Abstract
Breast cancer (BC) is characterized by high incidences of bone metastases. Current treatment strategies for BC bone metastases primarily focused on breaking the ‘vicious osteolytic cycle’. Platelet-activating factor (PAF) is a potent phospholipid mediator, which has previously reported biological activities in BC progression and osteoclast differentiation by activating its receptor PAF receptor (PTAFR). However, the role of PAF in the mediation of BC bone metastases remains elusive. In the present study, it was revealed that the upregulation of PTAFR was associated with an increased incidence of bone metastases. It was also revealed that PAF significantly enhanced the processes of BC cell migration and BC mediated osteoclastogenesis. These results suggest that PAF serves a promotion role in BC bone metastases. It was further demonstrated that the natural PAF antagonist Kadsurenone may effectively attenuate each process by partially blocking the PAF/PTAFR signaling pathway. Therefore, targeting PAF/PTAFR by Kadsurenone may be a promising treatment strategy for BC bone metastases.
Keywords: breast cancer, bone metastases, PAF, Kadsurenone, vicious osteolytic cycle, osteoclastogenesis
Introduction
Breast cancer (BC) is the most common malignant tumor in women patients (1,2). BC is responsible for the majority of skeletal metastases, and the incidence of bone metastases is 65–75% in advanced diseases (3). Bone metastases are a major cause for morbidity, the median survival of BC patients with bone metastases is 19–25 months (4). Bone metastases are characterized by severe pain, impaired mobility, pathologic fractures, spinal cord compression, bone marrow aplasia and hypercalcemia (5,6). Metastatic cancer cells could secrete a series of factors into the bone-tumor microenvironment and form an osteolytic lesion, and bone matrix released growth factors could reversely stimulate BC cell proliferation. The interactions of BC cells and bone formed a ‘vicious osteolytic cycle’ (7,8). With the advantages of modern science, treatment strategies for primary breast tumors are well developed, while clinical interventions for bone lesions are still unsatisfying (9,10). Therefore, finding targets which could prevent or cure bone metastases is a priority for cancer treatment.
Platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-snglycero-3-phosphocholine), a potent phospholipid mediator, has been shown to play a role in a number of biological pathways including inflammatory diseases, cardiovascular homeostasis as well as in cancer, by binding to the G-protein coupled receptor (GPCR) PAF receptor (PTAFR) (11). PAF play important roles in platelet aggregation, stimulation of neutrophils and macrophages, inflammation and allergic responses (12,13). It also play vital roles in tumor neo-angiogenesis by activation of nuclear factor-κB (NF-κB) (14,15). PAF has been reported highly expressed in BC cells but not in normal MECs. The Kaplan-Meier analysis showed that high levels of PAF expression were strongly associated with poor prognosis in BC (16) Bussolati et al reported that BC cell lines MDA-MB-231 and MCF-7 could secret PAF and increase the motility of cancer cells by an autocrine manner (17). The upregulation of PTAFR has also been observed in several BC cell lines. Recently, Anandi et al reported that PAF could promote motility in BC cells and distupts non-transformed breast acinar structures (18). All these findings highlighted the potential role of PAF/PTAFR signaling pathway in BC progression.
The bone microenvironment is a highly dynamic system balanced between osteoclastic breakdown and osteoblastic rebuilding under tightly control in a local, coordinated, and sequential manner (19). Interruption of the homeostasis of bone microenvironment by tumor cells is one of the most important mechanisms of BC bone metastases. In vitro studies has demonstrated that PAF could directly enhance osteoclast motility and resorptive activity (20,21). Clinical studies also indicates that higher plasma PAF levels are associated with increased risk of vertebral fracture and lower bone mineral density in postmenopausal women (22). Hence, blocking PAF induced osteoclastogenesis might be an effective strategy in treatment of osteoclast related diseases, including osteolytic BC bone metastases.
Kadsurenone is a natural product isolated from stems of Piper kadsura. This herb is used in traditional Chinese medicine for the relief of diseases as asthma and rheumatoid arthritis (RA) (23). Kadsurenone have been demonstrated as a natural PAF inhibitor which could stops or diminishes all unwanted reactions induced by PAF (24). In the current study, we revealed that the upregulation of PTAFR is associated with increased incidence of bone metastases. We also demonstrated that Kadsurenone could effectively inhibit PAF induced BC cell migration. Furthermore, Kadsurenone could also attenuate BC induced osteolytic bone metastases by blocking the PAF/PTAFR signaling pathway.
