Abstract
Context.
Investigators need novel methods for timely identification of patients with serious illness to test or implement new palliative care models.
Objectives.
The study’s aim was to develop an electronic health record (EHR) phenotype to identify patients with late-stage dementia for a clinical trial of palliative care consultation.
Methods.
We developed a computerized method to identify patients with dementia on hospital admission. Within a data warehouse derived from the hospital’s EHR, we used search terms of age, admission date, and ICD-9 and ICD-10 diagnosis codes to create an EHR dementia phenotype, followed by brief medical record review to confirm late-stage dementia. We calculated positive predictive value, false discovery rate, and false negative rate of this novel screening method.
Results.
The EHR phenotype screening method had a positive predictive value of 76.3% for dementia patients and 24.5% for late-stage dementia patients; a false discovery rate of 23.7% for dementia patients and 75.5% for late-stage dementia patients compared to physician assessment. The sensitivity of this screening method was 59.7% to identify hospitalized patients with dementia. Daily screening—including confirmatory chart reviews—averaged 20 minutes and was more feasible, efficient, and more complete than manual screening.
Conclusion.
A novel method using an EHR phenotype plus brief medical record review is effective to identify hospitalized patients with late-stage dementia. In health care systems with similar clinical data warehouses, this method may be applied to serious illness populations to improve enrollment in clinical trials of palliative care or to facilitate access to palliative care services.
Keywords: Geriatrics, family medicine, EHR, electronic health record, computable phenotype
Introduction
Specialty palliative care improves quality of life, reduces hospitalizations and invasive treatments, yet results in similar or better survival for patients with serious illnesses.1,2 Palliative care may benefit patients and families facing advanced-stage Alzheimer’s disease and other neurodegenerative dementias, most of whom prefer comfort-focused care.3 These incurable and progressive diseases affect more than 5 million Americans and have extraordinary societal and family costs.4,5 Dementia is associated with high rates of hospitalization, care transitions, and 30-day readmissions.6 Dementiais also the only leading cause of death in the U.S. with no meaningful preventive or curative intervention; as a result, prevalence is expected to double by 2030.7,8
From a population health perspective, specialty palliative care is most likely to benefit the subset of dementia patients who have symptomatic late-stage disease. Certain groups of seriously ill patients are clustered within a specific nursing unit, clinic or physician practice, but many serious illness populations are diffused across a hospital or health system. For example, while patients with advanced-stage cancer are found in oncology clinics or specialty hospital services, patients with congestive heart failure, chronic lung disease, or dementia are found in primary care and specialty care clinics and on various hospital services. Novel methods are needed to identify these patients for palliative care clinical services or research.9,10
Electronic Health Record Computable Phenotypes for Clinical Research
Electronic health records (EHRs) offer new potential to identify high-risk patient populations. Since 2000, EHR-based research has increased, particularly for chronic illnesses or for rare health conditions. This methodology requires systematic terminology and data presentation in electronic data.11,12 The EHR has been used to facilitate clinical trial enrollment for patients with chronic obstructive pulmonary disease (COPD) and chronic pain and to streamline multisite pragmatic trials.13–15
EHR computable phenotypes are customizable, structured queries of electronic health care data used by investigators to identify patients who meet criteria based on standardized terminology and Boolean logic.16–18 Existing EHR computable phenotypes largely capitalize on clinical laboratory values and other structured quantitative data. Building a clinically meaningful EHR phenotype requires extensive refinement and does not yet offer a reliable alternative to manual screening. For dementia, one existing, published algorithm is targeted to all patients with dementia at any stage, including using dementia-related medications as part of their phenotypes, which are more common in earlier stages.19 However, combining a sensitive and efficient EHR phenotype with more specific manual chart review may be an optimal way to find seriously ill patients from a much larger pool of patients served by a hospital or health system. This combined approach is known as serial testing.
Computable EHR Phenotype to Identify Patients With Late-Stage Dementia
To facilitate a clinical trial of palliative care consultation, we sought to identify late-stage dementia patients at the time of hospitalization. Our study’s aim was to develop an EHR computable phenotype to identify serious illness patients with late-stage dementia, promoting enrollment for this clinical trial.
