Abstract
In the present study, the effects of microRNA-29a (miRNA-29a) on colon cancer cell viability and the molecular mechanisms underlying the effects were investigated. The expression of miRNA-29a in colon cancer serum samples was notably downregulated, compared with in the normal group. First, miRNA-29a mimic was used to increase the expression of miRNA-29a in HCT-116 cells. Furthermore, upregulation of miRNA-29a suppressed cell viability, increased lactate dehydrogenase levels and apoptosis, and promoted caspase-3/9 activities and B-cell lymphoma 2-associated X protein and phosphatase and tensin homolog (PTEN) protein expression in colon cancer cells. Furthermore, upregulation of miRNA-29a decreased phosphoinositide 3-kinase, phosphorylated (p)-protein kinase B (Akt) and p-glycogen synthase kinase 3β (GSK3β) protein expression and suppressed the Wnt/β-catenin signaling pathway in colon cancer cells. The results of the present study verified that the protective effects of miRNA-29a suppress the PTEN/Akt/GSK3β and Wnt/β-catenin signaling pathways in colon cancer.
Keywords: microRNA-29a, colon cancer, phosphatase and tensin homolog/protein kinase B/glycogen synthase kinase 3β, Wnt/β-catenin
Introduction
Colon cancer is one of the primary malignant tumor types in the digestive system, with the highest incidence rate in developed countries, of which the total number of mortality (650,000) ranked second in China in 2012 (1). With the improvement in living standards, changes in diet, aging of the population and the census of colon cancer, colon cancer has been identified to exhibit an increasing trend in incidence in China in 2010, and is a serious threat to the health of the population (1). The survival and prognosis of patients with colon cancer depends on the time at which the tumor is detected (2). However, for >57% patients, the cancer has already metastasized upon diagnosis (1). In the last 20 years, a large number of studies regarding colon cancer have demonstrated favorable progress in the diagnosis and treatment, and the 5-year survival rate for the patients with an early stage of colon cancer is ~90%; however, the overall survival rate of the patients with advanced and metastatic colon cancer has not been increased significantly, at only 15% (1).
microRNAs (miRNAs) are a class of short (typically between 17 and 25 nucleotides) non-coding single-stranded RNAs, which are evolutionarily conservative (3). miRNAs serve an important regulatory function in cell metabolism, proliferation, differentiation, apoptosis and other biological processes involved in viral infections, as well as the occurrence, diagnosis and treatment of cardiovascular disease, nerve and muscle disorders and numerous other aspects (4). miRNAs also serve an important function in tumor biology, including tumor evolution, invasion, metastasis and angiogenesis (5).
Studies on the function of miRNA in diagnosis of cancer are based on the miRNA expression marks, i.e. miRNA expression profile studies (6). miRNA expression profiles consist of determining different miRNA expression levels in multiple tumor samples (7). Previous studies have demonstrated that miRNA expression profiles are able to identify the tumor type, the staging and the other clinical characteristics, in addition to distinguishing between tumor and normal tissues, by which a variety of tumor and normal tissue samples may be analyzed systematically (8,9). The diagnostic accuracy rate of a tumor based on specific miRNA expression profiles is ≤70% (8).
With an improved understanding of the cancer pathogenesis at the molecular level, an increasing number of tumor-associated signaling pathways have been identified, and the phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway is important (10). Gene expression of members of this pathway has been proved to be one of the most important prognostic markers for lung, breast and kidney cancer (11). To the best of our knowledge, there have been relatively few studies in this area regarding colon cancer, therefore research on the PTEN/PI3K/Akt signaling pathway is expected to lead to an improved understanding of the occurrence, individualized treatment and prognosis of colon cancer (10).
It has been indicated in previous research that glycogen synthase kinase 3β (GSK3β) is the major regulatory enzyme for numerous intracellular signal transduction pathways, including Wnt/β-catenin and nuclear factor-κB (12). It is able to participate in regulating cell proliferation and apoptosis by affecting the downstream nuclear transcription factor (13). However, the effect of GSK3β and GSK3β inhibitor on the biological characteristics of tumor cell remains controversial (13). GSK3β is the major regulatory enzyme of numerous intracellular signal transduction pathways (14). Regulating GSK3β activity is able to affect the growth and apoptosis of different tumor types, including colon, lung and breast cancer (15); however, experiments on the effect of regulating GSK3β activity on tumor cell proliferation have led to contrasting results (12–15).
