Abstract
Lichen sclerosus is a chronic and inflammatory disease. Extensive studies have focused on the epidermis, with the dermis or epidermis-dermis receiving less attention. To investigate the role of galectin-7, a keratinocyte protein, in vulvar lichen sclerosus (VLS) and its potential effects on dermal fibroblasts, immunohistochemical staining was performed with VLS tissue samples and normal control samples. The expression of galectin-7 was determined by evaluating the galectin-7 integrated density analysis, and further assessed by western blot analysis. Dermal fibroblasts were isolated from the normal tissue of the female anogenital region following sexual plastic surgery. A cell viability assay was performed on isolated dermal fibroblast cells in the presence or absence of galectin-7. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed to determine the transcriptional level of collagen I and collagen III in the response to different doses of galectin-7. In the immunohistochemical analysis, galectin-7 demonstrated a significantly elevated level in VLS, compared to control tissues, which was confirmed by western blot analysis. In the analysis of primary dermal fibroblast cells, galectin-7 significantly inhibited the viability rate of fibroblasts in a dose-dependent manner. RT-qPCR data revealed that the transcription level of collagen I and collagen III were positively associated with the galectin-7 treatment concentration. The overexpression of galectin-7 is associated with the progression of VLS in the epidermis, a high concentration of galectin-7 inhibits the viability of the primary vulvar dermal fibroblasts, and stimulates the accumulation of collagen I and collagen III in dermal fibroblast cultures, thus galectin-7 may serve as a drug target during VLS progression.
Keywords: lichen sclerosus, galectin-7, fibroblast, immunohistochemical, western blot, cell culture
Introduction
Lichen sclerosus (LS) is a chronic relapsing disease that predominantly affects the female anogenital region. It may occur at any age, but the majority of cases are reported in postmenopausal women (1). It evokes multiple symptoms, including intense itching, pain, burning, stenosis and dysuria, affecting the quality of normal life and sexual activity (1). Currently, there is no cure for LS; instead treatment is primarily aimed at the inhibition of itching, and prevention of scar formation and vulvar anatomical deformities. Previous studies have indicated that numerous factors are involved in LS, including autoimmune disturbance (2), abnormal hormone secretion (3), genetic factors and inflammation (4), although there is no consensus regarding their relative importance. Since the malignant potential of invasive vulvar carcinoma for LS is ~4%, ~60% of invasive squamous cell carcinoma are identified in the adjacent site of the LS (5), and thus it is considered as a precancerous lesion. The typical pathological changes of LS include skin epidermis atrophy over keratosis, a dermal layer of connective tissue fibrosis forming a homogeneous zone and lymphocyte infiltration. Genes associated with LS have been identified; these have primarily focused on the dysregulated epidermis, for the hyalinization of collagen fibers in fibroblast hinders further investigation in the superior dermis. The keratinocyte protein, galectin-7, is an apoptosis-associated protein that serves multiple roles in epidermal differentiation, maturation and regeneration (6). Although galectin-7 is not a canonical secreted protein that is transported through the vesicular biosynthetic pathway, it has the ability to reach the cell surface in a fully folded form via an ‘unconventional protein secretion’ mechanism (7). Thus, we hypothesized that galectin-7 may be secreted by keratinocytes and infiltrate the superior dermis, influencing the cellular activity of the dermal fibroblasts.
Patients and methods
Patient selection
The present study was approved by the Ethics Committee of China Medical University (Shenyang, China) and written informed consent as obtained from all patients. The present study involved 5 outpatients, 10 in-patients and 10 healthy biopsy tissues obtained from sexual plastic surgery between September 2011 and December 2013. The mean age of the 15 patients with VLS was 55.7±4.3 years (range, 34–65 years). Patients were diagnosed via the evident VLS morphology and samples were further confirmed by histopathological analysis. All patients had no comorbidities or other medical history and were comparable.