Methods
The Triggered Palliative Care for Dementia Study
The screening method was developed in the context of a pilot-randomized clinical trial of triggered palliative care consultation for patients with dementia. The study site was UNC Hospitals, a 929-bed acute care/tertiary care teaching hospital with 25,995 adult admissions in FY17 (excluding psychiatry, obstetrics, and rehabilitation). Patients were eligible if they had late-stage dementia, an acute illness hospitalization, and a family decision maker; patients with existing hospice or palliative care consultation were excluded. Patients and their family decision makers were randomized to the intervention (triggered specialty palliative care and postdischarge collaborative care) or control (educational materials) arms. Participants were followed for 60 days after enrollment to measure outcomes of hospitalization and emergency room transfers, goals-of-care communication, completion of advance care planning documentation, symptom distress, and referral to community-based hospice or palliative care services. All study procedures were approved by the University of North Carolina institutional review board and reviewed by a data safety monitor.
Developing a Method to Screen for Patients With Late-Stage Dementia in Hospital
The novel screening method consisted of an EHR computable phenotype of dementia followed by verification by research staff and palliative care physicians. To construct a computable phenotype, we used EHR data organized by Informatics for Integrating Biology and Bedside (i2b2; Boston, MA) software, designed initially to support integration of genomic research into clinical settings.20 The software searches the EHR based on prespecified parameters to select patients who meet criteria. We used i2b2 to build an initial EHR phenotype based on diagnoses at admission consistent with Alzheimer’s disease and other neurodegenerative dementias, variables indicating acute care admission, and age. We then linked this phenotype to patients’ records via the Carolina Data Warehouse, where information from the EHR is stored using IBM Business Objects (Armonk, NY). Each day, an automated report in Microsoft Excel was exported to a secure research server with medical record numbers and names of patients who met the algorithm’s parameters.
Each day, a trained and well-practiced research staff member received the automated report of hospitalized dementia patients and screened their medical records for evidence of late-stage dementia. Patients with potential late-stage dementia as determined by research staff were then sent to a palliative care physician who verified eligibility based on review of the EHR and conversation with the patient’s attending physician to confirm dementia diagnosis and Stage 5–7 using the Global Deterioration Scale (Fig. 1).21
Fig. 1.
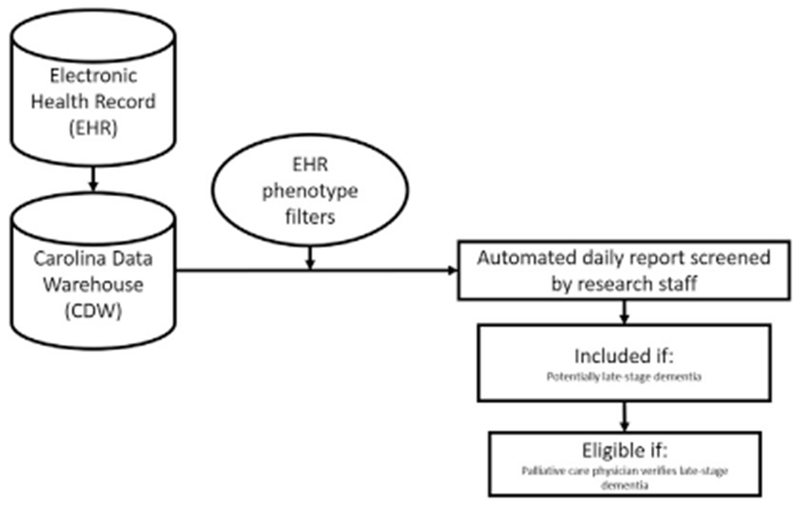
Data flow for the EHR phenotype. Novel screening method. EHR = electronic health record.
Feedback to Refine the EHR Phenotype
To refine the algorithm, our EHR phenotype was iteratively evaluated for its accuracy in detecting the target population and revised twice. We applied serial testing in which research staff reviewed EHR output and compared to the gold standard of physician-verified diagnosis of late-stage dementia. Starting with all 158 potential ICD-9 and ICD-10 diagnosis codes, we tested how much utility each had in identifying the first 82 patients with late-stage dementia who met eligibility criteria. The first iteration of the EHR phenotype retrieved 604 patients (3/29/16–7/13/16), 124 of whom were sent to a palliative care physician to determine that 82 had confirmed late-stage dementia. Screening all patient charts required an average of ~50 minutes/day from research team personnel using this mechanism (Appendix).