Materials and methods
Bioinformatics analysis
The online Oncomine database (www.oncomine.org) were used to reveal PTAFR expression patterns between normal breast tissues and invasive breast carcinomas. Involved expression data were deposited in the National Center for Biotechnology Information/Gene Expression Omnibus database entries GSE9014. Data for survival analysis of bone metastasis and PTAFR expression patterns between osteoclatogenic (OG) and non-OG cell lines were obtained from GSE2603 and GSE43811, respectively. Survival analyses were based on Kaplan-Meier method, cutoff value was determined by ROC curves.
Cells and culture conditions
MDA-MB-231, RAW 264.7 and 293T cells were all obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). MDA-MB-231 and 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplied with 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and 100 U/ml penicillin-streptomycin (P/S; Gibco; Thermo Fisher Scientific, Inc.). RAW264.7 cells and mouse bone marrow monocytes (BMMs) were cultured in α-minimum essential medium (α-MEM; Gibco; Thermo Fisher Scientific, Inc.), supplied with 10% FBS and 100 U/ml P/S. Cells were cultured in a humidified incubator (Thermo Fisher Scientific, Inc.) with 5% CO2 at 37°C.
Cell viability assay
The viability and cytotoxicity effect of Kadsurenone was determined by the MTS method following the manual of CellTiter 96 Aqueous One Solution Cell Proliferation assay (Promega Corporation, Madison, WI, USA) with VERSA max microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA) as described previously.
Transwell asssy
The transwell assay were performed with Boyden chambers (Corning Incorporated, Corning, NY, USA). MDA-MB-231 cells were collected and resuspend in blank DMEM after 12-h serum starve. 6×105 cells were plated in the top chambers with or without 200 nM PAF and different concentrations (0, 0.5, 1, 2.5 and 5 µM) of Kadsurenone. The bottom chambers were filled with 600 µl mediums supplemented with 2% FBS. After 8 h incubation, migrated cells were fixed with 4% paraformaldehyde (PFA) and stained with 1% crystal violet. Images were taken using an Olympus inverted microscope (Olympus Corporation, Tokyo, Japan) and migrated cells were counted using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Luciferase reporter gene assay
Dual luciferase assays were conducted in a 24-well plate format. Vectors were transfected into 70% confluent HEK293 cells, PAF or receptor activator for NF-κB ligand (RANKL) inducement and different concentrations of Kadsurenone were added. After 48-h transfection, frefly and renilla luciferase were quantified sequentially using the Dual Luciferase Assay kit (Promega Corporation) following the manufacturer's recommendations.
BC cells induced osteoclast differentiation assay
2×103 MDA-MB-231 cells and 5X103 RAW264.7 cells were pooled together and seeded into 24-well plates. Cells were cultured in α-MEM added with 10% FBS and different concentrations of Kadsurenone. After 5–7 days, cells were fixed with 4% PFA and permeabilized with 0.1% Triton-X 100 inPBS for 5 min, and subjected to tartrate-resistant acid phosphatase (Trap) staining with Leukocyte acid phosphatase kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Trap positive osteoclasts were photographed and counted thereafter.
Mouse BMMs isolation and osteoclast differentiation assay
Mouse BMMs were flushed and isolated from C57/BL6 mice as previously described. 8×103 BMMs were seeded into 96-well plates and incubated with 10 ng/ml M-CSF, 50 ng/ml RANKL and different concentrations of Kadsurenone. After 5–7 days, cells were fixed and subjected to Trap staining and photographed. All experimental protocols were approved by the Review Committee for the Use of Human or Animal Subjects of East China Normal University and Chang Zheng Hospital (Shanghai, China).
Acting ring formation assay
Mouse BMMs were isolation and induced for osteoclast differentiation for 5–7 days. Cells were then permeabilized with Triton X-100 and incubated with rhodamine conjugated phalloidin (Molecular Probes; Thermo Fisher Scientific, Inc.) to visualize F-actin.
RNA extraction and gene expression analysis
Mouse BMMs were isolated and induced for osteoclast differentiation with or without indicated concentrations of Kadsurenone. Cells were collected and total RNA were extracted with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Then, 500 ng total RNA were reverse transcribed with PrimeScript™ RT Master Mix (Takara Biotechnology Co., Ltd., Dalian, China), according to the manufacturer's instructions. The complementary DNA was used for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and a final volume of 20 µl was adopted as follows: 10 µl 2X SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.), 1 µl forward and reverse primers, 2.5 µM, 2 µl cDNA, 7 µl ddH2O. PCR and data collection were performed on Mx3000P QPCR system (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA) and data analysis was operated with the 2−ΔΔCq method normalized to the endogenous control β-actin. Primers used in RT-qPCR are listed in Table I.
Table I.
