Abstract
The present study aimed to identify new key genes as potential biomarkers for the diagnosis, prognosis or targeted therapy of clear cell renal cell carcinoma (ccRCC). Three expression profiles (GSE36895, GSE46699 and GSE71963) were collected from Gene Expression Omnibus. GEO2R was used to identify differentially expressed genes (DEGs) in ccRCC tissues and normal samples. The Database for Annotation, Visualization and Integrated Discovery was utilized for functional and pathway enrichment analysis. STRING v10.5 and Molecular Complex Detection were used for protein-protein interaction (PPI) network construction and module analysis, respectively. Regulation network analyses were performed with the WebGestal tool. UALCAN web-portal was used for expression validation and survival analysis of hub genes in ccRCC patients from The Cancer Genome Atlas (TCGA). A total of 65 up- and 164 downregulated genes were identified as DEGs. DEGs were enriched with functional terms and pathways compactly related to ccRCC pathogenesis. Seventeen hub genes and one significant module were filtered out and selected from the PPI network. The differential expression of hub genes was verified in TCGA patients. Kaplan-Meier plot showed that high mRNA expression of enolase 2 (ENO2) was associated with short overall survival in ccRCC patients (P=0.023). High mRNA expression of cyclin D1 (CCND1) (P<0.001), fms related tyrosine kinase 1 (FLT1) (P=0.004), plasminogen (PLG) (P<0.001) and von Willebrand factor (VWF) (P=0.008) appeared to serve as favorable factors in survival. These findings indicate that the DEGs may be key genes in ccRCC pathogenesis and five genes, including ENO2, CCND1, PLT1, PLG and VWF, may serve as potential prognostic biomarkers in ccRCC.
Keywords: clear cell renal cell carcinoma, bioinformatics, differentially expressed genes, biomarkers, Kaplan-Meier plot
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all human malignancies (1). It is estimated that more than 338,000 people are diagnosed with RCC each year, with a 22% increase projected by 2020; there are more than 140,000 RCC-related deaths per year (2). Clear cell renal cell carcinoma (ccRCC) is the most common (~75%), lethal subtype of RCC (3). Over the past decade, with improved surgical procedures and the application of specific targeted drugs, the survival of RCC patient has markedly improved (4). However, early accurate diagnosis of ccRCC is still a great challenge and chemotherapeutic or radiotherapeutic resistance remains (4).
A comprehensive understanding of ccRCC initiation, progression and metastasis contributes to early diagnosis and precise treatment. Previous studies have demonstrated that mutations of VHL are significant drivers of ccRCC by regulating various biological processes, and VHL alterations are considered as prognostic markers in ccRCC (5). Moreover, targeted therapies associated with the pVHL/HIF pathway have been tested in phase 3 trials (4). VHL alterations alone are insufficient to cause the cancer, as ccRCC is a systemic biological disease. Sequencing studies have identified some other specific molecular genetic alterations of ccRCC, such as mutations of TCEB1 (6), PBRM1 (7) and abnormal expression of miR-92 (8), miR-210 (9). Further insights into the molecular biology of ccRCC could help us find some novel molecular biomarkers and potential targets for early diagnosis and precise treatment.
Gene expression profiling arrays make it possible to identify numerous differentially expressed genes in tumor samples compared to non-tumor samples at the same time. In this study, we performed an integrated bioinformatics analysis of three gene expression profiles and identified several differentially expressed genes (DEGs) in ccRCC tissues compared with normal controls. We executed functional and pathway enrichment analysis, protein-protein interaction (PPI) network analysis of DEGs and employed the Kaplan-Meier method to analyze survival associated with hub genes. We intended to provide further insights into the complex molecular biology of ccRCC pathogenesis and to identify new key genes that may be candidates for diagnostic biomarkers, prognostic indicators or potential targets of precise therapy.
Materials and methods
Data collection
Three gene expression profiles (GSE36895, GSE46699 and GSE71963) were acquired from Gene Expression Omnibus (GEO) database, a free public genomics data repository for array- and sequence-based data.
The array data of GSE36895 included 29 ccRCC tumor samples and 23 matched adjacent normal kidney cortices (10). GSE46699 was comprised of 126 samples including 65 ccRCC tumors and 61 patient-matched adjacent-normal tissues (11). GSE71963 contained 32 ccRCC tumor samples and 16 normal kidney samples (12).
Data processing
GEO2R, a tool for online analysis of GEO series based on the R programming language (13), was used to screen DEGs between the normal kidneys and ccRCC samples. Adjusted P-value (adj. P) and |log Fold Change| (|log FC|) were used to select significant DEGs. adj. P<0.05 and |log FC| >2 were chosen as the cutoff criteria.
Functional and pathway enrichment analysis
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of DEGs was carried out using The Database for Annotation, Visualization and Integrated Discovery (DAVID) online (14,15). P<0.05 was selected as the cutoff value.
PPI network construction and significant module analysis
STRING v10.5 was utilized for functional interaction analysis to construct a PPI network (16). Confidence scores >0.7 were considered significant. Genes with degrees >10 were selected as hub genes. The PPI network was visualized by Cytoscape software, and module of PPI network was screened by the Molecular Complex Detection (MCODE) in Cytoscape. The parameters were set as follows: Degree cutoff: 2, node score cutoff: 0.2, k-core: 2, and max. depth: 100 (17). The functional and pathway enrichment analysis of the significant module was carried out by DAVID.
Regulation network analyses
The miRNAs and transcription factors (TFs) that potentially regulated the DEGs were predicted using Overrepresentation Enrichment Analysis (ORA) in WebGestal software (18). Then miRNA-target network and TF-target network were also visualized using Cytoscape software.