For the second iteration, we removed 92 codes that were not applied in the first iteration and did not represent a rare disease with progressive dementia, retaining 66 codes that identified at least one eligible patient. Through this process, we sought to improve the specificity of the algorithm without losing sensitivity.
The second iteration of the EHR phenotype identified 390 patients (7/14/16–10/31/16), 95 of whom had serious-enough illness to be reviewed by a palliative care physician. This yielded 53 patients, requiring 35 minutes of staff time per day. In the final iteration, we eliminated ICD-9 and ICD-10 codes for traumatic brain injury and stroke when presenting without co-occurrence of a neurodegenerative dementia code.
The final algorithm had 34 ICD codes (see Appendix) associated with dementia or cognitive impairment, hospital admission in last three days at a hospital participating in the study, and patient aged 65 years or older. This final EHR phenotype yielded 371 patients (11/1/16–3/31/17; out of 1615 adult patients admitted as inpatients during the time frame), 174 of whom were referred by research staff for final review by a palliative care physician, who verified late-stage dementia for 90. The final EHR phenotype required 20 minutes of staff time daily to complete all confirmatory chart reviews. Over the iterations, staff time decreased owing to decreases in the average number of patients derived from the EHR phenotype each month; the first iteration of the EHR phenotype yielded an average of 173 patients/month, the second iteration yielded an average of 111 patients/month, and the final EHR phenotype yielded an average of 74 patients/month. The EHR phenotype’s results over each refinement are presented in Table 1. The remainder of the results use data only from this final refinement period.
Table 1. Number of Patients Identified by the EHR Phenotype.
| Eligible Patients | Initial Method 3/29/16–7/13/16 | First Refinement 7/14/16–10/31/16 | Second Refinement 11/1/16–3/31/17 |
|---|---|---|---|
| Total reviewed (CDW list) | 604 | 390 | 371 |
| Sent to palliative care clinician for review (dementia) (% of total reviewed) | 124 (20.5) | 95 (24.4) | 174 (46.9) |
| Palliative care clinician confirm late-stage dementia (% of total reviewed) | 82 (13.6) | 53 (13.6) | 91 (24.5) |
EHR = electronic health record; CDW = Carolina Data Warehouse.
Measuring Feasibility and Efficiency
To assess feasibility and efficiency, research staff tracked the amount of time required to identify potential late-stage dementia patients in the entire hospital using the novel method compared to reviewing census lists for two inpatient services with the largest dementia populations.
Positive Predictive Value and False Discovery Rate
Positive predictive value (PPV) is presented as the percent of cases on the automated daily report that did have dementia of any stage. The false discovery rate (FDR; the proportion of cases that were not true dementia) is presented as the percent of cases on theautomated daily report without dementia. Because of the specific focus of this study on late-stage disease, we also calculated the PPV and FDR for late-stage dementia.
Measuring Sensitivity
During a five-month period, we measured the sensitivity of the EHR computable phenotype method (true positive/[true positive + false negative]) as a ratio of true cases of dementia found by the automated daily report compared to true cases of dementia who were and were not found by this method. False negative cases were identified through daily review of all patients aged 65 years and older on three daily census lists: palliative care consults, and two inpatient services with the highest proportion of dementia admissions (geriatrics and family medicine). Because of the study’s specific focus on late-stage dementia, we also calculated sensitivity for the late-stage population separately by examining clinician-confirmed cases of late-stage dementia.
Results
Feasibility and Efficiency of the Screening Method
We compared manual screening of two high-yield services to hospital-wide screening using the EHR phenotype during a five-month period (11/1/16–3/31/17). Manual screening of the daily census for two services with higher numbers of dementia cases (geriatrics and family medicine) took an average of 25 minutes/day. From a total of 541 admitted patients, this method yielded 236 patients with dementia and 70 patients with late-stage dementia, yielding PPVs of 44% and 13%, respectively.
By contrast, using the EHR phenotype followed by more selective manual screening across all hospital services averaged 20 minutes per day. This method screened 3174 admitted patients to yield 283 patients with dementia and 91 patients with late-stage dementia. The EHR phenotype presented patients within a median of three days after admission; it led to research enrollment within a median of four days. The average length of stay for these patients was nine days (median of five days).