Primer sequences for reverse transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence |
|---|---|---|
| β-actin | Forward | 5′-GTACGCCAACACAGTGCTG-3′ |
| Reverse | 5′-CGTCATACTCCTGCTTGCTG-3′ | |
| Ctsk | Forward | 5′-CTTCCAATACGTGCAGCAGA-3′ |
| Reverse | 5′-TCGGTTTCTTCTCCTCTGGA-3′ | |
| Trap | Forward | 5′-GCTGGAAACCATGATCACCT-3′ |
| Reverse | 5′-GAGTTGCCACACAGCATCAC-3′ | |
| Nfatc1 | Forward | 5′-TGGAGAAGCAGAGCACAGAC-3′ |
| Reverse | 5′-GCGGAAAGGTGGTATCTCAA-3′ |
Trap, tartrate-resistant acid phosphatase; Ctsk, cathepsin K; Nfatc1, nuclear factor of activated T cells 1.
Statistical analysis
Experiments were performed with 3 or more replicates. The results were reported as the mean ± standard error of the mean. The differences between control and experimental groups were determined using an unpaired Student's t-test or one-way analysis of variance followed by Tukey's post hoc test. Survival curves were analyzed by log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Bioinformatics analysis of PTAFR expression in BC tissues and osteoclastogenic (OG) cell lines
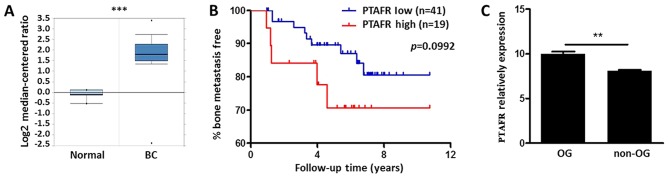
Using the Oncomine database (www.oncomine.org), we compared the expression patterns of PTAFR between normal breast tissues and invasive breast carcinomas PTAFR was significantly upregulated in cancer patients with a fold change of 3.88 (P<0.001; Fig. 1A). We further examined prognostic value of PTAFR expression level in BC bone metastasis by Minn's dataset (25). Although no significance was drown (P=0.0992, P<0.1), BC with higher expression levels of PTAFR (n=19) still indicated a tendency for bone metastases (Fig. 1B). We also revealed the expression patterns of PTAFR between OG and non-OG cell lines in GSE43811. PTAFR was significantly upregulated after RANKL stimulation in OG Raw264.7 cells (Fig. 1C).
Figure 1.
Bioinformatics analysis of PTAFR expression in BC tissues and OG cell lines. (A) Expression fold changes of PTAFR in patients with BS vs. normal breast tissues. The data were obtained from the Oncomine database. (B) Survival analysis using the PTAFR mRNA expression level for incidences of bone metastasis in Minn's dataset, P-values were calculated on the basis of a log-rank test. (C) Comparison of PTAFR mRNA relative expression in OG and non-OG cell lines following Rankl stimulation. Data were obtained from the GSE43811 dataset and are presented as the mean ± standard error of the mean (n=3). P-values were calculated using a Student's t-test. **P<0.01 and ***P<0.001. OG, osteoclastogenic; PTAFR, platelet-activating factor receptor; BC, breast cancer.
Kadsurenone inhibits PAF induced BC cell migration
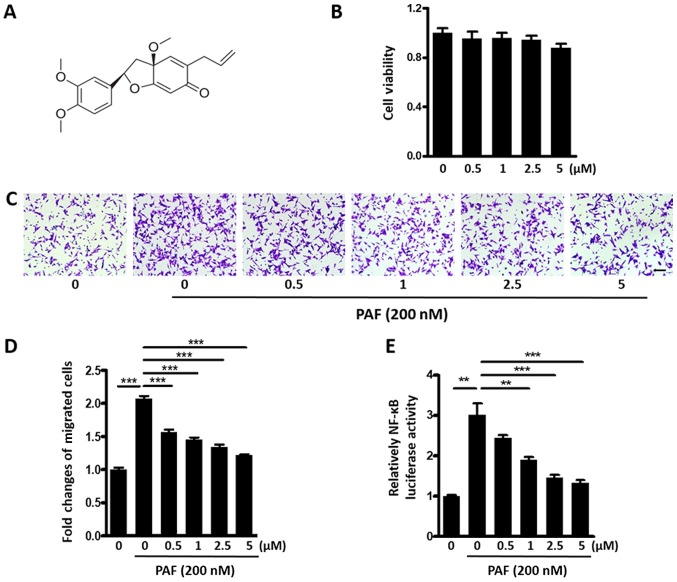
Kadsurenone is a natural product isolated from stems of Piper kadsura. Chemical structure of Kadsurenone was shown in Fig. 2A. We first tested cytotoxicity of Kadsurenone on BC cell line MDA-MB-231. No significant disturb of cell viability was observed with a highest concentration of 5 µM (Fig. 2B).
