Abstract
Gastric cancer is a serious threat to human health. Nuclear receptor subfamily 1 group D member 1 (REV-ERBα) is a member of the nuclear hormone receptor family that regulates lipid metabolism, inflammatory responses and circadian rhythms. However, the role of REV-ERBα in the pathogenesis of human gastric cancer is unclear. The present study employed gastric cancer tissues from 74 patients and determined the association between REV-ERBα expression with clinicopathological variables and prognosis. Furthermore, the association between REV-ERBα and apoptosis in undifferentiated and moderately differentiated human gastric cancer cells was determined. It was identified that REV-ERBα expression was decreased in gastric cancer, which was positively associated with poor differentiation (P=0.009), T stage (P=0.001), Tumor-Node-Metastasis (TMN) stage (P=0.001) and lymph node metastasis (P=0.007). In the survival analysis, the 3- and 5-year survival times of patients were significantly associated with REV-ERBα expression (P=0.009 and P=0.002, respectively). Low REV-ERBα expression was associated with poor prognosis (P<0.05). Concurrently, cleaved caspase-3 expression was downregulated, whereas expression levels of Bcl-2 and the Bcl-2/Bax ratio were upregulated in gastric cancer tissues compared with normal tissues. REV-ERBα activator GSK4112 caused apoptosis in SGC-7901 and BGC-823 cell lines. REV-ERBα levels were decreased in human gastric cancer, which was associated with poor differentiation, TMN stages and poor prognosis. REV-ERBα is a potential biomarker for tumor development and prognosis, and a potential therapeutic target for gastric cancer.
Keywords: nuclear receptor subfamily 1 group D member 1, gastric cancer, biomarker, prognosis, apoptosis
Introduction
Gastric cancer is the most common cancer, with the fourth highest incidence rate of all gastric adenocarcinomas and >723,000 mortalities every year worldwide (1). Despite the decreased incidence and mortality rates due to the major improved diagnosis and treatment, the ≤5-year survival rate is <20% (1). This may be due to lack of understanding of the mechanisms underlying the development and prognosis of gastric cancer. Therefore, it is urgent to develop effective therapeutic approaches for gastric cancer.
Nuclear receptor subfamily 1 group D member 1 (REV-ERBα) belongs to the nuclear hormone receptor family (2), which is abundantly expressed in liver, adipose, muscle and brain tissue. REV-ERBα serves an important role in regulating lipid metabolism, inflammatory responses and circadian rhythm (2–4). Previous studies have demonstrated that REV-ERBα may modulate the proliferation and apoptosis of HER2+ breast cancer cells, which is associated with poor clinical outcomes and survival (5,6). However, to the best of our knowledge, there are no studies regarding the regulation of REV-ERBα in gastric cancer. It is also unknown whether REV-ERBα alterations are associated with the clinicopathological factors and prognosis of human gastric cancer. We hypothesize that the levels of REV-ERBα in gastric cancer are altered compared with normal tissues, and is associated with the clinicopathological features and prognosis of this disease. To examine this hypothesis, samples from patients who were diagnosed with gastric cancer were utilized, and the REV-ERBα gene and protein levels in normal and cancer tissues in those patients were compared. The associations between REV-ERBα expression with the Tumor-Node-Metastasis (TMN) stages and survival times were also analyzed in patients with gastric cancer. Finally, human gastric cancer cells were treated with REV-ERBα activator GSK4112 to determine its effects on apoptosis. Conclusively, the reduction of REV-ERBα was associated with clinical features and prognosis in gastric cancer, and REV-ERBα agonist resulted in apoptosis in gastric cancer cells.
Materials and methods
Patients and tissues collection
All samples were obtained from 74 patients with diagnosed gastric cancer who underwent surgery (surgical resection) at The First Affiliated Hospital of Anhui Medical University in 2014, as previously described (7). The median age of the study population was 63.4 years (range, 33–84 years) and the sex distribution was 58 males and 16 females. The clinical characteristics are summarized in Table I. None of the patients had received any other therapies, including radiotherapy or chemotherapy prior to surgery. The hepatic, renal and bone marrow functions of all patients were normal, and the Eastern Cooperative Oncology Group Performance Status scores were between 0–2 (8). Patients with abnormal function rest results, and those who were pregnant or breast-feeding were excluded.
Table I.