TCGA verification and survival analysis of hub genes
UALCAN, a tool for in-depth analyses of The Cancer Genome Atlas (TCGA) data, was utilized to verify the differences in expression levels of hub genes (19). The correlation of hub genes with overall survival (OS) of ccRCC patients was examined by recruiting UALCAN as well. Patient data were categorized into two groups based on transcripts per million (TPM) value. The data with TPM greater than upper quartile were assigned to a high expression group and the others with TPM below upper quartile belonged to low/medium expression group. Survival analysis was performed by Kaplan-Meier method, and the log-rank test was carried out. P<0.05 was selected as the cutoff value.
Results
Identification of DEGs in ccRCC
A total of 591, 325 and 1118 genes were extracted from the GSE36895, GSE46699 and GSE71963 datasets, respectively. There were 229 genes consistently differentially expressed in all three datasets (Fig. 1), including 65 upregulated DEGs and 164 downregulated DEGs in ccRCC tissues compared with normal kidney tissues (Table I).
Figure 1.
Identification of DEGs in three mRNA expression profiles (GSE36895, GSE46699 and GSE71963). DEGs, differentially expressed genes.
Table I.
DEGs in ccRCC tissues compared with normal controls.
| DEGs | Gene name |
|---|---|
| Upregulated | TNFAIP6, PFKP, NDUFA4L2, CXCR4, NPTX2, C1QC, FLT1, LOX, PDK1, COL23A1, CDCA2, GAS2L3, KCNK3, NETO2, FABP7, RNASET2, ANGPTL4, GJC1, SCD, HILPDA, LOXL2, DGCR5, CA9, EGLN3, ENO2, TMEM45A, PPP1R3C, CAV2, VWF, CCND1, ST8SIA4, C3, DIRAS2, IGFBP3, FABP5, LAMA4, SAP30, CD36, CTHRC1, GAL3ST1, HK2, VEGFA, SCARB1, AHNAK2, CAV1, TGFBI, INHBB, ZNF395, PLOD2, TMCC1, PLXDC1, BHLHE41, CYP2J2, SPAG4, LPCAT1, CP, C1QB, FAM26F, APOC1, ENPP3, SLC6A3, ACKR3, ANGPT2, NOL3, ESM1 |
| Downregulated | PTGER3, ERBB4, RALYL, L1CAM, XPNPEP2, SLC4A1, MPPED2, EHF, HMGCS2, HPD, GGACT, SLC7A13, HRG, UGT3A1, GATA3, TMEM174, SLC13A1, PROM2, CALB1, SUSD2, KCNJ1, SLC12A3, CRYAA, HSD11B2, DEFB1, GPC5, CYP27B1, UCHL1, FABP1, TMEM30B, CYP4F2, NELL1, MTURN, FGF9, NPHS2, PSAT1, SLC4A9, TFCP2L1, ALDH4A1, SLC12A1, ERP27, ALDH8A1, SCIN, TSPAN8, KL, AZGP1, SLC22A6, EFHD1, LOC100505985, CRHBP, AQP2, ASS1, TACSTD2, PVALB, FOXI1, ABAT, TMEM52B, IRX2, MIOX, PIGR, ATP6V1G3, SEMA6D, S100A2, SCD5, MAL, FGF1, SORD, DMRT2, TFAP2B, GLDC, FBP1, RASD1, PLPPR1, CYP4F3, GSTM3, ESRRG, SLC47A2, KNG1, SLC34A1, MUC15, PTPRO, DPEP1, MECOM, ACSF2, CYP17A1, MT1G, PLG, UPP2, MFSD4A, SLC22A8, HAO2, ALDH6A1, MT1F, TMEM213, CHL1, EGF, DCXR, UMOD, ATP6V0D2, ANK2, HOGA1, DIO1, ELF5, SCNN1A, HSPA2, SOSTDC1, TYRP1, ENPP6, PCP4, GPC3, HS6ST2, CLDN8, PCK1, SLC5A2, NOX4, BMPR1B, G6PC, WNK4, ADH6, HEPA, CAM2, SOST, SH3GL2, SCNN1B, ALB, ALDOB, DCN, SCNN1G, KCNJ10, SLC13A3, SUCNR1, AFM, RAB25, ACPP, HPGD, FXYD4, DNER, RHCG, CYP4A11, CTXN3, KCNJ15, GRB14, PTH1R, GGT6, SLC26A7, C7, TMEM178A, OGDHL, ATP6V1B1, DUSP9, SERPINA5, SFRP1, CLCNKB, SLC7A8, SLC7A8, PIPOX, MAL2, PDE1A, TMPRSS2, GPAT3, PRODH2, FAM151A, EPCAM, MRO, ATP6V0A4 |
A total of 65 upregulated DEGs and 164 downregulated DEGs were identified in ccRCC tissues, compared with normal kidney tissues. The hub genes were shown in boldface. DEGs, differentially expressed genes; ccRCC, clear cell renal cell carcinoma.
GO analysis of DEGs in ccRCC
After performing GO analysis of DEGs with DAVID online, the DEGs were classified into three groups: biological process group, molecular function group and cellular component group. We found that the upregulated genes were mainly enriched in biological processes related to hypoxia, blood vessel morphogenesis and angiogenesis. The downregulated genes were commonly involved in functional terms associated with cellular components, metabolism and homeostasis.
Pathway enrichment analysis of DEGs in ccRCC
KEGG pathway enrichment analysis of DEGs was also conducted with DAVID online. KEGG results of the up- and downregulated genes were displayed in Tables II and III, respectively. The upregulated genes were mostly enriched in HIF-1 signaling pathway, PPAR signaling pathway, focal adhesion, coagulation cascades and AMPK signaling pathway. The downregulated genes were mainly enriched in metabolic pathways, collecting duct acid secretion, aldosterone-regulated sodium reabsorption, carbon metabolism and biosynthesis of antibiotics.