Figure 2.
Kadsurenone inhibits PAF induced BC cell migration. (A) Chemical structure of Kadsurenone. (B) Effect of Kadsurenone on the viability of BC cell line MDA-MB-231. MDA-MB-231 cells were treated with the indicated concentrations of Kadsurenone. (C) Transwell assay of MDA-MB-231 cells induced by PAF (200 nM) with the presence or absence of the indicated concentrations of Kadsurenone. (D) Graphs represent the mean fold changes of migrated cells from three independent experiments. (E) Relative NF-κB luciferase activity of MDA-MB-231 cells induced by PAF (200 nM) with the presence or absence of indicated concentrations of Kadsurenone. Data are presented as the mean ± standard error of the mean (n=3). Scale bar, 100 µm. P-values were calculated using one-way analysis of variance followed by Tukey's post hoc test. **P<0.01 and ***P<0.001. PAF, platelet-activating factor; BC, breast cancer; NF, nuclear factor.
We then examined effects of Kadsurenone on PAF induced MDA-MB-231 cells migration. Transwell assay shown that Kadsurenone could dose dependently inhibit PAF induced BC cells migration (Fig. 2C and D). Luciferase assay indicated that the NF-κB activity of MDA-MB-231 cells were significantly inhibited by Kadsurenone dose dependently (Fig. 2E). Our data proved that Kadsurenone did not disturb the cell viability significantly but suppressed cell migration in MDA-MB-231 cells, which indicated that PAF/PTAFR played key role in cell migration but not cell viability.
Kadsurenone inhibits BC cells induced osteoclastogenesis
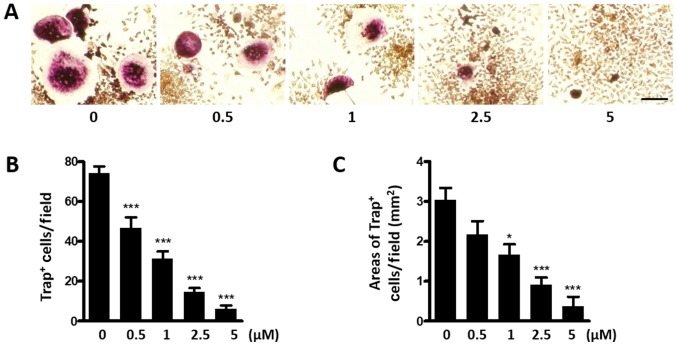
In the bone microenvironment, BC cells could stimulate osteoclast differentiation directly through excretion of cytokines such as interleukin (IL)-1β, tumor necrosis factor-α (TNFα). BC cells could also indirectly promote osteoclast differentiation via upregulation of RANKL by osteoblast. Therefore, we intended to study whether Kadsurenone could directly inhibit BCs induced osteoclastogenesis. MDA-MB-231 cells, which could secret PAF, were co-cultured with the OG cell line RAW264.7 cells. We proposed to use this co-culture system mimic the BC bone metastases microenvironment. BC cells could induce RAW264.7 cells differentiate into Trap positive osteoclasts. However, this process could be attenuated by Kadsurenone dose dependently (Fig. 3).
Figure 3.
Kadsurenone inhibits breast cancer cells induced osteoclastogenesis. (A) Representative images of Trap+ osteoclasts. MDA-MB-231 and RAW264.7 cells were co-cultured and treated with the indicated concentrations of Kadsurenone and stained on day 7. The (B) number and (C) area of Trap+ osteoclasts per field are reported. Scale bar, 100 µm. Data are presented as the mean ± standard error of the mean (n=3). P-values were calculated using one-way analysis of variance followed by Tukey's post hoc test. *P<0.05 and ***P<0.001 vs. the control. Trap, tartrate-resistant acid phosphatase.
Kadsurenone directly inhibits RANKL induced osteoclastogenesis
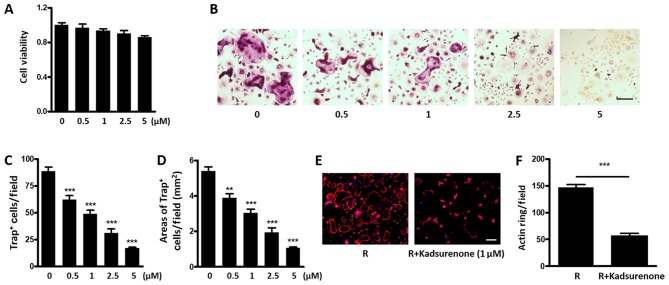
It is reported that BMMs could secret PAF as an autocrine factor and promote the OG process (26). Therefore, we intend to investigate whether Kadsurenone could directly affect osteoclastogenesis. We firstly confirmed that no significant cytotoxicity of Kadsurenone on mouse BMMs under a certain concentration (Fig. 4A). However, the process of RANKL induced Trap positive osteoclasts differentiated by BMMs were significantly attenuated by Kadsurenone dose dependently (Fig. 4B-D). Meanwhile, acting ring formation were also restrained (Fig. 4E and F).
