Abstract
Lung cancer has one of the highest mortality rates among malignancies globally, and smoking has been documented as the main cause of lung cancer. Nicotinic acetylcholine receptors (nAChRs) were initially identified as notable regulators of the nervous system. In addition to their function in the brain, accumulating evidence indicates that nAChRs perform a host of diverse functions in almost all non-neuronal mammalian cells. The homomeric α7nAChR, a subtype of nAChRs, is responsible for the proliferative, pro-angiogenic and pro-metastatic effects of nicotine in lung cancer. Provided the association of cigarette smoking with several disease types such as cardiovascular disease, the α7nAChR-mediated signaling pathway has been implicated in the pathophysiology of lung cancer. Currently, strategies that target the α7nAChR including α7nAChR antagonists are considered to be potentially useful anticancer drugs for therapeutic purposes. Thus, the present review assesses current understanding of the function and underlying molecular mechanisms of α7nAChR in lung cancer and evaluates how targeting α7nAChR may result in novel therapeutic methods.
Keywords: α7 nicotinic acetylcholine receptor, lung cancer, nicotine, proliferation, angiogenesis, metastasis
1. Introduction
Lung cancer is one of the most commonly occurring carcinoma types globally and has limited treatment options for advanced-stage disease (1). Lung cancer is a heterogeneous disease comprised of two main pathological types: Non-small-cell lung cancer (NSCLC) which accounts for 70–80% of all lung cancer cases and small-cell lung cancer (SCLC) which accounts for ~20% of all lung cancer cases (2). NSCLCs may be divided into three subtypes: Squamous-cell carcinoma (25–30% of all lung cancer cases), adenocarcinoma (~40% of all lung cancer cases) and large-cell carcinoma (10–15% of all lung cancer cases) (3). SCLC is the second most prevalent form of lung cancer, with a 5-year survival rate of <7% (4). Cigarette smoking is considered to be the main risk factor for lung cancer, and ~90% of all cases are associated with exposure to smoking and second-hand smoking (5). Other contributory factors include residential radon, occupational hazards including exposure to asbestos, arsenic and polycyclic aromatic hydrocarbons, radiation, coal smoke, indoor emission of fuel burning, outdoor pollution, previous non-malignant lung diseases in addition to a family history of tumors (6,7). Squamous-cell, large-cell and SCLC are the most commonly identified types of lung cancer present in smokers (8,9). In contrast, adenocarcinoma is the lung cancer type most commonly identified in non-smokers (10).
Cigarette smoke is a mixture of thousands of chemical compounds, a number of which have potent carcinogenic potential including polycyclic aromatic hydrocarbons, nicotine and the nicotine-derived nitrosamines 4-(methylnitrosamino)-1-(3-pyrydyl)-1-butanone (NNK) and N-nitrosonornicotine (11). The most harmful and addictive component is nicotine (11). These carcinogens and their metabolites may induce the formation of DNA adducts which result in mutations of a number of key cancer suppressor genes, including retinoblastoma tumor suppressor protein (Rb), KRAS proto-oncogene, GTPase and tumor protein p53 (11) and eventually contributing to tumorigenesis in different ways. Accumulating evidences have suggested that nicotine not only contributes to tumorigenesis but may also increase the spread of cancer in the body (12–14).
It has been demonstrated that nicotine exerts its biological effects through nicotinic acetylcholine receptors (nAChRs) in human lung cancer cells (15). nAChRs are pentameric proteins composed of homologous subunits, which are encoded by a large multigene family (16,17). This receptor family was initially identified as notable regulators of the nervous system (18). In addition to their function in neuromuscular and motor autonomous transmission, nAChRs perform numerous central functions in almost all non-neuronal mammalian cells (18). The α7 subtype (α7nAChR), a subtype of nAChRs, is expressed in a variety of cells, including in endothelial cells, glial cells, brain radial glial cells, synovial cells and thymus cells, lymphocytes, bone marrow cells, monocytes, macrophages and microglia (19–21). Numerous studies have revealed that α7nAChR serves a notable function in the oncogenic process (22–24). In accordance with this notion, α7nAChR has been implicated in the proliferative, pro-angiogenic and pro-metastatic effects of nicotine in lung cancer types (24–27). Therefore, the α7nAChR-associated signaling networks in tumor cells may represent a novel target for the therapy of nicotine-associated lung cancer types.
The present review overviews evidence of previous studies to demonstrate the effects and molecular mechanisms of α7nAChR in lung cancer, and then describes the potential association of these signaling pathways with cancer-associated processes.
