placement of breast prosthetics (5,6,8,9). This last type can occur associated with either saline or silicone prosthetics (6).
Pathophysiology of fibromatosis is still not completely understood, but it could result from a disturbance of cell proliferation following a trauma, a dysfunctional hormonal dependence of the local fibroblasts, or a genetic disorder in the regulation of fibroblast growth field (10). Alterations of the APC/β-catenin pathway with resultant nuclear translocation of β-catenin were described in the pathogenesis of both sporadic and FAP-associated breast fibromatosis (11) but mostly resulted from sporadic mutations in the β-catenin subunit (11). An unique case of bilateral fibromatosis in Gardner syndrome was reported in 1970 by Haggitt and Booth (12).
The relevance of illustrating the present case is to alert that, in classic FAP, the possibility of this serious complication must be considered in the decision to place breast implants. We also aim to contribute to the knowledge of the relationship between the APC gene mutation and the consequent phenotype, in order to prevent the development of deleterious conditions in FAP patients.
Case report
Clinical summary
We report a case of a 33-year-old woman with a classic FAP, with a cribriform-morular morphologic variant of papillary thyroid carcinoma (CMV-PTC) and also with bilateral breast fibromatosis in the context of silicone prosthetics. Previous family history was relevant, as her father had a colon cancer diagnosed at the age of 34 that was found to be in the context of FAP and died at the age of 38. At that time, at the age of 16 years old, our patient was referred to our Family Risk Consulting and underwent a flexible sigmoidoscopy that revealed few adenomas. A nonsense mutation (c.935C>A) at codon 935 in exon 15 of APC gene was found and the patient was also diagnosed with FAP.
The search of extra-colonic manifestations of the disease found a papillary thyroid carcinoma cribriform-morular variant treated with total thyroidectomy and with no signs of relapse until now. No other extra-colonic manifestations were found.
She underwent surveillance with annual colonoscopy and the polyps' number and size allowed to postponed prophylactic surgery. At the age of 23, the patient had about 80 colonic polyps and underwent a rectum-sparing total colectomy, with ileorectal anastomosis. Pathologic analysis of the colectomy specimen revealed more than 100 tubular and tubulovillous adenomas with low-grade dysplasia. The patient began to be followed with annual rectoscopy and upper endoscopy according to Spigelman classification for duodenal polyposis.
A breast augmentation surgery with retropectoral silicone prosthetics was performed for cosmetic purposes, at the age of 30 in another institution.
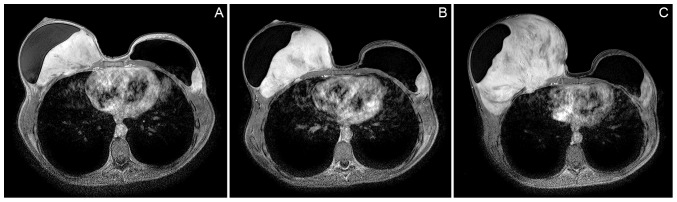
About one year later, she developed complaints of a growing tenderness in the right breast. The magnetic resonance imaging (MRI) showed a 10 cm longitudinal diameter mass in the right breast, limited anteriorly by the pectoralis major muscle and the silicone prosthetics, with invasion of the pectoralis minor muscle and with an intrathoracic component between the 4th and the 5th rib and also, in the left breast, a 4 cm mass was detected, without intrathoracic component. (Fig. 1A). The breast bilateral tumors were diagnosed in core biopsies as fibromatosis. The patient was under levothyroxine and an etonogestrel subdermal implant medication that was removed after the histological diagnosis of fibromatosis.
Figure 1.
(A) MRI at diagnosis. Lesion on the right breast with 10 cm in longitudinal diameter, limited anteriorly by the pectoralis major muscle and the silicone prosthetics. Invasion of the pectoralis minor muscle and an intrathoracic component between the 4th and the 5th rib was also observed. Lesion in the left breast with 4 cm was detected, without intrathoracic component. (B) MRI after 2 months of hormonal therapy with tamoxifen. Lesion on the right breast increased in size to 12 cm in transversal largest cross shaft. Lesion in the left breast did not experience increase in size (3,9×1,9 cm). (C) MRI before surgical treatment, one year after the diagnosis. Lesion on the right breast increased in size (13,7×9,6×14,5 cm). Lesion in the left breast is similar in size comparing with previous studies (6,4×5,4×3,1 cm).