To apply the final phenotype in research screening, we then built it into a prompt automatically generated if the criteria are met in the EHR. Instead of receiving a daily output, this mechanism quickly messages the research staff (within one day) within the EHR when a patient matching the phenotype enters the hospital. These prompts, often called “best practice advisories,” can be customized so that they alert only the research coordinator, not the care team.
The EHR alert was 100% reliable in delivering the list of potential dementia patients one day after admission, adding to time efficiency. That is, the list successfully fired to the research staff every day.
PPV, FDR, and Sensitivity of the Screening Method
Calculations and final rates appear in Table 2. The EHR phenotype screening method had a PPV for any stage dementia of 76.3%. The total number of physician-verified late-stage dementia patients was 91 out of 371 patients pulled by the final EHR phenotype, giving a PPV for late-stage disease of 24.5%. The FDR of the EHR phenotype screening method was 23.7%; the FDR for late-stage dementia was 75.5%. Simply stated, three of four patients identified by this method had dementia, and one of four had late-stage dementia.
Table 2. Calculations for Sensitivity and Specificity of the Final EHR Phenotype.
| Formula | Calculation of Formula Components With Project-Specific Data | Project-Specifi Result | Rate |
|---|---|---|---|
| Any dementia | |||
| Positive predictive value (PPV) = true positive (TP)/(true positive [TP] + false positive [FP]) | TP = EHR phenotype mild dementia and late-stage dementia | 283 | 76.3% |
| FP = patients from EHR phenotype with no dementia | 88 | ||
| False discovery rate (FDR) = false positive (FP)/(true positive [TP] + false positive [FP]) | FP = patients from EHR phenotype with no dementia | 88 | 23.7% |
| TP = EHR phenotype mild dementia and late-stage dementia | 283 | ||
| False negative rate = false negative (FN)/(false negative [FN] + true positive [TP]) | FN = mild and late-stage dementia palliative care consults, dementia on geriatrics or family medicine services and not from EHR phenotype | 191 | 40.3% |
| TP = EHR phenotype mild dementia and late-stage dementia | 283 | ||
| Sensitivity = true positive (TP)/(true positive [TP] + false negative [FN]) | TP = EHR phenotype mild dementia and late-stage dementia | 283 | 59.7% |
| FN = mild and late-stage dementia palliative care consults, dementia on geriatrics or family medicine services and not from EHR phenotype | 191 | ||
| Late-stage dementia | |||
| PPV for late-stage dementia = true positive late stage (TPLS)/(true positive [TP] + false positive [FP]) | TPLS = clinician-confirmed late-stage dementia from EHR phenotype | 91 | 24.5% |
| TP = EHR phenotype mild dementia and late-stage dementia | 283 | ||
| FP = patients from EHR phenotype with no dementia | 88 | ||
| FDR for late-stage dementia = false positive late stage (FPLS)/(true positive [TP] + false positive [FP]) | FPLS = all EHR phenotype patients—clinician-confirmed late-stage dementia | 280 | 75.5% |
| TP = EHR phenotype mild dementia and late-stage dementia | 283 | ||
| FP = patients from EHR phenotype with no dementia | 88 | ||
| False negative late-stage rate = false negative late stage (FNLS)/(false negative [FN] + true positive [TP]) | FNLS = palliative care consults with moderate/severe dementia or on geriatrics or family medicine services and not from EHR phenotype | 51 | 10.8% |
| FN = mild and late-stage dementia palliative care consults, dementia on geriatrics or family medicine services and not from EHR phenotype | 191 | ||
| TP = EHR phenotype mild dementia and late-stage dementia | 283 | ||
| Sensitivity = true positive late stage (TPLS)/(true positive late stage [TPLS] + false negative late stage [FNLS]) | TPLS = clinician-confirmed late-stage dementia from EHR phenotype | 91 | 64.1% |
| FNLS = palliative care consults with moderate/severe dementia or on geriatrics or family medicine services and not from EHR phenotype | 51 |
EHR = electronic health record.
The sensitivity of the final EHR screening method was 59.7% for any dementia; these were cases for which the EHR phenotype method endorsed real cases of dementia. For late-stage dementia, sensitivity was derived by dividing the number of physician-verified late-stage dementia from the EHR phenotype by the total number of physician-verified late-stage dementia from both the EHR phenotype and geriatric and family medicine census lists, yielding a sensitivity of 64.1%.