2. Epidemiology of lung cancer
Lung cancer is one of most deadly carcinoma types globally (28,29). Despite improvements in the diagnosis and treatment of this malignancy in previous years, the incidence and mortality rates of lung cancer are increasing. Based on Globocan 2012 estimates, lung cancer is the most commonly occuring cancer type among men in developed and developing countries and has exceeded breast cancer as the leading cause of cancer mortality amongst women in developed countries (30). In 2012, a total of ~1.8 million people were affected by this disease, and the estimated mortality rate was 1,098,700 and 491,200 for men and women, respectively (31). Amongst men, the highest lung cancer incidence rates were in Europe, Eastern Asia and Northern America, while the lowest incidence rates were in sub-Saharan Africa. Amongst women, the highest lung cancer rates were in Northern America, Northern and Western Europe, Australia/New Zealand and Eastern Asia (31). In China, lung cancer is the most commonly occurring cancer and the leading cause of cancer-associated mortality (32). Notably, this disease has one of the worst prognoses of all malignant tumor types and the overall 5-year survival rate is ~17.8% (33). Therefore, it is important to develop novel effective strategies in treatment of lung cancer.
Smoking is a key risk factor for lung cancer. The increase in lung cancer incidence globally parallels the rise of cigarette consumption (34). Particularly of note is that smoking is associated with 90% of SCLC and 60% of NSCLC cases and is responsible for ~80% of lung cancer mortality (35). A previous analysis revealed that passive smoking resulted in a higher risk of developing lung cancer compared with non-smokers (36). According to the U.S. Surgeon General, the risk of lung cancer in a non-smoker living with a smoker may be increased by 20–30% compared with a non-smoker living without a smoker (37). Notably, in countries where the tobacco epidemic has been established more recently, including in China, Indonesia and several countries in Africa, lung cancer rates are expected to continue to increase at least for the next few decades (38). Thus, apart from primary prevention programs including an effective tobacco-control policy, novel target molecules and the potential mechanisms of tobacco-associated lung cancer may attract more attention and should be further evaluated in future studies.
3. Expression of α7nAChR in lung cancer
Previous studies have revealed that nicotine-mediated tumor progression is initiated through the activation of nAChRs, specifically the α7 subunit (39–42). nAChRs belong to the superfamily of ligand-gated ion channels, including the excitatory 5HT3 receptor and the inhibitory receptors for glycine and γ-aminobutyric acid (43). To date, a number of nAChRs comprising various combinations of subunits have been identified (α1-α10, β1-β4, γ, δ and ε) (16,17). These receptors are activated by tumor cells contributing to the initiation of the non-adrenergic, non-cholinergic signaling, thereby promoting proliferation, angiogenesis and migration through autocrine and paracrine effects in lung cancer (44,45).
α7nAChR is expressed in several types of human lung cancer, including squamous cell lung cancer cells, lung adenocarcinoma and SCLC (11,46–49). Notably, the levels of α7nAChR expression are higher in squamous carcinoma compared with adenocarcinoma, particularly in smokers (50). In addition, there are different responses to cigarette smoking between women and men. α7nAChR expression is higher in male patients that smoked compared with female patients that smoked (51). Based on these observations, it has been proposed that α7nAChR upregulation in lung cancer cells may be involved in the nicotine-induced tumorigenic process (50,51). Future studies are required to explore the characteristics of α7nAChR which are emerging as a potential target for lung cancer therapy.
4. Roles and mechanisms of α7nAChR in lung cancer
Regulatory function of α7nAChR in lung cancer
Although nAChRs are widely expressed in non-neuronal and lung cancer cells, nicotine-mediated tumor progression is facilitated predominantly through α7nAChR (44,45). Consistent with this, α7nAChR levels have been revealed to be elevated in human squamous-cell lung cancer cells during sustained nicotine exposure (49). Similarly, the levels of α7nAChR in squamous cell carcinoma of lung tissues isolated from patients (who are active smokers) correlate with their smoking history (49). In addition, a previous study has revealed that α7nAChR levels were increased in mice that were administered nicotine (52), and nicotine-mediated effects on cell proliferation, invasion, migration and angiogenic tubule formation are abrogated in the presence of α7nAChR-specific inhibitors (53). Therefore, studying the role of α7nAChR and its underlying molecular mechanisms in lung cancer is clinically relevant.
A majority of mechanistic studies (27,47,49,51,53–69) focus on identifying the function of α7nAChR-mediated signaling in the regulation of the tumorigenic process including in proliferation, angiogenesis and metastasis in lung cancer (Table I). Notably, a number of α7nAChRs antagonists have been investigated to explore its influence on tumor progression (56,57,64–66,69). Provided that α7nAChR is a major genetic biomarker of nAChRs for lung cancer (70), strategies that target α7nAChR may be useful in the treatment of lung cancer for therapeutic purposes.
Table I.