The Wnt/β-catenin signaling pathway is associated with tumor development (15). In breast, liver, stomach, thyroid, lung and prostate cancer, as well as melanoma and other malignant tumor types, the abnormal activation of the Wnt/β-catenin signaling pathway and the downregulation of expression or the inactivation of the pathway inhibitory proteins, such as Dickkopf Wnt signaling pathway inhibitor 1 (DKKI) or Wnt inhibitory factor 1 (WIF), have been determined (15,16). The abnormal activation of the pathway is an early event in colorectal cancer, and also indicates its importance in development. The effects of miRNA-29a on colon cancer cell viability and the molecular mechanisms underlying the effects were investigated.
Materials and methods
Ethics statement
Serum samples from 12 patients (mean age 63.5±5.5 years, age range 58–69 years old, all male) and 6 normal healthy volunteers (mean age 60±7 years, age range 53–67 years old, all male) were obtained from General Surgery at June 2016 to July 2016, Beijing Chao-Yang Hospital, Capital Medical University (Beijing, China) and were stored at −70°C. The present study was approved by the Ethical Board of Beijing Chao-Yang Hospital, Capital Medical University. Written informed consent was provided by all patients and healthy volunteers for the use of their samples.
RNA isolation and quantification of mRNA expression
Total RNA was isolated from serum samples and HCT-116 (purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA (1 µg) was used for cDNA synthesis using a PrimeScript First Strand cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan). The reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed using Power SYBR® Green PCR Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.) using an ABI7700 system. PCR amplification was performed at 95°C for 3 min prior to 40 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 60 sec, followed by a final incubation at 72°C for 5 min. The primers for miRNA-29a were: 5′-GAGGATCCCCTCAAGGATACCAAGGGATGAAT-3′ (forward) and 5′-CTTCTAGAAGGAGTGTTTCTAGGTTCCGTCA-3′ (reverse). The primers for U6 were: 5′-CTCGCTTCGGCAGCACATATAC-3′ (forward) and 5′-GGAACGCTTCACGAATTTGC-3′ (reverse). Relative miRNA-29a expression was calculated using the 2−∆∆Cq method (17).
Cell culture and transfection
The HCT-116 cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere containing 5% CO2. A total of 100 ng Negative control (5′-CCCCCCCCCC-3′) and 100 ng miRNA-29a mimic (5′-ATGACTGATTTCTTTTGGTG-3′) were transfected into cells using Lipofectamine® RNAiMax reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Cell viability assay and lactate dehydrogenase (LDH) activity
Cells were plated at [(1–2)x103 cells/well] in 96-well plates, incubated overnight and transfected with negative control and miRNA-29a mimic at 37°C. After 24, 48 and 72 h, cell viability were determined using an MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 4 h. Dimethylsulfoxide was added to dissolve the resultant formazan crystals. Absorbance at 492 nm was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Pittsburg, PA, USA).
Cells were plated at [(1–20×103 cells/well] in 96-well plates, incubated overnight at 37°C and transfected with negative control and miRNA-29a mimic. After 48 h, the LDH activity of cells was determined using LDH activity kits (C0016; Beyotime Institute of Biotechnology, Haimen, China). Absorbance at 450 nm was determined using a NanoDrop ND-1000 spectrophotometer.
Apoptosis assay and caspase 3/9 activity assay
Cells were plated at [(1–2)x106 cells/well] in 6-well plates, incubated overnight and transfected with negative control and miRNA-29a mimic at 37°C. After 48 h, cells were stained with 5 µl annexin V-FITC and 10 µl propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA) for 30 min at 37°C. Apoptotic cells were measured using a Flow Cytometer (c6; BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using Flowjo 7.6.1 software (FlowJo LLC, Ashland, OR, USA). Cells were plated at [(1–2)x106 cells/well] in 6-well plates, incubated overnight at 37°C and transfected with negative control and miRNA-29a mimic. After 48 h, total protein extracts were prepared by lysing cells in Cell Lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA). Protein concentrations in the lysates were determined using a bicinchoninic acid (BCA) protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein extracts (50 µg) were incubated with caspase-3 (C1116) and caspase-9 activity kits (C1158; Beyotime Institute of Biotechnology) for 1 h at 37°C. Absorbance at 405 nm was determined using a NanoDrop ND-1000 spectrophotometer.
