Abstract
The only current curative treatment for patients with pancreatic ductal adenocarcinoma (PDA) is surgical resection, and certain patients still succumb to disease shortly after complete surgical resection. Wilms' tumor 1 (WT1) serves an oncogenic role in various types of tumors; therefore, in the present study, WT1 protein expression in patients with PDA was analyzed and the association with overall survival (OS) and disease-free survival (DFS) time in patients with PDA was assessed following surgical resection. A total of 50 consecutive patients with PDA who received surgical resection between January 2005 and December 2015 at the Jikei University Kashiwa Hospital (Kashiwa, Chiba, Japan) were enrolled. WT1 protein expression in PDA tissue was measured using immunohistochemical staining. Furthermore, laboratory parameters were measured within 2 weeks of surgery, and systemic inflammatory response markers were evaluated. WT1 protein expression was detected in the nucleus and cytoplasm of all PDA cells and in tumor vessels. WT1 exhibited weak staining in the nuclei of all PDA cells; however, the cytoplasmic expression of WT1 levels was classified into four groups: Negative (n=0), weak (n=19), moderate (n=23) and strong (n=8). In patients with PDA, it was demonstrated that the OS and DFS times of patients with weak cytoplasmic WT1 expression were significantly prolonged compared with those of patients with moderate-to-strong cytoplasmic WT1 expression, as determined by log-rank test (P=0.0005 and P=0.0001, respectively). Furthermore, an association between the density of WT1-expressing tumor vessels and worse OS/DFS times was detected. Multivariate analysis also indicated a significant association between the overexpression of WT1 in PDA tissue and worse OS/DFS times. To the best of our knowledge, the present study is the first to demonstrate that moderate-to-strong overexpression of WT1 in the cytoplasm of PDA cells is significantly associated with worse OS/DFS times. Therefore, overexpression of WT1 in the cytoplasm of PDA cells may impact the recurrence and prognosis of patients with PDA following surgical resection. The results further support the development of WT1-targeted therapies to prolong survival in all patients with PDA.
Keywords: Wilms' tumor 1, pancreatic cancer, prognosis
Introduction
The most common type of pancreatic cancer is pancreatic ductal adenocarcinoma (PDA), which presents as lethal solid tumors and is largely resistant to standard therapeutic modalities (1). The only curative treatment for PDA patients is surgical resection, and a limited number of patients present with resectable tumors at the time of diagnosis (2). Furthermore, even if patients with PDA undergo complete surgical resection, the majority of patients develop early recurrences or metastases, with a 5-year overall survival (OS) rate of ~5% (3–5). In total, >60% of surgically resected PDA patients develop recurrences within 2 years (3). Therefore, novel prognostic markers associated with early mortality are urgently required. However, prognostic factors for short-term survival following surgical resection have not been well characterized (6–8). Furthermore, understanding the prognostic factors associated with early recurrence following surgery can lead to insights into novel therapeutic targets.
The Wilms' tumor 1 (WT1) gene encodes a protein that consists of four zinc finger domains at the C terminus and a glutamine- and proline-rich domain at the N terminus (9). WT1 was initially defined as a tumor suppressor gene in Wilms' tumors, a childhood kidney neoplasm (10). However, subsequent research has revealed that WT1 also serves an oncogenic role in various other types of tumors through transcriptional regulation in tumorigenesis (11–13). Knockdown of WT1 can inhibit tumor cell proliferation (14), and WT1 activates transcription of the interleukin-10 gene, thereby promoting tumor escape from immune surveillance (15). Furthermore, previous studies have demonstrated that WT1 serves an important role in the prognosis of patients with tumors, including ovarian cancer (16,17), hepatocellular carcinoma (18), breast cancer (19) and acute leukemia (20), and WT1 protein overexpression is associated with a poor prognosis (21). Notably, WT1 was ranked as the top antigen in a list of 75 tumor-associated antigens by a National Cancer Institute prioritization project based on several factors, including the role of the antigen in tumorigenicity and the high expression level of WT1-positive tumor cells (22). Therefore, WT1 has been used as a target of cancer vaccines, and results from early clinical trials have demonstrated that WT1-targeted immunotherapy has the potential to treat patients with PDA (23–28). As only a small number of studies (29–31) have demonstrated WT1 protein expression in 30–70% of patients with PDA, WT1 expression at the protein level in PDA tissue and its role as a potential prognostic marker to predict OS and disease-free survival (DFS) times have not yet been documented.
Tumor-associated systemic inflammation promotes angiogenesis and tumor cell proliferation, resulting in recurrence and metastasis through the secretion of various chemokines and cytokines from tumor cells and proinflammatory cells (32). The systemic inflammatory response, which serves a crucial role in tumor development and progression, is reflected by increased levels of C-reactive protein (CRP), neutrophils, platelets, lymphocytes, leucocytes, monocytes and lactate dehydrogenase (LDH), and decreased levels of albumin (Alb) and hemoglobin (Hb) (33). Therefore, an increased neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR) and CRP/Alb ratio have been identified as easily accessible tools to assess the systemic inflammatory response and as indicators of a poor prognosis for various types of cancer (32,33). These reports led to the hypothesis that WT1 protein expression in PDA cells may be associated with specific systemic inflammatory markers that are indicative of disease progression. If such data were to confirm the prognostic value of WT1, significant advances may be made in the clinical translation of biomarkers, as well as the identification of patients with PDA at high risk for disease progression following surgical resection and of targets of PDA.
In the present study, the clinical implications of WT1 protein expression in PDA cells and systemic inflammatory markers in patients with PDA following surgical resection as prognostic parameters were investigated. An aim of the present study was to detect WT1 protein expression in the nuclei and cytoplasm of PDA cells from all 50 enrolled patients, and to perform an analysis using a modified immunohistochemical staining method. Furthermore, WT1 expression was also detected in specific tumor vessels in PDA stroma. We hypothesized that the overexpression of WT1 protein in the PDA cytoplasm would be significantly associated with worse OS and DFS times in PDA patients following surgical resection. However, further studies are required to confirm the role of cytoplasmic WT1 overexpression in PDA in order to identify clinical prognostic factors associated with early recurrence following surgery.
Materials and methods
PDA patients
Between January 2005 and December 2015, 50 consecutive patients with PDA (mean age, 65.4 years; age range, 33–79 years; sex distribution; 31 males and 19 females) who underwent macroscopically curative resection at the Jikei University Kashiwa Hospital (Kashiwa, Chiba, Japan) were enrolled in the present study. There is an increased incidence of postoperative mortality and complications among patients over the age of 80 (34). Furthermore, surgical resection is not superior to chemotherapy for octogenarian PDA patients; therefore, only select patients benefit from pancreatic resection amongst the elderly (35). As a result, PDA patients >80 years old were excluded from the present study. Information on the clinical features of the patients, including age, sex and tumor characteristics (e.g., location, size and pathology), were prospectively obtained from the patient's medical records. Tumor stage was assessed according to the American Joint Committee of Cancer (AJCC) pancreatic cancer Tumor-Node-Metastasis (TNM) staging system (36). A summary of the patients' profiles is shown in Table I. The present study was reviewed and approved by the Institutional Review Board of Jikei University. The procedures for the present study were conducted according to the Declaration of Helsinki. Due to the retrospective and non-interventional nature of the present study, the IRB waived the requirement for written informed consent from the participants for their clinical records and tissues to be used in the present study.
Table I.