Discussion
In this study, we found that a novel method using an EHR phenotype plus medical record review is effective to identify hospitalized patients with late-stage dementia. Using expert clinician case review as the gold standard, three of four patients identified by this method had dementia, and one of four had late-stage dementia. Daily screening—including confirmatory chart reviews—averaged 20 minutes and was more feasible, efficient, and more complete than manual screening.
Our EHR phenotype screening combined with selective manual review is also more complete than reliance on use of triggers by referring clinicians as it permits rapid review of an entire hospitalized population on a daily basis. We did trade off sensitivity for specificity as we refined the EHR phenotype to shorten time spent manually screening. A recent systematic review of palliative care implementation strategies described extensive use of outreach education, clinical triggers and feedback, and other interpersonal methods to improve access to specialty palliative care, but no prior studies of technology improvements or data mining in the electronic medical record. Thus, our approach is a novel method for identifying a seriously ill population to offer palliative care consultation or to research new clinical palliative care interventions.22
Across health care systems with similar clinical data warehouses, this method has the potential to screen for dementia and other serious illness and to facilitate access to palliative care services for those who can meaningfully benefit. Although a majority of patients who are hospitalized do not need palliative care consultations, palliative care remains underutilized for patients with serious and life-limiting illness.23 Earlier palliative care consultations are more effective to reduce overall cost of hospital care.24,25 We use the example of dementia patients, a population with limited research evidence for palliative intervention.26,27 This approach may be useful for other serious illness populations, including patients with congestive heart failure or cancer.28 The rapidity with which the final method identifies patients—within one day of admission—can also get palliative consultations to patients earlier, which may be the difference in getting a consult, given the relative short median lengths of stay.
This study also has implications for the pragmatic application of health care databases to improve palliative care research. Since its development, i2b2 software has been applied in diverse medical and biologic databases, including computable phenotypes for COPD and tracking of adverse laboratory values in chronic kidney disease.29,30 On a large scale, PCOR-net, a multicenter network of EHR data, uses similar mechanisms to identify patients with heart failure and multiple other conditions.31,32
Although our method worked quite well, there remain limitations to this approach that merit consideration. First, the EHR computable phenotype screening method is dependent on institutional investment in a searchable EHR clinical data warehouse and requires software and personnel to facilitate algorithmic and manual searches. Second, although this method proved more efficient and complete than manual screening of census lists, it could be even more efficient. Natural language processing is an emerging text search technology that could add efficiency and accuracy to the method we report.33,34 In particular, because variables distinguishing serious illness from chronic illness—disease stage and functional status—are often in text, natural language processing could optimize our methodology. Third, the gold standard for defining late-stage dementia remains imperfect owing to persistent clinical failure to record this diagnosis. Fourth, trends in dementia admissions and diagnoses might have changed over time, as the EHR computable phenotype was refined in a stage-wise fashion. The iterative refinements occurred in quick succession, potentially minimizing this risk, although the absence of a counter-factual remains a limitation.
Despite these limitations, our novel EHR phenotype screening method is a more accurate and efficient method to find late-stage dementia patients for a palliative care clinical trial. Although modest investments in technology and personnel are necessary to implement this approach in the clinical setting, it is feasible and enhances overall efficiency for palliative care research.
Disclosures and Acknowledgments
The authors would like to thank Jasper Benoit Becker, Joseph Dobgima Njinimbam, and Loretta Fearrington for their support in building, refining, and implementing the EHR phenotype. The authors would like to thank Emily Pfaff for providing early conceptual guidance for this article.
Ms. Ernecoff has nothing to disclose. Ms. Wessell has nothing to disclose. Ms. Gabriel has nothing to disclose. Dr. Carey reports grants from National Palliative Care Research Center, the National Institute on Aging, the Patient-Centered Outcomes Research Institute (PCORI), and the Duke Endowment during the conduct of the study. Dr. Hanson reports grants from National Palliative Care Research Center, grants from National Institute on Aging, during the conduct of the study.
The study was supported in part by a Pilot & Exploratory Project Support Grant from the National Palliative Care Research Center (NPCRC) and NIH-NIA: R21AG052140.