Western blot analysis
Total protein extracts were prepared by lysing cells in Cell Lysis buffer. Protein concentrations in the lysates were determined using a BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein extracts (50 µg) were separated by SDS-PAGE (8–12% gel) and transferred onto nitrocellulose membranes. The membranes were incubated with primary antibodies against B-cell lymphoma 2-associated X protein (Bax; 1:500; cat. no. sc-6236), PTEN (1:500; cat. no. sc-6817-R), phosphorylated (p)-Akt (1:300; cat. no. sc-7985-R), p-GSK3β (1:300; cat. no. sc-81496), Wnt (1:500; cat. no. sc-13962), β-catenin (1:500; cat. no. sc-515105) and GAPDH (1:500; cat. no. sc-25778) (all Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. Following washing with Tris-buffered saline containing Tween-20, membranes were probed with goat anti-rabbit or anti-mouse immunoglobulin G horseradish peroxidase-conjugated secondary antibodies (cat. nos. 7074 and 7076; 1:5,000; Cell Signaling Technology, Inc.) at 37°C for 1 h. The proteins designated were visualized using an enhanced chemiluminescence detection kit (GE Healthcare Life Sciences, Little Chalfont, UK) and quantified using Image_Lab_3.0 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Results are expressed as the mean ± standard deviation using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Comparison among groups was performed by one-way analysis of variance followed by Dunnett's t-test. P<0.01 was considered to indicate a statistically significant difference.
Results
Expression of miRNA-29a in colon cancer serum samples
First, miRNA-29a expression was analyzed in serum samples from patients with colon cancer. As presented in Fig. 1, miRNA-29a in colon cancer serum samples was significantly downregulated, compared with the normal group (P<0.01).
Figure 1.
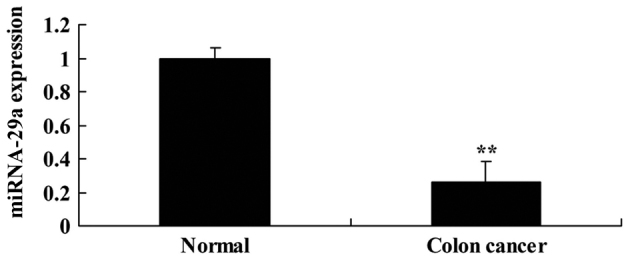
Expression of miRNA-29a in colon cancer serum samples. **P<0.01 vs. normal. miRNA-29a; microRNA-29a.
Upregulation of miRNA-29a suppresses viability of HCT-116 cells
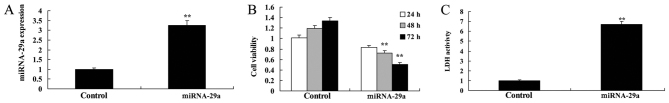
To further assess the effects of transfecting miRNA-29a into HCT-116 cells, cell viability and LDH activity were determined. miRNA-29a mimic led to increase of miRNA-29a expression, suppression of cell viability and an increase in LDH activity in HCT-116 cells, compared with the control group (Fig. 2).
Figure 2.
Upregulation of miRNA-29a suppresses the viability of HCT-116 cells. (A) miRNA-29a expression, (B) cell viability and (C) LDH activity. **P<0.01 vs. control. miRNA-29a, microRNA-29a; LDH, lactate dehydrogenase.
Upregulation of miRNA-29a increases apoptosis of HCT-116 cells
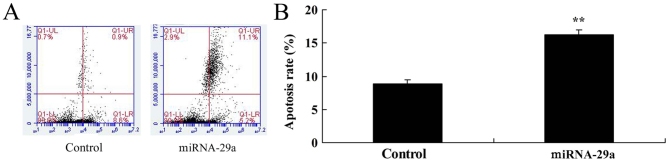
Furthermore, flow cytometry demonstrated that upregulation of miRNA-29a significantly increased apoptosis of HCT-116 cells, compared with the control group (P<0.01; Fig. 3).
Figure 3.
Upregulation of miRNA-29a increases apoptosis of HCT-116 cells. Upregulation of microRNA-29a increased apoptosis rate (A) determined by flow cytometry and (B) quantified. **P<0.01 vs. control. miRNA-29a, microRNA-29a.
Upregulation of miRNA-29a promotes caspase-3/9 activities of HCT-116 cells
Caspase-3/9 activities in HCT-116 cells by miRNA-29a were examined. As presented in Fig. 4, upregulation of miRNA-29a significantly promoted the caspase-3/9 activities of HCT-116 cells, compared with the control group (P<0.01).
Figure 4.