Treatment with 40 mg per day of tamoxifen was started one month later but no regression of the mammary fibromatosis masses was observed (Fig. 1B). Surgical treatment with bilateral mastectomy and removal of the prosthetics was performed one year after the diagnosis (Fig. 1C). For the right breast, resection of 4th and 5th costal arches and plastic surgical reconstruction of the thoracic wall with a myocutaneous retail from latissimus dorsi muscle was also carried out. Resection was made with negative surgical margins for both breast tumors.
A relapse in the right thoracic wall, confirmed by a core biopsy, occurred one year after and was again surgically removed.
Currently, regarding the colonic tumors, the patient is controlled and well and maintains appropriated surveillance for new relapses of fibromatosis 24 months after second surgery.
APC mutation analysis
Mutation analysis of exons 1–14 of the APC gene was performed by denaturing gradient gel electrophoresis (DGGE) as described previously and by direct sequencing for some exons (13,14). Exon 15 mutations were analyzed using the protein truncation test (PTT), according to a method formerly described (14). All DGGE and PTT fragments showing an aberrant electrophoretic banding pattern were sequenced using the Big Dye terminator cycle sequencing kit (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) on an automatic ABI Prism™ 310 Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.), in accordance with the manufacturer's instructions. Mutation was compared with the description of Genbank M74088 for APC gene.
Immunohistochemical studies
Immunostainings were performed by Ventana Bench Mark ULTRA. The peroxidase-indirect-polymer method Ventana Ultraview DAB, cat. no. 760-500, was used for primary antibodies anti-estrogen receptor (Ventana Rabbit Monoclonal SP1, cat. no. 790-4324; Ventana Medical Systems, Inc., Tucson, AZ, USA). For anti-β-catenin (Mouse Monoclonal cat. no. 5H10, ref. 80226, 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) and anti-cytokeratin (Dako Mouse Monoclonal AE1/AE3 cat. no. 3515, 1:100; Agilent Technologies, Inc., Santa Clara, CA, USA) Ventana Optiview DAB cat. no. 760-700 was used. Sections with 3 µm thick were cut, unto Superfrost plus slides from paraffin-embedded routine tissue blocks. The heat mediated antigen retrieval was Ventana CC1 52 min for primary antibodies anti-estrogen receptor; 40 min for anti-β-catenin; and a mixed CC1 16 min and Protease 3, 4 min for anti-AE1/AE3. As positive controls: A composite breast tissue was used for anti-estrogen receptor, a composite colon tissue for anti-β-catenin and a skin tissue for AE1/AE3. For negative controls, primary antibodies were omitted during the staining.
Mutational findings
A nonsense mutation (c.935C>A) at codon 935 in exon 15 of APC gene was found in this patient, resulting in a truncated protein.
Thyroid, total colon and bilateral breast surgical specimens-histopathologic and immunohistochemical findings
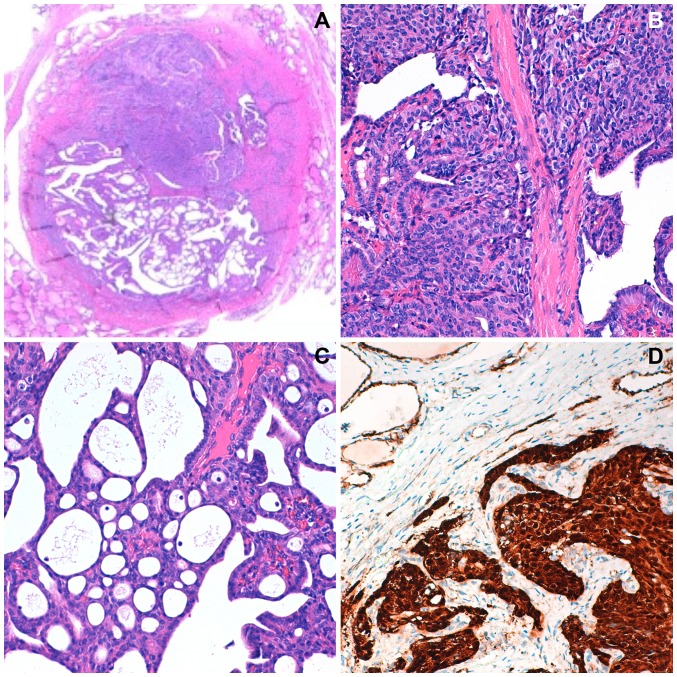
The total thyroidectomy showed identical right and left lobes, both with 3×3×2 cm. In the right lobe, a well delimitated intra-glandular, white nodule, with 6 mm was observed. This tumor presented histological features compatible with the CMV-PTC. Tumor cells showed strong cytoplasmic and nuclear expression with β-catenin (Fig. 2). Remaining thyroid parenchyma with no alterations.
