Abstract
DNA-damage regulated autophagy modulator 1 (DRAM1) is known as a target of TP53-mediated autophagy, and has been reported to promote the migration and invasion abilities of glioblastoma stem cells. However, the precise contribution of DRAM1 to cancer cell invasion and migration, and the underlying mechanisms remain unclear. In the present study, small interfering (si)RNA or short hairpin RNA mediated knockdown of DRAM1 was performed in hepatoblastoma cells and the migration and invasion abilities were detected in vitro and in vivo. To investigate the underlying mechanisms, western blotting and immunofluorescence were used to detect the expression of autophagy-associated proteins and epithelial-mesenchymal-transition (EMT)-associated markers. The results showed that DRAM1 knockdown by specific siRNA abrogated cell autophagy, as well as inhibited the migration and invasion of HepG2 cells in Transwell assays, which may be reversed by rapamycin treatment. In addition, DRAM1 knockdown increased the expression of E-Cadherin while decreased the expression of vimentin in HepG2 cells, which was also be reversed by rapamycin treatment. Taken together, these results suggest that DRAM1 is involved in the regulation of the migration and invasion of HepG2 cells via autophagy-EMT pathway.
Keywords: DRAM1, autophagy, migration, invasion, epithelial-mesenchymal-transition
Introduction
Hepatoblastoma (HB) is one of the most common solid tumors, which caused cancer-associated mortality worldwide (1). As the most common malignant liver tumor of childhood, the incidence peaks in the first two years of life, with the majority of cases presenting by 5 years of age. Slight male preponderance is observed. HB may be present at birth and prenatal cases have been reported (2,3). The molecular pathogensis of HB is remained to be elucidated. Different molecular alterations have been identified as being involved in the genesis of HB. Particularly, a deregulation of different signaling pathways has been described among which Wnt signaling (4–6), Sonic Hedgehog (7), Notch and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin are counted as the main players (8,9). It is of great importance to understand the molecular mechanisms to explore more targeted therapeutic strategies. HepG2 cells were isolated from a human liver biopsy of a 15-year old male with HB, which were shown to be a HB cell line (10–12).
DNA-damage regulated autophagy modulator 1 (DRAM1) is an evolutionarily conserved transmembrane protein, which localizes predominantly to lysosomes and acts as a target of tumor protein p53 (TP53)-mediated autophagy and programmed cell death (13). Recent reports have demonstrated that high levels of DRAM1 were associated with shorter overall survival in glioblastoma multiforme (GBM) patients, and knockdown of DRAM1 inhibited the migration and invasion abilities of glioblastoma stem cells (GSCs) (14). However, the precise contribution of DRAM1 to cancer cell invasion and migration and the underlying mechanisms remain unclear. Autophagy is an evolutionally conserved process in which amino acids, nutrients, and lipids are recycled when cells go through nutrient and oxygen deprivations (15). DRAM1 acts as a regulator of autophagy mediated by p53 in response to genotoxic stresses through regulation of the clearance of autophagosomes by promoting lysosomal acidification and inducing the activation of lysosomal enzymes (16).
Epithelial-mesenchymal-transition (EMT) represents a process of fast changes, during which the cell phenotype changes from epithelial to mesenchymal, the expression of mesenchymal markers were upregulated, the actin cytoskeleton was reorganization, cell-cell adhesion structures were destructed, and pseudopod formation emerges (17). During the EMT, the migratory ability of epithelial cells is enhanced (17). Autophagy is a catabolic process that mediates degradation of unnecessary or dysfunctional cellular components (18,19). These two important processes in cancer are linked in an intricate relationship. EMT requires autophagy to support viability of potentially metastatic cancer cells, while a number of additional evidence indicates that autophagy acts to prevent EMT and that the activation of the autophagic machinery may determine reversion of the EMT phenotype in cancer cells (20–24).
This study was designed to investigate the effect of DRAM1 on cell invasion and migration, as well as explore the underlying mechanisms involved in cell autophagy and EMT. We provided the evidence that DRAM1 knockdown inhibited cell invasion and migration abilities of HB cells by inhibiting the autophagy-EMT pathway.