Figure 4.
Kadsurenone directly inhibits RANKL induced osteoclastogenesis. (A) Effect of Kadsurenone on the viability of mouse BMMs. Mouse BMMs were isolated and treated with the indicated concentrations of Kadsurenone. (B) Representative images of Trap+ osteoclasts. Mouse BMMs were isolated and induced for osteoclastogenesis with RANKL (50 ng/ml). Cells were treated with thye indicated concentrations of Kadsurenone and stained on day 7. The (C) number and (D) area of Trap+ osteoclasts per field are indicated (E) Representative images of actin ring staining. Mouse BMMs were isolated and induced for osteoclastogenesis with RANKL (50 ng/ml). Cells were treated with the indicated concentrations of Kadsurenone and stained on day 7. (F) The bar chart represents the mean number of actin rings for three independent experiments. Scale bar, 100 µm. Data are presented as the mean ± standard error of the mean (n=3). P-values were calculated using one-way analysis of variance followed by Tukey's post hoc test. **P<0.01 and ***P<0.001 vs. the control. BMMs, bone marrow monocytes; RANKL, receptor activator for NF-κB ligand; Trap, tartrate-resistant acid phosphatase.
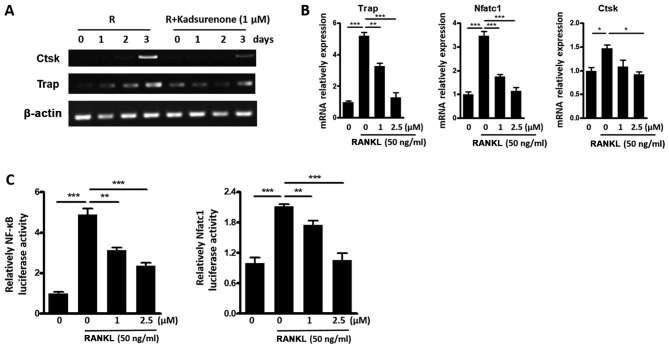
Kadsurenone inhibits RANKL induced OC marker genes expression by inhibiting the NF-κB pathway
To investigate potential mechanisms of the inhibitory function of Kadsurenone on BMMs differentiation. RT-PCR (Fig. 5A) and RT-qPCT (Fig. 5B) assay were performed. Results indicated that the expression of osteoclast marker genes Ctsk, Trap, and nuclear factor of activated T cells 1 (Nfatc1) were all inhibited by Kadsurenone. Luciferase assay indicated that the transcription factors NF-κB and Nfatc1 activities were all attenuated (Fig. 5C).
Figure 5.
Kadsurenone inhibits RANKL induce OC marker gene expression by inhibiting the NF-κB signaling pathway. (A) RT-qPCR analysis of the OC marker gene Ctsk and Trap expression. Mouse BMMs were isolated and induced for osteoclastogenesis with Rank l (50 ng/ml). Cells were treated with or without 1 µM Kadsurenone. mRNAs were harvested on the indicated days. (B) RT-qPCR analysis of the OC marker genes Trap, Nfatc1 and Ctsk expression. Mouse BMMs were isolated and induced for osteoclastogenesis with RANKL (50 ng/ml). Cells were treated with the indicated concentrations of Kadsurenone. mRNAs were harvested on day 3. (C) Relative NF-κB (left) and Nfatc1 (right) luciferase activity of mouse BMMs induced by RANKL (50 ng/ml) with the presence or absence of the indicated concentrations of Kadsurenone. Data are presented as the mean ± standard error of the mean (n=3). P-values were calculated using one-way analysis of variance followed by Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001 vs. the control. BMMs, bone marrow monocytess; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; OC, osteoclast; Ctsk, cathepsin K; Nfatc1, nuclear factor of activated T cells 1; Trap, tartrate-resistant acid phosphatase; RANKL, receptor activator for NF-κB ligand; NF, nuclear factor.