Figure 2.
Histological observation with hematoxylin-eosin staining of cribriform-morular papillary thyroid carcinoma (thyroidectomy specimen) at (A) magnification, ×40, (B) with solid and cribriform areas at magnification, ×100 and (C) magnification, ×400. (D) Solid area with strong nuclear and cytoplasmic positivity with β-catenin at magnification, ×400.
The rectum-sparing total colectomy measured 153 cm, had more than 100 tubular and tubulovillous adenomas <5 mm and included appendix with no alterations.
The resected specimen from the right breast mastectomy weighted 2,123 g, measured 24×19×11 cm and included two segments of ribcage, with 9,5 cm. The specimen removed from the left breast weighted 442 g and measured 17×11×6 cm. White, firm, fibrotic lesions with infiltrative margins, were identified. The prosthetics were intact. The excision was considered complete in both breasts.
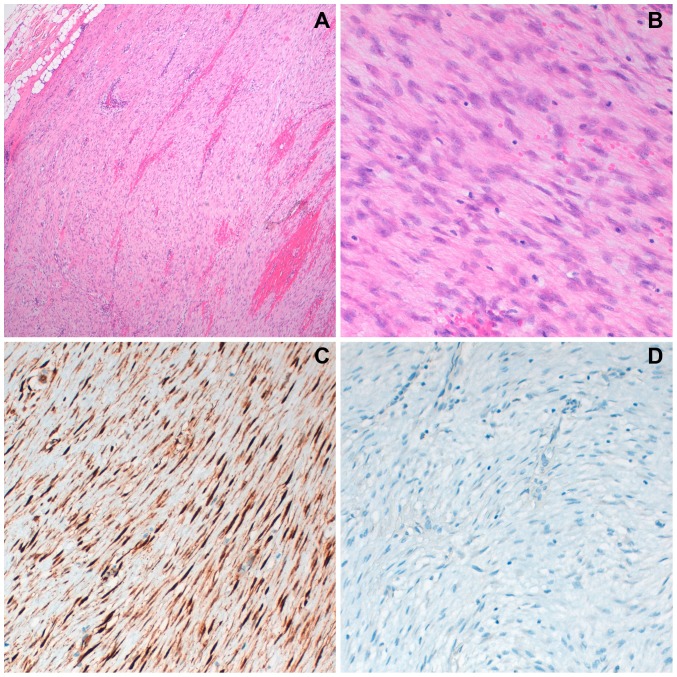
The histologic evaluation was similar to the previous biopsy (Fig. 3A and B). Immunohistochemistry with β-catenin and estrogen-receptors was reevaluated in the surgical specimen. Nuclear expression of β-catenin was detected (Fig. 3C) and estrogen-receptors immunostaining were negative (Fig. 3D).
Figure 3.
Histological observation of breast specimen with hematoxylin-eosin staining, consistent with desmoid-type fibromatosis (A) at magnification, ×40 and (B) at magnification, ×200: monotonous proliferation of spindle cells without atypia and absent mitosis. Immunostaining (breast specimen): nuclear positivity for β-catenin (C) and negative stain for estrogen-receptors (D), at magnification, ×200.
Discussion
The relation between the mutation of the APC gene and the consequent phenotype is important to establish an appropriate surveillance in a Family Risk Consulting, with a regular program of colonoscopy and upper gastrointestinal endoscopy to attempt evaluation of other FAP related lesions and also to prevent the development of life threatening conditions such as other neoplasms and the development of fibromatosis after surgical procedures.