Table II.
KEGG pathway enrichment analysis of 65 upregulated DEGs.
| Pathway | Name | P-value | Genes |
|---|---|---|---|
| hsa04066 | HIF-1 signaling pathway | 1.14×10−5 | PDK1, FLT1, VEGFA, EGLN3, ENO2, HK2, ANGPT2 |
| hsa03320 | PPAR signaling pathway | 4.19×10−4 | CD36, SCD, FABP7, FABP5, ANGPTL4 |
| hsa04510 | Focal adhesion | 7.01×10−4 | CAV2, VWF, LAMA4, CAV1, CCND1, FLT1, VEGFA |
| hsa04610 | Complement and coagulation cascades | 5.81×10−3 | C1QB, VWF, C3, C1QC |
| hsa04152 | AMPK signaling pathway | 2.70×10−2 | CCND1, CD36, SCD, PFKP |
| hsa05150 | Staphylococcus aureus infection | 3.35×10−2 | C1QB, C3, C1QC |
| hsa04151 | PI3K-Akt signaling pathway | 3.53×10−2 | VWF, LAMA4, CCND1, FLT1, VEGFA, ANGPT2 |
| hsa05230 | Central carbon metabolism in cancer | 4.57×10−2 | PDK1, PFKP, HK2 |
| hsa00010 | Glycolysis/Gluconeogenesis | 4.96×10−2 | ENO2, PFKP, HK2 |
The pathways were ranked by P-value. KEGG, Kyoto Encyclopedia of Genes and Genomes; DEGs, differentially expressed genes.
Table III.
KEGG pathway enrichment analysis of 164 downregulated DEGs.
| Pathway | Name | P-value | Genes |
|---|---|---|---|
| hsa01100 | Metabolic pathways | 2.40×10−5 | TYRP1, SORD, ASS1, OGDHL, ALDOB, UPP2, ADH6, ATP6V1B1, GPAT3, PIPOX, GLDC, CYP27B1, ALDH4A1, ATP6V0D2, HPD, ALDH6A1, KL, HOGA1, FBP1, PCK1, CYP4A11, CYP17A1, GGT6, G6PC, HMGCS2, HAO2, ABAT, PRODH2, CYP4F3, CYP4F2, ATP6V1G3, PSAT1, ATP6V0A4, DCXR |
| hsa04966 | Collecting duct acid secretion | 2.40×10−5 | CLCNKB, SLC4A1, ATP6V1G3, ATP6V1B1, ATP6V0A4, ATP6V0D2 |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 1.51×10−4 | FXYD4, HSD11B2, SCNN1G, SCNN1B, SCNN1A, KCNJ1 |
| hsa01200 | Carbon metabolism | 3.81×10−3 | ALDH6A1, OGDHL, ALDOB, HAO2, FBP1, PSAT1, GLDC |
| hsa01130 | Biosynthesis of antibiotics | 7.03×10−3 | HMGCS2, ASS1, OGDHL, ALDOB, HAO2, FBP1, PSAT1, PCK1, GLDC |
| hsa00010 | Glycolysis/gluconeogenesis | 1.19×10−2 | G6PC, ALDOB, FBP1, ADH6, PCK1 |
| hsa04742 | Taste transduction | 2.17×10−2 | PDE1A, SCNN1G, SCNN1B, SCNN1A |
| hsa05110 | Vibrio cholerae infection | 3.32×10−2 | ATP6V1G3, ATP6V1B1, ATP6V0A4, ATP6V0D2 |
| hsa00630 | Glyoxylate and dicarboxylate metabolism | 4.96×10−2 | HAO2, HOGA1, GLDC |
The pathways were ranked by P-value. KEGG, Kyoto Encyclopedia of Genes and Genomes; DEGs, differentially expressed genes.
PPI network construction and significant module analysis
A total of 169 genes of the 229 DEGs in all three datasets were filtered into the PPI network complex, containing 169 nodes and 432 edges (Fig. 2A). There were 44 upregulated genes and 125 downregulated genes among the 169 DEGs. Seventeen nodes with a degree >10 were identified as hub genes, such as ALB, VEGFA, EGF, AQP2, ENO2, PLG, FLT1,etc. (bold in Table I). The characteristic properties of the hub nodes based on analysis of the PPI network were tabulated in Table IV. These properties included degree, betweenness, closeness, stress and average shortest path length. After performing module analysis by MCODE, the most significant module was screened out from the PPI network of DEGs, composed of 15 nodes and 54 edges (Fig. 2B). Functional and pathway enrichment analysis of nodes in the module was displayed in Table V. Most of these nodes were enriched in the functional terms related to substance transport and the pathways associated with cancer.
Figure 2.
DEGs protein-protein interaction (PPI) network complex and one significant module obtained from PPI network. (A) DEGs PPI network containing 169 nodes and 432 edges. (B) One significant module composed of 15 nodes and 54 edges. Red nodes and green nodes stand for upregulated genes and downregulated genes, respectively. Lines represent the interaction between nodes. DEG, differentially expressed genes.
Table IV.