Baseline characteristics of patients with pancreatic ductal adenocarcinoma.
| Clinicopathological characteristics | n | % |
|---|---|---|
| Age at surgery, years | ||
| <65 | 18 | 36 |
| ≥65 | 32 | 64 |
| Sex | ||
| Male | 31 | 62 |
| Female | 19 | 38 |
| Tumor location | ||
| Head | 39 | 78 |
| Body-to-tail | 11 | 22 |
| Tumor differentiation | ||
| Well-to-moderate | 42 | 84 |
| Poor | 8 | 16 |
| Tumor stage | ||
| I and II | 34 | 68 |
| III | 16 | 32 |
| Tumor size, cm | ||
| <3 | 15 | 30 |
| ≥3 | 35 | 70 |
OS and DFS times
OS time was defined as the time from diagnosis to mortality from any cause. DFS time was defined as the time from the date of surgery to the first radiological evidence of recurrence or mortality without evidence of recurrence or a second primary cancer.
Laboratory parameters
All laboratory parameters, including the levels of leucocytes, lymphocytes, monocytes, neutrophils, Hb, platelets, CRP, Alb, LDH, amylase (AMY), carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), were measured within the 2 weeks prior to surgery. The systemic inflammatory response markers, including the NLR, PLR, LMR and CRP/Alb ratio prior to surgery, were also evaluated. All data were assessed to determine the prognostic impact on patients with PDA following surgical resection. Furthermore, the associations between WT1 protein expression in PDA cells and the various laboratory parameters were analyzed.
Immunohistochemistry
All PDA surgical tissues were routinely fixed in 10% formalin for 1 day at room temperature and embedded in paraffin. Immunohistochemical staining for WT1 was performed on the tissues. The paraffin sections were cut into 5-µm slices and placed on slides. Endogenous peroxidase activity was blocked in 10% H2O2 for 5 min at room temperature. Antigen retrieval was performed by heating (70°C for 20 min, followed by 110°C for 25 min) in Target Retrieval Solution [Tris/EDTA buffer (pH 9.0); Dako; Agilent Technologies GmbH, Waldbronn, Germany] to unmask the WT1 epitopes. The tissue slides were then incubated with mouse anti-human WT1 monoclonal antibody (clone 6F-H2; dilution 1:100; cat. no. M3561; Dako; Agilent Technologies GmbH) overnight at 4°C and washed with PBS. Immunostaining was performed for 30 min at room temperature using the EnVision detection system RUO kit (cat. no. K500711; Dako; Agilent Technologies GmbH) containing ready-to-use horseradish peroxidase-conjugated rabbit anti-mouse IgG and 3,3′-diaminobenzidine-peroxidase solution according to the manufacturer's protocol. As all cases exhibited diffuse or granular staining in the cytoplasm of the PDA cells, the intensity of WT1 protein expression in the PDA cells was classified as follows: i) Negative, no staining was observed in PDA cells; ii) weak, faint and barely perceptible cytoplasmic staining was observed in PDA cells under ×200 magnification; iii) moderate, moderate complete cytoplasmic staining was observed in PDA cells under ×40 magnification; and iv) strong, strong complete cytoplasmic staining was observed in PDA cells under low magnification. Additionally, the WT1-expressing tumor vessels (WT1-TV) were counted by light microscopy in 5 high-power fields (×200 magnification). The patients with PDA were divided into two groups; high (greater than the mean number of WT1-positive vessels) and low WT1-TV density (less than or equal to the mean number of WT1-positive vessels). Three investigators who were not privy to the requisite clinical information independently interpreted the WT1 protein expression results. Negative control staining was applied to all samples using a mouse immunoglobulin G (IgG) monoclonal antibody (dilution 1:100; cat. no. X0931; Dako; Agilent Technologies GmbH) overnight at 4°C. WT1-positive colonic gastrointestinal stromal tumor (GIST) localized at transverse colon obtained from an unenrolled patient was used as a positive control (37). The tissues fixed in 10% formalin for 1 day at room temperature and embedded in paraffin were cut into 5-µm slices and stained with hematoxylin (for 4 min at room temperature) and eosin (for 2 min at room temperature) for histopathological evaluation as previously described (38).
Statistical analysis
The results of immunohistochemical staining and laboratory data were assessed to determine their association with OS and DFS times. All statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA). The OS and DFS times were estimated using the Kaplan-Meier method and were compared using the log-rank test. Univariate and multivariate analyses were performed using a Cox proportional hazard model to evaluate the influence of multiple parameters, including lymphocyte numbers, NLR and CA19-9 as continuous variables, on independent predictors of OS and DFS times. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using Cox proportional hazard models. Differences in clinical and laboratory findings between the WT1 protein overexpression and non-overexpression groups were evaluated using the Mann-Whitney U test. The χ2 test was used to determine the association between cytoplasmic WT1 intensity and patient characteristics. P<0.05 was considered to indicate a statistically significant difference.
Results
PDA patient characteristics
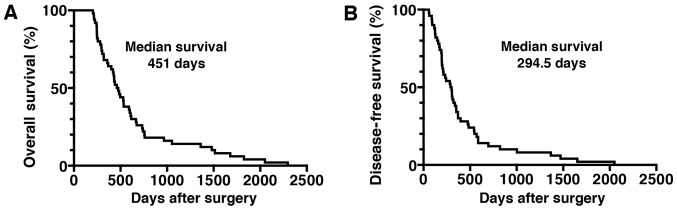
A total of 50 consecutive PDA patients with histologically confirmed invasive ductal adenocarcinoma who underwent complete surgical resection at the Jikei University Kashiwa Hospital between January 2005 and December 2015 were assessed. The clinical outcome and other characteristics of the PDA patients are summarized in Table I. The patient distribution consisted of 31 (62%) males and 19 (38%) females, with a mean age of 65.4 years (range, 33–79 years). The number of PDA patients classified as AJCC stage I/II was 34 (68%), whereas 16 were classified as stage III (32%). The majority of the PDA lesions (n=39; 78%) were located in the head of the pancreas (Table I). The median OS time of the patients was 451 days, and the median DFS time was 294.5 days (Fig. 1). The majority of patients (n=42; 84%) received chemotherapy following surgical resection. Laboratory parameters, including the levels of leucocytes, lymphocytes, monocytes, neutrophils, Hb, platelets, CRP, Alb, LDH, AMY and tumor markers (CEA and CA19-9), obtained within 2 weeks prior to surgery are shown in Table II. The systemic inflammatory response markers, such as the NLR, PLR, LMR and CRP/Alb ratio prior to surgery, are also shown in Table II.
Figure 1.
OS and DFS times in patients with PDA following surgical resection. Kaplan-Meier estimates of (A) OS and (B) DFS times for PDA patients following surgical resection. OS, overall survival; DFS, disease-free survival; PDA, pancreatic ductal adenocarcinoma.
Table II.