Immunohistochemistry
A total of 15 VLS and 10 normal vulva skin 4% formalin-fixed (Boster Biological Technology, Pleasanton, CA, USA) at room temperature overnight. The paraffin-embedded 4 µm sections of biopsies were examined using an optical microscope (magnification, ×200) for galectin-7 expression. Rabbit monoclonal anti-galectin-7 antibody (1:400; cat. no. AB108623; Abcam, Cambridge, UK) was used as the primary antibody at 4°C overnight. Alkaline phosphatase-conjugated goat monoclonal anti-rabbit antibody (1:625; cat. no. ZB-2301; OriGene Technologies, Inc., Beijing, China) was used as the secondary antibody at room temperature for 1 h. All immunohistochemical staining were also performed on control biopsy samples. The specificity of the primary antibody was confirmed with the negative signal obtained by substituting normal rabbit serum (ZLI-9023; OriGene Technologies, Inc.) for the primary antibody in the protocol. The pixel of integrated density from 6 sections/sample was measured using ImageJ 1.46 (National Institutes of Health, Bethesda, MD, USA), and a median pixel was calculated.
Western blot analysis
The protein from 10 paired VLS and healthy vulvar tissue samples were extracted using radioimmunoprecipitation assay buffer (P0013B; Beyotime Institute of Biotechnology, Shanghai, China) and quantified using a bicinchoninic acid assay. Loading samples were diluted with protein lysis buffer to produce a final concentration of 5 µg/µl. SDS-PAGE (15%) was performed to separate the protein samples (14 µl) by size. Following membrane transfer to a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA), was blocked with blocking buffer containing 5% non-fat milk in PBS with Tween-20 at room temperature for 2 h. Blocked membranes were incubated overnight at 4°C with a rabbit monoclonal antibody against galectin-7 (1:1,000; cat. no. AB108623; Abcam) and rabbit monoclonal β-actin (1:1,000; cat. no. SC-47778; Santa Cruz Biotechnology Inc., Dallas, TX, USA) at a dilution of 1:800. Signals were detected using horseradish-peroxidase-anti-rabbit secondary antibodies (1:1,000; cat. no. A0277) and an electrochemiluminescence detection kit (both Beyotime Institute of Biotechnology). Images were captured using a Quantity One v.4.62 software (Bio-Rad Laboratories, Inc.).
Isolation and cultivation of primary dermal fibroblasts
Samples were placed in 70% ethanol for 10 sec and washed five times with PBS. Then, samples were placed in Dulbecco's modified Eagle medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with antibiotics. Additional fat tissues were discarded. Subsequently, the samples were cut into 2–3 mm pieces, placed in a petri dish and incubated at 37°C in a humidified atmosphere with 5% CO2 and the medium was replaced every 3 days. Fibroblast outgrowths were harvested by trypsinization and re-seeded (100–110 cells/ml) in a T10 flask in DMEM. Cells were allowed to reach 90% confluence prior to freezing (−80°C) or splitting for use in the galectin-7 experiments.
Cell viability assay
After three passages, fibroblasts were seeded into 96-well plates at a density of 5×103 cells/well. After 24 h, different concentrations of galectin-7 were added (0.2, 0.5, 1, 2.0 and 5.0 µg/ml) to the DMEM. Cells were cultivated for another 48 h, then 20 µl MTS was added to 100 µl medium and the plate was incubated at 37°C avoiding exposure to light for 4 h. Optical density values were obtained with a plate reader at 490 nm, data were corrected with blank controls.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
qPCR primers were designed as follows: Collagen I forward, 5′-CCCCCTCCCCAGCCACAAAG-3′ and reverse, 5′-TCTTGGTCGGTGGGTGACTCT-3′ (product size, 360 bp); collagen III forward, 5′-CCAAACTCTATCTGAA-3′ and reverse, 5′-GGACTCATAGAATACA-3′ (product size, 449 bp); β-actin forward, 5′-ATCTGGCACCACACTTCTACA-3′ and reverse, 5′-GTTTCGTGGATGCCACAGGCT-3′ (product size, 577 bp).