Regulatory function of α7nAChR in lung cancer.
| Author, year | Targeted cell type(s) | Major outcome(s) associated with α7nAChR | (Refs.) |
|---|---|---|---|
| Zhang et al, 2016 | NSCLCs | NSCLC cell invasion, migration and epithelial-mesenchymal transition were mediated by α7nAChR and MEK/ | (27) |
| ERK signaling pathway induced by nicotine | |||
| Dasgupta et al, 2006 | NSCLCs | α7nAChR regulated the oncogenic process which depends on proliferation and survival-associated genes induced by nicotine | (47) |
| Brown et al, 2013 | SCCLs | Upregulation of α7nAChRs accelerated tumor proliferation and progression through binding GATA4 or GATA6 stimulated by nicotine | (49) |
| Paleari et al, 2008 | NSCLCs | α7nAChR promoted tumor cell growth by activating the Rb-Raf-1/phospho-ERK/phospho-p90RSK pathway | (51) |
| Medjber et al, 2015 | NSCLCs | α7nAChR regulated cell growth and stimulated tumor invasion depending on the differentiation status of the tumor in NSCLCs | (53) |
| Al-Wadei et al, 2012 | NSCLCs | α7nAChR promoted proliferation in nicotine-treated NSCLC cells by upregulating the stress neurotransmitter noradrenaline | (54) |
| Zovko et al, 2013 | NSCLCs | APS8 inhibited cell growth and triggered the intrinsic apoptotic pathways | (55) |
| Paleari et al, 2009 | NSCLCs (A549 cells) | α-CbT specifically inhibited the α7nAChR-mediated survival pathway | (56) |
| Grozio et al, 2008 | NSCLCs (A549 cells) | α-CbT may reduce the tumor cell growth factors of nicotine | (57) |
| Sheppard et al, 2000 | SCLCs | Activation of Ca2+ influx contributed to the development of SCLCs by binding α7nAChR induced by NNK | (58) |
| Jull et al, 2001 | SCLCs | NNK regulated the SCLCs growth by initiating the Raf-1/MAPK/c-myc kinase pathway in vitro | (59) |
| Hung et al, 2009 | CL1.0 lung cancer cells | NNK activated α7nAChR downstream signaling pathways of Akt and ERK | (60) |
| Zhong et al, 2015 | NSCLCs | PGE2 increased the expression of α7nAChR by activating signals of JNK, PI3K and PKA through upregulating c-Jun | (61) |
| Sun et al, 2009 | NSCLCs | Nicotine upregulated the expression of PPARβ/δ through α7nAChR-mediated activation of PI3K/mTOR signals and suppression of AP-2α protein expression and DNA binding activity in the PPARβ/δ gene promoter | (62) |
| Chernyavsky et al, 2015 | SCCLs (SW900) | Activation of α7nAChR is associated with EGF and VEGF receptors in cell membrane | (63) |
| Brown et al, 2012 | SCLCs | MG624 inhibited the angiogenesis of human SCLC tumor types followed by the suppression of nicotine-induced FGF2 | (64) |
| Shen et al, 2012 | Lung cancer cell lines (i.e. H1299, H82, H157 | α-BTX blocked the tyrosine phosphorylation of c-Src, PKCι and FAK and prevented metastatic tumor types induced by NNK cells and H460 cells) | (65) |
| Iskandar et al, 2016 | Lung cancer cells | BCX restrains the migration and invasion of α7nAChR-positive lung cancer cells through the downregulation of α7nAChR/PI3K signaling | (66) |
| Zhang et al, 2017 | NSCLCs (H1299) | Blocking α7nAChRs suppresses nicotine-induced H1299 cell proliferation are mediated through the de-phosphor ylation of the MEK signaling pathway in H1299 cells | (67) |
| Mucchietto et al, 2017 | NSCLCs (A549 cells) | In A549 cells, α7 nAChR not only regulate nicotine-induced cell proliferation but also the activation of the Akt and ERK pathways | (68) |
| Yan et al, 2017 | NSCLCs (A549 cells) | The methyllycaconitine citrate hydrate MLA and rL-RVG (the rabies virus glycoprotein) treatments significantly inhibited proliferation and migration and promoted apoptosis in the lung cancer cells | (69) |
α7nAChR, α7 nicotinic acetylcholine receptor; NSCLC, non-small-cell lung carcinoma; SCCL, small cell carcinoma of the lung; SCLC, small-cell lung carcinoma; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; Rb, retinoblastoma tumor suppressor protein; Raf-1, RAF proto-oncogene serine/threonine-protein kinase; p90RSK, MAPK-activated protein kinase-1; GATA, GATA binding protein; APS8, an analog of 3-alkylpyridinium polymers with a defined alkyl chain length and molecular size; α-CbT, α-cobratoxin; NNK, nicotine-derived nitrosamines 4-(methylnitrosamino)-1-(3-pyrydyl)-1-butanone; MAPK, mitogen-activated protein kinase; c-myc, MYC proto-oncogene, BHLH transcription factor; Akt, protein kinase B; PGE2, prostaglandin E2; JNK, c-Jun N-terminal kinase; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PPAR, peroxisome proliferator-activated receptors; mTOR, mammalian target of rapamycin; AP-2α, transcription factor AP-2 α; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; MG624, an α7-nicotinic receptor antagonist; α-BTX, α-Bungarotoxin; MLA, an α7 nAChR antagonist; FGF2, fibroblast growth factor 2; c-Src, proto-oncogene tyrosine-protein kinase Src; PKCι, protein kinase Cι; FAK, focal adhesion kinase; BCX, β-cryptoxanthin.
