Abstract
Epithelial-mesenchymal transition (EMT) serves an important role in the metastasis of prostate cancer. Juglone is a natural compound isolated from plants that is reported to possess potent cytotoxic properties. However, there are no studies on the anti-EMT effect of juglone in prostate cancer, or its potential underlying mechanisms of action. In the present study, the effect of juglone on the EMT of prostate cancer cells was investigated. Transwell assays were used to demonstrate that juglone inhibits the migration and invasion of the prostate cancer (PC) LNCaP and LNCaP-AI cell lines. Results from western blot analysis demonstrated that juglone increases the expression of the epithelial marker E-cadherin while decreasing the expression of mesenchymal markers (N-cadherin and Vimentin) in a dose-dependent manner. The data from the present study also revealed that juglone downregulates the expression of Snail, a repressor of E-cadherin and an inducer of EMT. Furthermore, juglone prevented inactivation of glycogen synthase kinase-3β (GSK-3β), an endogenous inhibitor of Snail in a dose-dependent manner. Lithium chloride (LiCl), a GSK-3β inhibitor, prevented juglone-mediated downregulation of Snail expression and upregulation of E-cadherin. In addition, phosphorylation and subsequent activation of protein kinase B (Akt), which is known to phosphorylate GSK-3β at serine 9 (Ser9), leading to its inhibition, were significantly decreased by juglone in LNCaP and LNCaP-AI cells. Inhibition of the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt pathway by LY294002 augmented juglone-mediated GSK-3β activity by inhibiting Ser9 phosphorylation. These findings indicated that juglone suppresses EMT via the Akt/GSK-3β/Snail pathway, consequently decreasing the invasiveness of PC cells.
Keywords: juglone, prostate cancer, epithelial-mesenchymal transition, metastasis, protein kinase B, glycogen synthase kinase-3β, Snail
Introduction
Prostate cancer (PC) is the second leading cause of cancer-associated mortality among males in Western countries. The principal difficulty with PC is its metastasis to the bone, which results in significant morbidity (1,2). The 5-year survival rate for PC patients with skeletal involvement is <1% (3). Therefore, there is an urgent requirement for novel PC treatments.
Tumor metastasis is a complicated process that involves cellular detachment from the local microenvironment and degradation of the surrounding extracellular matrix and relocation to a distal site. All these processes are regulated by multiple factors and molecular pathways. Epithelial-mesenchymal transition (EMT) is a differentiation program by which cells switch from an epithelial to a mesenchymal phenotype; the latter is characterized by an increased ability to migrate and increased invasiveness and is associated with metastasis (4). EMT events have been observed following androgen withdrawal therapy in PC, leading to increased migration and invasiveness of tumor cells and ultimately, a significant metastatic burden (5). Therefore, the ability to target primary tumor cells that are more likely to undergo EMT would benefit patients at risk of disease progression. A group of transcription factors, including Snail, Slug, Zinc finger E-box-binding homeobox 1 (ZEB1) and Twist, have been revealed to serve a crucial role in modulating E-cadherin expression and ultimately in promoting EMT (6,7). Furthermore, protein kinase B (Akt) and glycogen synthase kinase-3β (GSK-3β) have been linked to EMT in cancer. Akt activation leads to increased phosphorylation of the 9th residue of GSK3β, which facilitates GSK3β ubiquitination and prevents its participation in the degradation of Snail (8,9).
Juglone (5-hydroxy-1, 4-naphthoquinone) is a natural compound that is isolated from plants and has been reported to possess potent cytotoxic properties in vitro against human leukemia, cervical carcinoma and pancreatic cancer cell lines (10–12). In an earlier study, the authors of the present study reported the effect of juglone on ovarian cancer cells and concluded that juglone likely induced apoptosis via the mitochondrial pathway and reduced cell invasiveness by decreasing matrix metalloproteinase expression (13). In the present study, the effects of juglone on PC cell invasion and EMT and the underlying mechanisms of action were investigated.