We describe a peculiar case of bilateral breast fibromatosis after the placement of breast silicone prosthetics in a woman diagnosed with classic FAP (≥100 tubular adenomas in the colorectal specimen) with a nonsense mutation (c.935C>A) at codon 935 in exon 15 of APC gene. She also had antecedents of thyroidectomy for a pT1 cribriform-morular papillary thyroid carcinoma.
Thyroid carcinoma was identified after the diagnosis of germinal mutation, at the age of 16 years old. The search of extra-colonic manifestations allowed the identification of a single and rather small CMV-PTC, at an early age. CMV-PTC is a rare variant of thyroid carcinoma (0.1–0.2% of all papillary carcinomas) almost always associated with FAP (15). It affects female patients at 35 years' age or younger, being exceptional in pediatric patients, and sometimes preceding the colon manifestations (15). Characteristically presents distinctive morphological features with a cribriform and morular architecture, lack of nuclear atypia, mitosis or necrosis, and strong nuclear and cytoplasmic expression for β-catenin, that allows the diagnosis, and harbors germline mutations in the APC gene (exon 15), in more than 85% of patients. The majority of these germline mutations are found before codon 1220 and outside the mutation cluster region (codons 1286 to 1513) (16) and the overall prognosis of this variant is similar to that of classical variant of PTC.
The surge of bilateral breast fibromatosis was not anticipated, as this patient's mutation, located in exon 15 (c.935C>A), is seldom associated to fibromatosis; although recent studies demonstrated that fibromatosis can develop irrespective of the APC gene mutations site (1). In a genetic context of FAP, the surgical trauma of the colocation of breast implants, the continuous trauma of the prosthetics, seems to be concurrent factors for the development of bilateral fibromatosis, with an aggressive course and a rapid relapse.
All fibromatosis tumors, either superficial (fascial) or deep (muscle-aponeurotic), have a morphological similar pattern, corresponding to a proliferation of relatively monomorphic population of fibroblasts and myofibroblasts, almost without mitotic figures, arranged in long fascicles with dense collagen, with nuclear expression of β-catenin in about 67–80% of cases (4). Cytokeratin's expression, CD34 and S100 protein are negative (5). Some of the cases are positive for estrogen-receptors and may express smooth muscle actin and calponin (4). As breast fibromatosis is very rare (5,8) the histological differential diagnosis is challenging (7), mainly in core biopsies.
The differential diagnosis includes nodular fasciitis, neurofibroma, fibroadenoma, phyllodes tumor and low-grade fibromatosis-like spindle cell metaplastic carcinoma of breast (5). MRI is particularly important in the evaluation of margins and the eventual chest wall involvement, helping to plan surgery, but it does not assists in the differential diagnosis (5). MRI can also be used for assessing the response to medical treatments (5).
The clinical behavior may be indolent or progressive and there is no histological discriminator to predict the clinical behavior of fibromatosis.
In FAP patients' colon reducing risk surgery is effective being this patient controlled and well at present, but regarding fibromatosis, the best therapeutic approach is controversial in all locations (4,17–19).
Surgery, radiotherapy, anti-inflammatory drugs, hormone therapy, doxorubicin (DOX) or tyrosine kinase inhibitors (TKI) and a wait and watch approach have been used.
Previously, radical surgical resection with negative margins and radiotherapy were the first-line treatments (4,17,19). According to some authors, the clinical indolent or progressive behavior of fibromatosis could require different surgical approaches and different importance on negative margins (4,17). Surgery may be a problematic solution, because the growth factors released could lead to the activation of β-catenin, acting as a tumor enhancer factor for fibromatosis (17).
Radiotherapy has been used in extra-abdominal fibromatosis, as an adjuvant therapy after surgery or as the primary treatment in cases of non-resectable fibromatosis, with controversial results (4,17).
Systemic treatment regimens became more frequent in the last years (4,17). The most commonly used are nonsteroidal anti-inflammatory drugs like sulindac and indomethacin (4,17). Antiestrogen, most often tamoxifen, and anthracycline-containing regimes appear to be associated with higher response rates than TKI (17,19). Some studies suggest that tamoxifen combination with anti-inflammatory drugs is more effective than tamoxifen alone (17). Regarding TKI, imatinib and sorafenib have been tested, with better responses for sorafenib (4,17). Other drugs, like DOX and vinca alkaloids are reserved for rapidly progressive disease (4,17).