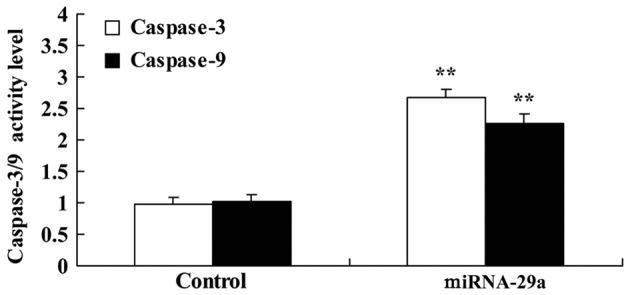
Upregulation of miRNA-29a promotes caspase-3/9 activities of HCT-116 cells. **P<0.01 vs. control. miRNA-29a, microRNA-29a.
Upregulation of miRNA-29a promotes Bax and PTEN protein expression in HCT-116 cells
Next, Bax and PTEN protein expression in HCT-116 cells were determined following miRNA-29a transfection for 48 h. Bax and PTEN protein expression following miRNA-29a upregulation were significantly increased, compared with the control group (P<0.01; Fig. 5).
Figure 5.
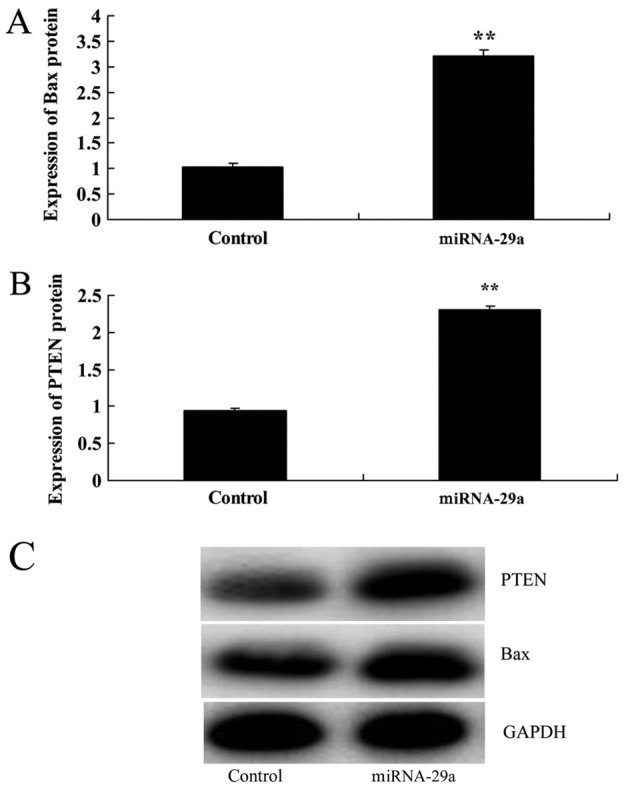
Upregulation of miRNA-29a promotes Bax and PTEN protein expression in HCT-116 cells. Upregulation of miRNA-29a promoted (A) Bax and (B) PTEN protein expression in HCT-116 cells, as determined by (C) western blot analysis. **P<0.01 vs. control. miRNA-29a, microRNA-29a; Bax, B-cell lymphoma 2-associated X protein; PTEN, phosphatase and tensin homolog.
Upregulation of miRNA-29a decreases PI3K, p-Akt and p-GSK3β expression in HCT-116 cells
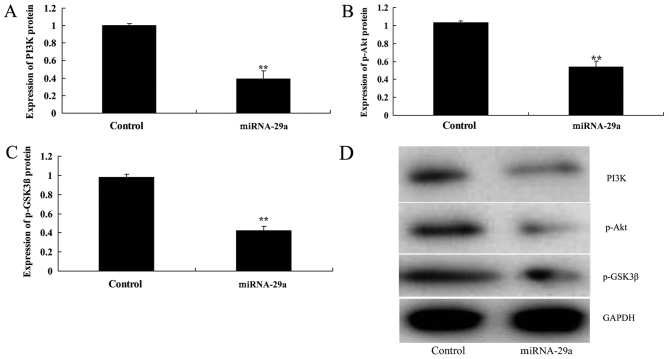
Western blot analysis indicated that upregulation of miRNA-29a significantly suppressed PI3K, p-Akt and p-GSK3β expression levels in HCT-116 cells, compared with the control group (P<0.01; Fig. 6).
Figure 6.