Briefly, cells were harvested following incubation with different galectin-7 concentrations. According to the results of cell viability assay, the cells were assigned as normal human fibroblasts group (N-HF), low concentration group (≤1 µg/ml, L-galectin) and high concentration group (>1 µg/ml, H-galectin). The RNA was isolated with TRIzol (Life Technologies; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol from duplicate or triplicate cell samples. cDNA synthesis was performed on 1 µg RNA with SuperScript™ III First-Strand system (Invitrogen; Thermo Fisher Scientific, Inc.) and oligo(dT) primers, according to the manufacturers protocols. The cDNA was diluted to a final volume of 200 and 1 µl was added to 500 nM of the forward and reverse primers in a final volume of 10 µl per PCR reaction. qPCR was performed with SYBR® Green (Takara Biotechnology Co., Ltd., Dalian, China) under the following conditions: Pre-denature 95°C for 1 min; then denature at 95°C for 30 sec; annealing 44°C for 30 sec; elongation 72°C for 1 min (35 cycles). Melting curve data was collected following amplification. The negative control was loaded with ddH2O. Data were analyzed using the ΔΔCq method (8). Charts were plotted using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA).
Statistical analysis
Statistical analyses were conducted using SPSS v.17.0 software (SPSS Inc., Chicago, IL, USA). Independent samples t-tests were used to compare the expression difference of Gelectin-7 between vulvar normal skin and VLS. Statistical comparisons among groups in the cell viability assay and qPCR were analyzed using one-way analysis of variance with the least significant difference post hoc test. All data are presented as the mean ± standard error of the mean. P<0.05 was considered to indicate a statistically significant difference.
Results
High galectin-7 level in VLS tissues
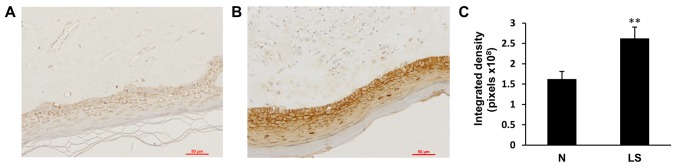
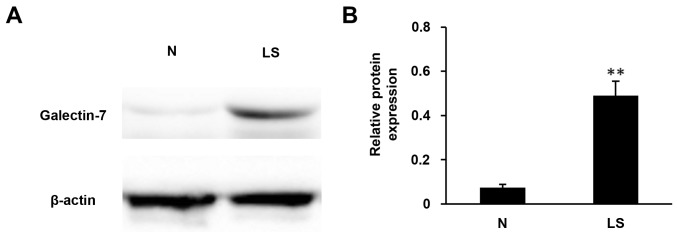
To determine the expression of galectin-7 in the epidermis, immunohistochemical staining of galectin-7 was performed in healthy control biopsy and VLS samples. In the healthy control skin, moderate expression of galectin-7 was detected in the inner part of epidermis, with an even distribution in the nucleus and cytoplasm. In contrast, VLS tissue exhibited significantly elevated immune expression of galectin-7 in the superficial epidermis (Fig. 1; P<0.01). To further confirm the immunohistochemical staining results, the protein content was extracted from tissues and western blotting was performed with the galectin-7 antibody. The results demonstrated >5-fold upregulation in galectin-7 expression in VLS tissue compared with healthy tissue (Fig. 2; P<0.01). These data indicated a positive association between galectin-7 expression and VLS.
Figure 1.
Representative image of immunohistochemical staining of galectin-7 in (A) normal vulvar tissue and (B) vulvar lichen sclerosus (magnification, ×200). (C) galectin-7 was significantly elevated in the vulvar lichen sclerosus samples compared with the normal vulvar tissues (**P<0.01). Date was presented as the mean ± standard deviation.
Figure 2.
Expression levels of galectin-7 were analyzed using western blot analysis. (A) Expression of galectin-7 in 10 pairs of lichen sclerosus (LS) and normal vulvar tissues (N). β-actin was used as an internal standard. (B) Galectin-7 was significantly elevated in the VLS samples compared to the normal vulvar tissues (**P<0.01). VLS, vulvar lichen sclerosus; N, normal vulvar tissues; LS, lichen sclerosus. Data was presented as the mean ± standard deviation.