Materials and methods
Cell culture
The androgen-dependent human LNCaP PC cells, were obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were maintained in phenol red-containing RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The castration-resistant PC LNCaP-AI cell line was established by culturing LNCaP cells in phenol red-free RPMI-1640 medium supplemented with 10% dextran/charcoal-treated FBS (DCC-FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) for 6 months (14). All cells were cultured at 37°C in a humidified environment of 5% CO2 in air, and the medium was replenished twice weekly.
MTT assay
Cell viability was assessed by measuring the cells' ability to metabolize MTT. Cells (2×104/well) were seeded in 96-well plates and cultured at 37°C with 5% CO2 for 24 h. Juglone (97% purity; Sigma-Aldrich; Merck KgaA, Darmstadt, Germany) was added at a final concentration of 0, 6.25, 12.5, 25, 50 or 100 µM. Each sample was assayed in triplicate. Following incubation for 24 h, 20 µl MTT solution was added to each well and the cells were incubated for another 4 h. The resulting formazan crystals were dissolved with dimethyl sulfoxide and the absorbance was read at 570 nm in an ELISA reader (Thermo Fisher Scientific, Inc.). The percentage of cell viability was calculated as follows: Cell viability (%) = (OD of treatment/OD of control) ×100.
Migration and invasion
Cell migration and invasion assays were performed in 24-well plates with 8-µm pore size filters (Corning Incorporated, Corning, NY, USA). Following pre-treatment with juglone for 24 h, the LNCaP and LNCaP-AI cells were suspended in RPMI-1640 medium containing 0.1% FBS and 0.1% DCC-FBS, respectively. For the migration assays, the cells (5×104/well; 200 µl) were seeded with the medium into the upper chamber on top of an uncoated membrane. For the invasion assays, the cells (1×105/well, 200 µl) were seeded into the upper chamber on top of a Matrigel-coated membrane. The bottom chambers were filled with 600 µl RPMI-1640 medium containing 10% FBS and 10% DCC-FBS for the LNCaP and LNCaP-AI assays, respectively. Following incubation for 24 h, the remaining cells in the upper chamber were gently removed with a cotton swab. The cells that had moved to the bottom chamber were stained with 0.1% crystal violet for 20 min at room temperature and observed using light microscopy (magnification, ×200; Olympus Corporation, Tokyo, Japan).
Western blotting
Cells were washed twice with phosphate-buffered saline and then lysed with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Nanjing, Jiangsu, China) containing a cocktail of protease inhibitors (Roche Diagnostics GmbH, Mannheim, Germany). To extract specific protein compartments, a Compartmental Protein Extraction kit (EMD Millipore, Billerica, MA, USA) was used according to the manufacturer's protocol. Protein concentration was determined using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, Nanjin, Jiangsu, China). Equal amounts of protein (30 µg) were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (EMD Millipore) and then blocked with 5% fat-free milk in PBS containing 0.05% Tween-20 at room temperature for 2 h. The membranes were incubated with rabbit monoclonal antibodies against human p-Akt (Ser 473; cat. no. 4060), p-GSK-3β (Ser 9; cat. no. 9322), E-cadherin (cat. no. 3195), N-cadherin (cat. no. 13116), Vimentin (cat. no. 5741) and Snail (cat. no. 3879) all at a dilution of 1:1,000 (Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. β-actin was used as a loading control (1:2,000; cat. no. 4970; Cell Signaling Technology, Inc.). Primary antibody binding was detected using a horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:3,000; cat. no. A0208; Beyotime Institute of Biotechnology) and was visualized using an enhanced chemiluminescence detection system (Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
The data are presented as the mean ± standard deviation. One-way analysis of variance, followed by Holm's method was used to compare significant differences in the means among all treatment groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Suppressive effect of juglone on viability of LNCaP and LNCaP-AI cells
LNCaP and LNCaP-AI cells were treated with increasing doses of juglone for 24 h and examined for cell viability using the MTT assay. As revealed in Fig. 1, juglone treatment markedly decreased cell viability in a dose-dependent manner. The IC50 dose of juglone at 24 h was 32.2 µM for LNCaP cells and 43.1 µM for LNCaP-AI cells (data not shown). Based on the IC50 of juglone, subsequent experiments were performed using 12.5 or 25 µM of juglone in order to reduce the influence of drug-induced apoptosis on the experimental results.