A treatment algorithm has been suggested by Bonvalot et al (17) and by the Consensus on sporadic desmoid-type fibromatosis, according with the indolent or progressive behavior of the fibromatosis (20).
In the present case, a wait and watch approach was initially used and the contraceptive subdermal implant was removed. After 1 month, the tumor continued to grow according to MRI evaluation. The estrogen-receptors immunostaining was negative, but a relative recent review (21) found that approximately half of the patients respond to tamoxifen, irrespective of the estrogen receptor status. Thus, tamoxifen treatment was initiated, after carefully weighing this therapy with the patient.
As the tumor continued to progress (Fig. 1C), one year after the diagnosis, another treatment strategy was advised and surgical excision with a thoracic wall reconstruction was performed. The relapsing tumor on right thoracic wall, one year after, was again approached by surgical excision. Currently the patient remains in surveillance 24 months after this surgery, without evidence of recurrence.
In conclusion, this is the first case reported in the literature of a patient with classic FAP and with antecedents of a cribriform-morular papillary thyroid carcinoma that developed an aggressive breast bilateral fibromatosis after breast silicone prosthetics. The knowledge of the relationship between the mutation of the APC gene and the consequent phenotype is important to establish appropriate surveillance protocols in FAP patients and to prevent the development of life threatening conditions.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- FAP
familial adenomatous polyposis
- APC
adenomatous polyposis coli
- CMV-PTC
cribriform-morular morphologic variant of papillary thyroid carcinoma
- MRI
magnetic resonance imaging
- DGGE
denaturing gradient gel electrophoresis
- PTT
protein truncation test
- DOX
doxorubicin
- TKI
tyrosine kinase inhibitors
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
SS made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, was involved in drafting the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RA and FC provided clinical data and contribute to the general discussion. PL contributed with familial and genetic data and to the general discussion. AC analyzed the data and participated in the discussion. AF analyzed the data, participated in the discussion and did the paper's review. SA made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, was involved in drafting the manuscript and in revising it critically, gave final approval of the version to be published, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Also, she got the funding for publication and supervised the research group. All authors approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from the patient in this clinical case to authorize its publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Calvert GT, Monument MJ, Burt RW, Jones KB, Randall RL. Extra-abdominal desmoid tumors associated with familial adenomatous polyposis. Sarcoma. 2012;2012:726537. doi: 10.1155/2012/726537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plawski A, Banasiewicz T, Borun P, Kubaszewski L, Krokowicz P, Skrzypczak-Zielinska M, Lubinski J. Familial adenomatous polyposis of the colon. Hered Cancer Clin Pract. 2013;11:15. doi: 10.1186/1897-4287-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colaco RJ, Menasce LP, Ranson M, Sobrinho Simoes M, Cameselle Teijeiro J, Vinjamuri S, Yap BK. A clinical case report of cribriform-morular variant of papillary thyroid carcinoma with neuroendocrine differentiation and aggressive behaviour in a patient with familial adenomatous polyposis coli. Thyroid Sci. 2009;4:1–3. [Google Scholar]
- 4.Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol. 2012;23:562–569. doi: 10.1093/annonc/mdr386. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahim L, Parry J, Taylor DB. Fibromatosis of the breast: A pictorial review of the imaging and histopathology findings. Clin Radiol. 2014;69:1077–1083. doi: 10.1016/j.crad.2014.05.105. [DOI] [PubMed] [Google Scholar]