Galectin-7 inhibits the viability of primary dermal fibroblast cells
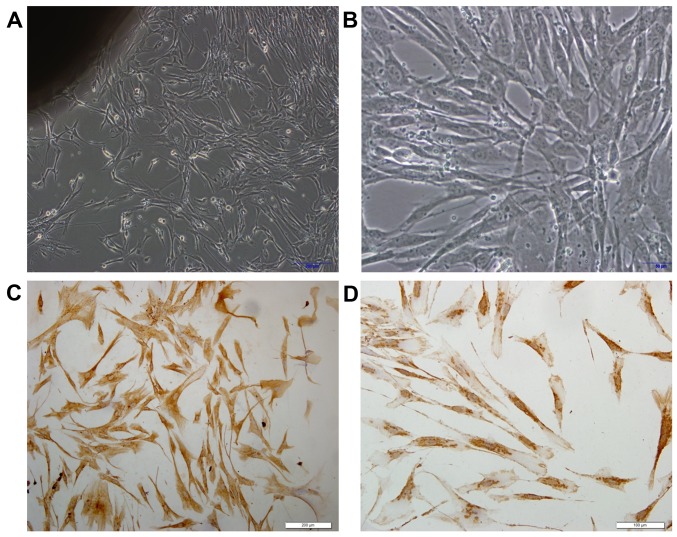
To determine whether galectin-7 influences vulvar dermis fibroblasts, primary dermal fibroblast cells were isolated from healthy control biopsy tissue. Phase contrast microscopy demonstrated spindle shaped fibroblasts, whereby the cells were bipolar and refractile, with enlarged nuclei (Fig. 3A and B). To confirm that the cells were fibroblasts, the typical mesoderm marker Vimentin, which is expressed in fibroblasts was used for staining (9). It was observed that all the isolated fibroblast cells exhibited Vimentin expression (Fig. 3C and D). Whether galectin-7 serves a role in the growth rate of the isolated primary dermal fibroblasts was then investigated. There were significant overall group effects in the result of the MTS cell viability assay [F(5,12)=7.631; P=0.002]. It revealed that galectin-7 exerted a significantly inhibitory effect on fibroblasts at concentrations >1 µg/ml. Compared with that of untreated fibroblasts, fibroblast viability was significantly impaired at the concentrations of 2 µg/ml (P<0.05) and 5 µg/ml (P<0.01) (0.82±0.03 vs. 0.63±0.81 and 0.60±0.11, respectively), indicating that galectin-7 serves a negative role on the growth of primary dermal fibroblasts (Fig. 4).
Figure 3.
Fibroblasts in primary culture and verification. Fibroblast primary culture at (A) magnification, ×100 and (B) ×200. Vimentin immunocytochemistry images at (C) magnification, ×200 and (D) ×400, demonstrating that the cells were positive for vimentin, and thus fibroblasts.
Figure 4.
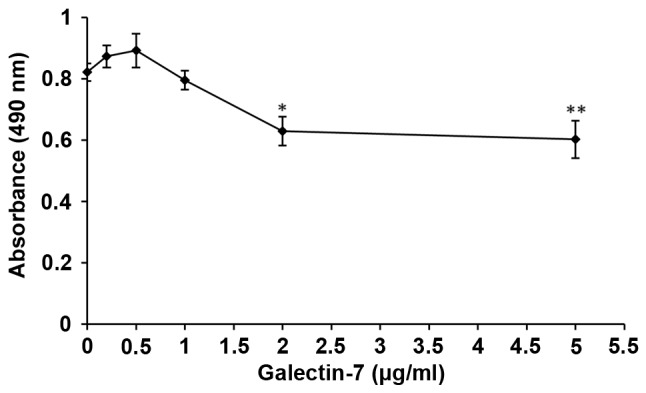
Effect of different concentrations of galectin-7 on the viability of human skin fibroblasts. *P<0.05, **P<0.01 vs. the point at 0 µg/ml. Data was presented as the mean ± standard deviation.
Galectin-7 regulates Collagen I and collagen III at transcriptional level
A typical feature of VLS is the accumulation and dis-organization of collagen I and collagen III in the dermal fibroblasts (10). We hypothesized that galectin-7 may contribute to VLS through regulating the dermal fibroblast function and influencing collagen levels. Isolated primary dermal fibroblasts were seeded in the presence of galectin-7 and a qPCR analysis was performed to determine the transcriptional level of collagen I and collagen III. The results demonstrated that the levels of collagen I [F(2.6)=6.267; P=0.034] and collagen III [F(2,6)=26.669; P=0.001] were significantly elevated following the administration of galectin-7 in a dose-dependent manner (Fig. 5). It would be interesting to explore in the future whether the accumulated collagens are irregularly distributed in the cytoplasm of fibroblasts, and whether they contribute to the hyalinization encountered in VLS.