Upregulation of miRNA-29a decreases PI3K, p-Akt and p-GSK3β expression levels in HCT-116 cells. Upregulation of miRNA-29a decreased (A) PI3K, (B) p-Akt and (C) p-GSK3β protein expression in HCT-116 cells, as determined by (D) western blot analysis. **P<0.01 vs. control. miRNA-29a, microRNA-29a; p-, phosphorylated; GSK3β, glycogen synthase kinase 3β; PI3K, phosphoinositide 3-kinase.
Upregulation of miRNA-29a suppresses the Wnt/β-catenin signaling pathway of HCT-116 cells
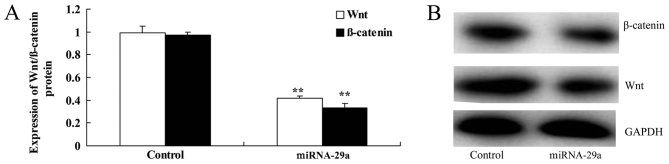
As presented in Fig. 7, upregulation of miRNA-29a significantly suppressed Wnt and β-catenin protein expression in HCT-116 cells, compared with the control group (P<0.01).
Figure 7.
Upregulation of miRNA-29a suppresses the Wnt/β-catenin signaling pathway in HCT-116 cells. Upregulation of miRNA-29a promoted (A) Wnt and β-catenin protein expression in HCT-116 cells, as determined by (B) western blot analysis. **P<0.01 vs. control. miRNA-29a, microRNA-29a.
Discussion
As a common malignant, colon cancer is a common malignancy, which ranks third in total morbidity and mortalities (2814,000) in the USA in 2015 (18). In China, colon cancer is the third most common cancer and ranks fifth for its mortality rate (19). The average age for a patient with colon cancer is ~40 years, with being between 50 and 60 years, 10 years less compared with the average age of Western countries (19). It was demonstrated that miRNA-29a in colon cancer serum samples was significantly downregulated, compared with the normal group. These results revealed that miRNA-29a may be an important element in colon cancer. The effect of miRNAs on cancer makes them an important target for therapeutic intervention. Gene therapy is able to prevent colon cancer cell growth by modulating the expression of tumor suppressor miRNA or miRNA promoters (20). This adjustment is able to control the tumor growth rate, which has the potential for the treatment of early- and late-stage cancer. This indicates that a number of the factors may reverse miRNA expression, and it may be possible to transform cancerous tissue into normal tissue (21). miRNA may serve a function in cancer chemoprevention, which may have the ability to decrease tumor size and metastasis, to lead to the discovery of novel therapeutic drugs (21). Although the experimental efficacy of miRNA appears to be promising, it must also be validated with different patients in future clinical practice (22). These results indicated that upregulation of miRNA-29a suppressed cell viability, increased apoptosis, and promoted caspase-3/9 activities and Bax protein expression of colon cancer cells. Therefore, miRNA-29a may have induced colon cancer cell death through Bax/caspase-3/9.
The PTEN/PI3K/Akt signaling pathway is composed of PTEN, PI3K, Akt and its downstream effector molecules (11). PTEN is another important tumor suppressor gene following p53, which has a phosphatase activity, and serves an important role in regulating the cell cycle and inducing the apoptosis of tumor cells (11). PI3K is an important intracellular kinase, and excessive activation of which serves an important function in the activation of tumor occurrence (11). Akt is markedly homologous with the viral oncogene v-Akt, which induces leukemia in mice, and the major effector molecules of PI3K, and overactivation may inhibit or activate the downstream target proteins, leading to the infinite proliferation of cells though numerous mechanisms (20). PI3K is activated by a variety of mechanisms, resulting in the production of important molecules, including phosphatidylinositiol 3,4,5-trisphosphate (PIP3); binding Akt; phosphorylating Akt, and changing the conformation as an intracellular second messenger, which furthermore leads to the translocation of Akt from the cytoplasm to the plasma membrane, thereby activating the downstream target proteins and mediating growth factors, such as insulin, promoting cell survival (20). Suppressor gene PTEN generates phosphatidylinositol 4,5-bisphosphate through the dephosphorylation by the catalysis of PIP3, to inhibit the Akt translocation and conformational change so as to decrease the activity of Akt, thereby antagonizing the signaling pathway to serve a tumor-suppressing function (20). When PTEN is mutated or inactivated, the suppressive effect on PIP3 ceases, leading to intracellular accumulation of a large amount of PIP3, Akt overactivation, cell immortalization (23,24). In this report, upregulation of miRNA-29a decreased PTEN, PI3K, p-Akt and p-GSK3β protein expression in colon cancer cells. Li et al (25) indicated that miRNA-29a induced apoptosis of papillary thyroid carcinoma cells through Akt expression.