Discussion
Bone metastasis is a deleterious and debilitating aspect of BC patients. Up to 70% of BC patients who succumb to disease present with bone metastases on autopsy (27). Bone metastases lesions are primarily osteolytic since BC cells could regulate the bone-tumor microenvironment (28–30). Osteoclasts were over activated by BC cells while BC cells were reversely promoted by cytokines released from bone matrix degradation. Current treatment strategies for BC bone metastases mainly focused on breaking such ‘vicious osteolytic cycle’ (31–33). Bioinformatics analysis provided clues that the PAF/PTAFR signaling pathway may play important roles in regulating BC bone metastasis and osteoclastogenesis. In the current study, we demonstrated that PAF could promote BC cell migration and induce BMMs differentiation. Besides, it has been reported that absence of PAFR protects mice from osteoporosis following ovariectomy, and PAF could enhance osteoclast differentiation directly. Kadsurenone could attenuate both processes, and could be considered as a potential therapeutic reagent for BC bone metastases.
PAF can be synthesized within variety of cells like platelets, macrophages, eosinophils, basophils and endothelial cells by de novo or remodeling pathways and cause different physiological reactions (34,35). The expression of PAF and its receptor PTAFR in BC cell lines have also been demonstrated (17,18). In the tumor microenvironment, PAF is secreted and accumulated by infiltrated inflammation cells and tumor cells per se, which forms an autocrine loop. PTAFR is a GPCR. PAF/PTAFR activation resulted complex cell responses including increase of cell motility, upregulation of IL-6 and matrix metalloproteinase (MMP) expression, and activation of NF-κB signaling (34). The increased migration ability is an important characteristic for cancer cells forming metastatic lesions, including bone metastases (36). Both of published literatures and our results revealed increased cell migration ability of BC cells after PAF treatment. To avoid the harmful effects of PAF, mostly PAF inhibitors are used to abolish or attenuate the PAF/PTAFR signaling activation (37,38). Comparing to synthetic PAF inhibitors, natural inhibitors are preferred due to the safety reason (34). In the current study, we used the natural herbal extraction Kadsurenone effectively abolished PAF induced BC migration, and little cytotoxicity manifested.
The great majority of BC produces osteolytic bone metastases (9,39,40). This process is characterized by over activated osteoclastogenesis and subsequent bone destruction. BC cells could form a complex and multigenic programed ‘vicious osteolytic cycle’ to remold the bone microenvironment and promote cancer progression after, or even before, bone colonization (28,36,41). Cytokines secreted by BC cells like IL-6, parathyroid hormone like hormone (PTHRP) and TNFα could stimulate osteoblasts and upregulate the RANKL/osteoprotegerin (OPG) ratio, which further stimulates the development of osteoclasts from myeloid precursors. At the same time, other cytokines such as IL-11 and osteopontin (OPN) could directly promote ostoclastogenesis. Activated osteoclasts degrade the bone matrix, and release transforming growth factor β (TGFβ), bone morphogenetic protein (BMPs) and insulin-like growth factor (IGFs), which are mainly stored in bone matrix, reversely stimulate BC progression.
Comparing to the ‘vicious osteolytic cycle’, PAF could promote BMMs differentiate into osteoclasts by two parallel manners. On one hand, PAF could stimulate BC cells upregulate downstream signaling such as IL-6, IL-11, MMPs and NF-κB, which further activate osteoclastogenesis (34). On the other hand, PAF could also directly activate BMMs differentiate into osteoclasts (20,21). In the current study, we demonstrated that Kadsurenone could inhibit both the BC induced or PAF directly stimulated osteoclastogenesis. Expression of osteoclast differentiation markers are all downregulated after Kadsurenone treatment. It is known that NFATc1 is the master transcription factor for osteoclast differentiation. NF-κB regulates RANKL induced osteoclast differentiation mainly through promoting NFATc1 transcription activity. NF-κB is a critical signal for OC differentiation downstream of RANKL, Mice lacking the subunit p50 and p52 of NF-κB signal pathway have been shown to be osteopetrotic p50-/- or p52-/- precursors fail to form OCs. The results in our study indicated that PAF might partially inhibited NF-κB activation and osteoclast differentiation via blocking NFATc1 transcription activity.