Baseline characteristics of laboratory parameters of patients with pancreatic ductal adenocarcinoma.
| Laboratory parameters | Median | Range |
|---|---|---|
| Leucocytes, count/µl | 5,150 | 2,500–12,000 |
| Lymphocytes, count/µl | 1,400 | 500-2,800 |
| Monocytes, count/µl | 300 | 100–900 |
| Neutrophils, count/µl | 3,050 | 1,500–9,000 |
| Hemoglobin, g/dl | 12.5 | 9.5–16.5 |
| Platelets, ×104 count/µl | 23.2 | 12.1–80.6 |
| C-reactive protein, mg/dl | 0.1 | 0.1–3.8 |
| Albumin, g/dl | 3.9 | 2.0–4.9 |
| Lactate dehydrogenase, IU/l | 181.5 | 112–319 |
| Amylase, IU/l | 93 | 15–1556 |
| Carcinoembryonic antigen, ng/ml | 4.7 | 1.3–43.7 |
| Carbohydrate antigen 19-9, U/ml | 114 | 1–5739 |
| Neutrophil/lymphocyte ratio, % | 2.1 | 1.00–7.60 |
| Platelet/lymphocyte ratio, % | 174.4 | 61.8–424.2 |
| Lymphocyte/monocyte ratio, % | 5.12 | 0.6–11.0 |
| C-reactive protein/albumin, % | 0.031 | 0.02–1.07 |
WT1 protein expression in PDA
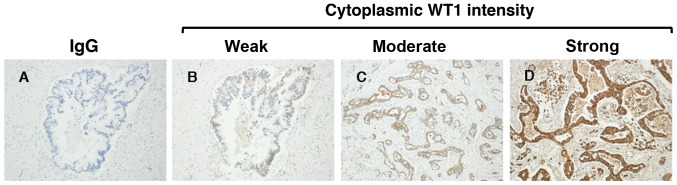
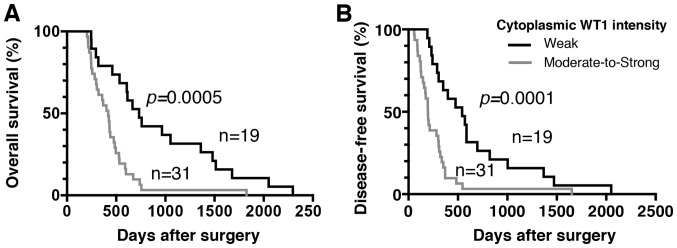
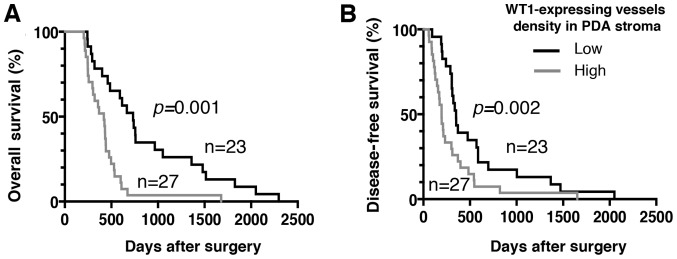
Regarding the immunohistochemical staining of WT1, granular or diffuse nucleo-cytoplasmic staining in PDA cells is considered positive (29,30). In the present study, WT1 protein expression was detected in the nuclei and cytoplasm of PDA samples from all patients, whereas no expression was detected in corresponding normal pancreatic ductal cells (Figs. 2 and 3). There was weak nuclear immunostaining using WT1-specific monoclonal antibodies; however, WT1 proteins were predominantly localized to the cytoplasm in all cases. Therefore, the patients with PDA were subdivided into four groups based on the cytoplasmic WT1 staining intensity: Negative (0%), weak (38%), moderate (46%) and strong (16%) (Fig. 2). In addition, no staining was observed in any samples stained with the non-reactive mouse IgG (negative control). In the present study, in order to assess the prognostic significance of WT1 expression in patients with PDA, patients were classified as having either a weak or moderate-to-strong cytoplasmic WT1 intensity. The associations between cytoplasmic WT1 intensity and the various clinicopathological parameters of PDA are illustrated in Table III. There were no statistically significant associations between cytoplasmic WT1 intensity and clinicopathological parameters, including age at surgical resection, sex or tumor characteristics (e.g., location, pathology, size and stage). The median survival time for patients with PDA with weak and moderate-to-strong cytoplasmic WT1 expression was 732 and 419 days, respectively. Furthermore, the median DFS time for patients with PDA with weak and moderate-to-strong cytoplasmic WT1 expression was 543 and 196 days, respectively. Importantly, the OS and DFS times of PDA patients with weak cytoplasmic WT1 expression were significantly prolonged compared with those of patients with moderate-to-strong cytoplasmic WT1 expression (P=0.0005 and P=0.0001, respectively) (Fig. 4). Univariate and multivariate analyses of OS and DFS times according to the cytoplasmic WT1 intensity and various clinicopathological parameters were also performed. The cytoplasmic WT1 intensity in PDA, which was revealed to be significant by univariate analysis, was further analyzed by multivariate analysis (Tables IV and V). Multivariate analysis also revealed that the cytoplasmic WT1 intensity in PDA cells was a significant prognostic factor independent of the other prognostic factors (Tables IV and V). Furthermore, the HRs of the cytoplasmic WT1 intensity in relation to OS and DFS times based on the multivariate analysis (3.972 and 4.117, respectively) were greater than those observed in the univariate analysis (2.992 and 2.952, respectively). These data suggest that moderate-to-strong WT1 immunostaining in the cytoplasm of PDA cells may serve as a surrogate marker for the poor prognosis of patients with PDA following surgical resection. Furthermore, WT1 expression was also detected in certain tumor vessels in the PDA stroma. Therefore, the PDA patients were divided into two groups according to WT1-TV density. The mean WT1-TV density was 4.03 vessels, confirming the stroma-rich and hypovascular features of PDA. The mean WT1-TV count was used as the cutoff value between low and high density. An association between a high density of WT1-TV (>4.03 vessels) and worse OS or DFS times was also demonstrated (Fig. 5).
Figure 2.
Intensity of cytoplasmic WT1 intensity in pancreatic ductal adenocarcinoma patients following surgical resection. (A) Negative control samples were stained with IgG monoclonal antibody. Cells positive for cytoplasmic WT1, as determined using an anti-human WT1 monoclonal antibody (6F-H2), were graded as (B) weak, (C) moderate or (D) strong (magnification, ×200). WT1, Wilms' tumor 1; IgG, immunoglobulin G.
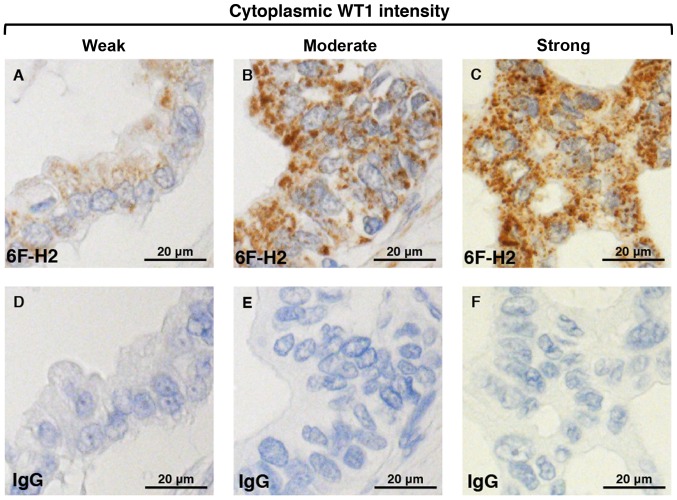
Figure 3.
Intensity of nucleo-cytoplasmic WT1 staining in PDA patients following surgical resection. The cytoplasmic WT1 intensity, as determined using the anti-human WT1 monoclonal antibody (6F-H2), in PDA is shown in the upper panel and is categorized as (A) weak, (B) moderate or (C) strong. Weak nucleic WT1 expression in the PDA samples is also shown in the upper panel. (D, E and F) Samples were also stained with a non-specific IgG monoclonal antibody as a negative control. WT1, Wilms' tumor 1; PDA, pancreatic ductal adenocarcinoma; IgG, immunoglobulin G.