- 6.Balzer BL, Weiss SW. Do biomaterials cause implant-associated mesenchymal tumors of the breast? Analysis of 8 new cases and review of the literature. Hum Pathol. 2009;40:1564–1570. doi: 10.1016/j.humpath.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Ünal B, Erdoğan G, Karaveli FŞ. Step by step approach to rare breast lesions containing spindle cells. Springerplus. 2015;4:678. doi: 10.1186/s40064-015-1480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mátrai Z, Tóth L, Gulyás G, Szabó E, Szentirmay Z, Kásler M. A desmoid tumor associated with a ruptured silicone breast implant. Plast Reconstr Surg. 2011;127:1e–4e. doi: 10.1097/PRS.0b013e3181f958ba. [DOI] [PubMed] [Google Scholar]
- 9.Jeong WS, Oh TS, Sim HB, Eom JS. Desmoid tumor following augmentation mammoplasty with silicone implants. Arch Plast Surg. 2013;40:470–472. doi: 10.5999/aps.2013.40.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amourak S, Alaoui FF, Jayi S, Chaara H, Melhouf MA. Desmoid fibromatosis of the breast: A case report on and a review of the literature. Pan Afr Med J. 2015;21:88. doi: 10.11604/pamj.2015.21.88.7124. (In French) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham SC, Reynolds C, Lee JH, Montgomery EA, Baisden BL, Krasinskas AM, Wu TT. Fibromatosis of the breast and mutations involving the APC/beta-catenin pathway. Hum Pathol. 2002;33:39–46. doi: 10.1053/hupa.2002.30196. [DOI] [PubMed] [Google Scholar]
- 12.Haggitt RC, Booth JL. Bilateral fibromatosis of the breast in Gardner syndrome. Cancer. 1970;25:161–166. doi: 10.1002/1097-0142(197001)25:1<161::AID-CNCR2820250123>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Filipe B, Baltazar C, Albuquerque C, Fragoso S, Lage P, Vitoriano I, Mão de Ferro S, Claro I, Rodrigues P, Fidalgo P, et al. APC or MUTYH mutations account for the majority of clinically well-characterized families with FAP and AFAP phenotype and patients with more than 30 adenomas. Clin Genet. 2009;76:242–255. doi: 10.1111/j.1399-0004.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- 14.Albuquerque C, Cravo M, Cruz C, Lage P, Chaves P, Fidalgo P, Suspiro A, Nobre Leitão C. Genetic characterisation of patients with multiple colonic polyps. J Med Genet. 2002;39:297–302. doi: 10.1136/jmg.39.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perea Del Pozo E, Ramirez Plaza C, Padillo Ruiz J, Martos Martínez JM. Cribiform variant of papillary thyroid cancer and familial adenomatous polyposis. Int J Surg Case Rep. 2015;16:192–194. doi: 10.1016/j.ijscr.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao X, Eu KW, Seow-Choen F, Zao Y, Cheah PY. APC mutation and phenotypic spectrum of Singapore familial adenomatous polyposis patients. Eur J Hum Genet. 2000;8:42–48. doi: 10.1038/sj.ejhg.5200397. [DOI] [PubMed] [Google Scholar]
- 17.Bonvalot S, Desai A, Coppola S, Le Péchoux C, Terrier P, Dômont J, Le Cesne A. The treatment of desmoid tumors: A stepwise clinical approach. Ann Oncol. 2012;23(Suppl 10):x158–x166. doi: 10.1093/annonc/mds298. [DOI] [PubMed] [Google Scholar]
- 18.Roussin S, Mazouni C, Rimareix F, Honoré C, Terrier P, Mir O, Dômont J, Le Péchoux C, Le Cesne A, Bonvalot S. Toward a new strategy in desmoid of the breast? Eur J Surg Oncol. 2015;41:571–576. doi: 10.1016/j.ejso.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Bonvalot S, Ternès N, Fiore M, Bitsakou G, Colombo C, Honoré C, Marrari A, Le Cesne A, Perrone F, Dunant A, Gronchi A. Spontaneous regression of primary abdominal wall desmoid tumors: More common than previously thought. Ann Surg Oncol. 2013;20:4096–4102. doi: 10.1245/s10434-013-3197-x. [DOI] [PubMed] [Google Scholar]
- 20.Kasper B, Baumgarten C, Bonvalot S, Haas R, Haller F, Hohenberger P, Moreau G, van der Graaf WT, Gronchi A, Desmoid Working Group Management of sporadic desmoid-type fibromatosis: A European consensus approach based on patients' and professionals' expertise-a sarcoma patients EuroNet and European Organisation for research and treatment of cancer/soft tissue and bone sarcoma group initiative. Eur J Cancer. 2015;51:127–136. doi: 10.1016/j.ejca.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Bocale D, Rotelli MT, Cavallini A, Altomare DF. Anti-oestrogen therapy in the treatment of desmoid tumours: A systematic review. Colorectal Dis. 2011;13:e388–e395. doi: 10.1111/j.1463-1318.2011.02758.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.