Function and mechanisms of α7nAChR on cell proliferation
At present, α7nAChR has been proposed to mediate nicotine-induced survival rate and proliferation in cancer cells in vitro and in vivo (22,70). It was revealed that proliferative signaling via α7nAChR required the scaffolding protein β-arrestin, while the ablation of β-arrestin or disruption of the Rb-RAF proto-oncogene serine/threonine-protein kinase (Raf-1) interaction blocked the nicotine-induced proliferation of NSCLCs (47). Furthermore, the α7nAChR-induced release of noradrenaline significantly stimulated NSCLC proliferation associated with the induction of phosphorylated (p)-extracellular signal-regulated kinases (ERK) and p-cAMP response element-binding protein signaling, suggesting that α7nAChR represents an attractive target for developing more effective intervention strategies for NSCLC (54). A previous study demonstrated that exposure to nicotine resulted in α7nAChRs upregulation in human squamous cell lung cancer via the Sp1 transcription factor/GATA binding protein pathway, which accelerates tumor proliferation and progression (49). However, several signals underlying α7nAChR-induced cell proliferation included the activation of Ca2+ influx (58), Raf-1 (51,59), mitogen-activated protein kinase/ERK (27,51,59,60), c-Jun N-terminal kinase, phosphoinositide-3 kinase (PI3K)/protein kinase B (Akt), protein kinase A (PKA) pathway (60–62), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) receptors (63), and mitogen-activated protein kinase kinase (MEK)/ERK (67). In nicotine-induced lung cancer cells, Chernyavsky et al (63) revealed that the activation of cell membrane α7nAChR resulted in the association with EGF receptors, whereas activated mitochondrial α7nAChR physically associated with the intramitochondrial protein kinases PI3K and Src. Zhang et al (67) demonstrated that the blockade of α7nAChR specifically inhibited nicotine-stimulated tumor growth in NSCLC through the MEK/ERK signaling pathway. It has also been reported that α7nAChRs mediate the pro-proliferative effects of nicotine through activating Akt and ERK pathways, and blocking α7nAChRs eliminates nicotine-induced proliferation and signaling in A549 cells (68). These findings indicate that the expression of α7nAChR is associated with cellular survival rate and proliferation in lung cancer. A potential strategy may be to use α7nAChR as a biomarker to inhibit tumor proliferation and progression in lung cancer. Based on this information, α7nAChRs antagonists were revealed to attenuate the proliferative effects of nicotine in lung cancer (22). An analog of 3-alkylpyridinium polymers with a defined alkyl chain length and molecular size (APS8) may inhibit tumor may inhibit tumor growth and trigger the intrinsic apoptotic pathways in NSCLCs (55). Another study has confirmed that α7nAChRs antagonists including d-tubocurarine and α-cobratoxin (α-CbT) may reduce tumor cell growth factors stimulated by nicotine (56,57). Yan et al (69) revealed that methyl lycaconitine citrate hydrate (a α7nAChR antagonist) and rabies virus glycoprotein treatments significantly inhibited proliferation and promoted apoptosis in A549 lung adenocarcinoma cells.
Function and mechanisms of α7nAChR on angiogenesis
Angiogenesis is widely known as a typical characteristic in cancer to sustain tumor growth (71). Angiogenesis is necessary for primary tumor progression (72). Surprisingly, there is a limited study focusing on the angiogenic activity of α7nAChR in lung cancer. A previous study has demonstrated that the small-molecule antagonist for α7nAChR (MG624), inhibited angiogenesis effects in SCLCs followed by the suppression of nicotine-induced fibroblast growth factor 2 (64). Since α7nAChR upregulation by cancer cells stimulates tumor progression, it can be used in future studies to further explore its effects on angiogenesis.
Function and mechanisms of α7nAChR on metastasis
Metastasis is the major cause of mortality in cancer (73). The process of metastasis may be classically divided into a number of steps: Invasion of tumor cells into the surrounding tissues, penetration of vessels and migration toward distant sites of the body away from the primary sites (74). At present, several clinical studies in humans revealed an association between smoking and an increase in the metastasis of lung cancer (75–78). The α7nAChR is expressed in SCLC and NSCLC cells (24). Nicotine has a high affinity with α7nAChR in lung cancer cells (50). Thus, it would be useful to understand the mechanism of α7nAChR in metastasis in nicotine-associated lung cancer types (79). α7nAChR may regulate cell growth and stimulate tumor invasion depending on the differentiation status of the tumor in NSCLCs (53). The pro-proliferative activity of poorly-differentiated NSCLC was stimulated by nicotine, whereas it was suppressed in well-differentiated cells (53). Nicotine may also induce NSCLC cells invasion, migration and mesenchymal transition, which were mediated by α7nAChR involving the MEK/ERK signaling pathway (27). Meanwhile, the effects induced by nicotine may be suppressed by pharmacological intervention using α7nAChR selective antagonists or by genetic intervention using α7nAChR small interfering RNAs (55,68). α-bungarotoxin appeared to be one of the specific inhibitor for α7nAChR, which blocked metastatic tumors by NNK-induced tyrosine phosphorylation of proto-oncogene tyrosine-protein kinase Src, protein kinase Cι and focal adhesion kinase (65). In addition, β-cryptoxanthin treatment restrained the migration and invasion of α7nAChR-positive lung cancer cells through the downregulation of α7nAChR/PI3K signaling (66). All the aforementioned results suggest that α7nAChR enhances the metastasis of lung cancer cells, although the underlying molecular mechanisms require further investigation.