Table III.
Associations between cytoplasmic WT1 intensity and patient characteristics.
| Cytoplasmic WT1 intensity, n (%) | |||
|---|---|---|---|
| Clinicopathological characteristics | Weak | Moderate-to-strong | P-value |
| Age at surgery, years | 0.764 | ||
| ≥65 | 13 (68.4) | 19 (61.3) | |
| <65 | 6 (31.6) | 12 (38.7) | |
| Sex | 0.556 | ||
| Male | 13 (68.4) | 18 (58.1) | |
| Female | 6 (31.6) | 13 (41.9) | |
| Tumor location | 0.293 | ||
| Body-to-tail | 6 (31.6) | 5 (16.1) | |
| Head | 13 (68.4) | 26 (83.9) | |
| Tumor differentiation | 0.659 | ||
| Poor | 2 (10.5) | 6 (19.4) | |
| Well-to-moderate | 17 (89.5) | 25 (80.6) | |
| Tumor stage | 0.549 | ||
| I/II | 14 (73.7) | 20 (64.5) | |
| III/IV | 5 (26.3) | 11 (35.5) | |
| Tumor size, cm | 0.205 | ||
| <3 | 8 (42.1) | 7 (22.6) | |
| ≥3 | 11 (57.9) | 24 (77.4) | |
WT1, Wilm's tumor 1.
Figure 4.
Association of cytoplasmic WT1 intensity in PDA cells with OS and DFS times. (A) Kaplan-Meier estimates of OS time for PDA patients with weak or moderate-to-strong expression of WT1 proteins. The OS time in PDA patients with moderate-to-strong overexpression of WT1 proteins was significantly worse than that in patients with weak expression of WT1 proteins (P=0.0005). (B) Kaplan-Meier estimates of DFS time for PDA patients with weak or moderate-to-strong expression of WT1 proteins. The DFS time in PDA patients with moderate-to-strong overexpression of WT1 proteins was significantly worse than that in patients with weak expression of WT1 proteins (P=0.0001). WT1, Wilms' tumor 1; PDA, pancreatic ductal adenocarcinoma; OS, overall survival; DFS, disease-free survival.
Table IV.
Univariate and multivariate analysis of overall survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Clinicopathological characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age at surgery (≥65 vs. <65 years) | 0.855 | 0.482–1.565 | 0.601 | 0.990 | 0.460–2.130 | 0.980 |
| Tumor location (body-tail vs. head) | 0.762 | 0.366–1.465 | 0.438 | 0.559 | 0.219–1.428 | 0.224 |
| Pathology (poor vs. well-to-moderate) | 1.863 | 0.789–3.922 | 0.123 | 0.923 | 0.378–2.254 | 0.860 |
| Tumor stage (III/IV vs. I/II) | 0.928 | 0.491–1.680 | 0.809 | 1.041 | 0.469–2.311 | 0.921 |
| Lymphocyte (per 100 cells/µl) | 1.001 | 0.943–1.061 | 0.961 | 0.938 | 0.861–1.021 | 0.141 |
| NLR (per unit) | 0.962 | 0.790–1.143 | 0.680 | 0.923 | 0.670–1.272 | 0.625 |
| CA19-9 (per 10 U/ml) | 1.001 | 0.997–1.003 | 0.574 | 1.002 | 0.999–1.005 | 0.230 |
| Cytoplasmic WT1 intensity (moderate-to-strong vs. weak) | 2.992 | 1.609–5.749 | <0.001 | 3.972 | 1.877–8.404 | <0.001 |
WT1, Wilm's tumor 1; HR, hazard ratio; CI, confidence interval; NLR, neutrophil/lymphocyte ratio; CA19-9, carbohydrate antigen 19-9.
Table V.
Univariate and multivariate analysis of disease-free survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Clinicopathological characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age at surgery (≥65 vs. <65 years) | 0.859 | 0.482–1.574 | 0.614 | 0.968 | 0.477–1.964 | 0.927 |
| Tumor location (body-tail vs. head) | 0.790 | 0.382–1.504 | 0.496 | 0.797 | 0.339–1.877 | 0.604 |
| Pathology (poor vs. well-to-moderate) | 2.204 | 0.927–4.690 | 0.053 | 1.350 | 0.560–3.252 | 0.504 |
| Tumor stage (III/IV vs. I/II) | 0.946 | 0.503–1.704 | 0.856 | 0.833 | 0.387–1.794 | 0.641 |
| Lymphocyte (per 100 cells/µl) | 0.992 | 0.933–1.052 | 0.781 | 0.939 | 0.866–1.018 | 0.129 |
| NLR (per unit) | 0.995 | 0.813–1.187 | 0.962 | 1.017 | 0.751–1.378 | 0.912 |
| CA19-9 (per 10 U/ml) | 1.000 | 0.997–1.002 | 0.879 | 1.001 | 0.998–1.004 | 0.471 |
| Cytoplasmic WT1 intensity (moderate-to-strong vs. weak) | 2.952 | 1.610–5.580 | <0.001 | 4.117 | 1.922–8.817 | <0.001 |
WT1, Wilm's tumor 1; HR, hazard ratio; CI, confidence interval; NLR, neutrophil/lymphocyte ratio; CA19-9, carbohydrate antigen 19-9.
Figure 5.
Association of density of WT1-expressing tumor vessels with OS and DFS times. (A) Kaplan-Meier estimates of OS time for PDA patients with a high or low density of WT1-expressing tumor vessels. The OS time in PDA patients with a high density of WT1-expressing tumor vessels was significantly worse than that in patients with a low-density of such tumor vessels (P=0.001). (B) Kaplan-Meier estimates of DFS time for PDA patients with a high or low density of WT1-expressing tumor vessels. The DFS time of PDA patients with a high density of WT1-expressing tumor vessels was significantly worse than that of patients with a low density of such tumor vessels (P=0.002). WT1, Wilms' tumor 1; PDA, pancreatic ductal adenocarcinoma; OS, overall survival; DFS, disease-free survival.
Association of laboratory data with OS and DFS times in PDA cells
The association between prognostic and laboratory data [e.g., leucocytes, lymphocytes, monocytes, neutrophils, Hb, platelets, CRP, Alb, LDH, AMY and tumor markers (CEA and CA19-9)] and systemic inflammatory response markers (e.g., NLR, PLR, LMR and CRP/Alb ratio) obtained within 2 weeks prior to surgery were also analyzed in the present study (Tables III and IV). The OS and DFS times were estimated using the Kaplan-Meier method. All laboratory data were not found to be significantly associated with a poor prognosis (data not shown). Univariate and multivariate analysis also revealed that lymphocyte numbers, NLR and CA19-9, all of which have been reported as prognostic markers, were not significant prognostic factors in the patient cohort of the present study following surgical resection (Tables IV and V).