Materials and methods
Antibodies and reagents
Antibodies for E-Cadherin and Vimentin were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies for DRAM1, P62 and LC3 were purchased from Abcam (Cambridge, MA, USA). Antibody for β-actin was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rapamycin was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Transwell chamber was purchased from Corning Incorporated (Corning, NY, USA). Matrigel was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Cell culture
Human HB derived HepG2 cells were obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (DMEM; 11965500; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; 086150008; Wisten Inc., Anthem, AZ, USA), 100 IU/ml penicillin, and 100 IU/ml streptomycin in a humidified incubator at 37°C under 5% CO2 atmosphere, and passaged at preconfluent densities using 0.25% trypsin solution every 2 to 3 days. Cells were stored and used within 3 months after resuscitation from cryopreservation status.
Transfection and RNA interference
The DRAM1 shRNA (shDRAM1: Tracking number TRCN0000161451, clone ID NM 018370.1–1356s1c1) were purchased from Sigma-Aldrich and the small interfering (si)RNA targeting DRAM1 was purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). For establish stable DRAM1 knockdown cells, HepG2 cells were transfected with DRAM1 shRNA and were selected in cell culture medium containing 1.5 mg/ml poromycin for 1 week. Cells were then cultured in culture medium for in vivo experiments. For transfection, cells were plated in 6-well plates at 30% confluency, and siRNA duplexes were introduced into the cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's recommendation. The siRNAs targeting sense sequences were as follows: DRAM1-1, 5′-CCACGAUGUAUACAAGAUA-3′; DRAM1-2, 5′-CCACGAAAUCAAUGGUGA-3′; Negative control, 5′-UAAGGCUAUGAAGAGAUAC-3′. The knockdown efficiency of specific proteins was determined by western blot analysis 48 h after shRNA or siRNA treatment.
Short hairpin RNA (shRNA) lentiviruses production and transduction
A shRNA against DRAM1 was cloned into the pLKO.1 vector according to the manufacturer's protocol (Addgene, Inc., Cambridge, MA, USA). pLKO.1, scrambled shRNA (negative control), pMD2.G (used for viruspackaging) and psPAX2 (used for virus packaging) were purchased from Addgene, Inc. All constructs were verifed by sequencing. Lentiviruses were produced by co-transfecting 293FT cells (Invitrogen; Thermo Fisher Scientifc, Inc.) in 10-cm dishes with 10 µg pLKO.1-shRNA, 2.5 µg pMD2.G and 7.5 µg psPAX2 using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). HepG2 cells were infected with DRAM1 shRNA. Virus-containing medium was removed after 16 h and replaced with fresh DMEM. After 24 h, the cells were used experimentally.
Transwell assay
The migration and invasion abilities were detected using Transwell chambers with 8 µm pore filters. The filters used for invasion assays were coated with 30 µl pre-diluted Matrigel (diluted at a ratio of 1:6 with serum-free DMEM medium). Cells (1×105 cell/well for migration assay, 2.5×105 cell/well for invasion assay) were appropriately added to the upper chambers. And then 0.6 ml DMEM medium supplemented with 10% FBS was added to the lower chambers as a chemoattractant. After incubation for 24 h, migrated or invaded cells were stained with DAPI. The cell number was counted under an optical microscope (5 different visual fields were randomly selected for each membrane and observed under ×600 magnification lenses). All experiments were performed at least in triplicate.