Association between REV-ERBα expression and clinicopathological variables.
| REV-ERBα expression level, n | ||||
|---|---|---|---|---|
| Clinicopathological variables | Numbers of patients, n (n=74) | Low (n=43) | High (n=31) | P-value |
| Sex | ||||
| Male | 58 | 35 | 23 | 0.322 |
| Female | 16 | 8 | 8 | |
| Age, years | ||||
| <60 | 23 | 16 | 7 | 0.138 |
| ≥60 | 51 | 27 | 24 | |
| Primary tumor site | ||||
| Gastric cardia or fundus | 36 | 22 | 14 | 0.392 |
| Gastric antrum or body | 38 | 21 | 17 | |
| Diameter of tumor, cm | ||||
| <5 | 31 | 18 | 13 | 0.995 |
| ≥5 | 43 | 25 | 18 | |
| Level of differentiation | ||||
| Moderate | 22 | 8 | 14 | 0.009 |
| Poor | 52 | 37 | 15 | |
| T stage | ||||
| T1-T2 | 21 | 5 | 16 | 0.001 |
| T3-T4 | 53 | 39 | 14 | |
| TNM stage | ||||
| I–II | 18 | 4 | 14 | 0.001 |
| III–IV | 56 | 40 | 16 | |
| Lymph node metastasis | ||||
| Present | 58 | 41 | 17 | 0.007 |
| Absent | 16 | 5 | 11 | |
TNM, Tumor-Node-Metastasis; REV-ERBα, nuclear receptor subfamily 1 group D member 1.
All patients provided written informed consent. The study protocol was approved by the Clinical Research Ethics Committee of Anhui Medical University (Hefei, China). All methods were performed in accordance with the human ethics guidelines in the clinical research project (9).
Cell culture
The human gastric cancer SGC-7,901 [American Type Culture Collection (ATCC), Manassas, VA, USA] and BGC-823 (ATCC) cell lines and human normal gastric epithelial GES-1 (ATCC) cell line were employed to detect REV-ERBα expression. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing high glucose, 1 mmol/l L-glutamine, pyridoxine hydrochloride, 110 mg/l sodium pyruvate and bicarbonate. Additionally, 10% heat inactivated foetal calf serum (Clark Bioscience, Richmond, VA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin were added to the DMEM. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2.
Immunohistochemistry
REV-ERBα expression in human gastric cancer and normal tissues (5 cm from the tumor site) was measured by immunohistochemistry as described previously (7). Immunohistochemistry was performed on 4-µm-thick sections from 10% formalin-fixed paraffin-embedded tissue specimens. Sections were deparaffinized with 100% xylene at 25°C for 10 min and removed xylene through a graded series (100, 85 and 70% ethanol) and were subjected to microwaving with 10 mm citrate buffer (pH=6.0) at 100°C for 5 min. Briefly, the sections were blocked with 3% hydrogen peroxide and 10% normal goat serum (Clark Bioscience) each for 10 min at room temperature, and then incubated with REV-ERBα rabbit antibody (cat no. AB174309; 1:100 dilution; Abcam, Cambridge, MA, USA) overnight at 4°C. Following incubation with a biotin-conjugated secondary antibody (cat. no. PV6000; 1:100 dilution; ZSGB-BIO; OriGene Technologies, Inc., Beijing, China), the tissue slides were incubated with a streptavidin-biotin horseradish peroxidase complex for 30 min at room temperature, followed by incubation with 3,3′-diaminobenzidine (ZSGB-BIO; OriGene Technologies, Inc.) for 5 min at room temperature. The counterstaining with 20% hematoxylin was then performed for 60 sec at room temperature, and images of stained samples were captured in a single-blinded manner under a fluorescent microscope at a magnification of ×200. The relative protein expression of all images was calculated using mean optical density units, and the sequences were analyzed using IPWIN Application software version 6.0.0260 (Media Cybernetics, Inc., Rockville, MD, USA). The staining intensity was scored according to the number of cells: 0, no staining; 1 (≤25%), weakly stained; 2 (25–50%), moderately stained; or 3 (≥50%), markedly stained. A low REV-ERBα expression was defined as score ‘0’, ‘1’ or ‘2’, and a high REV-ERBα expression was defined as score ‘3’. The patients were divided into two groups: The low expression group (n=43) and high expression group (n=31), as summarized in Table I.