Figure 5.
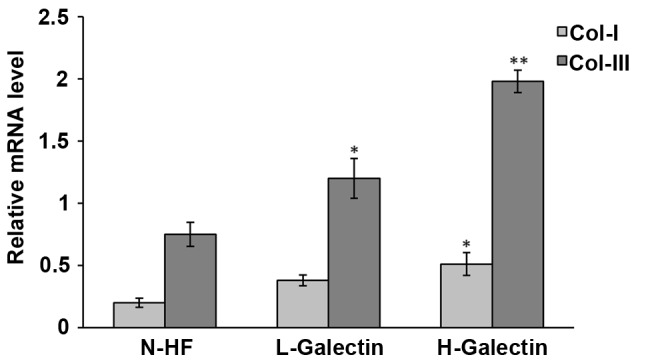
Expression levels of type I and II procollagen mRNA in fibroblasts cultured with different concentrations of galectin-7. *P<0.05, **P<0.01 vs. N-HF. N-HF, normal human fibroblasts; L-galectin concentration, ≤1.0 µg/ml; H-galectin concentration, >1.0 µg/ml. Data was presented as the mean ± standard deviation.
Discussion
In the current study, it was observed that galectin-7 serves important roles in primary dermal fibroblasts, including an inhibitory role on their growth rate, as well as a promoter role on the transcription of collagen I and collagen III. The increased expression of galectin-7 in patients with VLS significantly influenced the viability of fibroblasts.
Members of the galectin family are recognized for their ability to bind β-galactosides via a highly conserved carbohydrate recognition domain. They serve an important role in several physiological processes, including embryonic development, intercellular adhesion, host-pathogen interactions, cell migration and the immune response (11). Galectin-7 belongs to the galectin family and was initially described as a marker of epithelial differentiation, due to its expression in the stratified epithelium of various tissues. It is induced by p53, thus it is also known as p53-induced gene 1 (12). The induction of galectin-7 triggers the release of cytochrome c and increases the activity of JUN N-terminal kinase (JNK), triggering programmed apoptosis (13), which provides evidence of the inhibitory effect on cell viability. The results of the present study demonstrated that galectin-7 was significantly upregulated in VLS tissue from patients compared with healthy tissue, indicating that a high level of galectin-7 may increase the apoptosis rate of epidermal cells and inhibit their viability, leading to the shrinking and loss of epidermal tissue. There is evidence that the epidermis of extracellular superoxide dismutase (EC-SOD) transgenic mice produces more galectin-7 compared with wild type animals, and furthermore the epidermis of EC-SOD transgenic mice is thinner compared with that in their wild type counterparts (14). Transgenic mice were developed specifically overexpressing galectin-7 to aid in research into the basal epidermal keratinocytes. It was reported that an excess of galectin-7 causes a destabilization of the adherens junctions associated with defects in epidermal repair (15). Another study demonstrated that galectin-7 is highly expressed in the epidermis of patients with actinic keratosis, and that galectin-7 upregulates the apoptosis of the epidermis via T lymphocytes (16). The galectin-7 gene was reported to be upregulated by 8-fold in bronchial epithelial cells from asthmatic children following RSV infection in vitro, compared with tissues from the healthy control patients, indicating that this protein may be associated with bronchial epithelial cell apoptosis in asthma (17). These findings suggest that the overexpression of galectin-7 is associated with VLS epidermal atrophy.
In our previous study, it was demonstrated that galectin-7 was downregulated in the early stage of vulvar squamous cell carcinoma (18). Since it is a pro-apoptosis protein, this may be associated with the progression of malignancy. Other reports have revealed a high expression of galectin-7 in certain cancer cell lines, including in breast cancer (19), cervical cancer (20), and late and poorly differentiated vulvar squamous cell carcinoma (18). Galectin-7 has also been associated with a poor prognosis in ovarian cancer (21). In summary, the role of galectin-7 in apoptosis may be organ- or tissue-dependent, and may be associated with the methylation pattern of galectin-7 itself (22,23).