Western blot analysis
Protein was extracted from cells using cell lysis solutions containing protease inhibitors and phosphorylase inhibitors. Equal amounts of protein were fractionated on Tris-glycine SDS-polyacrylamide gels and subjected to electrophoresis and transferred to NC membranes. Membranes were blocked with 5% non-fat milk with Tris buffered Saline-Tween-20 (TBS-T), and then incubated with primary antibodies against DRAM1, P62, LC3, E-Cadherin, or Vimentin. After washing in TBST, membranes were incubated with fluorescent secondary antibodies. β-actin was used as the loading control. Immunoreactivity was detected using ODYSSEY INFRARED IMAGER (Li-COR Biosciences, Nebraska, NE, USA). The signal intensity of primary antibody binding was quantitatively analyzed with Image J software (W.S. Rasband, Image J; National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
The HepG2 cells were seeded onto cover glass in 24 well plates and cultured to the appropriate confluency. Thereafter, cells were washed with phosphate-buffered saline (PBS) for 5 min ×3 times. Then cells were treated with precooled alcohol for 15 min before blocked in PBS containing 1% BSA and 0.1% Triton X-100 for 1 h at room temperature. Then the cells were incubated with primary antibody overnight at 4°C. After washed 3 times with PBS for 10 min, the cells were incubated with Cy3-conjugated donkey anti-rabbit IgG for 1 h at room temperature. After another 10 min × 3 times of washing with PBS, cells were incubated with DAPI for 10 min, and dehydrated in increasing grades of ethanol and cover-slipped with Fluoromount Aqueous Mounting Medium (Sigma F4680; Sigma-Aldrich; Merck KGaA). The slices were analyzed with a laser scanning confocal unit (Zeiss LSM 710; Carl Zeiss, Jena, Germany).
In vivo tumor growth analysis
The control shRNA-transfected cells and DRAM1 shRNA-transfected cells (1×107) were intravenously injected into the tail vein of 6-week-old female athymic nude mice (Shanghai SLAC Laboratory Animal Co. Ltd., Shanghai, China). At 4 weeks later, the mice were anesthetized and photographed. After the mice were sacrificed, the livers were removed and photographed. All animal procedures were approved and monitored by the local Animal Care and Use Committee in Soochow University (License no. Syxk; Su-0062).
Statistical analysis
All data were presented as means ± SEM. Data were subjected to one-way ANOVA using the GraphPad Prism software statistical package (GraphPad Software; GraphPad Software, Inc., La Jolla, CA, USA). When a significant group effect was found, post hoc comparisons were performed using the Student-Newman-Keuls test to examine special group differences. Independent group t-tests were used for comparing two groups. Significant differences with P<0.05, 0.01, and 0.001 are indicated by *, **, ***, respectively. All calculations were performed using the 14.0 SPSS software package (SPSS, Inc., Chicago, IL, USA).
Results
DRAM1 knockdown inhibits the invasion and metastasis of HepG2 cells in vivo and in vitro
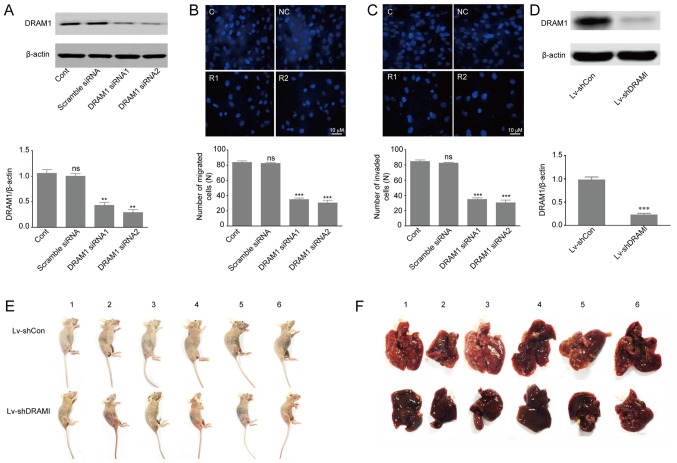
Previous studies reported that high expression of DRAM1 indicated poor prognosis in GBM tumors, and that inhibition of DRAM1 expression reduced invasion and metastasis of GSCs (14,25). In order to further investigate the effects of DRAM1 on invasion and metastasis of HepG2 cells, we designed two different DRAM1 siRNA molecules (DRAM1 siRNA1, DRAM1 siRNA2) to knockdown DRAM1 expression. HepG2 cells were transfected with scramble siRNA, DRAM1 siRNA1 or DRAM1 siRNA2 at the concentration of 80 nM for 48 h. Western blot analysis of DRAM1 protein levels showed 57 and 71% of silence efficiency with DRAM1 siRNA1 and DRAM1 siRNA2, respectively (Fig. 1A). Transwell chambers for migratory and invasive culture system were used to detect the migration and invasion abilities of HepG2 cells after DRAM1 was knocked down. The results showed that knockdown of DRAM1 significantly reduced the number of migrated (Fig. 1B) and invaded (Fig. 1C) cells, which indicated that DRAM1 knockdown inhibited migration and invasion abilities of HepG2 cells. Moreover, we transduced HepG2 cells with vectors expressing shRNAs against DRAM1 or the scramble shRNA (Fig. 1D) and grafted these cells into nude mice by tail vein injection (1×107 cells per nude mouse). 4 weeks later the DRAM1 knockdown cells exhibited slower growth and lower metastasis compared to the control shRNA-transfected cells (Fig. 1E and F). Collectively, these results suggested that knockdown of DRAM1 inhibited the migration and invasion abilities of HepG2 cells both in vitro and in vivo.