Associations between cytoplasmic WT1 intensity and laboratory parameters
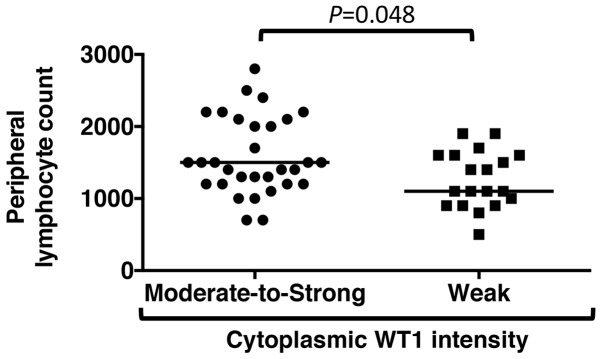
The associations between WT1 staining intensity in the cytoplasm and laboratory parameters were then assessed in patients with PDA. Table VI shows that laboratory data, with the exception of peripheral lymphocyte numbers, demonstrated no associations with the WT1 reaction intensity in PDA cytoplasm (Table VI); PDA patients with moderate-to-strong WT1 staining in the cytoplasm showed high peripheral blood lymphocyte numbers obtained within 2 weeks prior to surgery compared with those with weak WT1 staining (P=0.048) (Fig. 6).
Table VI.
Associations between cytoplasmic WT1 intensity and laboratory parameters in patients with pancreatic duct adenocarcinoma.
| WT1 intensity | |||
|---|---|---|---|
| Laboratory parameters | Weak | Moderate-to-strong | P-value |
| Leucocytes, count/µl | 5,100 (2,880–7,140) | 5,200 (3,300–8,800) | 0.246 |
| Lymphocytes, count/µl | 1,100 (770–1,900) | 1,500 (850–2,450) | 0.048 |
| Monocytes, count/µl | 300 (100–450) | 300 (200–450) | 0.238 |
| Neutrophils, count/µl | 3,100 (1,590–5,670) | 3,000 (1,650–5,800) | 0.764 |
| Hemoglobin, g/dl | 12.5 (10.2–14.3) | 12.4 (10.4–15.0) | 0.548 |
| Platelets ×104 count/µl | 20.4 (12.9–35.3) | 24.9 (13.4–45.8) | 0.259 |
| C-reactive protein, mg/dl | 0.1 (0.1–1.7) | 0.1 (0.1–2.8) | 0.851 |
| Albumin, g/dl | 3.9 (2.9–4.5) | 3.8 (3.0–4.5) | 0.873 |
| Lactate dehydrogenase, IU/l | 168 (117–233) | 185 (145–257) | 0.171 |
| Amylase, IU/l | 100 (29–381) | 93 (24–862) | 0.852 |
| Carcinoembryonic antigen, ng/ml | 3.9 (1.9–24.1) | 6.1 (2.2–24.3) | 0.224 |
| Carbohydrate antigen 19-9, U/ml | 93 (20–1759) | 139 (1–743) | 0.479 |
| Neutrophil/lymphocyte ratio, % | 2.0 (1.3–6.1) | 2.1 (1.0–4.3) | 0.569 |
| Platelet/lymphocyte ratio, % | 170 (107–302) | 176 (73–307) | 0.484 |
| Lymphocyte/monocyte ratio, % | 5.3 (2.5–9.2) | 5.0 (3.0–11.0) | 0.96 |
| C-reactive protein/albumin, % | 0.05 (0.02–0.47) | 0.03 (0.02–0.78) | 0.936 |
All data are presented as the median (5–95th percentile). WT1, Wilm's tumor 1.
Figure 6.
Associations between cytoplasmic WT1 intensity and peripheral blood lymphocyte count. The peripheral blood lymphocyte count in pancreatic ductal adenocarcinoma patients with moderate-to-strong cytoplasmic WT1 intensity and those with weak cytoplasmic WT1 intensity are shown. The bar indicates the median of the peripheral blood lymphocyte counts. WT1, Wilms' tumor 1.
Discussion
The data from the current study showed that nucleo-cytoplasmic expression of the WT1 protein was present in all 50 examined patients with PDA. Notably, the cytoplasmic overexpression of WT1 protein was significantly associated with worse OS and DFS times in patients with PDA undergoing complete surgical resection. Furthermore, an association was detected between a high density of tumor vessels expressing WT1 proteins and worse OS and DFS times.
Currently, few studies are available regarding WT1 protein expression in patients with PDA (29–31). Therefore, the present study attempted to assess whether WT1 protein expression could be detected in PDA cells from formalin-fixed and paraffin-embedded tissues. In order to identify the epitopes, antigen retrieval was performed using a combination of high-temperature heating and a Tris/EDTA buffer (pH 9.0), which are well suited for use on formalin-fixed, paraffin-embedded tissue sections mounted on glass slides. Previous studies have reported that WT1 proteins were detected in 30 of 40 patients (75%) using a polyclonal (C-19) antibody raised against the C terminus (amino acids 431–450) of the WT1 protein (29) and in 10 of 15 samples (66.7%) using a monoclonal (6F-H2) antibody targeting the N terminus (amino acids 1–181) (30). Another study also detected WT1 expression using the monoclonal (6F-H2) antibody in 19 out of 63 (30.2%) PDA cells (31). In the present study, the WT1 monoclonal (6F-H2) antibody was selected due to its increased specificity for the WT1 protein compared with a polyclonal (C-19) antibody (29,30). Although citrate buffer (pH 6.0) was previously used to expose the WT1 epitopes on tissue sections mounted on glass slides (29,30), the present study implemented a combination method using Tris/EDTA buffer (pH 9.0) at a high temperature. According to the manufacturer, compared with citrate buffer (pH 6.0), Tris/EDTA buffer (pH 9.0) can significantly improve the staining results for numerous antigens. Furthermore, immunostaining was performed using a newly developed immunohistochemical detection system, EnVision, which has an extremely high sensitivity and was recently made available to the Institute of Clinical Medicine and Research, The Jikei University School of Medicine (Chiba, Japan). The modified antigen retrieval and highly sensitive detection methods are, at least in part, why the nucleo-cytoplasmic expression of WT1 proteins in all 50 patients with PDA were detected using the 6F-H2 antibody. It is an urgent requirement to establish a uniform validated method for the analysis of WT1 reactions in PDA cells.
WT1 was detected in the granular or diffuse nucleo-cytoplasmic staining of all the tested PDA cells. Although the cytoplasmic staining of 6F-H2 antibodies in certain adenocarcinomas and glioblastomas (30,39) has been reported, specific nuclear immunoreactivity to 6F-H2 in leukemia blast cells has also been demonstrated (40). The nucleo-cytoplasmic staining of WT1 in PDA cells observed in the present study suggests that in tumorigenesis, WT1 has complex functions outside its traditional role as a transcription factor, such as functions as a tumor suppressor or oncogene. Although nuclear immunostaining was weak in all the PDA cells, moderate-to-strong cytoplasmic WT1 expression in PDA cells (62%) was detected in the present study. It has been reported that the WT1 protein shuttles between the nucleus and the cytoplasm in several types of tumors, including rhabdomyosarcomas, certain breast cancer types and colorectal adenocarcinomas, through the alternative splicing of WT1 mRNA (41,42). Although the WT1 protein is predominantly localized to the nucleus in several tumors, this expression pattern is altered upon protein kinase A phosphorylation of the DNA-binding domain of WT1, resulting in the inhibition of DNA binding and the cytoplasmic sequestration of WT1 (43). Furthermore, WT1 can also undergo nucleo-cytoplasmic shuttling in PDA cells following exposure to the standard chemotherapeutic agent gemcitabine via activation of nuclear factor κB (44). It is hypothesized that WT1 protein localization may depend on tumor cell conditions, suggesting the complexity of the role of WT1 in tumorigenesis through transcriptional and post-transcriptional regulation (45). As WT1 has multiple isoforms, alternative WT1 transcripts may primarily localize in the PDA cytoplasm depending on the cellular context. The results of the present study demonstrated nucleo-cytoplasmic WT1 staining patterns in all patients with PDA, which also indicates a complex role of WT1 in tumorigenesis. However, the clinical implications of the localization of WT1 expression in PDA cells remain unknown.