Western blot analysis
Gastric tissues and cells were lysed in lysis buffer (25 mM HEPES, 2 mM MgCl2, 2 mM DTT, 1 mM EDTA, 1 mM PSMF, 5 µg/ml leupeptin, pH 7.4). Subsequent to freeze-thawing the suspension liquid containing the extracted protein 3 times, the lysates were centrifuged at 32,869.2 × g at 4°C for 30 min. The concentration of all the extracted protein was determined by the BCA assay. The protein extracts (10 µl) were separated by 12% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Following non-specific blocking with 5% skimmed milk at room temperature for 2 h, PVDF membranes were washed 2 times with TBST (TBS contained 0.1% Tween-20) for 10 min each time. The membranes were then incubated with primary antibodies against: REV-ERBα (rabbit; cat no. AB174309; 1:500 dilution; Abcam); cleaved caspase-3 (mouse; cat no. SC70497; 1:500 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA); B-cell lymphoma 2 (Bcl-2; mouse; cat no. SC23960; 1:500 dilution; Santa Cruz Biotechnology, Inc.); Bcl-2-associated X protein (Bax; mouse; cat no. SC23959; 1:500 dilution; Santa Cruz Biotechnology, Inc.); and β-actin (mouse; cat no. AB8226; 1:1,000 dilution; Abcam) overnight at 4°C, and then washed 3 times with TBST for 10 min each time. The membranes were incubated with the corresponding Goat anti-mouse horseradish peroxidase-conjugated secondary antibody (mouse; cat no. AP124P; 1:1,000 dilution; EMD Millipore, Billerica, MA, USA) or rabbit anti-Goat horseradish peroxidase-conjugated secondary antibody (rabbit; cat no. AP106P; 1:1,000 dilution; EMD Millipore) for 2 h at 20°C, and then washed 3 times with TBST again for 10 min each time. Finally, the detection of the molecules of interest was performed using enhanced chemiluminescence (Beyotime Institute of Biotechnology, Haimen, China). The bands were quantified to calculate relative protein expression levels using Quantity one software version 4.99.5.2.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from human tissues and cells using TRIzol® (Life Technologies; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Total RNA was reverse transcribed by cDNA synthesis using a PrimeScript RT Reagent kit with gDNA Eraser (Perfect Real Time; Takara Bio, Inc., Otsu, Japan) at 37°C for 30 min and 85°C for 5 sec. qPCR was performed using a 7,900 Thermal Cycler (ABI, Applied Biosystems; Thermo Fisher Scientific, Inc.) with GoTaq® Green Master Mix (Promega Corporation, Madison, WI USA) at an initial denaturation at 95°C for 30 sec, followed by 40 cycles of denaturation for 5 sec at 95°C, annealing for 30 sec at 60°C and extension for 15 sec at 72°C. The qPCR primers for REV-ERBα were 5′-ACAGAATCGAACTCTGCACTTCT-3′ (forward) and 5′-GGGGAGGGAGGCAGGTATT-3′ (reverse) (10). The primers for β-actin were 5′-CATGTACGTTGCTATCCAGGC-3′ (forward) and 5′-CTCCTTAATGTCACGCACGAT-3 (reverse). The cycle threshold (Cq) values were obtained in each sample. Relative levels of mRNA were determined using the 2−ΔΔCq method (10). β-actin was used as an internal gene for normalization.
Morphological measurement of apoptosis
The morphological changes associated with apoptosis were assayed using fluorescence microscopy following Hoechst33258 staining. The number of cells was counted in five random fields under a fluorescence microscope at a magnification of ×200. Briefly, SGC-7901 and BGC-823 cells (3,500 cells/well) were fixed for 30 min at room temperature in 70% ethanol, followed by Hoechst33258 (10 µg/ml) staining for 2 min at 37°C and then visualization using the UV fluorescence microscope. Apoptotic cells were considered as cells exhibiting nuclear and cytoplasmic shrinkage, chromatin condensation and apoptotic bodies. A minimum of 400 cells were counted, and the percentage of apoptotic cells, or the apoptotic index, was calculated as described previously (11).
Statistical analysis
Data are expressed as the mean ± standard deviation. Data analysis was performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Comparison between different groups was performed using analysis of variance. The Student-Newman-Keuls test was the post-hoc test used following analysis of variance. The χ2 test was utilized to analyze the associations between REV-ERBα expression levels and clinicopathological variables and survival time. A Kaplan-Meier test was utilized to describe survival curves and the log-rank test was used to analyze survival curves. P<0.05 was considered to indicate a statistically significant difference.