Figure 1.

Juglone decreases LNCaP and LNCaP-AI cell viability. MTT assay was used to assess LNCaP and LNCaP-AI cell viability with increasing doses of juglone for 24 h. *P<0.05, **P<0.01 vs. the without juglone treated cells as control group. Values shown are the mean ± standard deviation of three experiments.
Juglone inhibits the migration and invasion of LNCaP and LNCaP-AI cells
To investigate whether juglone inhibits the motility of LNCaP and LNCaP-AI cells, Transwell assays were performed with un-coated and Matrigel-coated membranes to characterize juglone's effect on cell migration and invasion, respectively. The cells were treated with 0, 12.5 or 25 µM juglone for 24 h and then assessed for their ability to migrate through the membranes. The Transwell chamber migration assays revealed that juglone inhibited LNCaP and LNCaP-AI cell migration (Fig. 2A). Similarly, juglone inhibited cell invasion compared with that of control cells without juglone treatment (Fig. 2B). The results of these Transwell assays demonstrated that juglone suppresses the migration and invasion of LNCaP and LNCaP-AI cells.
Figure 2.
Juglone inhibits the migration and invasion of LNCaP and LNCaP-AI cells. Effect on (A) migration and (B) invasion of LNCaP and LNCaP-AI cells after 24h treatment with 0, 12.5 or 25 µM juglone. *P<0.05, **P<0.01 vs. without juglone treated cells as the control group. Values shown are the mean ± standard deviation of three experiments.
Juglone alters the expression of EMT markers in LNCaP and LNCaP-AI cells
To determine whether juglone inhibits EMT, western blot analysis was used to investigate the effect of 24 h treatment with juglone (0, 12.5 or 25 µM) on the expression of the EMT markers E-cadherin, N-cadherin and Vimentin in LNCaP and LNCaP-AI cells. As demonstrated in Fig. 3, E-cadherin expression increased significantly, whereas the expression of N-cadherin and vimentin decreased in juglone-treated LNCaP and LNCaP-AI cells. Collectively, these observations suggested that inhibition of migration and invasion by juglone in LNCaP and LNCaP-AI cells may be associated with inhibition of EMT.
Figure 3.
Juglone suppresses the EMT of LNCaP and LNCaP-AI cells. (A) The expression of the epithelial marker E-cadherin and the mesenchymal markers N-cadherin and vimentin after 24 h treatment with juglone (0, 12.5 or 25 µM) is shown. Statistical analysis of the western blot results in (B) LNCaP or (C) LNCaP-AI cells is also shown. *P<0.05, **P<0.01 vs. the control group. Values shown are the mean ± standard deviation of three experiments.
Juglone induces downregulation of Snail via GSK-3β activation in LNCaP and LNCaP-AI cells
LNCaP and LNCaP-AI cells were treated with various concentrations of juglone (0, 12.5 or 25 µM) for 24 h and the expression of Snail and phosphorylated (p-)GSK-3β was assessed. As demonstrated in Fig. 4A, juglone significantly inhibited the expression of Snail and p-GSK-3β in a dose-dependent manner. Therefore, the effects of juglone on the association between GSK-3β and Snail were further investigated. LNCaP and LNCaP-AI cells were pretreated with LiCl, a GSK-3β inhibitor, for 2 h prior to the addition of juglone. As demonstrated in Fig. 4B, treatment with lithium chloride (LiCl) significantly attenuated the inhibitory effect of juglone on Snail expression, leading to downregulation of E-cadherin. Taken together, these data suggested that treating LNCaP and LNCaP-AI cells with juglone causes activation of GSK-3β, leading to the degradation of Snail and upregulation of E-cadherin protein expression.
Figure 4.