Due to the multifunctional of the galectin family, its sub-cellular localization has been extensively studied. Based on the fact that galectins are able to be detected in intracellular and extracellular locations, as well as in the plasma of patients with tumors, recombinant galectins have been used widely in various in vitro models (11). All lectins, whether dimers or oligomers, bind to cell surface receptors, including growth factor receptors and integrins (24,25). The results of the immunohistochemistry assay in the present study revealed that galectin-7 was expressed exclusively in the epidermis. Galectin-7 is specifically synthesized and secreted by keratinized epithelial cells. Non-keratinizing cells can only be obtained through membrane surface receptors (12). In the immunohistochemistry experiments in the present study, galectin-7 was not detected in the dermis. It was hypothesized that galectin-7 formed in the epidermis can reach the dermis via paracrine and bind to the surface receptor of dermal fibroblast cell membrane, which affects cell function. Therefore, in subsequent cell experiments, recombinant galectin-7 was added to the culture medium to detect changes in collagen fibers. A previous study reported that recombinant galectin-3 binds to the glycol-receptor and stimulates the expression of matrix metalloproteinase 9 (26). We hypothesized that the elevated galectin-7 expression in the VLS epidermis may be released to the dermal part of the skin and bind to fibroblasts, and thus, galectin-7 may modulate the function of dermal fibroblasts.
In the dermis layer, dermal fibroblasts generate connective tissue allowing the skin to recover from injury. One of the primary functions of dermal fibroblasts is to produce proteins which form the extracellular matrix (27); among these proteins, collagen I and collagen III are the main collagens generated (28). To the best of our knowledge, no suitable animal models for Lichen sclerosus have been produced previously (29). Therefore, primary dermal fibroblasts isolated from the healthy control tissues through biopsy were cultured to mimic the in vivo progression of VLS. As revealed by the results of the present study, a high concentration of galectin-7 (>1 µg/ml) significantly inhibited the viability of fibroblasts. Notably, exposure to a low dose (<1 µg/ml) slightly promoted the viability of fibroblasts, although this was not significant. It is not known whether this is associated with different levels of galectin-7 expression in the epidermis. Due to the characteristics of pathological tissues, it is difficult to perform further research, and there have been no literature regarding any change in the number of dermal cells in VLS.
In the present study, the expression of collagens and its association with galectin-7 in primary dermal fibroblasts were also analyzed. Collagen I and collagen III, the primary collagens generated by dermal fibroblasts, were upregulated by galectin-7 in a dose-dependent manner, suggesting that galectin-7 may be an essential regulator for the hyalinization mediated by fibroblasts in VLS. Thus, galectin-7 has the potential of being a target in VLS therapy. Galectin-7 might positively regulate the collagen expression through the JNK pathway since the apoptotic role of Galcetin-7 is mediated by accelerating the release of cytochrome C and the upregulation of JNK. Hashimoto et al (30) reported that tumor growth factor-β promoted the transdifferentiation of fibroblasts into myofibroblasts through JNK and that also led to the production of extracellular matrix proteins, leading to pulmonary fibrosis (30). In VLS skin, the hyalinized collagen is found below the epithelium. Whether galectin-7 has a role in the irregular distribution of collagen fibers still needs to be clarified. Certain data have indicated that galectin-7 induces the viability of T cells, primarily the cluster of differentiation 8+ sub-population (31), and possibly function as an accelerant of T-cell responses (32). A previous study described the autoimmune phenotype in VLS, including high levels of Th1-specific cytokines as well as a dense infiltration of T cells (33). All these data suggest that galectin-7 may serve a role in the elevated immune response encountered in VLS.
In conclusion, the present study identified that the expression of galectin-7 was significantly upregulated in VLS tissues. In addition, galectin-7 significantly inhibited the viability of dermal fibroblasts and stimulated the production of collagen, thus galectin-7 may be a potential drug target for the treatment of patients with VLS.
Acknowledgements
This manuscript has been edited by the professional English writing editor, Dr. Ewen MacDonald from the University of Eastern Finland.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 30973190 and 81400202).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YZ drafted the manuscript and performed the immunohistochemical and western blot analysis. SZ conducted the statistical analyses. HL performed the PCR. XQ performed the cell culturing. XW has provided conceptualization and approved the final version to be published. All authors made substantial contributions to this manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of China Medical University (Shenyang, China) and written informed consent as obtained from all patients.