5. Conclusions
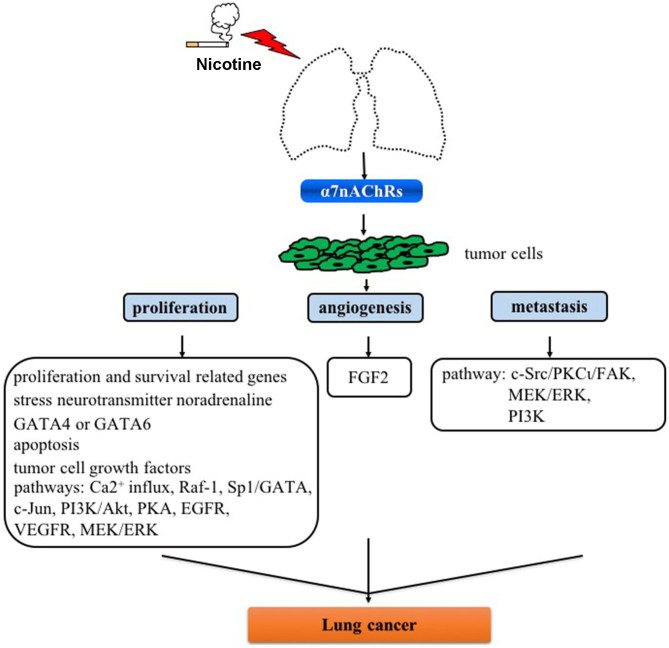
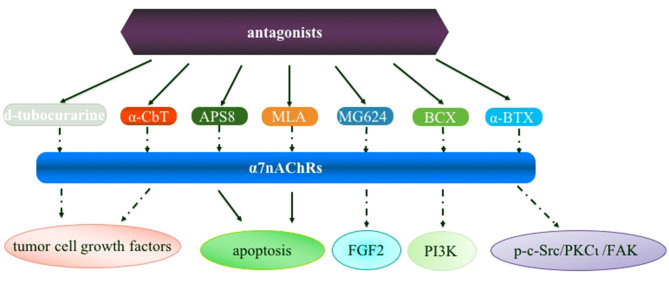
Despite efforts that have been reported focusing on α7nAChR as a molecular target in human diseases including lung cancer, a number of issues remain be addressed in future studies: i) Currently available evidence indicates that α7nAChR activation activates signaling pathways involved in the proliferation, angiogenesis and metastasis for developing lung cancer (Fig. 1), and thus it is crucial to analyze the difference of α7nAChR expression and underlying mechanisms in SCLC and NSCLC cells; ii) Little is known on the roles of these pathways in cell types including macrophages and other immune cells which are also very important in tumorigenesis; iii) α7nAChR expression is activated in the process of nicotine-mediated cancer; however, how α7nAChR antagonists (e.g., α-CbT treatment) are regulated in lung cancer is uclear (Fig. 2). Nevertheless, although there are several limitations for α7nAChR-based drug therapy for clinical use for lung cancer or other diseases, these potential mechanisms are inevitably the foundation of designing novel anticancer drugs in lung cancer.
Figure 1.
Role of α7nAChR on proliferation, angiogenesis and metastasis in lung cancer. α7nAChR, α7 nicotinic acetylcholine receptor; GATA, GATA binding protein; Raf-1, Raf-1 proto-oncogene, serine/threonine kinase; Sp1, Sp1 transcription factor; c-Jun N-terminal kinase; FGF2, fibroblast growth factor 2; c-Src, proto-oncogene tyrosine-protein kinase Src; PKCι, protein kinase Cι; FAK, focal adhesion kinase; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinases; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; EGFR, epidermal growth factor; VEGFR, vascular endothelial growth factor receptors.
Figure 2.
Overview of the α7nAChR antagonists in lung cancer. α7nAChR, α7 nicotinic acetylcholine receptor; α-CbT, α-cobratoxin; APS8, an analog of 3-alkylpyridinium polymers with a defined alkyl chain length and molecular size; MLA, an α7 nAChR antagonist; MG624, an α7-nicotinic receptor antagonist; BCX, β-cryptoxanthin; α-BTX, α-Bungarotoxin; FGF2, fibroblast growth factor 2; PI3K, phosphoinositide 3-kinase; p-c-Src, phosphorylated proto-oncogene tyrosine-protein kinase Src; PKCι, protein kinase Cι; FAK, focal adhesion kinase.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- nAChRs
nicotinic acetylcholine receptors
- NNK
nicotine-derived nitrosamines 4-(methylnitrosamino)-1-(3-pyrydyl)-1-butanone
- NSCLC
non-small-cell lung cancer
- SCLC
small-cell lung cancer
- α-CbT
α-cobratoxin
Funding
This study was supported by the Medical and Health Science Technology plan project of Zhejiang province, China (no. 2017KY431).