GSK-3β regulates the expression of Snail in juglone-treated LNCaP and LNCaP-AI cells. (A) Expression of p-GSK-3β and Snail in LNCaP and LNCaP-AI cells treated with juglone (0, 12.5 or 25 µM) for 24 h. (B) Expression of E-cadherin and Snail following juglone treatment for 24 h (25 µM) with or without LiCl pre-treatment (20 mM). *P<0.05, **P<0.01 vs. the cells without juglone treatment as the control group. #P<0.05, ##P<0.01 vs. cells treated with juglone. Values are the mean ± standard deviation of three experiments. p-, phosphorylated-; GSK-3β, glycogen synthase kinase-3β; Akt, protein kinase B; LiCl, lithium chloride.
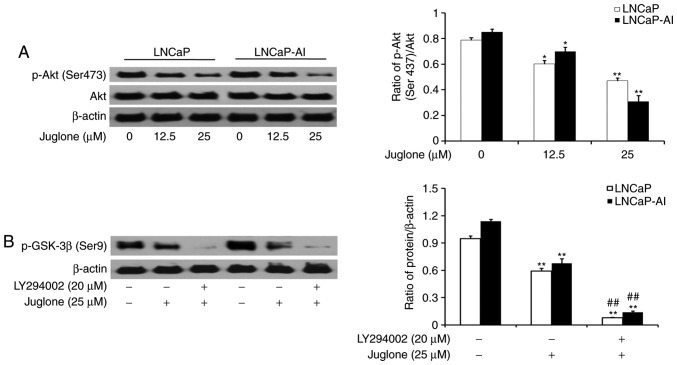
Juglone modulates GSK-3β phosphorylation via the PI3K/Akt pathway in LNCaP and LNCaP-AI cells
LNCaP and LNCaP-AI cells were treated with various concentrations of juglone (0, 12.5 or 25 µM) for 24 h and the phosphorylation of Akt was assessed by western bolt analysis. The results revealed that juglone inhibited the expression of p-Akt in a dose-dependent manner (Fig. 5A). To further investigate the role of the PI3K/Akt pathway in juglone-mediated regulation of p-GSK-3β expression, LY294002, a specific inhibitor of PI3K, was applied to LNCaP and LNCaP-AI cells. As revealed in Fig. 5B, LY294002 further enhanced the juglone-induced inhibition of GSK3-β phosphorylation. The results demonstrated that juglone inhibits p-Akt expression, thereby enhancing GSK-3β activity.
Figure 5.
Juglone inhibits EMT via the Akt/GSK-3β signaling pathway in LNCaP and LNCaP-AI cells. (A) Expression of p-Akt in LNCaP and LNCaP-AI cells treated with juglone (0, 12.5 or 25 µM) for 24 h. (B) Expression of p-GSK-3β following juglone treatment for 24 h (25 µM) ± LY294002 pre-treatment (20 µM). *P<0.05, **P<0.01 vs. cell without juglone treatment as the control group. ##P<0.01 vs. cells treated with juglone. Values shown are the mean ± standard deviation of three experiments. p-, phosphorylated-; GSK-3β, glycogen synthase kinase-3β; Akt, protein kinase B.
Discussion
Metastasis is the leading cause of cancer-associated morbidity and mortality (15). Therefore, it is important to seek effective drugs to suppress cancer metastasis. EMT is one of the early steps in the metastatic process and is an important mechanism contributing toward the increased invasive and metastatic potential of cancer cells. An increasing number of studies have reported that EMT serves a role in metastatic progression of PC and treatment resistance (16,17). Previous data have indicated that juglone may be a useful anti-proliferative and anti-invasive agent for certain types of cancer (10–12). In this study, it was demonstrated that juglone can modulate the migration and invasion of PC cells. It was further revealed that juglone suppresses mesenchymal markers (vimentin and N-cadherin) and promotes the expression of the epithelial marker E-cadherin in LNCaP and LNCaP-AI cells. Notably, juglone-induced inhibition of E-cadherin expression was increased in LNCaP-AI cells. LNCaP-AI cells have been revealed to have a more aggressive phenotype than LNCaP cells (18), and androgen independence has been linked to cell growth and EMT (5); therefore, it is imperative for therapeutic agents to be active in androgen-dependent and independent PC cells. The results of the present study suggested that juglone inhibits EMT in prostate cancer cells.