GSK3β is a multifunctional serine/threonine kinase. It is involved in numerous important physiological processes, including intracellular glycometabolism, cell proliferation, differentiation and apoptosis (26). Abnormal GSK3β expression and dysfunction may induce a series of insuperable diseases, including cancer, diabetes and Alzheimer's disease (27). Therefore, GSK3β has become a focus of research. The present study determined that upregulation of miRNA-29a suppressed p-GSK3β protein expression in colon cancer cells. Shen et al (28) demonstrated that miRNA-29a contributes to drug resistance of breast cancer cells to Adriamycin via the GSK3β signaling pathway. There results indicated that miRNA-29a regulates the PTEN/PI3K/Akt/GSK3β signaling pathway to induce the apoptosis of colon cancer cells.
The Wnt/β-catenin signaling pathway is markedly conserved during evolution, serving an important function during embryonic development from fruitflies to humans (26). The Wnt/β-catenin pathway has multiple sites of action, which is subject to the regulation of multiple signaling pathways (27). The change in any component in this pathway may cause an abnormality in the signal transduction, resulting in body dysplasia or neoplasia (27). In a variety of tumor types, including breast cancer, prostate cancer, melanoma, colorectal cancer and lung cancer, abnormalities have been determined in this pathway (29). The downregulation in expression of signaling pathway antagonist proteins, such as DKKI or WIF, may be determined in a variety of human tumor types, indirectly causing abnormal regulation of Wnt/β-catenin, which serves an important function in the occurrence and the development of colorectal cancer (30). The abnormal regulation of Wnt/β-catenin and its upstream signals result in intracellular accumulation of β-catenin and translocation, and, following translocation into the nucleus, it activates T-cell factor/lymphoid enhancer factor transcriptional activity, causing the abnormal expression of downstream genes (31). For >90% patients with colorectal cancer, the activation of the Wnt/β-catenin signaling pathway can be determined, ~80% exhibited increased expression of β-catenin and abnormal expression in the cytoplasm and nucleus, and the key protein β-catenin that inhibits the Wnt/β-catenin pathway in colorectal cells is able to inhibit tumor growth and progression (32). The results of the present study also indicated that upregulation of miRNA-29a suppressed the Wnt/β-catenin signaling pathway in colon cancer cells. Nagano et al (33) indicated that miRNA-29a induces resistance to gemcitabine of pancreatic cancer cells through the Wnt/β-catenin signaling pathway, therefore it was considered that miRNA-29a induced apoptosis of colon cancer through the suppression of the Wnt/β-catenin signaling pathway.
In conclusion, the results of the present study demonstrated a significant association between miRNA-29a expression and the response to apoptosis in colon cancer cell. The results demonstrated that the miRNA-29a-induced apoptosis of colon cancer is mediated by activation of the PTEN/PI3K/Akt/GSK3β signaling pathway and suppression of the Wnt/β-catenin signaling pathway (Fig. 8).
Figure 8.
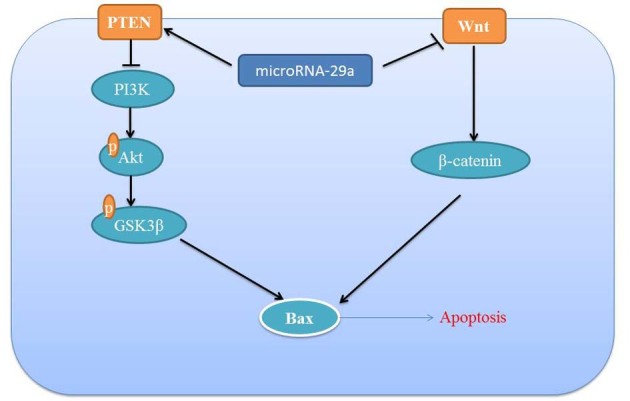
MicroRNA-29a inhibits colon cancer growth by regulation of the PTEN/Akt/GSK3β and Wnt/β-catenin signaling pathways. PTEN, phosphatase and tensin homolog; PI3K, phosphoinositide 3-kinase; GSK3, glycogen synthase kinase; Bax, B-cell lymphoma 2-associated X protein; p, phosphorylated; Akt, protein kinase B.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZG designed the experiment. XH, JZ and YW performed the experiment. ZG and XH analyzed the data. ZG wrote the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethical Board of Beijing Chao-Yang Hospital. Written informed consent was provided by all patients and healthy volunteers for the use of their samples.