Consent for publication
Written informed consents from all patients were obtained. There is no identifying information in the article.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808–817. doi: 10.1097/01.grf.0000179635.64663.3d. [DOI] [PubMed] [Google Scholar]
- 2.Regauer S, Reich O, Beham-Schmid C. Monoclonal gamma-T-cell receptor rearrangement in vulvar lichen sclerosus and squamous cell carcinomas. Am J Pathol. 2002;160:1035–1045. doi: 10.1016/S0002-9440(10)64924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birenbaum DL, Young RC. High prevalence of thyroid disease in patients with lichen sclerosus. J Reprod Med. 2007;52:28–30. [PubMed] [Google Scholar]
- 4.Hantschmann P, Sterzer S, Jeschke U, Friese K. P53 expression in vulvar carcinoma, vulvar intraepithelial neoplasia, squamous cell hyperplasia and lichen sclerosus. Anticancer Res. 2005;25:1739–1745. [PubMed] [Google Scholar]
- 5.Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: Review of the literature and current recommendations for management. J Urol. 2007;178:2268–2276. doi: 10.1016/j.juro.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto N, Asano C, Ono K, Tajima S. Verruciform Xanthoma results from epidermal apoptosis with galectin-7 overexpression. J Eur Acad Dermatol Venereol. 2013;27:922–923. doi: 10.1111/j.1468-3083.2012.04664.x. [DOI] [PubMed] [Google Scholar]
- 7.Cummings RD, Liu FT. Galectins. Cold Spring Harbor Laboratory Press. 2009;33 [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Nishioka M, Venkatesan N, Dessalle K, Mogas A, Kyoh S, Lin TY, Nair P, Baglole CJ, Eidelman DH, Ludwig MS, Hamid Q. Fibroblast-epithelial cell interactions drive epithelial-mesenchymal transition differently in cells from normal and COPD patients. Respir Res. 2015;16:72. doi: 10.1186/s12931-015-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godoy CA, Teodoro WR, Velosa AP, Garippo AL, Eher EM, Parra ER, Sotto MN, Capelozzi VL. Unusual remodeling of the hyalinization band in vulval lichen sclerosus by type V collagen and ECM 1 protein. Clinics (Sao Paulo) 2015;70:356–362. doi: 10.6061/clinics/2015(05)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA. When galectins recognize glycans: From biochemistry to physiology and back again. Biochemistry. 2011;50:7842–7857. doi: 10.1021/bi201121m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 13.Barkan B, Cox AD, Kloog Y. Ras inhibition boosts galectin-7 at the expense of galectin-1 to sensitize cells to apoptosis. Oncotarget. 2013;4:256–268. doi: 10.18632/oncotarget.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS, Lee Y, Jeon B, Jeon Y, Yoo H, Kim TY. EC-SOD induces apoptosis through COX-2 and galectin-7 in the epidermis. J Dermatol Sci. 2012;65:126–133. doi: 10.1016/j.jdermsci.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Gendronneau G, Sanii S, Dang T, Deshayes F, Delacour D, Pichard E, Advedissian T, Sidhu SS, Viguier M, Magnaldo T, Poirier F. Overexpression of galectin-7 in mouse epidermis leads to loss of cell junctions and defective skin repair. PLoS One. 2015;10:e0119031. doi: 10.1371/journal.pone.0119031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi T, Hiromasa K, Kabashima-Kubo R, Yoshioka M, Nakamura M. Galectin-7, induced by cis-urocanic acid and ultraviolet B irradiation, down-modulates cytokine production by T lymphocytes. Exp Dermatol. 2013;22:840–842. doi: 10.1111/exd.12268. [DOI] [PubMed] [Google Scholar]
- 17.Yin GQ, Zhao SY, Guo SP, Zhao YH, Liu XC, Jiang ZF. Galectin-7 is associated with bronchial epithelial cell apoptosis in asthmatic children. Zhonghua Er Ke Za Zhi. 2006;44:523–526. (In Chinese) [PubMed] [Google Scholar]