Snail, a zinc finger protein, is a crucial transcription factor in the regulation of EMT through its role in downregulating E-cadherin, a hallmark of the EMT process (6). The data from the present study demonstrated that juglone treatment results in downregulation of Snail in LNCaP and LNCaP-AI cells. In particular, low concentrations of juglone (12.5 µM) could inhibit Snail expression in LNCaP-AI cells with concomitant upregulation of E-cadherin. It is known that Snail is phosphorylated by GSK-3β, which induces its degradation (8,9). The present study revealed significant changes in p-GSK-3β and Snail protein levels following treatment of LNCaP and LNCaP-AI cells with juglone. To confirm that the GSK-3β signaling pathway was involved in juglone-induced inhibition of EMT in LNCaP and LNCaP-AI cells, GSK-3β activity was blocked using LiCl. It was revealed that the suppression of GSK-3β activity by LiCl prevented juglone-induced E-cadherin expression and promoted Snail expression. Taken together, these data indicated that GSK-3β activation serves an important role in the progression of EMT and that the effect of juglone is GSK-3β/Snail-dependent.
Previous studies have reported the role of Akt/GSK-3β in regulating metastasis of various types of tumors via modulation of EMT, including PC (8,9,19). It is known that the PI3K/Akt pathway is involved in the phosphorylation of the Ser9 in GSK-3β, inhibiting its activity (8,9). The present study therefore investigated whether this pathway is involved in the juglone-mediated effects on p-GSK-3β expression. It was revealed that the expression of p-Akt and p-GSK-3β decreased with juglone treatment. It was also demonstrated that inhibition of the PI3K/Akt pathway with LY294002 further decreased p-GSK-3β expression, suggesting that juglone acted on GSK-3β via the PI3K/Akt pathway.
In summary, the present study described a novel role for juglone in the inhibition of metastasis in PC cells. The results of the present study demonstrated that juglone inhibits the migration and invasion of PC cells by suppressing EMT. In addition, in LNCaP and LNCaP-AI cells, juglone affected the expression of proteins involved in EMT regulation through the Akt/GSK-3β/Snail signaling pathway. Taken together, the results of the present study demonstrated that juglone is a promising agent for PC therapy.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- EMT
epithelial-mesenchymal transition
- GSK-3β
glycogen synthase kinase-3β
- PC
prostate cancer
- FBS
fetal bovine serum
- PI3K
phosphatidylinositol-3-kinase
Funding
The present study was supported by grants from the China National Natural Science Foundation (grant no. 81202031), the Scientific Research Project of Jilin Province Science and Technology Department (grant no. 20160204033YY), the Technical Innovation Project of Jilin Province Health Department (grant no. 2016J103), the Technical Innovation Project of Jilin Science and Technology Bureau (grant no. 20163306) and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (grant no. 201713743006).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
LW conceived and designed the study. FF and SC contributed toward the data acquisition and analysis and drafted the manuscript. JM and JC were involved in data acquisition. QL and GM contributed toward data acquisition and revised the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ibrahim T, Flamini E, Mercatali L, Sacanna E, Serra P, Amadori D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116:1406–1418. doi: 10.1002/cncr.24896. [DOI] [PubMed] [Google Scholar]