Results
REV-ERBα expression is decreased in human gastric cancer tissues
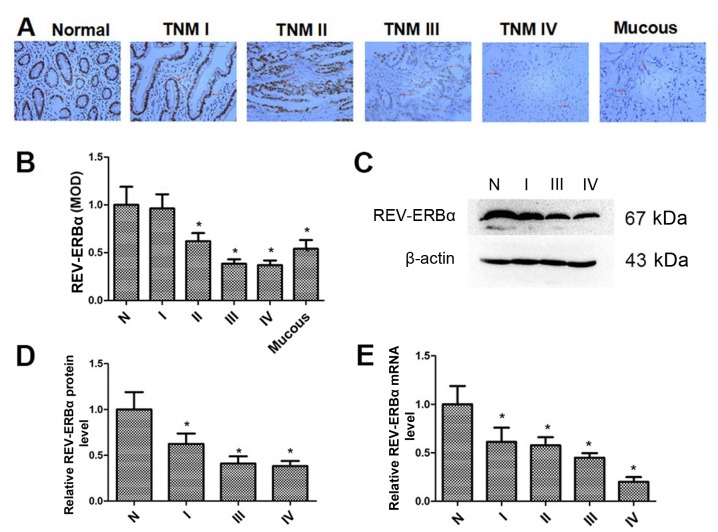
The REV-ERBα expression levels in normal gastric and mucous gastric cancer tissues, and gastric cancer tissues with different TNM stages, were determined by immunohistochemistry. As demonstrated in Fig. 1A and B, REV-ERBα protein levels were decreased in gastric cancer tissues, which was associated with increased TNM stage. Additionally, REV-ERBα expression in mucous gastric cancer tissues was also decreased compared with normal gastric tissues. Furthermore, the REV-ERBα expression in normal gastric and gastric cancer tissues was confirmed by western blot analysis (Fig. 1C and D). The mRNA levels of REV-ERBα were also measured by RT-qPCR (Fig. 1E). It was identified that the levels of REV-ERBα mRNA were also decreased in gastric cancer tissues, which was associated with incremental TNM stage. These results suggest that REV-ERBα levels are decreased significantly in gastric cancer tissues with higher TNM stages.
Figure 1.
REV-ERBα is decreased in human gastric cancer tissues. (A) The expression of REV-ERBα in normal gastric and mucous gastric cancer tissues, and gastric cancer tissues from patients with TNM I to IV stages was determined by immunohistochemistry. Scale bars=100 µm (original magnification, ×200). (B) The MOD indicated the changes in the expression levels of REV-ERBα by immunohistochemistry. (C) The expression of REV-ERBα in normal gastric and gastric cancer tissues from patients with TNM I to IV stages was measured by western blot analysis. β-actin was used a loading control. (D) Relative protein expression of REV-ERBα was normalized to that of β-actin in normal gastric and gastric cancer tissues. (E) The mRNA levels of REV-ERBα in normal gastric and gastric cancer tissues from patients with TNM I to IV stages were measured by reverse transcription quantitative polymerase chain reaction. Data are presented as the mean ± standard deviation. n=3. *P<0.05 vs. normal gastric tissues. N, normal gastric tissues; Mucous, mucous gastric cancer tissues; TNM, Tumor-Node-Metastasis; MOD, mean optical density; REV-ERBα, nuclear receptor subfamily 1 group D member 1.
Association between REV-ERBα expression and clinicopathological factors in gastric cancer
The association between the REV-ERBα expression and clinicopathological factors was analyzed using immunohistochemistry data (Table I). A low expression of REV-ERBα was significantly associated with poor differentiation (P=0.009), T stage (P=0.001), TMN stage (P=0.001) and lymph node metastasis (P=0.007). The results indicated that REV-ERBα level is associated with the progression of gastric cancer.
Association between REV-ERBα expression and survival time of patients with gastric cancer
The different survival times of patients with low and high REV-ERBα expression levels are summarized in Table II. The 3- and 5-year survival times of patients were significantly associated with REV-ERBα expression (P=0.009 and P=0.002, respectively). The patients with low REV-ERBα expression exhibited poor prognosis (P<0.05) compared with patients with high REV-ERBα expression (Fig. 2).
Table II.
Association between REV-ERBα expression and survival time of patients with gastric cancer.
| 3-year survival time | 5-year survival time | |||||
|---|---|---|---|---|---|---|
| REV-ERBα expression | Survival, n | Mortality, n | P-value | Survival, n | Mortality, n | P-value |
| Low | 12 | 31 | 0.009 | 8 | 35 | 0.002 |
| High | 18 | 13 | – | 16 | 15 | – |
REV-ERBα, nuclear receptor subfamily 1 group D member 1.