Consent for publication
Consent was received from the patients for publication of this study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhang HX, Wang ZT, Lu XX, Wang YG, Zhong J, Liu J. NLRP3 gene is associated with ulcerative colitis (UC), but not Crohn's disease (CD), in Chinese Han population. Inflamm Res. 2014;63:979–985. doi: 10.1007/s00011-014-0774-9. [DOI] [PubMed] [Google Scholar]
- 2.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, Galsgaard J, Turino SY, Brodersen JB, Rashid S, Rasmussen BK, et al. Genetically determined high activity of IL-12 and IL-18 in ulcerative colitis and TLR5 in Crohns disease were associated with non-response to anti-TNF therapy. Pharmacogenomics J. 2018;18:87–97. doi: 10.1038/tpj.2016.84. [DOI] [PubMed] [Google Scholar]
- 4.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 5.Engler DB, Leonardi I, Hartung ML, Kyburz A, Spath S, Becher B, Rogler G, Müller A. Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm Bowel Dis. 2015;21:854–861. doi: 10.1097/MIB.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 6.Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu C, Chen Y, Cai W, Wu J. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget. 2016;7:83951–83963. doi: 10.18632/oncotarget.13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, Drillenburg P, van Deventer SJ, Chvatchko Y, Te Velde AA. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001;121:1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Mizushima T, Wu X, Okuzaki D, Kambara N, Ishikawa S, Wang J, Qian Y, Hirose H, Yokoyama Y, et al. A miR-29b byproduct sequence exhibits potent tumor-suppressive activities via inhibition of NF-kappaB signaling in KRAS-mutant colon cancer cells. Mol Cancer Ther. 2018;17:977–987. doi: 10.1158/1535-7163.MCT-17-0850. [DOI] [PubMed] [Google Scholar]
- 9.Wang N, Zhang Y, Liang H. microRNA-598 inhibits cell proliferation and invasion of glioblastoma by directly targeting metastasis associated in colon cancer-1. Oncol Res. 2018. Feb 14. Doi: 10.3727/096504018X15185735627746. [DOI] [PMC free article] [PubMed]
- 10.Bruusgaard A, Andersen RB. Chenodeoxycholic-acid treatments of rheumatoid arthritis. Lancet. 1976;1:700. doi: 10.1016/S0140-6736(76)92827-0. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Hu X, Xuan Y, Ying J, Fei Y, Rong J, Zhang Y, Zhang J, Liu C, Liu Z. Kaempferol protects ethanol-induced gastric ulcers in mice via pro-inflammatory cytokines and NO. Acta Biochim Biophys Sin (Shanghai) 2018;50:246–253. doi: 10.1093/abbs/gmy002. [DOI] [PubMed] [Google Scholar]
- 12.Park GB, Chung YH, Gong JH, Jin DH, Kim D. GSK-3β-mediated fatty acid synthesis enhances epithelial to mesenchymal transition of TLR4-activated colorectal cancer cells through regulation of TAp63. Int J Oncol. 2016;49:2163–2172. doi: 10.3892/ijo.2016.3679. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Martínez E, Martín-Ruiz A, Martín P, Calvo V, Provencio M, García JM. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3beta signaling pathway. Oncotarget. 2016;7:68781–68791. doi: 10.18632/oncotarget.11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jendželovský R, Koval J, Mikeš J, Papčová Z, Plšíková J, Fedoročko P. Inhibition of GSK-3beta reverses the pro-apoptotic effect of proadifen (SKF-525A) in HT-29 colon adenocarcinoma cells. Toxicol In Vitro. 2012;26:775–782. doi: 10.1016/j.tiv.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Jamwal G, Singh G, Dar MS, Singh P, Bano N, Syed SH, Sandhu P, Akhter Y, Monga SP, Dar MJ. Identification of a unique loss-of-function mutation in IGF1R and a crosstalk between IGF1R and Wnt/β-catenin signaling pathways. Biochim Biophys Acta. 2018;1865:920–931. doi: 10.1016/j.bbamcr.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Li Y, Chen Y, Chen H, Zhu P, Xu M, Wang H, Wu M, Yang Z, Hoffman RM, Gu Y. Ethanolic extract of traditional chinese medicine (TCM) gamboge inhibits colon cancer via the Wnt/beta-catenin signaling pathway in an orthotopic mouse model. Anticancer Res. 2018;38:1917–1925. doi: 10.21873/anticanres.12429. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Lazaridis LD, Pistiki A, Giamarellos-Bourboulis EJ, Georgitsi M, Damoraki G, Polymeros D, Dimitriadis GD, Triantafyllou K. Activation of NLRP3 inflammasome in inflammatory bowel disease: Differences between crohn's disease and ulcerative colitis. Dig Dis Sci. 2017;62:2348–2356. doi: 10.1007/s10620-017-4609-8. [DOI] [PubMed] [Google Scholar]