- 2.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez Uzquiza A, Lopez Haber C, Jernigan DL, Fatatis A, Kazanietz MG. PKCε is an essential mediator of prostate cancer bone metastasis. Mol Cancer Res. 2015;13:1336–1346. doi: 10.1158/1541-7786.MCR-15-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Shiota M, Itsumi M, Takeuchi A, Imada K, Yokomizo A, Kuruma H, Inokuchi J, Tatsugami K, Uchiumi T, Oda Y, Naito S. Crosstalk between epithelial-mesenchymal transition and castration resistance mediated by Twist1/AR signaling in prostate cancer. Endocr Relat Cancer. 2015;22:889–900. doi: 10.1530/ERC-15-0225. [DOI] [PubMed] [Google Scholar]
- 6.Kaufhold K, Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: A target for therapeutic intervention. J Exp Clin Cancer Res. 2014;33:62. doi: 10.1186/s13046-014-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banyard J, Bielenberg DR. The role of EMT and MET in cancer dissemination. Connect Tissue Res. 2015;56:403–413. doi: 10.3109/03008207.2015.1060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Z, Guo Y, Yang Y, Kan J, Dai S, Helian M, Li B, Xu J, Liu C. Nitidine chloride suppresses epithelial-to-mesenchymal transition in osteosarcoma cell migration and invasion through Akt/GSK-3β/Snail signaling pathway. Oncol Rep. 2016;36:1023–1029. doi: 10.3892/or.2016.4846. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8:e56664. doi: 10.1371/journal.pone.0056664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu HL, Yu XF, Qu SC, Qu XR, Jiang YF, Sui da Y. Juglone, from Juglans mandshruica Maxim, inhibits growth and induces apoptosis in human leukemia cell HL-60 through a reactive oxygen species dependent mechanism. Food Chem Toxicol. 2012;50:590–596. doi: 10.1016/j.fct.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Liu A, Li Y, Zhao X, Lv S, Zhu W, Jin Y. Anticancer activity and mechanism of juglone on human cervical carcinoma Hela cells. Can J Physiol Pharmacol. 2012;90:1553–1558. doi: 10.1139/y2012-134. [DOI] [PubMed] [Google Scholar]
- 12.Avci E, Arikoğiu H, Erkoç Kaya D. Investigation of juglone effects on metastasis and angiogenesis in pancreatic cancer cells. Gene. 2016;588:74–78. doi: 10.1016/j.gene.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Fang F, Qin Y, Qi L, Fang Q, Zhao L, Chen S, Li Q, Zhang D, Wang L. Juglone exerts antitumor effect in ovarian cancer cells. Iran J Basic Med Sci. 2015;18:544–548. [PMC free article] [PubMed] [Google Scholar]
- 14.Kong DY, Yang DM, Chen YY, Wang Y, Zhang JQ, Meng G, Fang F. Establishment of androgen independent human prostate cancer cell line model LNCaP-AI. J Jilin Med College. 2012;33:361–363. (In Chinese) [Google Scholar]
- 15.Hurst DR, Welch DR. Metastasis suppressor genes: At the interface between the environment and tumor cell growth. Int Rev Cell Mol Biol. 2011;286:107–180. doi: 10.1016/B978-0-12-385859-7.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bitting RL, Schaeffer D, Somarelli JA, Garcia-Blanco MA, Armstrong AJ. The role of epithelial plasticity in prostate cancer dissemination and treatment resistance. Cancer Metastasis Rev. 2014;33:441–468. doi: 10.1007/s10555-013-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marín-Aguilera M, Codony-Servat J, Reig Ò, Lozano JJ, Fernández PL, Pereira MV, Jiménez N, Donovan M, Puig P, Mengual L, et al. Epith elial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol Cancer Ther. 2014;13:1270–1284. doi: 10.1158/1535-7163.MCT-13-0775. [DOI] [PubMed] [Google Scholar]
- 18.Yu P, Duan X, Cheng Y, Liu C, Chen Y, Liu W, Yin B, Wang X, Tao Z. Androgen-independent LNCaP cells are a subline of LNCaP cells with a more aggressive phenotype and androgen suppresses their growth by inducing cell cycle arrest at the G1 phase. Int J Mol Med. 2017;40:1426–1434. doi: 10.3892/ijmm.2017.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu ZC, Wang HS, Zhang G, Liu H, Chen XH, Zhang F, Chen DY, Cai SH, Du J. AKT/GSK-3β regulates stability and transcription of Snail which is crucial for bFGF-induced epithelial-mesenchymal transition of prostate cancer cells. Biochim Biophys Acta. 2014;1840:3096–3105. doi: 10.1016/j.bbagen.2014.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.