Topology properties of 17 hub genes.
| Genes name | Degree | Betweenness centrality | Closeness centrality | Clustering coefficient | Stress | Average shortest path length |
|---|---|---|---|---|---|---|
| ALB | 50 | 0.42 | 0.50 | 0.10 | 30,746 | 2.00 |
| VEGFA | 35 | 0.14 | 0.42 | 0.15 | 11,030 | 2.40 |
| EGF | 26 | 0.14 | 0.45 | 0.25 | 11,990 | 2.23 |
| AQP2 | 19 | 0.20 | 0.41 | 0.23 | 15,956 | 2.44 |
| ENO2 | 17 | 0.08 | 0.39 | 0.13 | 6,610 | 2.60 |
| PLG | 16 | 0.01 | 0.38 | 0.45 | 1,644 | 2.62 |
| CAV1 | 15 | 0.05 | 0.39 | 0.29 | 4,140 | 2.57 |
| KNG1 | 15 | 0.04 | 0.38 | 0.45 | 3,414 | 2.62 |
| CXCR4 | 15 | 0.02 | 0.38 | 0.45 | 3,020 | 2.62 |
| FLT1 | 15 | 0.01 | 0.39 | 0.51 | 1,474 | 2.58 |
| VWF | 14 | 0.00 | 0.37 | 0.52 | 582 | 2.67 |
| GLDC | 13 | 0.06 | 0.34 | 0.15 | 5,708 | 2.96 |
| DCN | 12 | 0.09 | 0.37 | 0.26 | 6,442 | 2.69 |
| CCND1 | 12 | 0.04 | 0.38 | 0.47 | 2,944 | 2.65 |
| SLC12A1 | 12 | 0.03 | 0.38 | 0.42 | 3,942 | 2.62 |
| ALDH4A1 | 12 | 0.03 | 0.31 | 0.21 | 3,776 | 3.20 |
| FGF1 | 11 | 0.02 | 0.37 | 0.53 | 1,592 | 2.67 |
The genes were ranked by degree.
Table V.
Functional and pathway enrichment analyses of nodes in the significant module.
| Term | Description | Count | P-value |
|---|---|---|---|
| GO:0006811 | Ion transport | 12 | 6.36×10−10 |
| GO:0034220 | Ion transmembrane transport | 10 | 1.07 ×10−08 |
| GO:0007588 | Excretion | 5 | 1.97 ×10−08 |
| GO:0016324 | Apical plasma membrane | 7 | 4.25 ×10−08 |
| GO:0015672 | Monovalent inorganic cation transport | 8 | 4.97 ×10−08 |
| GO:0050878 | Regulation of body fluid levels | 8 | 6.29 ×10−08 |
| GO:0030001 | Metal ion transport | 9 | 7.29 ×10−08 |
| GO:0016324 | Apical plasma membrane | 7 | 1.68 ×10−07 |
| GO:0055085 | Transmembrane transport | 10 | 1.70 ×10−07 |
| GO:0006812 | Cation transport | 9 | 2.94 ×10−07 |
| KEGG:hsa04960 | Aldosterone-regulated sodium reabsorption | 4 | 1.94×10−05 |
| KEGG:hsa04510 | Focal adhesion | 5 | 1.40×10−04 |
| KEGG:hsa05219 | Bladder cancer | 3 | 1.50×10−03 |
| KEGG:hsa04742 | Taste transduction | 3 | 1.81×10−03 |
| KEGG:hsa05212 | Pancreatic cancer | 3 | 3.73×10−03 |
| KEGG:hsa04066 | HIF-1 signaling pathway | 3 | 8.32×10−03 |
| KEGG:hsa04151 | PI3K-Akt signaling pathway | 4 | 1.14×10−02 |
| KEGG:hsa05205 | Proteoglycans in cancer | 3 | 3.22×10−02 |
| KEGG:hsa04015 | Rap1 signaling pathway | 3 | 3.52×10−02 |
| KEGG:hsa04014 | Ras signaling pathway | 3 | 4.03×10−02 |
| KEGG:hsa04060 | Cytokine-cytokine receptor interaction | 3 | 4.16×10−02 |
Two GO categories including GO FAT and GO Direct was used for GO analysis. The top 10 GO terms were selected by P-value. If the term was filtered out by GO DIRECT and GO FAT at the same time, the more significant one would be selected. The GO terms and pathways were ranked by P-value. GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
TF-DEG regulatory network
The DEG-associated transcriptional regulatory network was shown in Fig. 3A. A total of 90 nodes with 135 edges were contained in this regulation network, including 61 downregulated genes, 19 upregulated genes and 10 TFs.
Figure 3.
Regulatory network complex. (A) TF-DEG regulatory network containing 61 downregulated genes, 19 upregulated genes and 10 TFs. (B) miRNA-DEG regulatory network containing 31 nodes and 28 edges. Red nodes, green nodes, blue nodes and yellow nodes stand for upregulated genes, downregulated genes, TFs and miRNAs respectively. TF, transcription factor; miR, miRNA; DEG, differentially expressed genes.
miRNA-DEG regulatory network
In total, 6 miRNAs were filtered out (miR-144, miR-96, miR-503, miR-150, miR-383 and miR-338) (Fig. 3B). A total of 31 nodes and 28 edges were included in this regulatory network.