- 19.Itani S, Watanabe T, Nadatani Y, Sugimura N, Shimada S, Takeda S, Otani K, Hosomi S, Nagami Y, Tanaka F, et al. NLRP3 inflammasome has a protective effect against oxazolone-induced colitis: A possible role in ulcerative colitis. Sci Rep. 2016;6:39075. doi: 10.1038/srep39075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi EM. Kaempferol protects MC3T3-E1 cells through antioxidant effect and regulation of mitochondrial function. Food Chem Toxicol. 2011;49:1800–1805. doi: 10.1016/j.fct.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Yan L, Guo Z, Chen Y, Li M, Huang C, Chen Z, Meng X. Chenodeoxycholic acid attenuates high-fat diet-induced obesity and hyperglycemia via the G protein-coupled bile acid receptor 1 and proliferator-activated receptor γ pathway. Exp Ther Med. 2017;14:5305–5312. doi: 10.3892/etm.2017.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano Y, Hirano F, Fujii H, Makino I. Fibrates suppress chenodeoxycholic acid-induced RANTES expression through inhibition of NF-kappaB activation. Eur J Pharmacol. 2002;448:19–26. doi: 10.1016/S0014-2999(02)01902-7. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Qian J, Wang L, Li J, Zhao Y, Han J, Khan Z, Chen X, Wang J, Liang G. Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine. 2018;60:83–94. doi: 10.1007/s12020-018-1525-4. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Park JG, Lee J, Yang WS, Park GW, Kim HG, Yi YS, Baek KS, Sung NY, Hossen MJ, et al. The dietary flavonoid Kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediators Inflamm. 2015;2015:904142. doi: 10.1155/2015/904142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Liu J, Li Q, Chen G, Yu X. miR-29a suppresses growth and metastasis in papillary thyroid carcinoma by targeting AKT3. Tumour Biol. 2016;37:3987–3996. doi: 10.1007/s13277-015-4165-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang Z, Ye G, Huang B. Kaempferol Alleviates the Interleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-kappaB. Med Sci Monit. 2017;23:3925–3931. doi: 10.12659/MSM.902491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng X, Yang YL, Yang H, Wang YH, Du GH. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int Immunopharmacol. 2018;56:29–35. doi: 10.1016/j.intimp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Shen H, Li L, Yang S, Wang D, Zhong S, Zhao J, Tang J. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway. Gene. 2016;593:84–90. doi: 10.1016/j.gene.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Basu A, Das AS, Sharma M, Pathak MP, Chattopadhyay P, Biswas K, Mukhopadhyay R. STAT3 and NF-kappaB are common targets for kaempferol-mediated attenuation of COX-2 expression in IL-6-induced macrophages and carrageenan-induced mouse paw edema. Biochem Biophys Rep. 2017;12:54–61. doi: 10.1016/j.bbrep.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merhi A, de Mees C, Abdo R, Victoria Alberola J, Marini AM. Wnt/β-catenin signaling regulates the expression of the ammonium permease gene RHBG in human cancer cells. PLoS One. 2015;10:e0128683. doi: 10.1371/journal.pone.0128683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mervai Z, Sólyomváry A, Tóth G, Noszál B, Molnár-Perl I, Baghy K, Kovalszky I, Boldizsár I. Endogenous enzyme-hydrolyzed fruit of Cirsium brachycephalum: Optimal source of the antiproliferative lignan trachelogenin regulating the Wnt/beta-catenin signaling pathway in the SW480 colon adenocarcinoma cell line. Fitoterapia. 2015;100:19–26. doi: 10.1016/j.fitote.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Voloshanenko O, Erdmann G, Dubash TD, Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T, Anchang B, et al. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat Commun. 2013;4:2610. doi: 10.1038/ncomms3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagano H, Tomimaru Y, Eguchi H, Hama N, Wada H, Kawamoto K, Kobayashi S, Mori M, Doki Y. MicroRNA-29a induces resistance to gemcitabine through the Wnt/β-catenin signaling pathway in pancreatic cancer cells. Int J Oncol. 2013;43:1066–1072. doi: 10.3892/ijo.2013.2037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.