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through grant award number UL1TR001111. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix
ICD Codes Before and After Refinement.
| ICD-9 Codes | ICD-10 Codes | First Round (3/29/16–7/13/16) | Second Round (7/14/16–10/31/16) | Final Round (11/1/16–3/31/17) | |
|---|---|---|---|---|---|
| 290 | Dementias | ||||
| 290.4 | F01.50 F01.51 | Vascular dementia | |||
| 290.41 | Dementia, vascular with delirium | ||||
| 291.2 | F10.27 F10.97 | Alcohol-induced persisting dementia | |||
| 294.11 | Dementia in conditions classified elsewhere with behavioral disturbance | ||||
| 294.21 | F03.91 | Dementia, unspecified, with behavioral disturbance | |||
| 294.8 | F99 | Mental disorder, other | |||
| 331 | G30.0 G30.1 G30.8 G30.9 | Alzheimer’s disease | |||
| 331.1 | G31.09 | Frontotemporal disease | |||
| F02.80 F02.81 | Dementia in conditions classified elsewhere | ||||
| F03.90 | Dementia, unspecified, without behavioral disturbance | ||||
| G31.83 | Dementia w/lewy Bodies | ||||
| I67.89 | Cerebrovascular disease | ||||
| I69.81 | Cerebrovascular disease | ||||
| 294.1 | Dementia in conditions classified elsewhere without behavioral disturbance | ||||
| 294.2 | Dementia, unspecified without behavioral disturbance | ||||
| 331.82 | Lewy body dementia | ||||
| A81.00 | Creutzfeldt-Jakob disease, unspecified | ||||
| G10 | Huntington’s disease | ||||
| G31.01 | Pick’s disease | ||||
| 290 | F03.90 | Senile dementia, uncomplicated | |||
| 331.2 | G31.1 | Senile degeneration of brain | |||
| 331.83 | G31.84 | Mild cognitive impairment | |||
| 436 | I63.9 | Cerebrovascular accident | |||
| 438.89 | I67.9 | Cerebrovascular disease | |||
| 780.93 | Memory loss | ||||
| 799.59 | Cognition sign/symptom NEC | ||||
| I69.91 | Cerebrovascular disease | ||||
| 290.1 | Presenile dementia | ||||
| 290.2 | Senile dementia with delusional or depressive features | ||||
| 290.3 | Senile dementia with delirium | ||||
| 290.42 | Dementia, vascular with delusions | ||||
| 290.8 | Other specified senile psychotic conditions | ||||
| 290.9 | Unspecified senile psychotic condition | ||||
| 310 | R41.844 | Frontal lobe syndrome | |||
| I69.80 | Cerebrovascular disease | ||||
| G46.80 | Cerebrovascular disease | ||||
| ZI68.8 | Cerebrovascular disease | ||||
| 46.19 | Creutzfeldt-Jakob disease NEC/NOS | ||||
| 291.1 | Alcohol amnestic disorder | ||||
| 300.9 | Nonpsychotic disorder, not otherwise specified | ||||
| 333.4 | Huntington’s chorea | ||||
| 434.91 | Cerebral artery occlusion unspecified with cerebral infarction | ||||
| 437.1 | AC cerebrovascular insufficiency, NOS | ||||
| 437.8 | Cerebrovascular disease NEC | ||||
| 437.9 | Cerebrovascular disease NOS | ||||
| 438 | Late effects of cerebrovascular disease: cognitive deficits | ||||
| G93.1 | Anoxic brain damage, not elsewhere classified | ||||
| S06.9X0A | Unspecified intracranial injury without loss of consciousness, initial encounter | ||||
| S06.9X0D | Unspecified intracranial injury without loss of consciousness, subsequent encounter | ||||
| S06.9X0S | Unspecified intracranial injury without loss of consciousness, sequela | ||||
| S09.90XA | Unspecified injury of head, initial encounter | ||||
| S09.90XD | Unspecified injury of head, subsequent encounter | ||||
| S09.90XS | Unspecified injury of head, sequela | ||||
| I61.9 | Nontraumatic intracerebral hemorrhage, unspecified | ||||
| I63.511 | Cerebral infarction due to unspecified occlusion or stenosis of right middle cerebral artery | ||||
| I63.521 | Cerebral infarction due to unspecified occlusion or stenosis of right anterior cerebral artery | ||||
| I63.9 | Cerebral infarction, unspecified | ||||
| I65.29 | Occlusion and stenosis of unspecified carotid artery |
Shading indicates that the code was included.