TCGA validation and the Kaplan-Meier plot
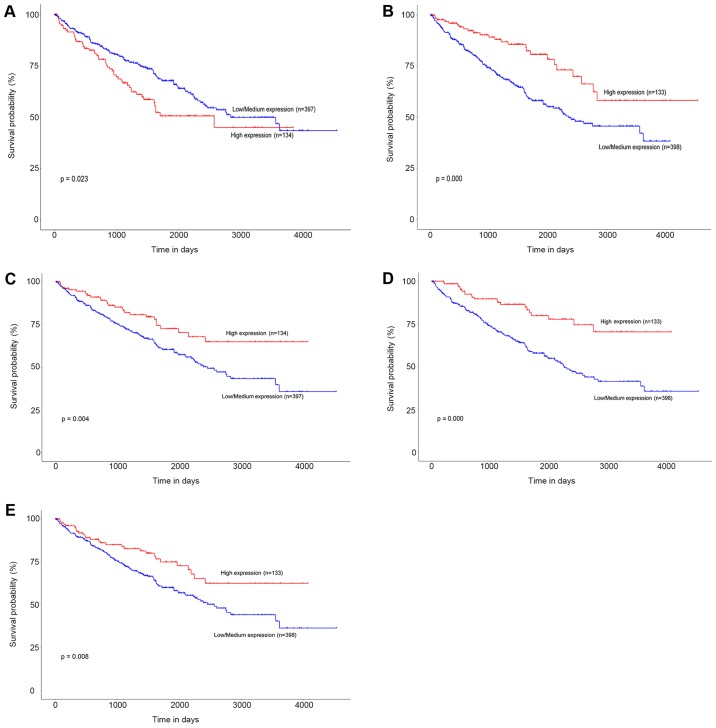
TCGA data of ccRCC patients were used via the UALCAN data portal. The hub genes identified from the PPI network were differentially expressed between ccRCC tissues and normal tissues (Fig. 4). The expression trends were identical within the three GEO datasets. Kaplan-Meier curve for overall survival of TCGA patients with ccRCC was obtained according to the low and high expression of each gene. The results showed that patients in the high mRNA expression group for ENO2 had significantly worse OS than those in the low/medium expression group (P=0.023) (Fig. 5A). While high mRNA expression level of CCND1 was associated with longer OS for ccRCC patients (P=0.000), as well as FLT1 (P=0.004), PLG (P=0.000), and VWF (P=0.008) (Fig. 5B-E).
Figure 4.
Boxplots showing the expression of the 17 hub genes in healthy controls (n=72) and ccRCC tissues (n=533) of TCGA samples. The t-test was performed on the relevant results (*P<0.05 and ***P<0.001). ccRCC, clear cell renal cell carcinoma; TCGA, The Cancer Genome Atlas.
Figure 5.
Kaplan-Meier survival curve for (A) ENO2, (B) CCND1, (C) FLT1, (D) PLG and (E) VWF expression levels in TCGA patients with ccRCC. The log-rank test was carried out on the relevant results. ccRCC, clear cell renal cell carcinoma; TCGA, The Cancer Genome Atlas.
Discussion
The prognosis remains uncertain in ccRCC patients. Identifying novel potential biomarkers for early diagnosis, prognostic evaluation or targeted therapy may improve patient outcomes. Here we performed an in-depth analysis of three expression profiles (with 126 ccRCC tissues and 100 normal controls) using bioinformatics method and identified 65 up- and 164 downregulated genes. Then we constructed a PPI network of DEGs and extracted 17 hub genes and one significant module from the PPI network. GO and KEGG pathway analysis revealed that the DEGs were commonly involved in functional terms and pathways related to the progression and prognosis of ccRCC. For example, hypoxia and HIF-1 pathway alterations are critical for the initiation and metastasis of ccRCC (20). Hypoxia could induce a series of tumor-related aberrations within cellular metabolism, apoptosis, migration and angiogenesis through dysregulation of HIF target genes (20). Drugs targeting the HIF-1 pathway have proven to be effective in treating ccRCC patients (21). In addition, metabolic pathways play a critical role in ccRCC progression according to previous studies, as well as glycolysis/gluconeogenesis, AMPK signaling pathway, and PI3K-Akt signaling pathway (22).
Interestingly, the Staphylococcus aureus infection pathway was found to be significant in our study. Growing evidence has indicated that bacterial infection is highly associated with certain human malignancies (23). It has been reported that lipoteichoic acids from S. aureus induce proliferation of two human non-small-cell lung cancer cell lines, A549 and H226 (24). However, the role of S. aureus infection in ccRCC still remains to be detected.
Using a Kaplan-Meier plot for survival analysis, the mRNA expression levels of ENO2, CCND1, PLT1, PLG and VWF were found to be significantly correlated with OS in ccRCC.
Enolase 2 (ENO2) encodes an enolase isoenzyme which is considered as a sensitive and specific biomarker for small-cell lung cancer (25,26). According to our KEGG results, ENO2 was involved in several pathways compactly related to ccRCC pathogenesis such as glycolysis/gluconeogenesis, HIF-1 signaling pathway and metabolic pathways. In addition, ENO2 is found to be induced by HIF-2a although suppression of its mRNA expression alone does not significantly inhibit the growth of the ccRCC cell line 786-O (27). Combining our survival analysis, we infer that ENO2 may be an indicator in the diagnosis and prognosis rather than a potential target for therapy.
Cyclin D1 (CCND1) encodes an essential protein in the cell cycle which shows dual functions in cell growth. It is well-established that CCND1 regulates the cell cycle transition from G1 to S phase by binding to CK4 and CDK6 (28,29). Previous studies suggest that the overexpression of CCND1 promotes cell growth in many malignancies (30–34). Other studies have shown an apoptotic induction effect of CCND1. Consistent expression of an exogenous CCND1 significantly inhibits cell proliferation (35) and induces apoptosis in mammary epithelial cell lines (36). Upregulated CCND1 induces apoptosis of fibroblasts (37) and has a positive correlation with a high apoptotic index in squamous cell carcinomas (38). Our analysis and previous studies show that CCND1 is upregulated in ccRCC patients (39). Furthermore, it has been reported that reducing CCND1 expression leads to a suppression of tumor growth in ccRCC (27). CCND1 is considered as an oncogene in ccRCC. Interestingly, our results showed that high expression of CCND1 was associated with favorable prognosis in ccRCC. Similarly, CCND1 is elevated and has a favorable effect on disease-free survival in papillary superficial bladder cancer (40). Two independent studies have shown that colon cancer patients with higher CCND1 expression have better outcomes (41,42). The molecular mechanism of CCND1 in cancer awaits further investigation.