Figure 2.
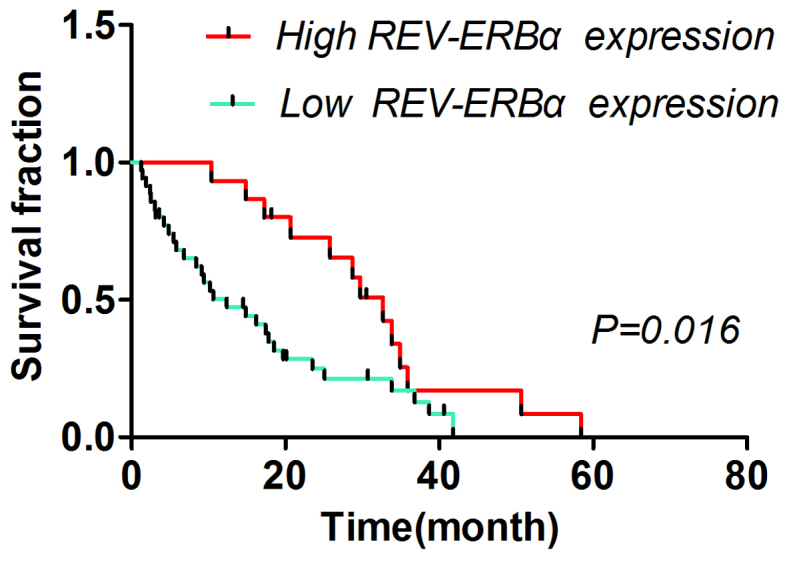
Log-rank test analysis of the association between REV-ERBα expression and survival time of patients with gastric cancer. Patients with high REV-ERBα expression survived (median survival, 32.70 months) significantly longer compared with patients with low REV-ERBα expression (median survival, 12.40 months). REV-ERBα, nuclear receptor subfamily 1 group D member 1.
Cleaved caspase-3 expression is downregulated, whereas the Bcl-2 expression and Bcl-2/Bax are upregulated, in gastric cancer tissues
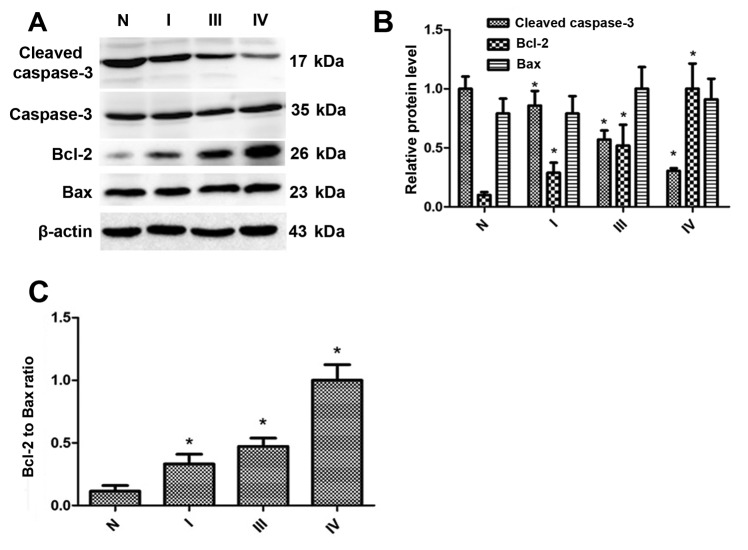
To determine whether REV-ERBα was associated with the expression of cleaved caspase-3, Bcl-2 and Bax, western blot analysis was employed to detect the levels of these proteins. As indicated in Fig. 3A and B, the expression levels of cleaved caspase-3 were downregulated in gastric cancer tissues, which was associated with enhanced TNM stage. The Bcl-2 expression levels were upregulated in gastric cancer tissues, which were also associated with enhanced TNM stage. Additionally, the ratio of Bcl-2 to Bax, according to the densitometry of the western blot analysis bands (Fig. 3C), was increased in gastric cancer tissues compared with normal gastric tissues. Therefore, these results suggest that the level of REV-ERBα is decreased in human gastric cancer, which is associated with decreased levels of apoptosis.
Figure 3.