The present study also sought to investigate the associations between WT1 expression and the prognosis of PDA patients following complete surgical resection, and the cytoplasmic WT1 intensity was thus graded as negative, weak, moderate or strong. Of the 8 PDA patients with an OS time of >1,000 days, 7 (87.5%) exhibited weak cytoplasmic WT1 expression. At the Jikei University School of Medicine, Kashiwa Hospital (Chiba, Japan), the median OS time is 451 days for patients with PDA following surgical resection, which indicates that an OS time of >1,000 days represents significantly extended survival. To clarify the association between cytoplasmic WT1 expression and prognostic significance, PDA patients were divided into 2 groups: Weak and moderate-to-strong. Patients with PDA with weak cytoplasmic WT1 expression presented with significantly longer OS and DFS times compared with those patients with moderate-to-strong cytoplasmic WT1 expression. Furthermore, patients with strong cytoplasmic WT1 overexpression exhibited shorter OS and DFS times than those with weak-to-moderate WT1 expression (data not shown). Therefore, cytoplasmic WT1 overexpression may be involved in PDA pathogenesis and have a potential prognostic impact on patients with PDA. The association between WT1 overexpression and worse OS and DFS times has been previously demonstrated in solid tumors, including serous epithelial ovarian carcinoma, endometrial cancer, non-small cell lung cancer, colorectal cancer, osteogenic sarcoma, uterine sarcoma, glioblastoma, melanomas, malignant pleural mesothelioma, prostate cancer, breast cancer, hepatocellular carcinoma, soft-tissue sarcoma and astrocytoma (21,46–48). Despite the clinical development of WT1-targeted therapies, no report has demonstrated an association between WT1 protein expression and the prognosis of PDA patients following surgical resection. The results of the present study, to the best of our knowledge, are the first to suggest that strong immunostaining of WT1 in the PDA cytoplasm may be a potentially useful marker to predict the risk of relapse and progression in patients with PDA. Recently, it was reported that the overexpression of WT1-associated protein (WTAP), a ubiquitously expressed nuclear protein, reduced the OS time of PDA patients via a WTAP-WT1 axis (49). This study supports the present findings that cytoplasmic WT1 overexpression in PDA is associated with a worse prognosis. WTAP and WT1 have recently been reported as oncogenic factors associated with the regulation of tumor migration and invasion (50). However, the function of the WTAP-WT1 axis is not yet clear, and the mechanism by which WT1 is regulated by WTAP requires further study. PDA patients with cytoplasmic overexpression of WT1 may require standard chemotherapy combined with a WT1-targeted therapy rapidly following surgical resection in order to overcome early mortality. Previous studies have produced WT1-based cancer vaccines, including WT1 peptide vaccines (25,51) and dendritic cells pulsed with WT1 peptides (24,52), to extend the survival time of patients with advanced PDA (53). The results from these clinical trials demonstrated that patients with PDA who received WT1-based cancer vaccines exhibited delayed type hypersensitivity and significantly improved OS time compared with the negative control patients. These results also support the development of WT1-targeted cancer therapies for patients either with inoperable PDA or following surgical resection to improve their prognosis.
It has been reported that WT1 serves an important role in tumorigenesis via expansion of tumor vessels and metastasis formation (54,55). Therefore, the present study also examined WT1-TV in the PDA stroma. According to the density of WT1-TV, patients with PDA were classified into 2 groups: High density (greater than the mean number of vessels) and low density (less than or equal to the mean number of vessels). An association between the high-density group and worse OS/DFS times was observed. These results support previous findings that WT1 acts as a critical regulator of tumor progression via tumor vascularization (55). Therefore, the overexpression of cytoplasmic WT1 proteins in PDA cells may be associated with a higher density of WT1-TV. Inhibition of tumor angiogenesis has been suggested as a therapeutic option for cancer therapy (55,56). WT1 may have the potential to act as a therapeutic target in patients with PDA by regulating WT1-expressing PDA cells and tumor vessels implicated in tumor progression (55,56).
Previous studies have reported that laboratory data and systemic inflammatory response markers (e.g., NLR, PLR, LMR and CRP/Alb ratio) can serve as prognostic factors for patients with several types of cancer (57–59). Therefore, the association of the prognosis of patients with PDA who received surgical resection and laboratory data obtained within 2 weeks prior to surgery were analyzed in the present study. However, in this experimental setting, no biomarkers associated with the prognosis of PDA patients following surgical resection were detected. A total of 31 patients with moderate-to-strong cytoplasmic WT1 overexpression in their PDA cells showed high peripheral blood lymphocyte numbers obtained within 2 weeks prior to surgery compared with that in 19 PDA patients with weak WT1 staining (P=0.048). However, the PDA sample number collected following surgical resection is relatively low and may be too small to assess the laboratory data and identify potential prognostic markers. Therefore, further studies are required to assess the associations between peripheral lymphocyte numbers and WT1 expression in PDA cytoplasm. Although previous studies have indicated that the WT1 protein is involved in tumor cell growth (14,29), no associations were observed between WT1 intensity and clinicopathological characteristics in patients with PDA who received surgery in the present study. Multiple factors, including KRAS proto-oncogene GTPase wild-type, human epidermal growth factor receptor 2 amplification, BRCA2 DNA repair-associated mutation and ATM serine/threonine kinase mutation may be involved in PDA tumorigenesis (60). In certain patients with PDA who exhibit weak cytoplasmic expression of WT1 proteins, the WT1 gene may not be associated with PDA cell growth (29), which may be the reason why the WT1 protein staining intensity and clinicopathological characteristics were not significantly associated in the present study.
In conclusion, the results of the present study suggest that immunostaining of WT1 in PDA cytoplasm and tumor vessels may serve as a marker to predict the risk of relapse and progression in patients with PDA following complete surgical resection. A limitation of the present study is the relatively small sample size used to evaluate the indicative value of WT1 for PDA-specific mortality. Furthermore, the present study analyzed retrospective data collected from a single institution. Therefore, a large prospective cohort study to evaluate the specific association between WT1 expression and prognosis in patients with PDA is strongly recommended. Future results may expand the body of knowledge presently available on the clinical implications of WT1-targeting cancer vaccines for all patients with PDA.
Acknowledgements
The authors would like to thank clinical technologist Mrs Tsuuse Bito (Division of Gastroenterology and Hepatology, Department of Internal Medicine, The Jikei University School of Medicine, Kashiwa Hospital) for providing technical support.
Funding
The present study was supported, in part, by Grants in Aid for Scientific Research (C) from the Ministry of Education, Cultures, Sports, Science and Technology of Japan (grant no. 15K09050).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YO, SN, SS, MO, HS and SK were responsible for study conception and design and developed the methodology. The acquisition, analysis, and interpretation of data was undertaken by TK, ZI, YO, MSu, KT, MK, MSa, SF, TM, TA, HY and SK, and administrative, technical or material support was provided by TK, YO, SN, SF, TM, TA and SK. The writing, review and/or revision of the manuscript was the responsibility of TK and SK. All authors approved the final version of this manuscript.
Ethics approval and consent to participate
The present study was reviewed and approved by the Institutional Review Board (IRB) of the Jikei University [IRB no: 27–071 (7956)].