Figure 1.
Knockdown of DRAM1 inhibited invasion and metastasis of HepG2 cells in vivo and in vitro. (A) Knockdown efficiency of DRAM1 in HepG2 cells. Transient transfection of siRNAs was applied to knockdown DRAM1 expression for 48 h. The protein levels of DRAM1 were detected with Western blotting. β-actin protein was used as a loading control. Quantitative analysis was performed with Image J. (B) Migration and (C) invasion assay of HepG2 cells with or without DRAM1 siRNA. Invasion assay was performed with transwell-inserts coated with Matrigel. Images were taken with a microscope (magnification, ×600). Values were means ± SEM from 3 independent experiments. **P<0.01; ***P<0.001; ns, P>0.05 vs. control group. (D) Lentivirus-mediated shRNA decreased DRAM1 expression in HepG2 cells. Analysis of DRAM1 knockdown efficiency in HepG2 cells. Values were means ± SD from 3 independent experiments. ***P<0.001, compared with Lv-shCon. (E) In vivo tumor metastasis assay of DRAM1 knockdown cells. HepG2 cells were intravenously injected into the tail vein of nude mice for 4 weeks. (F) The mice were sacrificed and examined for tumor metastases. DRAM1, DNA-damage regulated autophagy modulator 1; SD, standard deviation; SEM, standard error of the mean.
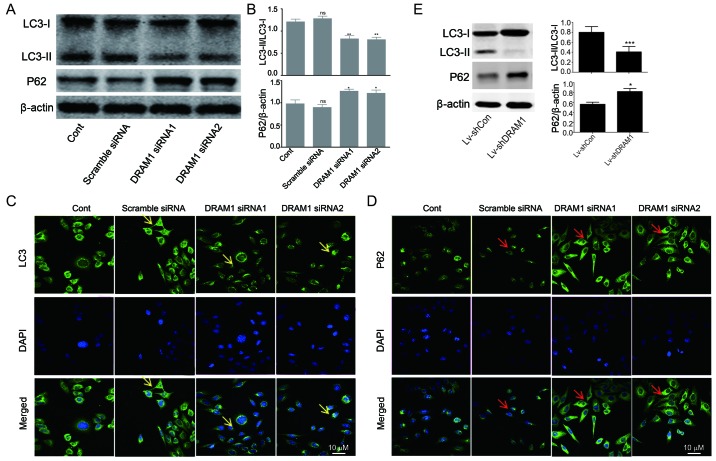
DRAM1 knockdown inhibits autophagy in HepG2 cells
In order to investigate the mechanisms underlying the inhibitory effects of DRAM1 knockdown on cell invasion and metastasis, the levels of autophagy-related proteins were detected. As shown in Fig. 2A and B, decreased transformations of LC3I to LC3II as well as increased expressions of p62 were observed in DRAM1 siRNA groups, indicating that autophagy was inhibited by DRAM1 knockdown (Fig. 2A and B). We next performed immunofluorescence to further detect the distribution of LC3 and p62 in HepG2 cells. In consistent with Western blot analysis, the results showed decreased distribution of LC3II and increased expression of p62 in the cytoplasm in DRAM1 knockdown cells (Fig. 2C and D), which suggested that DRAM1 knockdown inhibited cell autophagy. Also, similar result was observed in knockdown cells by shDRAM1 (Fig. 2E).
Figure 2.