In gastric cancer tissues, the cleaved caspase-3 expression level is downregulated, whereas the expression levels of Bcl-2 and the Bcl-2/Bax ratio are upregulated. (A) Expression of cleaved caspase-3, Bcl-2 and Bax in normal gastric tissues and gastric cancer tissues from patients with TNM I to IV stages was evaluated by western blot analysis. β-actin was used as a loading control. (B) Relative protein expression of cleaved caspase-3, Bcl-2 and Bax was normalized to that of β-actin. (C) Densitometry of Bcl-2/Bax ratio according to western blot analysis bands. Data are represented as the mean ± standard deviation. n=3. *P<0.05 vs. normal gastric tissues. N, normal; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; TNM, Tumor-Node-Metastasis; REV-ERBα, nuclear receptor subfamily 1 group D member 1.
REV-ERBα expression is decreased in human gastric cancer cells, and activation of REV-ERBα causes apoptosis in gastric cancer cells
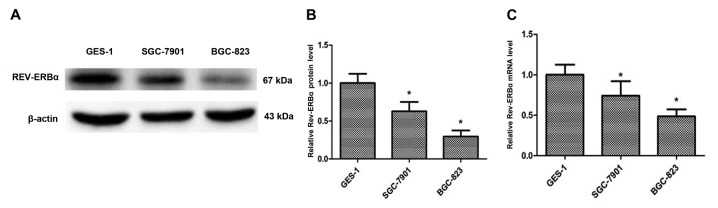
To additionally investigate the REV-ERBα expression in gastric cancer, GES-1, SGC-7901 and BGC-823 cell lines were employed to determine the expression level of REV-ERBα (Fig. 4A and B). The REV-ERBα expression levels were significantly decreased in SGC-7901 (moderately differentiated) and BGC-823 (undifferentiated) compared with the GES-1 cell line. The mRNA levels of REV-ERBα were also decreased in SGC-7901 and BGC-823 cells compared with the GES-1 cell line (Fig. 4C). Furthermore, SGC-7901 and BGC-823 cells were treated with the REV-ERBα activator GSK4112 (20 and 40 µM; MedChemExpress, Monmouth Junction, NJ, USA), and it was identified that GSK4112 treatment for 48 h at 37°C caused an increase in apoptosis in a dose-dependent manner (Fig. 5). Therefore, these results suggest that REV-ERBα activation induces apoptosis in human gastric cancer cells.
Figure 4.
Expression of REV-ERBα is decreased in human gastric cells. (A) Expression of REV-ERBα in the normal gastric epithelial GES-1 cell line and human gastric cancer SGC-7901 and BGC-823 cell lines was measured by western blot analysis. β-actin was used as a loading control. (B) Relative protein expression of REV-ERBα was normalized to that of β-actin. (C) The mRNA levels of REV-ERBα was measured by reverse transcription quantitative polymerase chain reaction in the normal gastric epithelial GES-1 cell line and human gastric cancer SGC-7901 and BGC-823 cell lines. Data are presented as the mean ± standard deviation. n=3. *P<0.05 vs. normal gastric epithelial GES-1 cell line. REV-ERBα, nuclear receptor subfamily 1 group D member 1.
Figure 5.
Activation of REV-ERBα causes apoptosis in SGC-7901 and BGC-823 cell lines. SGC-7901 and BGC-823 cells were treated with a REV-ERBα activator GSK4112 (20 and 40 µM) for 48 h. Hoechst33258 staining was performed to detect apoptotic cells that exhibited nuclear and cytoplasmic shrinkage, chromatin condensation and apoptotic bodies using UV fluorescence microscopy (original magnification, ×200) and were illustrated using red arrows. Data are represented as the mean ± standard deviation. n=4. ***P<0.001 vs. Veh. Veh, vehicle; REV-ERBα, nuclear receptor subfamily 1 group D member 1.
Discussion
REV-ERBs were originally regarded as orphan receptors to regulate gene transcription in response to multifarious environmental stimuli (12). The members of REV-ERB family, including REV-ERBα and REV-ERBβ, exhibit abundant and overlapping expression in adipose, muscle, brain and liver tissues (13). However, REV-ERBα is broadly expressed at the similar level in a number of different tissues, whereas REV-ERBβ is highly expressed in parts of the brain, including the pineal gland and prefrontal cortex, thyroid, uterus and pituitary (12).
REV-ERBα modulates the circadian rhythm by directly activating the expression of key circadian clock genes, including Clock circadian regulator, Brain and muscle ANRT-like 1, Cryptochrome and Period (14–17). Epidemiological data indicate that disruption of the circadian clock is associated with an increased risk of development of breast cancer (18,19). Dysregulation of the circadian clock genes may have complex effects on energy homeostasis, potentially resulting in metabolic disorders including cancer (20,21). The present study identified that REV-ERBα expression was significantly decreased in human gastric cancer tissues, which was associated with different clinicopathological stages. This was in agreement with the results that the levels of REV-ERBα were additionally decreased in undifferentiated BGC-823 cells compared with moderately differentiated SGC-7,901 cells. These data suggest the possibility of REV-ERBα as a biomarker of gastric cancer.