- 18.Jiang Y, Tian R, Yu S, Zhao YI, Chen Y, Li H, Qiao Y, Wu X. Clinical significance of galectin-7 in vulvar squamous cell carcinoma. Oncol Lett. 2015;10:3826–3831. doi: 10.3892/ol.2015.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosset AA, Poirier F, Gaboury L, St-Pierre Y. Galectin-7 expression potentiates her-2-positive phenotype in breast cancer. PLoS One. 2016;11:e0166731. doi: 10.1371/journal.pone.0166731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higareda-Almaraz JC, Ruiz-Moreno JS, Klimentova J, Barbieri D, Salvador-Gallego R, Ly R, Valtierra-Gutierrez IA, Dinsart C, Rabinovich GA, Stulik J, et al. Systems-level effects of ectopic galectin-7 reconstitution in cervical cancer and its microenvironment. BMC Cancer. 2016;16:680. doi: 10.1186/s12885-016-2700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Jeon HK, Lee JK, Sung CO, Do IG, Choi CH, Kim TJ, Kim BG, Bae DS, Lee JW. Clinical significance of galectin-7 in epithelial ovarian cancer. Anticancer Res. 2013;33:1555–1561. [PubMed] [Google Scholar]
- 22.Demers M, Couillard J, Giglia-Mari G, Magnaldo T, St-Pierre Y. Increased galectin-7 gene expression in lymphoma cells is under the control of DNA methylation. Biochem Biophys Res Commun. 2009;387:425–429. doi: 10.1016/j.bbrc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Hwang JA, Ro JY, Lee YS, Chun KH. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget. 2013;4:1461–1471. doi: 10.18632/oncotarget.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol. 2007;17:513–520. doi: 10.1016/j.sbi.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dange MC, Agarwal AK, Kalraiya RD. Extracellular galectin-3 induces MMP9 expression by activating p38 MAPK pathway via lysosome-associated membrane protein-1 (LAMP1) Mol Cell Biochem. 2015;404:79–86. doi: 10.1007/s11010-015-2367-5. [DOI] [PubMed] [Google Scholar]
- 27.Quan T, Little E, Quan H, Qin Z, Voorhees JJ, Fisher GJ. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J Invest Dermatol. 2013;133:1362–1366. doi: 10.1038/jid.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lochner K, Gaemlich A, Sudel KM, Südel KM, Venzke K, Moll I, Knott A, Stäb F, Wenck H, Döring O, et al. Expression of decorin and collagens I and III in different layers of human skin in vivo: A laser capture microdissection study. Biogerontology. 2007;8:269–282. doi: 10.1007/s10522-006-9070-6. [DOI] [PubMed] [Google Scholar]
- 29.Canady J, Karrer S, Fleck M, Bosserhoff AK. Fibrosing connective tissue disorders of the skin: Molecular similarities and distinctions. J Dermatol Sci. 2013;70:151–158. doi: 10.1016/j.jdermsci.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T. Transforming growth Factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir Crit Care Med. 2001;163:152–157. doi: 10.1164/ajrccm.163.1.2005069. [DOI] [PubMed] [Google Scholar]
- 31.Rossi NE, Reiné J, Pineda-Lezamit M, Pulgar M, Meza NW, Swamy M, Risueno R, Schamel WW, Bonay P, Fernández-Malavé E, Regueiro JR. Differential antibody binding to the surface alphabetaTCR.CD3 complex of CD4+ and CD8+ T lymphocytes is conserved in mammals and associated with differential glycosylation. Int Immunol. 2008;20:1247–1258. doi: 10.1093/intimm/dxn081. [DOI] [PubMed] [Google Scholar]
- 32.Luo Z, Ji Y, Zhou H, Huang X, Fang J, Guo H, Pan T, Chen ZK. Galectin-7 in cardiac allografts in mice: Increased expression compared with isografts and localization in infiltrating lymphocytes and vascular endothelial cells. Transplant Proc. 2013;45:630–634. doi: 10.1016/j.transproceed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Terlou A, Santegoets LA, van der Meijden WI, Heijmans-Antonissen C, Swagemakers SM, van der Spek PJ, Ewing PC, van Beurden M, Helmerhorst TJ, Blok LJ. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: A Th1 response and high levels of microRNA-155. J Invest Dermatol. 2012;132:658–666. doi: 10.1038/jid.2011.369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.