The importance of VEGF in RCC progression is well established and several VEGFR inhibitors such as sunitinib and sorafenib have proven to be significantly beneficial for progression-free survival (PFS) and OS in phase 3 trials (43,44). Recent research has demonstrated that FLT1 (also known as VEGFR-1) protein expression in the tumor epithelium of localized ccRCC patients has a negative effect on prognosis (45). Other studies have found that high mRNA expression level of FLT1 is significantly related to favorable PFS in metastatic ccRCC patients treated with sunitinib (46). In this study, we found that higher mRNA expression levels of FLT1 in ccRCC tissue were associated with longer OS. The implication of FLT1 in ccRCC remains unclear. It should be noted that FLT1 can be generated as a transmembrane form and a soluble form. Soluble FLT1 (sFlt1) lacks transmembrane and intracellular domains in contrast to the primary form, a full-length transmembrane receptor (47). Additionally, sFlt1 is thought to be a natural antagonist of VEGF. Recent studies have found that sFlt1 has an antitumor effect on several cancer cells (48–50). Enhanced sFlt1 expression in the serum of breast cancer patients inhibits circulating tumor cells entering the peripheral blood, which may contribute to favorable outcomes (51). Herein we hypothesize that not transmembrane FLT1 but sFLT1 may have an antitumor effect on ccRCC and the value of sFlt1 in patient serum or urine may be worthy of further evaluation.
More and more evidence has demonstrated that plasminogen-plasmin system components are involved in tumor growth, invasion and metastasis by regulating angiogenesis and cell migration (52). The high levels of uPA, uPAR or PAI-1 expression have proven to be prognostic biomarkers of poor outcome in many cancers, such as ovary cancer, breast cancer and renal cancer (53). The mRNA expression level of PLG in ccRCC patients was found to be downregulated in our analysis and other studies (54). Our results revealed that the ccRCC patients with a higher PLG mRNA expression had longer OS. Similar results have been reported in advanced ovarian cancer recently, and PLG was identified to be a favorable prognostic biomarker in this disease (55).
Another favorable biomarker in our analysis is Von Willebrand Factor (VWF), which shows dual functions in angiogenesis and cancer metastasis according to previous data (56). VWF exhibits a pro-apoptotic effect on 769P, a ccRCC-derived cell line (57). While others have found that serum VWF levels are notably higher in progressive RCC patients compared with stable RCC patients (58). More studies should be done to clarify the link between VWF and ccRCC.
The main limitation of our study is that exploration is done at a bioinformatics level, in silico. Future studies, especially biological experiments in vitro and in vivo are needed to validate the function of these DEGs in ccRCC.
In conclusion, through an integrated bioinformatics analysis of three gene profiles, we identified 229 DEGs, which may contain key genes in ccRCC pathogenesis. Five of the 17 hub genes including ENO2, CCND1, PLT1, PLG and VWF were filtered out through our analysis and may be potential prognostic biomarkers in ccRCC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guangdong Obers Blood Purification Academician Work Station (grant no. 2013B090400004); the Guangzhou Entrepreneurial Leader Talent (grant no. LCY201215); and the Guangdong Provincial Center for Clinical Engineering of Blood Purification (grant no. 507204531040).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BH, LY and TL designed the study; TL, XC, SZ, BG, BH, YL and CY performed data analysis; YM, FL and TW performed literature research and data acquisition and participated in the data analysis; TL and XC wrote the manuscript; BH and LY revised the manuscript; YL and CY edited the manuscript and approved the final version of the manuscript; LY obtained funding. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- 1.Li P, Znaor A, Holcatova I, Fabianova E, Mates D, Wozniak MB, Ferlay J, Scelo G. Regional geographic variations in kidney cancer incidence rates in European countries. Eur Urol. 2015;67:1134–1141. doi: 10.1016/j.eururo.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan R, Ricketts CJ, Sourbier C, Linehan WM. New strategies in renal cell carcinoma: Targeting the genetic and metabolic basis of disease. Clin Cancer Res. 2015;21:10–17. doi: 10.1158/1078-0432.CCR-13-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387:894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 5.Gossage L, Eisen T. Alterations in VHL as potential biomarkers in renal-cell carcinoma. Nat Rev Clin Oncol. 2010;7:277–288. doi: 10.1038/nrclinonc.2010.42. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibragimova I, Maradeo ME, Dulaimi E, Cairns P. Aberrant promoter hypermethylation of PBRM1, BAP1, SETD2, KDM6A and other chromatin-modifying genes is absent or rare in clear cell RCC. Epigenetics. 2013;8:486–493. doi: 10.4161/epi.24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valera VA, Walter BA, Linehan WM, Merino MJ. Regulatory effects of microRNA-92 (miR-92) on VHL gene expression and the hypoxic activation of miR-210 in clear cell renal cell carcinoma. J Cancer. 2011;2:515–526. doi: 10.7150/jca.