It has also been demonstrated that the REV-ERBα expression leading to apoptosis was affected by circadian rhythms (22). REV-ERBα controls the circadian clock genes and regulates Early growth response 1 expression, causing extensive expression of Tumor protein p73 (p73) (23). p73 activates the transcription of Bax and Bcl-2, causing the release of cytochrome c from the mitochondria (24). Apoptosome assembly, and the eventual cleavage and activation of transducer caspase-9, in turn cleaves and activates executioner caspase-3. Hence, the circadian clock has a regulating role in the expression of apoptosis factors (Caspase-3, Bcl-2 and Bax) and the activation of the apoptosis pathway (24). The present study identified that REV-ERBα expression was decreased in human gastric cancer, and that this decrease may protect against apoptosis by decreasing the levels of cleaved caspase-3 and increasing Bcl-2 levels. However, one limitation of the present study is that there were only 74 patients included. A larger human cohort is required to confirm these results. It was also unknown how REV-ERBα regulates the apoptosis pathway and expression of apoptosis-associated factors, including cleaved caspase-3, Bcl-2 and Bax, in gastric cancer.
It has been demonstrated previously that REV-ERBα regulates lipid metabolism during proliferation and apoptosis (2,25,26). The preliminary data from the present study suggested that REV-ERBα decreased glycolysis levels in gastric cancer cells (data not shown). Nevertheless, it is unclear whether a REV-ERBα-mediated decrease of glycolysis is beneficial for therapy in gastric cancer.
In summary, the present study employed immunohistochemistry, western blot analysis and RT-qPCR methods, and identified that REV-ERBα expression was decreased in human gastric cancer tissues. The data indicated that REV-ERBα expression was significantly associated with poor differentiation, T stage, TMN stage and lymph node metastasis in human gastric cancer. Additionally, the survival time of patients was significantly associated with REV-ERBα expression, suggesting that REV-ERBα may be an independent prognosis factor in gastric cancer. Furthermore, REV-ERBα activation induced apoptosis in human gastric cancer cells. Therefore, REV-ERBα is a potential biomarker for tumor development and prognosis, and a potential therapeutic target for gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation of Anhui Province (grant no. 1608085MH182).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XSW and ZW designed the study. NW performed the immunohistochemistry to determine the expression of REV-ERBα. XSW and XW conducted the western blot analysis. XW also performed the cell proliferation and morphological measurements of apoptosis. HY performed RT-qPCR to detect the expression of REV-ERBα. XSW drafted the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Clinical Research Ethics Committee of Anhui Medical University (Hefei, China). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Written informed consent was obtained from all patients for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzoccoli G, Cai Y, Liu S, Francavilla M, Giuliani F, Piepoli A, Pazienza V, Vinciguerra M, Yamamoto T, Takumi T. REV-ERBα and the clock gene machinery in mouse peripheral tissues: A possible role as a synchronizing hinge. J Biol Regul Homeost Agents. 2012;26:265–276. [PubMed] [Google Scholar]
- 4.Vieira E, Merino B, Quesada I. Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas. Diabetes Obes Metab. 2015;17(Suppl 1):S106–S114. doi: 10.1111/dom.12522. [DOI] [PubMed] [Google Scholar]