Availability of data and materials
Not applicable.
Authors' contributions
SCW and YH conceived and wrote the paper. YH reviewed and made final approval of the version to be published. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Schwartz AG, Cote ML. Epidemiology of lung cancer. Adv Exp Med Biol. 2016;893:21–41. doi: 10.1007/978-3-319-24223-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83:355–367. doi: 10.4065/83.3.355. [DOI] [PubMed] [Google Scholar]
- 3.Kuribayashi K, Funaguchi N, Nakano T. Chemotherapy for advanced non-small cell lung cancer with a focus on squamous cell carcinoma. J Cancer Res Ther. 2016;12:528–534. doi: 10.4103/0973-1482.174185. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhao Y, Li C, Zhu L, Liu C, Liu L. The revision of 8th edition TNM stage criteria is more accurate in prediction postoperative survival for SCLC patients. Int J Surg. 2017;48:83–85. doi: 10.1016/j.ijsu.2017.09.072. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society . Cancer facts and figures 2015. American Cancer Society; Atlanta, GA; 2015. [Google Scholar]
- 6.Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, International Agency for Research on Cancer Monograph Working Group IARC The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. doi: 10.1016/S1470-2045(13)70487-X. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 8.Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control. 2008;17:198–204. doi: 10.1136/tc.2007.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muscat JE, Stellman SD, Zhang ZF, Neugut AI, Wynder EL. Cigarette smoking and large cell carcinoma of the lung. Cancer Epidemiol Biomarkers Prev. 1997;6:477–480. [PubMed] [Google Scholar]
- 10.Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers-a review. Eur J Cancer. 2012;48:1299–1311. doi: 10.1016/j.ejca.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Schaal C, Chellappan S. Nicotine-mediated regulation of nicotinic acetylcholine receptors in non-small cell lung adenocarcinoma by E2F1 and STAT1 transcription factors. PLoS One. 2016;11:e015645. doi: 10.1371/journal.pone.0156451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardinale A, Nastrucci C, Cesario A, Russo P. Nicotine: Specific role in angiogenesis, proliferation and apoptosis. Crit Rev Toxicol. 2012;42:68–89. doi: 10.3109/10408444.2011.623150. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 15.Improgo MR, Scofield MD, Tapper AR, Gardner PD. The nicotinic acetylcholine receptor CHRNA5/A3/B4 gene cluster: Dual role in nicotine addiction and lung cancer. Prog Neurobiol. 2010;92:212–226. doi: 10.1016/j.pneurobio.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 17.Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- 18.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxico. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 19.Papke RL, Bagdas D, Kulkarni AR, Gould T, AlSharari SD, Thakur GA, Damaj MI. The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology. 2015;91:34–42. doi: 10.1016/j.neuropharm.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias HR, Richards VE, Ng D, Ghafoori ME, Le V, Mousa SA. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int J Biochem Cell Biol. 2009;41:1441–1451. doi: 10.1016/j.biocel.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Russo P, Taly A. α7-Nicotinic acetylcholine receptors: An old actor for new different roles. Curr Drug Targets. 2012;13:574–578. doi: 10.2174/138945012800398874. [DOI] [PubMed] [Google Scholar]
- 22.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: Multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–158. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Ritzenthaler JD, Roman J, Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol. 2007;37:681–690. doi: 10.1165/rcmb.2007-0051OC. [DOI] [PubMed] [Google Scholar]
- 24.Schuller HM. Regulatory role of the α7nAChR in cancer. Curr Drug Targets. 2012;13:680–687. doi: 10.2174/138945012800398883. [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillai S, Chellappan S. α7 nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition. Curr Drug Targets. 2012;13:671–679. doi: 10.2174/138945012800398847. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Ding XP, Zhao QN, Yang XJ, An SM, Wang H, Xu L, Zhu L, Chen HZ. Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget. 2016;7:59199–59208. doi: 10.18632/oncotarget.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifert U, Schlanstedt-Jahn U, Klug SJ. Screening for cancer. Internist (Berl) 2015;56:1114–1123. doi: 10.1007/s00108-015-3738-4. [DOI] [PubMed] [Google Scholar]
- 29.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 30.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 31.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute SEER cancer statistics review, 1975–2011. https://seer.cancer.gov/archive/csr/1975_2011/ 2014. https://seer.cancer.gov/archive/csr/1975_2011/ Updated December 17.