Patient consent for publication
Due to the retrospective and non-interventional nature of the present study, the IRB waived the requirement for written informed consent from the participants for their clinical records and tissue to be used in the present study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 2.Zuckerman DS, Ryan DP. Adjuvant therapy for pancreatic cancer: A review. Cancer. 2008;112:243–249. doi: 10.1002/cncr.23174. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, Cameron JL, Pawlik TM, Schulick RD, Wolfgang CL, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: The Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Adham M, Jaeck D, Le Borgne J, Oussoultzouglou E, Chenard-Neu MP, Mosnier JF, Scoazec JY, Mornex F, Partensky C. Long-term survival (5–20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: A series of 30 patients collected from 3 institutions. Pancreas. 2008;37:352–357. doi: 10.1097/MPA.0b013e31818166d2. [DOI] [PubMed] [Google Scholar]
- 7.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, Strasberg S, Hanna S, Taylor B, Langer B, Gallinger S. Prognostic factors in resected pancreatic adenocarcinoma: Analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Shimada K, Sakamoto Y, Nara S, Esaki M, Kosuge T, Hiraoka N. Analysis of 5-year survivors after a macroscopic curative pancreatectomy for invasive ductal adenocarcinoma. World J Surg. 2010;34:1908–1915. doi: 10.1007/s00268-010-0570-9. [DOI] [PubMed] [Google Scholar]
- 9.Huff V. Wilms' tumours: About tumour suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer. 2011;11:111–121. doi: 10.1038/nrc3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990;60:509–520. doi: 10.1016/0092-8674(90)90601-A. [DOI] [PubMed] [Google Scholar]
- 11.Osaka M, Koami K, Sugiyama T. WT1 contributes to leukemogenesis: Expression patterns in 7,12-dimethylbenz[a]anthracene (DMBA)-induced leukemia. Int J Cancer. 1997;72:696–699. doi: 10.1002/(SICI)1097-0215(19970807)72:4<696::AID-IJC23>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Han Y, Saiz Suarez F, Minden MD. A tumor suppressor and oncogene: The WT1 story. Leukemia. 2007;21:868–876. doi: 10.1038/sj.leu.2404770. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama H. WT1 (Wilms' Tumor Gene 1): Biology and cancer immunotherapy. Jap J Clin Oncol. 2010;40:377–387. doi: 10.1093/jjco/hyp194. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi N, Oji Y, Tsuji N, Tsuda A, Higashio M, Aoyagi S, Fukuda I, Ito K, Nakamura J, Takashima S, et al. Wilms' tumor gene WT1-shRNA as a potent apoptosis-inducing agent for solid tumors. Int J Oncol. 2008;32:701–711. [PubMed] [Google Scholar]
- 15.Sciesielski LK, Kirschner KM, Scholz H, Persson AB. Wilms' tumor protein Wt1 regulates the Interleukin-10 (IL-10) gene. FEBS Lett. 2010;584:4665–4671. doi: 10.1016/j.febslet.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Taube ET, Denkert C, Sehouli J, Kunze CA, Dietel M, Braicu I, Letsch A, Darb-Esfahani S. Wilms tumor protein 1 (WT1)-not only a diagnostic but also a prognostic marker in high-grade serous ovarian carcinoma. Gynecol Oncol. 2015;140:494–502. doi: 10.1016/j.ygyno.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Köbel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008;5:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sera T, Hiasa Y, Mashiba T, Tokumoto Y, Hirooka M, Konishi I, Matsuura B, Michitaka K, Udaka K, Onji M. Wilms' tumour 1 gene expression is increased in hepatocellular carcinoma and associated with poor prognosis. Eur J Cancer. 2008;44:600–608. doi: 10.1016/j.ejca.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Qi XW, Zhang F, Yang XH, Fan LJ, Zhang Y, Liang Y, Ren L, Zhong L, Chen QQ, Zhang KY, et al. High Wilms' tumor 1 mRNA expression correlates with basal-like and ERBB2 molecular subtypes and poor prognosis of breast cancer. Oncol Rep. 2012;28:1231–1236. doi: 10.3892/or.2012.1906. [DOI] [PubMed] [Google Scholar]
- 20.Lapillonne H, Renneville A, Auvrignon A, Flamant C, Blaise A, Perot C, Lai JL, Ballerini P, Mazingue F, Fasola S, et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol. 2006;24:1507–1515. doi: 10.1200/JCO.2005.03.5303. [DOI] [PubMed] [Google Scholar]
- 21.Qi XW, Zhang F, Wu H, Liu JL, Zong BG, Xu C, Jiang J. Wilms' tumor 1 (WT1) expression and prognosis in solid cancer patients: A systematic review and meta-analysis. Sci Rep. 2015;5:8924. doi: 10.1038/srep08924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y, Ueno H, Kondo S, Morizane C, Ikeda M, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother. 2011;34:92–99. doi: 10.1097/CJI.0b013e3181fb65b9. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195–205. doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 25.Nishida S, Koido S, Takeda Y, Homma S, Komita H, Takahara A, Morita S, Ito T, Morimoto S, Hara K, et al. Wilms tumor gene (WT1) peptide-based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother. 2014;37:105–114. doi: 10.1097/CJI.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi M, Shimodaira S, Nagai K, Ogasawara M, Takahashi H, Abe H, Tanii M, Okamoto M, Tsujitani S, Yusa S, et al. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: A multicenter analysis. Cancer Immunol Immunother. 2014;63:797–806. doi: 10.1007/s00262-014-1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms' tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20:4228–4239. doi: 10.1158/1078-0432.CCR-14-0314. [DOI] [PubMed] [Google Scholar]
- 28.Mayanagi S, Kitago M, Sakurai T, Matsuda T, Fujita T, Higuchi H, Taguchi J, Takeuchi H, Itano O, Aiura K, et al. Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci. 2015;106:397–406. doi: 10.1111/cas.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oji Y, Nakamori S, Fujikawa M, Nakatsuka S, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, et al. Overexpression of the Wilms' tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci. 2004;95:583–587. doi: 10.1111/j.1349-7006.2004.tb02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatsuka S, Oji Y, Horiuchi T, Kanda T, Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M, et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol. 2006;19:804–814. doi: 10.1038/modpathol.3800588. [DOI] [PubMed] [Google Scholar]
- 31.Naitoh K, Kamigaki T, Matsuda E, Ibe H, Okada S, Oguma E, Kinoshita Y, Takimoto R, Makita K, Ogasawara S, Goto S. Immunohistochemical analysis of WT1 antigen expression in various solid cancer cells. Anticancer Res. 2016;36:3715–3724. [PubMed] [Google Scholar]