In conclusion, Kadsurenone maybe an effectively strategy to attenuate BC formed osteolytic bone metastases by blocking the PAF/PTAFR signaling. Nonetheless, many questions pertaining to be answered. Further studies are requested to reveal detailed mechanisms of Kadsurenone function and in vivo studies are needed.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- BC
breast cancer
- PAF
platelet-activating factor
- PTAFR
platelet-activating factor receptor
- OG
osteoclatogenic
- NF-κB
nuclear factor-κB
- P/S
penicillin-streptomycin
- BMMs
bone marrow monocytes
- α-MEM
α-minimum essential medium
- Trap
tartrate-resistant acid phosphatase
- RANKL
receptor activator for NF-κB ligand
- GPCR
G-protein coupled receptor
- IL
interleukin
- MMP
matrix metalloproteinase
- PTHRP
parathyroid hormone like hormone
- TNFα
tumor necrosis factor-α
- OPG
osteoprotegerin
- OPN
osteopontin
- TGFβ
transforming growth factor-β
- BMP
bone morphogenetic protein
- IGF
insulin-like growth factor
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HT performed the experiments and drafted the manuscript. LY, LS and ZC performed the data analyses and wrote the manuscript. JY, WD, TL, ZM, XW, QM and WZ performed the experiments. ZJ and WH helped perform the data analysis. LZ contributed to the conception of the study. XJ contributed to the conception of the study and revised the manuscript critically for important intellectual content.
Ethics approval and consent to participate
All experimental protocols were approved by the Review Committee for the Use of Human or Animal Subjects of East China Normal University and Chang Zheng Hospital.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 4.Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: A review. Crit Rev Oncol Hematol. 2005;56:365–378. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe P, Connock M, Shyangdan D, Court R, Kandala NB, Clarke A. A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol Assess. 2013;17:1–274. doi: 10.3310/hta17420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly ML, Kshettry VR, Rosenbaum BP, Seicean A, Weil RJ. Effect of a randomized controlled trial on the surgical treatment of spinal metastasis, 2000 through 2010: A population-based cohort study. Cancer. 2014;120:901–908. doi: 10.1002/cncr.28497. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen DX, Bos PD, Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland A, Forsyth A, Cong Y, Grant L, Juan TH, Lee JK, Klimowicz A, Petrillo SK, Hu J, Chan A, et al. The role of prolactin in bone metastasis and breast cancer cell-mediated osteoclast differentiation. J Natl Cancer Inst. 2015;108 doi: 10.1093/jnci/djv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Moos R, Sternberg C, Body JJ, Bokemeyer C. Reducing the burden of bone metastases: Current concepts and treatment options. Support Care Cancer. 2013;21:1773–1783. doi: 10.1007/s00520-013-1755-1. [DOI] [PubMed] [Google Scholar]
- 10.Li BT, Wong MH, Pavlakis N. Treatment and prevention of bone metastases from breast cancer: A comprehensive review of evidence for clinical practice. J Clin Med. 2014;3:1–24. doi: 10.3390/jcm3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiani E, Ullrich SE. Understanding the connection between platelet-activating factor, a UV-induced lipid mediator of inflammation, immune suppression and skin cancer. Prog Lipid Res. 2016;63:14–27. doi: 10.1016/j.plipres.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pałgan K, Bartuzi Z. Platelet activating factor in allergies. Int J Immunopathol Pharmacol. 2015;28:584–589. doi: 10.1177/0394632015600598. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Shields LBE, Gao Z, Wang Y, Zhang YP, Chu T, Zhu Q, Shields CB, Cai J. Current understanding of platelet-activating factor signaling in central nervous system diseases. Mol Neurobiol. 2017;54:5563–5572. doi: 10.1007/s12035-016-0062-5. [DOI] [PubMed] [Google Scholar]
- 14.McHowat J, Gullickson G, Hoover RG, Sharma J, Turk J, Kornbluth J. Platelet-activating factor and metastasis: Calcium-independent phospholipase A2β deficiency protects against breast cancer metastasis to the lung. Am J Physiol Cell Physiol. 2011;300:C825–C832. doi: 10.1152/ajpcell.00502.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JT, Kral JG. The NF-kappaB/IkappaB signaling system: A molecular target in breast cancer therapy. J Surg Res. 2005;123:158–169. doi: 10.1016/j.jss.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Jung YS, Jun S, Lee S, Wang W, Schneider A, Oh Sun Y, Lin SH, Park BJ, Chen J, et al. PAF-Wnt signaling-induced cell plasticity is required for maintenance of breast cancer cell stemness. Nat Commun. 2016;7:10633. doi: 10.1038/ncomms10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bussolati B, Biancone L, Cassoni P, Russo S, Rola-Pleszczynski M, Montrucchio G, Camussi G. PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. Am J Pathol. 2000;157:1713–1725. doi: 10.1016/S0002-9440(10)64808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anandi VL, Ashiq KA, Nitheesh K, Lahiri M. Platelet-activating factor promotes motility in breast cancer cells and disrupts non-transformed breast acinar structures. Oncol Rep. 2016;35:179–188. doi: 10.3892/or.2015.4387. [DOI] [PubMed] [Google Scholar]