- 34.Warren GW, Cummings KM. Tobacco and lung cancer: Risks, trends, and outcomes in patients with cancer. Am Soc Clin Oncol Educ Book: 2013. pp. 359–364. [DOI] [PubMed]
- 35.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 36.Whitrow MJ, Smith BJ, Pilotto LS, Pisaniello D, Nitschke M. Environmental exposure to carcinogens causing lung cancer: Epidemiological evidence from the medical literature. Respirology. 2003;8:513–521. doi: 10.1046/j.1440-1843.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 37.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . Centers for Disease Control and Prevention (US) Atlanta, GA; 2014. The health consequences of smoking-50 years of progress: A report of the surgeon general. [PubMed] [Google Scholar]
- 38.Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655–664. doi: 10.1038/nrc2703. [DOI] [PubMed] [Google Scholar]
- 39.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cattaneo MG, D'Atri F, Vicentini LM. Mechanisms of mitogen-activated protein kinase activation by nicotine in small-cell lung carcinoma cells. Biochem J. 1997;328:499–503. doi: 10.1042/bj3280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song P, Sekhon HS, Proskocil B, Blusztajn JK, Mark GP, Spindel ER. Synthesis of acetylcholine by lung cancer. Life Sci. 2003;72:2159–2168. doi: 10.1016/S0024-3205(03)00078-X. [DOI] [PubMed] [Google Scholar]
- 42.Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, Mark GP, Grando SA, Spindel ER. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–3944. doi: 10.1158/0008-5472.CAN-06-2484. [DOI] [PubMed] [Google Scholar]
- 43.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 44.Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, Proskosil BJ, Gravett C, Lindstrom J, Mark GP, et al. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008;68:4693–4700. doi: 10.1158/0008-5472.CAN-08-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- 46.Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci USA. 2006;103:6332–6337. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;16:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S, Tin VP, Chung LP, Wong MP, Shay JW, et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638–4647. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- 49.Brown KC, Perry HE, Lau JK, Jones DV, Pulliam JF, Thornhill BA, Crabtree CM, Luo H, Chen YC, Dasgupta P. Nicotine induces the up-regulation of the α7-nicotinic receptor (α7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway. J Biol Chem. 2013;288:33049–33059. doi: 10.1074/jbc.M113.501601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bordas A, Cedillo JL, Arnalich F Esteban-Rodriguez I, Guerra-Pastrián L, de Castro J, Martín-Sánchez C, Atienza G, Fernández-Capitan C, Rios JJ, Montiel C. Expression patterns for nicotinic acetylcholine receptor subunit genes in smoking-related lung cancers. Oncotarget. 2017;8:67878–67890. doi: 10.18632/oncotarget.18948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paleari L, Catassi A, Ciarlo M, Cavalieri Z, Bruzzo C, Servent D, Cesario A, Chessa L, Cilli M, Piccardi F, et al. Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation. Cell Prolif. 2008;41:936–959. doi: 10.1111/j.1365-2184.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, Chellappan S. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medjber K, Freidja ML, Grelet S, Lorenzato M, Maouche K, Nawrocki-Raby B, Birembaut P, Polette M, Tournier JM. Role of nicotinic acetylcholine receptors in cell proliferation and tumour invasion in broncho-pulmonary carcinomas. Lung Cancer. 2015;87:258–264. doi: 10.1016/j.lungcan.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: A novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zovko A, Viktorsson K, Lewensohn R, Kološa K, Filipič M, Xing H, Kem WR, Paleari L, Turk T. APS8, a polymeric alkylpyridinium salt blocks α7 nAChR and induces apoptosis in non-small cell lung carcinoma. Mar Drugs. 2013;11:2574–2594. doi: 10.3390/md11072574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paleari L, Sessa F, Catassi A, Servent D, Mourier G, Doria-Miglietta G, Ognio E, Cilli M, Dominioni L, Paolucci M, et al. Inhibition of non-neuronal alpha7-nicotinic receptor reduces tumorigenicity in A549 NSCLC xenografts. Int J Cancer. 2009;125:199–211. doi: 10.1002/ijc.24299. [DOI] [PubMed] [Google Scholar]
- 57.Grozio A, Paleari L, Catassi A, Servent D, Cilli M, Piccardi F, Paganuzzi M, Cesario A, Granone P, Mourier G, Russo P. Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: A promising prospective in anti-cancer drug development. Int J Cancer. 2008;122:1911–1915. doi: 10.1002/ijc.23298. [DOI] [PubMed] [Google Scholar]
- 58.Sheppard BJ, Williams M, Plummer HK, Schuller HM. Activation of voltage-operated Ca2+-channels in human small cell lung carcinoma by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int J Oncol. 2000;16:513–518. doi: 10.3892/ijo.16.3.513. [DOI] [PubMed] [Google Scholar]
- 59.Jull BA, Plummer HK, III, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–717. doi: 10.1007/s004320100289. [DOI] [PubMed] [Google Scholar]