- 5.Kourtidis A, Jain R, Carkner RD, Eifert C, Brosnan MJ, Conklin DS. An RNA interference screen identifies metabolic regulators NR1D1 and PBP as novel survival factors for breast cancer cells with the ERBB2 signature. Cancer Res. 2010;70:1783–1792. doi: 10.1158/0008-5472.CAN-09-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ka NL, Na TY, Na H, Lee MH, Park HS, Hwang S, Kim IY, Seong JK, Lee MO. NR1D1 recruitment to sites of DNA damage inhibits repair and is associated with chemosensitivity of breast cancer. Cancer Res. 2017;77:2453–2463. doi: 10.1158/0008-5472.CAN-16-2099. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Xiong F, Wang X, Qi Y, Yu H, Zhu Y, Zhu H. Nuclear receptor retinoid-related orphan receptor alpha promotes apoptosis but is reduced in human gastric cancer. Oncotarget. 2017;8:11105–11113. doi: 10.18632/oncotarget.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan SQ, Nie RC, Chen YM, Qiu HB, Li XP, Chen XJ, Xu LP, Yang LF, Sun XW, Li YF, et al. Glasgow Prognostic Score is superior to ECOG PS as a prognostic factor in patients with gastric cancer with peritoneal seeding. Oncol Lett. 2018;15:4193–4200. doi: 10.3892/ol.2018.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Si X, Xu A, Meng X, Gao S, Qi Y, Zhu L, Li T, Li W, Dong L. Activation of STAT3 in human gastric cancer cells via interleukin (IL)-6-type cytokine signaling correlates with clinical implications. PLoS One. 2013;8:e75788. doi: 10.1371/journal.pone.0075788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) methods. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Dong L, Zhen Y, Wang Y, Qi D, Xu A, Meng X, Li W. Astragalus extract inhibits proliferation but enhances apoptosis in gastric cancer. Pak J Pharm Sci. 2016;29:1473–1482. [PubMed] [Google Scholar]
- 12.Sitaula S, Zhang J, Ruiz F, Burris TP. Rev-erb regulation of cholesterologenesis. Biochem Pharmacol. 2017;131:68–77. doi: 10.1016/j.bcp.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Qi Y, Liu H, Wang X, Zhu H, Wang Z. AMPK activator AICAR promotes 5-FU-induced apoptosis in gastric cancer cells. Mol Cell Biochem. 2016;411:299–305. doi: 10.1007/s11010-015-2592-y. [DOI] [PubMed] [Google Scholar]
- 14.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marciano DP, Chang MR, Corzo CA, Goswami D, Lam VQ, Pascal BD, Griffin PR. The therapeutic potential of nuclear receptor modulators for treatment of metabolic disorders: PPARγ, RORs, and Rev-erbs. Cell Metab. 2014;19:193–208. doi: 10.1016/j.cmet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Lee S, Chung S, Park N, Son GH, An H, Jang J, Chang DJ, Suh YG, Kim K. Identification of a novel circadian clock modulator controlling BMAL1 expression through a ROR/REV-ERB-response element-dependent mechanism. Biochem Biophys Res Commun. 2016;469:580–586. doi: 10.1016/j.bbrc.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Mazzoccoli G, de Cata A, Piepoli A, Vinciguerra M. The circadian clock and the hypoxic response pathway in kidney cancer. Tumour Biol. 2014;35:1–7. doi: 10.1007/s13277-013-1076-5. [DOI] [PubMed] [Google Scholar]
- 18.Reszka E, Przybek M. Circadian genes in breast cancer. Adv Clin Chem. 2016;75:53–70. doi: 10.1016/bs.acc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Kojetin D, Burris TP. Anti-proliferative actions of a synthetic REV-ERBα/β agonist in breast cancer cells. Biochem Pharmacol. 2015;96:315–322. doi: 10.1016/j.bcp.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu L, Kettner NM. The circadian clock in cancer development and therapy. Prog Mol Biol Transl Sci. 2013;119:221–282. doi: 10.1016/B978-0-12-396971-2.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez-Martinez P, Rossi DJ, Beerman I. DNA damage and aging around the clock. Trends Mol Med. 2016;22:635–637. doi: 10.1016/j.molmed.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chomez P, Neveu I, Mansén A, Kiesler E, Larsson L, Vennström B, Arenas E. Increased cell death and delayed development in the cerebellum of mice lacking the rev-erbA(alpha) orphan receptor. Development. 2000;127:1489–1498. doi: 10.1242/dev.127.7.1489. [DOI] [PubMed] [Google Scholar]
- 23.Tao W, Wu J, Zhang Q, Lai SS, Jiang S, Jiang C, Xu Y, Xue B, Du J, Li CJ. EGR1 regulates hepatic clock gene amplitude by activating Per1 transcription. Sci Rep. 2015;5:15212. doi: 10.1038/srep15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Sancar A. Circadian clock disruption improves the efficacy of chemotherapy through p73-mediated apoptosis. Proc Natl Acad Sci USA. 2011;108:10668–10672. doi: 10.1073/pnas.1106284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y, Lin F, Chen Y, Tan Z, Bai D, Zhao Q. Overexpression of the circadian clock gene rev-erbα affects murine bone mesenchymal stem cell proliferation and osteogenesis. Stem Cells Dev. 2015;24:1194–1204. doi: 10.1089/scd.2014.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.