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick RI, Blick C, Ragoussis J, Schoedel J, Mole DR, Young AC, Selby PJ, Banks RE, Harris AL. miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal cancer, regulates ISCU and correlates with good prognosis. Br J Cancer. 2013;108:1133–1142. doi: 10.1038/bjc.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng0912-1072b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckel-Passow JE, Serie DJ, Bot BM, Joseph RW, Cheville JC, Parker AS. ANKS1B is a smoking-related molecular alteration in clear cell renal cell carcinoma. BMC Urol. 2014;14:14. doi: 10.1186/1471-2490-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi M, Tsukamoto Y, Kai T, Tokunaga A, Nakada C, Hijiya N, Uchida T, Daa T, Nomura T, Sato F, et al. Downregulation of WDR20 due to loss of 14q is involved in the malignant transformation of clear cell renal cell carcinoma. Cancer Sci. 2016;107:417–423. doi: 10.1111/cas.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, et al. NCBI GEO: Archive for functional genomics data sets-update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 15.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res. 2013;41:W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schödel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, Mole DR. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016;69:646–657. doi: 10.1016/j.eururo.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escudier B, Szczylik C, Porta C, Gore M. Treatment selection in metastatic renal cell carcinoma: Expert consensus. Nat Rev Clin Oncol. 2012;9:327–337. doi: 10.1038/nrclinonc.2012.59. [DOI] [PubMed] [Google Scholar]
- 22.Ciccarese C, Brunelli M, Montironi R, Fiorentino M, Iacovelli R, Heng D, Tortora G, Massari F. The prospect of precision therapy for renal cell carcinoma. Cancer Treat Rev. 2016;49:37–44. doi: 10.1016/j.ctrv.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhu C, Wang Y, Cai C, Cai Q. Bacterial infection and associated cancers. Adv Exp Med Biol. 2017;1018:181–191. doi: 10.1007/978-981-10-5765-6_11. [DOI] [PubMed] [Google Scholar]
- 24.Hattar K, Reinert CP, Sibelius U, Gökyildirim MY, Subtil FSB, Wilhelm J, Eul B, Dahlem G, Grimminger F, Seeger W, Grandel U. Lipoteichoic acids from Staphylococcus aureus stimulate proliferation of human non-small-cell lung cancer cells in vitro. Cancer Immunol Immunother. 2017;66:799–809. doi: 10.1007/s00262-017-1980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fizazi K, Cojean I, Pignon JP, Rixe O, Gatineau M, Hadef S, Arriagada R, Baldeyrou P, Comoy E, Le Chevalier T. Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: An early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer. 1998;82:1049–1055. doi: 10.1002/(SICI)1097-0142(19980315)82:6<1049::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Oremek GM, Sauer-Eppel H, Bruzdziak TH. Value of tumour and inflammatory markers in lung cancer. Anticancer Res. 2007;27:1911–1915. [PubMed] [Google Scholar]
- 27.Zhang T, Niu X, Liao L, Cho EA, Yang H. The contributions of HIF-target genes to tumor growth in RCC. PLoS One. 2013;8:e80544. doi: 10.1371/journal.pone.0080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLachlan TK, Sang N, Giordano A. Cyclins, cyclin-dependent kinases and cdk inhibitors: Implications in cell cycle control and cancer. Crit Rev Eukaryot Gene Expr. 1995;5:127–156. doi: 10.1615/CritRevEukarGeneExpr.v5.i2.20. [DOI] [PubMed] [Google Scholar]
- 29.Alao JP. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arber N, Hibshoosh H, Moss SF, Sutter T, Zhang Y, Begg M, Wang S, Weinstein IB, Holt PR. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 31.Gautschi O, Ratschiller D, Gugger M, Betticher DC, Heighway J. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung Cancer. 2007;55:1–14. doi: 10.1016/j.lungcan.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Bosch F, Jares P, Campo E, Lopez-Guillermo A, Piris MA, Villamor N, Tassies D, Jaffe ES, Montserrat E, Rozman C, et al. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: A highly specific marker of mantle cell lymphoma. Blood. 1994;84:2726–2732. [PubMed] [Google Scholar]
- 33.Faust JB, Meeker TC. Amplification and expression of the bcl-1 gene in human solid tumor cell lines. Cancer Res. 1992;52:2460–2463. [PubMed] [Google Scholar]
- 34.Buckley MF, Sweeney KJ, Hamilton JA, Sini RL, Manning DL, Nicholson RI, deFazio A, Watts CK, Musgrove EA, Sutherland RL. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 35.Han EK, Sgambato A, Jiang W, Zhang YJ, Santella RM, Doki Y, Cacace AM, Schieren I, Weinstein IB. Stable overexpression of cyclin D1 in a human mammary epithelial cell line prolongs the S-phase and inhibits growth. Oncogene. 1995;10:953–961. [PubMed] [Google Scholar]
- 36.Han EK, Begemann M, Sgambato A, Soh JW, Doki Y, Xing WQ, Liu W, Weinstein IB. Increased expression of cyclin D1 in a murine mammary epithelial cell line induces p27kip1, inhibits growth, and enhances apoptosis. Cell Growth Differ. 1996;7:699–710. [PubMed] [Google Scholar]
- 37.Sofer-Levi Y, Resnitzky D. Apoptosis induced by ectopic expression of cyclin D1 but not cyclin E. Oncogene. 1996;13:2431–2437. [PubMed] [Google Scholar]