Knockdown of DRAM1 inhibited autophagy in HepG2 cells. (A and B) Western blot analysis of autophagy related protein LC3I, LC3II and p62 in HepG2 cells, in response to knockdown of DRAM1. (C and D) Immunofluorescence analysis of LC3II and p62 in HepG2 cells, in response to knockdown of DRAM1. Values are means ± SEM from 3 independent experiments. *P<0.05; **P<0.01; ns, P>0.05 vs. control group. LC3 fluorescence signal: Yellow arrow; p62 fluorescence signal: Red arrow (E) HepG2 cells were transduced with lentiviruses expressing a scramble oligo and a sequence targeting DRAM1 (shDRAM1). Western blot analysis of autophagy related protein LC3I, LC3II and p62 in HepG2 cells, in response to knockdown of DRAM1. Values are means ± SEM from 3 independent experiments. *P<0.05; ***P<0.001. DRAM1, DNA-damage regulated autophagy modulator 1; SEM, standard error of the mean.
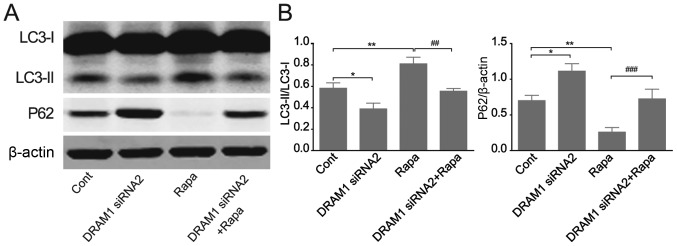
DRAM1 knockdown abrogates RAPA-induced autophagy in HepG2 cells
We continued to verify the effects of DRAM1 knockdown on cell autophagy by applying RAPA, an autophagy inducer. HepG2 cells were transfected with DRAM1 siRNAs for 48 h with or without RAPA treatment and autophagy related protein levels were detected by Western blots. The results showed that the autophagy induced by RAPA stimulation was inhibited by DRAM1 knockdown. RAPA treatment caused an increased transformation of LC3I to LC3II and a reduced expression of p62 (Fig. 3A and B), and the stimulatory effects of RAPA on cell autophagy were obviously inhibited when DRAM1 was knocked down. These results further verified the inhibitory potential of DRAM1 knockdown on cell autophagy.
Figure 3.
DRAM1 knockdown abrogates RAPA-induced autophagy in HepG2 cells. HepG2 cells treated with 200 nM RAPA for 12 h. (A and B) Western blot analysis of the autophagy related protein. Values were means ± SEM from 3 independent experiments. *P<0.05; **P<0.01 vs. control group, ##P<0.01; ###P<0.001 vs. RAPA. DRAM1, DNA-damage regulated autophagy modulator 1; SEM, standard error of the mean.
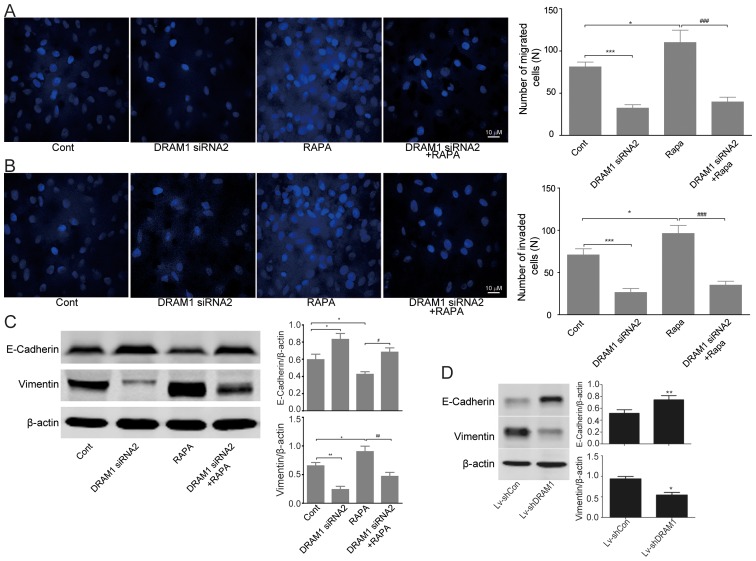
Invasion and migration of HepG2 cells were inhibited through autophagy-EMT pathway when DRAM1 was knocked down
We next set out to discover the mechanisms underlying the inhibitory effects of DRAM1 knockdown on the cell invasion and metastasis. Transwell chamber migratory and invasive culture systems were adopted to detect the migratory and invasive ability of HepG2 cells after DRAM1 was knocked down. As showed in Fig. 4A and B, cell invasion and metastasis were inhibited after DRAM1 knockdown. Moreover, the stimulatory effects of RAPA on cell invasion and metastasis were also obviously rescued by DRAM1 knockdown (Fig. 4A and B).