- 19.Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8:225–235. doi: 10.1177/1759720X16670154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng ZG, Wood DA, Sims SM, Dixon SJ. Platelet-activating factor stimulates resorption by rabbit osteoclasts in vitro. Am J Physiol. 1993;264:E74–E81. doi: 10.1152/ajpendo.1993.264.1.E74. [DOI] [PubMed] [Google Scholar]
- 21.Wood DA, Hapak LK, Sims SM, Dixon SJ. Direct effects of platelet-activating factor on isolated rat osteoclasts. Rapid elevation of intracellular free calcium and transient retraction of pseudopods. J Biol Chem. 1991;266:15369–15376. [PubMed] [Google Scholar]
- 22.Kim H, Kim BJ, Ahn SH, Lee SH, Koh JM. Higher plasma platelet-activating factor levels are associated with increased risk of vertebral fracture and lower bone mineral density in postmenopausal women. J Bone Miner Metab. 2015;33:701–707. doi: 10.1007/s00774-014-0634-2. [DOI] [PubMed] [Google Scholar]
- 23.Huang SP, Lin LC, Wu YT, Tsai TH. Pharmacokinetics of kadsurenone and its interaction with cyclosporin A in rats using a combined HPLC and microdialysis system. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:247–252. doi: 10.1016/j.jchromb.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, Li R, Yu H, Shi D, Dong N, Zhang S, Wang H. Development of an LC-MS/MS method for quantification of kadsurenone in rat plasma and its application to a pharmacokinetic study. Biomed Chromatogr. 2013;27:1754–1758. doi: 10.1002/bmc.2989. [DOI] [PubMed] [Google Scholar]
- 25.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hikiji H, Ishii S, Shindou H, Takato T, Shimizu T. Absence of platelet-activating factor receptor protects mice from osteoporosis following ovariectomy. J Clin Invest. 2004;114:85–93. doi: 10.1172/JCI20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RW, Finger EC, Olcina MM, Vilalta M, Aguilera T, Miao Y, Merkel AR, Johnson JR, Sterling JA, Wu JY, Giaccia AJ. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol. 2016;18:1078–1089. doi: 10.1038/ncb3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 29.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 30.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, Goncalves F. Bone metastases: An overview. Oncol Rev. 2017;11:321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trémollieres FA, Ceausu I, Depypere H, Lambrinoudaki I, Mueck A, Pérez-López FR, van der Schouw YT, Senturk LM, Simoncini T, Stevenson JC, et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas. 2017;95:65–71. doi: 10.1016/j.maturitas.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Van Acker HH, Anguille S, Willemen Y, Smits EL, Van Tendeloo VF. Bisphosphonates for cancer treatment: Mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24–40. doi: 10.1016/j.pharmthera.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Santini D, Stumbo L, Spoto C, D'Onofrio L, Pantano F, Iuliani M, Fioramonti M, Zoccoli A, Ribelli G, Virzì V, et al. Bisphosphonates as anticancer agents in early breast cancer: preclinical and clinical evidence. Breast Cancer Res. 2015;17:121. doi: 10.1186/s13058-015-0634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh P, Singh IN, Mondal SC, Singh L, Garg VK. Platelet-activating factor (PAF)-antagonists of natural origin. Fitoterapia. 2013;84:180–201. doi: 10.1016/j.fitote.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Tsoupras AB, Fragopoulou E, Nomikos T, Iatrou C, Antonopoulou S, Demopoulos CA. Characterization of the de novo biosynthetic enzyme of platelet activating factor, DDT-insensitive cholinephosphotransferase, of human mesangial cells. Mediators Inflamm. 2007;2007:27683. doi: 10.1155/2007/27683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: A fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet-activating factor (PAF): A review of its role in asthma and clinical efficacy of PAF antagonists in the disease therapy. Recent Pat Inflamm Allergy Drug Discov. 2008;2:72–76. doi: 10.2174/187221308783399306. [DOI] [PubMed] [Google Scholar]
- 38.Imrie CW, McKay CJ. The possible role of platelet-activating factor antagonist therapy in the management of severe acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:357–364. doi: 10.1053/bega.1999.0030. [DOI] [PubMed] [Google Scholar]
- 39.Waning DL, Guise TA. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin Cancer Res. 2014;20:3071–3077. doi: 10.1158/1078-0432.CCR-13-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox TR, Gartland A, Erler JT. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 2016;76:188–192. doi: 10.1158/0008-5472.CAN-15-2306. [DOI] [PubMed] [Google Scholar]
- 41.Cox TR, Rumney RM, Schoof EM, Perryman L, Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.