- 38.Kotelnikov VM, Coon JS, IV, Mundle S, Kelanic S, LaFollette S, Taylor S, IV, Hutchinson J, Panje W, Caldarelli DD, Preisler HD. Cyclin D1 expression in squamous cell carcinomas of the head and neck and in oral mucosa in relation to proliferation and apoptosis. Clin Cancer Res. 1997;3:95–101. [PubMed] [Google Scholar]
- 39.Tang SW, Chang WH, Su YC, Chen YC, Lai YH, Wu PT, Hsu CI, Lin WC, Lai MK, Lin JY. MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett. 2009;273:35–43. doi: 10.1016/j.canlet.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 40.Sgambato A, Migaldi M, Faraglia B, de Aloysio G, Ferrari P, Ardito R, de Gaetani C, Capelli G, Cittadini A, Trentini GP. Cyclin D1 expression in papillary superficial bladder cancer: Its association with other cell cycle-associated proteins, cell proliferation and clinical outcome. Int J Cancer. 2002;97:671–678. doi: 10.1002/ijc.10055. [DOI] [PubMed] [Google Scholar]
- 41.Holland TA, Elder J, McCloud JM, Hall C, Deakin M, Fryer AA, Elder JB, Hoban PR. Subcellular localisation of cyclin D1 protein in colorectal tumours is associated with p21(WAF1/CIP1) expression and correlates with patient survival. Int J Cancer. 2001;95:302–306. doi: 10.1002/1097-0215(20010920)95:5<302::AID-IJC1052>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Ogino S, Nosho K, Irahara N, Kure S, Shima K, Baba Y, Toyoda S, Chen L, Giovannucci EL, Meyerhardt JA, Fuchs CS. A cohort study of cyclin D1 expression and prognosis in 602 colon cancer cases. Clin Cancer Res. 2009;15:4431–4438. doi: 10.1158/1078-0432.CCR-08-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 44.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 45.Klatte T, Seligson DB, LaRochelle J, Shuch B, Said JW, Riggs SB, Zomorodian N, Kabbinavar FF, Pantuck AJ, Belldegrun AS. Molecular signatures of localized clear cell renal cell carcinoma to predict disease-free survival after nephrectomy. Cancer Epidemiol Biomarkers Prev. 2009;18:894–900. doi: 10.1158/1055-9965.EPI-08-0786. [DOI] [PubMed] [Google Scholar]
- 46.Beuselinck B, Jean-Baptiste J, Schöffski P, Couchy G, Meiller C, Rolland F, Allory Y, Joniau S, Verkarre V, Elaidi R, et al. Validation of VEGFR1 rs9582036 as predictive biomarker in metastatic clear-cell renal cell carcinoma patients treated with sunitinib. BJU Int. 2016;118:890–901. doi: 10.1111/bju.13585. [DOI] [PubMed] [Google Scholar]
- 47.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyake T, Kumasawa K, Sato N, Takiuchi T, Nakamura H, Kimura T. Soluble VEGF receptor 1 (sFLT1) induces non-apoptotic death in ovarian and colorectal cancer cells. Sci Rep. 2016;6:24853. doi: 10.1038/srep24853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takano S, Ishikawa E, Matsuda M, Sakamoto N, Akutsu H, Yamamoto T, Matsumura A. The anti-angiogenic role of soluble-form VEGF receptor in malignant gliomas. Int J Oncol. 2017;50:515–524. doi: 10.3892/ijo.2016.3810. [DOI] [PubMed] [Google Scholar]
- 50.Niu J, Wang Y, Wang J, Bin L, Hu X. Delivery of sFIT-1 engineered MSCs in combination with a continuous low-dose doxorubicin treatment prevents growth of liver cancer. Aging (Albany NY) 2016;8:3520–3534. doi: 10.18632/aging.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilsmaier T, Rack B, Janni W, Jeschke U, Weissenbacher T, SUCCESS Study Group Angiogenic cytokines and their influence on circulating tumour cells in sera of patients with the primary diagnosis of breast cancer before treatment. BMC Cancer. 2016;16:547. doi: 10.1186/s12885-016-2612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwaan HC, McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res. 2009;148:43–66. doi: 10.1007/978-0-387-79962-9_4. [DOI] [PubMed] [Google Scholar]
- 53.McMahon BJ, Kwaan HC. Components of the plasminogen-plasmin system as biologic markers for cancer. Adv Exp Med Biol. 2015;867:145–156. doi: 10.1007/978-94-017-7215-0_10. [DOI] [PubMed] [Google Scholar]
- 54.Schrödter S, Braun M, Syring I, Klümper N, Deng M, Schmidt D, Perner S, Müller SC, Ellinger J. Identification of the dopamine transporter SLC6A3 as a biomarker for patients with renal cell carcinoma. Mol Cancer. 2016;15:10. doi: 10.1186/s12943-016-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao S, Dorn J, Napieralski R, Walch A, Diersch S, Kotzsch M, Ahmed N, Hooper JD, Kiechle M, Schmitt M, Magdolen V. Plasmin(ogen) serves as a favorable biomarker for prediction of survival in advanced high-grade serous ovarian cancer. Biol Chem. 2017;398:765–773. doi: 10.1515/hsz-2016-0282. [DOI] [PubMed] [Google Scholar]
- 56.Mojiri A, Stoletov K, Carrillo MA, Willetts L, Jain S, Godbout R, Jurasz P, Sergi CM, Eisenstat DD, Lewis JD, Jahroudi N. Functional assessment of von Willebrand factor expression by cancer cells of non-endothelial origin. Oncotarget. 2017;8:13015–13029. doi: 10.18632/oncotarget.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mochizuki S, Soejima K, Shimoda M, Abe H, Sasaki A, Okano HJ, Okano H, Okada Y. Effect of ADAM28 on carcinoma cell metastasis by cleavage of von Willebrand factor. J Natl Cancer Inst. 2012;104:906–922. doi: 10.1093/jnci/djs232. [DOI] [PubMed] [Google Scholar]
- 58.Braybrooke JP, O'Byrne KJ, Propper DJ, Blann A, Saunders M, Dobbs N, Han C, Woodhull J, Mitchell K, Crew J, et al. A phase II study of razoxane, an antiangiogenic topoisomerase II inhibitor, in renal cell cancer with assessment of potential surrogate markers of angiogenesis. Clin Cancer Res. 2000;6:4697–4704. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.