Figure 4.
DRAM1 knockdown inhibits invasion and migration of HepG2 cells. (A and B) Migration and invasion assay of HepG2 cells with or without DRAM1 siRNA. Invasion assay was performed with transwell-inserts coated with Matrigel. Images were taken with a microscope (magnification, ×600), *P<0.05; ***P<0.001 vs. control; ###P<0.001 vs. RAPA. (C) Western blot analysis of E-cadherin, and vimentin in HepG2 cells transfected with DRAM1 siRNA. Values were means ± SEM from 3 independent experiments. *P<0.05; **P<0.01 vs. control group; #P<0.05; ##P<0.01 vs. RAPA. (D) In vivo tumor metastasis assay of DRAM1 knockdown cells. HepG2 cells were intravenously injected into the tail vein of nude mice for 4 weeks. The mice were sacrificed and their livers were harvested. Liver specimens were examined for the protein levels of E-cadherin and Vimentin by western blotting. Values were means ± SEM from 3 independent experiments. *P<0.05; **P<0.01 vs. control group. DRAM1, DNA-damage regulated autophagy modulator 1; SEM, standard error of the mean.
Since we have observed significant inhibition of DRAM1 knockdown on the invasion and metastasis abilities of HepG2 cells both in vitro and in vivo, we next detected the expression of EMT related proteins by western blots. The results showed an increased expression of E-cadherin and a decreased expression of vimentin in DRAM1 knockdown HepG2 cells, which indicated an inhibitory effect of DRAM1 knockdown on EMT. On the contrary, RAPA treatment caused a decreased expression of E-cadherin and an increased expression of vimentin, both of which were significantly reversed by the combined treatment of DRAM1 siRNA (Fig. 4C). Similar result was obtained by shDRAM1 (Fig. 4D). Collectively, these results suggested that the inhibitory effects of DRAM1 knockdown on the invasion and migration abilities of HepG2 cells likely worked through the autophagy-EMT pathway.
Discussion
HepG2 cells were shown to be a HB cell line, which did not affected the outcomes of the study. HB is the most common liver malignant tumor diagnosed by the age of 4 years, accounting for 80% of liver cancers in children under the age of 15 years, among patients with localized HB, surgical resection is a common treatment option (26,27). Liver transplantation is considered to be the only curative therapy, however, the majority of patients with advanced HB are not suitable for transplantation (1). Therefore, a more comprehensive understanding of the regulatory mechanisms of HB invasion and migration is beneficial for the survival improvement of HB patients. Previous reports have shown that DRAM1 played an important role in the migration and invasion of GSCs (14). Our previous results also revealed that DRAM1 was highly expressed in intestinal cancer. However, little is known about the precise contribution of DRAM1 in the migration and invasion of HB cells. In this study, we found that DRAM1 knockdown could inhibit the migration and invasion of HepG2 cells in the setting of transwell assay, which was further confirmed by animal experiments.
EMT is a natural process in which epithelial cells obtain characteristics of mesenchymal cells, which is recognized as an important procedure in cell invasion and metastasis. EMT happens in normal cellular processes, however, it may also be exploited by cancer cells, which triggers them to invade and form metastases at distant sites. EMT was reported to motivate the expression of mesenchymal markers in epithelial cells (28). The molecular regulators involved in EMT include specific molecules which distinguish epithelial cells from mesenchymal cells, and other constituents that can drive cells towards the targeted sites. Cells undergoing EMT typically show an increase in the expression of vimentin, fibronectin and integrin αvβ6, as well as a decrease in the expression of E-cadherin and cytokeratins. In our present study, with the application of rapamycin, the expression of vimentin was significantly upregulated while E-cadherin was downregulated in HepG2 cells. Meanwhile, the concomitant knockdown of DRAM1 reversed the alterations of these EMT markers. Our results suggested that DRAM1 knockdown regulated EMT thus inhibiting the invasion and metastasis of HepG2 cells.