- 32.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 33.Gu L, Li H, Chen L, Ma X, Li X, Gao Y, Zhang Y, Xie Y, Zhang X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget. 2016;7:31926–31942. doi: 10.18632/oncotarget.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukharamwala P, Thoens J, Szuchmacher M, Smith J, DeVito P. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: A meta-analysis and systematic review. HPB (Oxford) 2012;14:649–657. doi: 10.1111/j.1477-2574.2012.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita S, Sho M, Yanagimoto H, Satoi S, Akahori T, Nagai M, Nishiwada S, Yamamoto T, Hirooka S, Yamaki S, et al. Potential role of surgical resection for pancreatic cancer in the very elderly. Pancreatology. 2015;15:240–246. doi: 10.1016/j.pan.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 37.Bing Z, Pasha TL, Acs G, Zhang PJ. Cytoplasmic overexpression of WT-1 in gastrointestinal stromal tumor and other soft tissue tumors. Appl immunohistochem Mol Morphol. 2008;16:316–321. doi: 10.1097/PAI.0b013e31815c2e02. [DOI] [PubMed] [Google Scholar]
- 38.Martina JD, Simmons C, Jukic DM. High-definition hematoxylin and eosin staining in a transition to digital pathology. J Pathol Inform. 2011;2:45. doi: 10.4103/2153-3539.86284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oji Y, Suzuki T, Nakano Y, Maruno M, Nakatsuka S, Jomgeow T, Abeno S, Tatsumi N, Yokota A, Aoyagi S, et al. Overexpression of the Wilms' tumor gene W T1 in primary astrocytic tumors. Cancer Sci. 2004;95:822–827. doi: 10.1111/j.1349-7006.2004.tb02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menssen HD, Renkl HJ, Rodeck U, Kari C, Schwartz S, Thiel E. Detection by monoclonal antibodies of the Wilms' tumor (WT1) nuclear protein in patients with acute leukemia. Int J Cancer. 1997;70:518–523. doi: 10.1002/(SICI)1097-0215(19970304)70:5<518::AID-IJC5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Vajjhala PR, Macmillan E, Gonda T, Little M. The Wilms' tumour suppressor protein, WT1, undergoes CRM1-independent nucleocytoplasmic shuttling. FEBS Lett. 2003;554:143–148. doi: 10.1016/S0014-5793(03)01144-X. [DOI] [PubMed] [Google Scholar]
- 42.Niksic M, Slight J, Sanford JR, Caceres JF, Hastie ND. The Wilms' tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum Mol Genet. 2004;13:463–471. doi: 10.1093/hmg/ddh040. [DOI] [PubMed] [Google Scholar]
- 43.Ye Y, Raychaudhuri B, Gurney A, Campbell CE, Williams BR. Regulation of WT1 by phosphorylation: Inhibition of DNA binding, alteration of transcriptional activity and cellular translocation. EMBO J. 1996;15:5606–5615. [PMC free article] [PubMed] [Google Scholar]
- 44.Takahara A, Koido S, Ito M, Nagasaki E, Sagawa Y, Iwamoto T, Komita H, Ochi T, Fujiwara H, Yasukawa M, et al. Gemcitabine enhances Wilms' tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response. Cancer Immunol Immunother. 2011;60:1289–1297. doi: 10.1007/s00262-011-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magro G, Salvatorelli L, Vecchio GM, Musumeci G, Rita A, Parenti R. Cytoplasmic expression of Wilms tumor transcription factor-1 (WT1): A useful immunomarker for young-type fibromatoses and infantile fibrosarcoma. Acta Histochem. 2014;116:1134–1140. doi: 10.1016/j.acthis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Oji Y, Tatsumi N, Shimizu S, Kanai Y, Nakazawa T, Asada M, Jomgeow T, Aoyagi S, Nakano Y, et al. Antiapoptotic function of 17AA(+)WT1 (Wilms' tumor gene) isoforms on the intrinsic apoptosis pathway. Oncogene. 2006;25:4217–4229. doi: 10.1038/sj.onc.1209455. [DOI] [PubMed] [Google Scholar]
- 47.Jomgeow T, Oji Y, Tsuji N, Ikeda Y, Ito K, Tsuda A, Nakazawa T, Tatsumi N, Sakaguchi N, Takashima S, et al. Wilms' tumor gene WT1 17AA(−)/KTS(−) isoform induces morphological changes and promotes cell migration and invasion in vitro. Cancer Sci. 2006;97:259–270. doi: 10.1111/j.1349-7006.2006.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oji Y, Tatsumi N, Kobayashi J, Fukuda M, Ueda T, Nakano E, Saito C, Shibata S, Sumikawa M, Fukushima H, et al. Wilms' tumor gene WT1 promotes homologous recombination-mediated DNA damage repair. Mol Carcinog. 2015;54:1758–1771. doi: 10.1002/mc.22248. [DOI] [PubMed] [Google Scholar]
- 49.Li BQ, Huang S, Shao QQ, Sun J, Zhou L, You L, Zhang TP, Liao Q, Guo JC, Zhao YP. WT1-associated protein is a novel prognostic factor in pancreatic ductal adenocarcinoma. Oncol Lett. 2017;13:2531–2538. doi: 10.3892/ol.2017.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin DI, Lee SW, Han ME, Kim HJ, Seo SA, Hur GY, Jung S, Kim BS, Oh SO. Expression and roles of Wilms' tumor 1-associating protein in glioblastoma. Cancer Sci. 2012;103:2102–2109. doi: 10.1111/cas.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishida S, Ishikawa T, Egawa S, Koido S, Yanagimoto H, Ishii J, Kanno Y, Kokura S, Yasuda H, Oba MS, et al. Combination gemcitabine and WT1 peptide vaccination improves progression-free survival in advanced pancreatic ductal adenocarcinoma: A phase II randomized study. Cancer Immunol Res. 2018 Jan 22; doi: 10.1158/2326-6066.CIR-17-0386. Doi: 10.1158/2326-6066.CIR-17-0386. [DOI] [PubMed] [Google Scholar]
- 52.Koido S, Homma S, Okamoto M, Takakura K, Gong J, Sugiyama H, Ohkusa T, Tajiri H. Chemoimmunotherapy targeting Wilms' tumor 1 (WT1)-specific cytotoxic T lymphocyte and helper T cell responses for patients with pancreatic cancer. Oncoimmunology. 2014;3:e958950. doi: 10.4161/21624011.2014.958950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koido S, Okamoto M, Shimodaira S, Sugiyama H. Wilms' tumor 1 (WT1)-targeted cancer vaccines to extend survival for patients with pancreatic cancer. Immunotherapy. 2016;8:1309–1320. doi: 10.2217/imt-2016-0031. [DOI] [PubMed] [Google Scholar]
- 54.Wagner N, Michiels JF, Schedl A, Wagner KD. The Wilms' tumour suppressor WT1 is involved in endothelial cell proliferation and migration: Expression in tumour vessels in vivo. Oncogene. 2008;27:3662–3672. doi: 10.1038/sj.onc.1211044. [DOI] [PubMed] [Google Scholar]
- 55.Wagner KD, Cherfils-Vicini J, Hosen N, Hohenstein P, Gilson E, Hastie ND, Michiels JF, Wagner N. The Wilms' tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun. 2014;5:5852. doi: 10.1038/ncomms6852. [DOI] [PubMed] [Google Scholar]
- 56.Folkman J. Tumor angiogenesis: Therapeutic implications. New Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 57.Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J Gastroenterol. 2015;21:2807–2815. doi: 10.3748/wjg.v21.i9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15:145–150. doi: 10.1016/j.pan.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, Dong J, Deng X, Gao J, Li X, et al. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer. 2014;134:2646–2655. doi: 10.1002/ijc.28584. [DOI] [PubMed] [Google Scholar]
- 60.Chantrill LA, Nagrial AM, Watson C, Johns AL, Martyn-Smith M, Simpson S, Mead S, Jones MD, Samra JS, Gill AJ, et al. Precision medicine for advanced pancreas cancer: The individualized molecular pancreatic cancer therapy (IMPaCT) trial. Clinical Cancer Res. 2015;21:2029–2037. doi: 10.1158/1078-0432.CCR-15-0426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.