- 60.Hung YH, Hung WC. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) enhances invasiveness of lung cancer cells by up-regulating contactin-1 via the alpha7 nicotinic acetylcholine receptor/ERK signaling pathway. Chem Biol Interact. 2009;179:154–159. doi: 10.1016/j.cbi.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 61.Zhong X, Fan Y, Ritzenthaler JD, Zhang W, Wang K, Zhou Q, Roman J. Novel link between prostaglandin E2 (PGE2) and cholinergic signaling in lung cancer: The role of c-Jun in PGE2-induced α7 nicotinic acetylcholine receptor expression and tumor cell proliferation. Thorac Cancer. 2015;6:488–500. doi: 10.1111/1759-7714.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun X, Ritzenthaler JD, Zhong X, Zheng Y, Roman J, Han S. Nicotine stimulates PPARbeta/delta expression in human lung carcinoma cells through activation of PI3K/mTOR and suppression of AP-2alpha. Cancer Res. 2009;69:6445–6453. doi: 10.1158/0008-5472.CAN-09-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chernyavsky AI, Shchepotin IB, Grando SA. Mechanisms of growth-promoting and tumor-protecting effects of epithelial nicotinic acetylcholine receptors. Int Immunopharmacol. 2015;29:36–44. doi: 10.1016/j.intimp.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 64.Brown KC, Lau JK, Dom AM, Witte TR, Luo H, Crabtree CM, Shah YH, Shiflett BS, Marcelo AJ, Proper NA, et al. MG624, an α7-nAChR antagonist, inhibits angiogenesis via the Egr-1/FGF2 pathway. Angiogenesis. 2012;15:99–114. doi: 10.1007/s10456-011-9246-9. [DOI] [PubMed] [Google Scholar]
- 65.Shen J, Xu L, Owonikoko TK, Sun SY, Khuri FR, Curran WJ, Deng X. NNK promotes migration and invasion of lung cancer cells through activation of c-Src/PKCι/FAK loop. Cancer Lett. 2012;318:106–113. doi: 10.1016/j.canlet.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iskandar AR, Miao B, Li X, Hu KQ, Liu C, Wang XD. β-cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by downregulating nicotinic acetylcholine receptor α7 signalingg. Cancer Prev Res (Phila) 2016;9:875–886. doi: 10.1158/1940-6207.CAPR-16-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Yu P, Zhu L, Zhao Q, Lu X, Bo S. Blockade of α7 nicotinic acetylcholine receptors inhibit nicotine-induced tumor growth and vimentin expression in non-small cell lung cancer through MEK/ERK signaling way. Oncol Rep. 2017;38:3309–3318. doi: 10.3892/or.2017.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mucchietto V, Fasoli F, Pucci S, Moretti M, Benfante R, Maroli A, Di Lascio S, Bolchi C, Pallavicini M, Dowell C, et al. α9- and α7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line. Br J Pharmacol. 2017. Jul 20. (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 69.Yan Y, Su C1, Hang M, Huang H, Zhao Y, Shao X, Bu X. Recombinant Newcastle disease virus rL-RVG enhances the apoptosis and inhibits the migration of A549 lung adenocarcinoma cells via regulating alpha 7 nicotinic acetylcholine receptors in vitro. Virol J. 2017;14:190. doi: 10.1186/s12985-017-0852-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paleari L, Cesario A, Fini M, Russo P. alpha7-Nicotinic receptor antagonists at the beginning of a clinical era for NSCLC and Mesothelioma? Drug Discov Today. 2009;14:822–836. doi: 10.1016/j.drudis.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 71.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 72.Cavallaro U, Christofori G. Molecular mechanisms of tumor angiogenesis and tumor progression. J Neurooncol. 2000;50:63–70. doi: 10.1023/A:1006414621286. [DOI] [PubMed] [Google Scholar]
- 73.Wu MY, Li CJ, Yiang GT, Cheng YL, Tsai AP, Hou YT, Ho YC, Hou MF, Chu PY. Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem. 2018;46:1423–1438. doi: 10.1159/000489184. [DOI] [PubMed] [Google Scholar]
- 74.Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Shenker RF, McTyre ER, Ruiz J, Weaver KE, Cramer C, Alphonse-Sullivan NK, Farris M, Petty WJ, Bonomi MR, Watabe K, et al. The Effects of smoking status and smoking history on patients with brain metastases from lung cancer. Cancer Med. 2017;6:944–952. doi: 10.1002/cam4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014;15:e568–e580. doi: 10.1016/S1470-2045(14)70266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gopalakrishna R, Chen ZH, Gundimeda U. Tobacco smoke tumor promoters, catechol and hydroquinone, induce oxidative regulation of protein kinase C and influence invasion and metastasis of lung carcinoma cells. Proc Natl Acad Sci USA. 1994;91:12233–12237. doi: 10.1073/pnas.91.25.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshino I, Maehara Y. Impact of smoking status on the biological behavior of lung cancer. Surg Today. 2007;37:725–734. doi: 10.1007/s00595-007-3516-6. [DOI] [PubMed] [Google Scholar]
- 79.Schuller HM. Neurotransmitter receptor-mediated signaling pathways as modulators of carcinogenesis. Prog Exp Tumor Res. 2007;39:45–63. doi: 10.1159/000100045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.