Growing evidence has suggested that autophagy is closely related to human physiology and diseases including cancers. Pervious researches revealed that DRAM1 was a lysosomal protein associated with cell autophagy (13,29,30). Therefore, we speculated that autophagy may be involved in the inhibition of DRAM1 knockdown on the migration and invasion of HB cells. The results in this study demonstrated that the elevation of LC3-II and the decline of P62 stimulated by rapamycin treatment were blocked by DRAM1 knockdown, which suggested that cell autophagy was inhibited after DRAM1 knockdown. However, it is still controversial on the function of autophagy in regulating cell migration and invasion. Robin and colleagues showed that autophagy inhibition significantly reduced the invasion of tumor cells (31). Zhan et al reported that autophagy induced by the activation of toll-like receptor 3 or 4 could enhance the production of various cytokines and thereby facilitating the migration and invasion of lung cancer cells (32). On the contrary, there were also several researches suggested that autophagy could inhibit tumor cell migration and invasion (22,33). In this study, we found that DRAM1 knockdown significantly decreased the levels of EMT markers and inhibited the migration and invasion of HepG2 cells, which could be reversed by rapamycin treatment. It needs to be clarified in future studies whether we could reverse the inhibition of migration and invasion in DRAM1-silenced HepG2 cells by activating autophagy.
In summary, DRAM1 played an important role in the regulation of HB migration and invasion. Our study demonstrated that DRAM1 knockdown inhibited cell invasion and migration by inhibiting the autophagy-EMT pathway, which could provide basic knowledge for the development of new therapies for HB in clinical practice.
Acknowledgements
Not applicable.
Funding
This work was supported by the Medicine and Technology Project For Youth Changshu (CSWSQ201707) and the National Natural Science Foundation of China (grant no. 81602613).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CC contributed to the idea conception, experimental work and manuscript preparation. QYL, HKC, PFW, ZYF, XMM and HRW contributed to the experimental work and manuscript preparation. GQZ guided the idea conception, experimental work and manuscript preparation. All authors reviewed the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avni FE, Massez A, Cassart M. Tumours of the fetal body: A review. Pediatr Radiol. 2009;39:1147–1157. doi: 10.1007/s00247-009-1160-6. [DOI] [PubMed] [Google Scholar]
- 3.Catanzarite V, Hilfiker M, Daneshmand S, Willert J. Prenatal diagnosis of fetal hepatoblastoma: Case report and review of the literature. J Ultrasound Med. 2008;27:1095–1098. doi: 10.7863/jum.2008.27.7.1095. [DOI] [PubMed] [Google Scholar]
- 4.Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. Beta-catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1578–G1585. doi: 10.1152/ajpgi.00359.2006. [DOI] [PubMed] [Google Scholar]
- 5.Monga SP. Role and regulation of β-catenin signaling during physiological liver growth. Gene Expr. 2014;16:51–62. doi: 10.3727/105221614X13919976902138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, Michalopoulos GK, Kaestner KH, Monga SP. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann W, Küchler J, Koch A, Friedrichs N, Waha A, Endl E, Czerwitzki J, Metzger D, Steiner S, Wurst P, et al. Activation of phosphatidylinositol-3′-kinase/AKT signaling is essential in hepatoblastoma survival. Clin Cancer Res. 2009;15:4538–4545. doi: 10.1158/1078-0432.CCR-08-2878. [DOI] [PubMed] [Google Scholar]
- 9.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 10.Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282:615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- 11.López-Terrada D, Cheung SW, Finegold MJ, Knowles BB. Hep G2 is a hepatoblastoma-derived cell line. Hum Pathol. 2009;40:1512–1515. doi: 10.1016/j.humpath.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA, Reddel RR, Freshney RI. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer. 2010;127:1–8. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 13.Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Galavotti S, Bartesaghi S, Faccenda D, Shaked-Rabi M, Sanzone S, McEvoy A, Dinsdale D, Condorelli F, Brandner S, Campanella M, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32:699–712. doi: 10.1038/onc.2012.111. [DOI] [PubMed] [Google Scholar]
- 15.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XD, Qi L, Wu JC, Qin ZH. DRAM1 regulates autophagy flux through lysosomes. PLoS One. 2013;8:e63245. doi: 10.1371/journal.pone.0063245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: A cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv Q, Wang W, Xue J, Hua F, Mu R, Lin H, Yan J, Lv X, Chen X, Hu ZW. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res. 2012;72:3238–3250. doi: 10.1158/0008-5472.CAN-11-3832. [DOI] [PubMed] [Google Scholar]
- 21.Qiang L, Zhao B, Ming M, Wang N, He TC, Hwang S, Thorburn A, He YY. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc Natl Acad Sci USA. 2014;111:9241–9246. doi: 10.1073/pnas.1322913111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Catalano M, D'Alessandro G, Lepore F, Corazzari M, Caldarola S, Valacca C, Faienza F, Esposito V, Limatola C, Cecconi F, Di Bartolomeo S. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol Oncol. 2015;9:1612–1625. doi: 10.1016/j.molonc.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G, Li CX, Xia M, Ritter JK, Gehr TW, Boini K, Li PL. Enhanced epithelial-to-mesenchymal transition associated with lysosome dysfunction in podocytes: Role of p62/sequestosome 1 as a signaling hub. Cell Physiol Biochem. 2015;35:1773–1786. doi: 10.1159/000373989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gugnoni M, Sancisi V, Gandolfi G, Manzotti G, Ragazzi M, Giordano D, Tamagnini I, Tigano M, Frasoldati A, Piana S, Ciarrocchi A. Cadherin-6 promotes EMT and cancer metastasis by restraining autophagy. Oncogene. 2017;36:667–677. doi: 10.1038/onc.2016.237. [DOI] [PubMed] [Google Scholar]
- 25.Humbert M, Mueller C, Fey MF, Tschan MP. Inhibition of damage-regulated autophagy modulator-1 (DRAM-1): Impairs neutrophil differentiation of NB4 APL cells. Leuk Res. 2012;36:1552–1556. doi: 10.1016/j.leukres.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Herzog CE, Andrassy RJ, Eftekhari F. Childhood cancers: Hepatoblastoma. Oncologist. 2000;5:445–453. doi: 10.1634/theoncologist.5-6-445. [DOI] [PubMed] [Google Scholar]
- 27.Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology. 2003;38:560–566. doi: 10.1053/jhep.2003.50375. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crighton D, Wilkinson S, Ryan KM. DRAM links autophagy to p53 and programmed cell death. Autophagy. 2007;3:72–74. doi: 10.4161/auto.3438. [DOI] [PubMed] [Google Scholar]
- 30.Mah LY, O'Prey J, Baudot AD, Hoekstra A, Ryan KM. DRAM-1 encodes multiple isoforms that regulate autophagy. Autophagy. 2012;8:18–28. doi: 10.4161/auto.8.1.18077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macintosh RL, Timpson P, Thorburn J, Anderson KI, Thorburn A, Ryan KM. Ryan, Inhibition of autophagy impairs tumor cell invasion in an organotypic model. Cell Cycle. 2012;11:2022–2029. doi: 10.4161/cc.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan Z, Xie X, Cao H, Zhou X, Zhang XD, Fan H, Liu Z. Autophagy facilitates TLR4- and TLR3-triggered migration and invasion of lung cancer cells through the promotion of TRAF6 ubiquitination. Autophagy. 2014;10:257–268. doi: 10.4161/auto.27162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Xie F, Wang H, Liu Z, Liu X, Sun L, Niu Z. Ubenimex inhibits cell proliferation, migration and invasion in renal cell carcinoma: The effect is autophagy-associated. Oncol Rep. 2015;33:1372–1380. doi: 10.3892/or.2014.3693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.