References
- 1. Gade G Venohr I Conner D et al. Impact of an inpatient palliative care team: a randomized controlled trial J Palliat Med 2008. 11 180–190 [DOI] [PubMed] [Google Scholar]
- 2. Temel JS Greer JA Muzikansky A et al. Early palliative care for patients with metastatic non–small-cell lung cancer N Engl J Med 2010. 363 733–742 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell SL Palmer JA Volandes AE Hanson LC Habtemariam D Shaffer ML Level of care preferences among nursing home residents with advanced dementia J Pain Symptom Manage 2017. 54 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hebert LE Weuve J Scherr PA Evans DA Alzheimer disease in the United States (2010-2050) estimated using the 2010 census Neurology 2013. 80 1778–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurd MD Martorell P Delavande A Mullen KJ Langa KM Monetary costs of dementia in the United States N Engl J Med 2013. 368 1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callahan CM Arling G Tu W et al. Transitions in care for older adults with and without dementia J Am Geriatr Soc 2012. 60 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tinetti ME McAvay GJ Murphy TE Gross CP Lin H Allore HG Contribution of individual diseases to death in older adults with multiple diseases J Am Geriatr Soc 2012. 60 1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prince M Bryce R Albanese E Wimo A Ribiero W Ferri CP The global prevalence of dementia: a systematic review and meta-analysis Alzheimers Dement 2013. 9 63–75 [DOI] [PubMed] [Google Scholar]
- 9. Fadul N Elsayem A Palmer JL Zhang T Braiteh F Bruera E Predictors of access to palliative care services among patients who died at a Comprehensive Cancer Center J Palliat Med 2007. 10 1146–1152 [DOI] [PubMed] [Google Scholar]
- 10. Nelson JE Curtis JR Mulkerin C et al. Choosing and using screening criteria for palliative care consultation in the ICU: a report from the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board Crit Care Med 2013. 41 2318–2327 [DOI] [PubMed] [Google Scholar]
- 11. Dean BB Lam J Natoli JL Butler Q Aguilar D Nordyke RJ Review: use of electronic medical records for health outcomes research a literature review Med Care Res Rev 2009. 66 611–638 [DOI] [PubMed] [Google Scholar]
- 12.Aickin M. Patient-centered research from electronic medical records. Perm J. 2011;15:89. doi: 10.7812/tpp/11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkhenini HF, Davis KJ, Stein ND. et al. Using an electronic medical record (EMR) to conduct clinical trials: Salford Lung Study feasibility. BMC Med Inform Decis Mak. 2015;15:1. doi: 10.1186/s12911-015-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian TY Zlateva I Anderson DR Using electronic health records data to identify patients with chronic pain in a primary care setting J Am Med Inform Assoc 2013. 20 e275–e280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joseph CL Ownby DR Zoratti E et al. Recruitment experience for a pragmatic randomized controlled trial: using EMR initiatives and minimizing research infrastructure Clin Res Regul Aff 2016. 33 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mo H Thompson WK Rasmussen LV et al. Desiderata for computable representations of electronic health records-driven phenotype algorithms J Am Med Inform Assoc 2015. 22 1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu J Rasmussen LV Shaw PL et al. Review and evaluation of electronic health records-driven phenotype algorithm authoring tools for clinical and translational research J Am Med Inform Assoc 2015. 22 1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berg RL Pacheco JA Peissig P Rasmussen L Weston N Chute CG Analyzing the heterogeneity and complexity of Electronic Health Record oriented phenotyping algorithms AMIA Annu Symp Proc 2011. 2011 274–283 [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson C, Group Health Cooperative. Dementia. PheKB; [Accessed November 27, 2017]. 2012. Available from https://phekb.org/phenotype/10. [Google Scholar]
- 20. [Accessed June 21, 2017]; Available from https://www.i2b2.org/about.
- 21. Reisberg B Ferris SH de Leon MJ Crook T The Global Deterioration Scale for assessment of primary degenerative dementia Am J Psychiatry 1982. 139 1136–1139 [DOI] [PubMed] [Google Scholar]
- 22.van Riet Paap J, Vernooij-Dassen M, Sommerbakk R. et al. Implementation of improvement strategies in palliative care: an integrative review. Implement Sci. 2015;10:1. doi: 10.1186/s13012-015-0293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Connor NR Moyer ME Behta M Casarett DJ The impact of inpatient palliative care consultations on 30-day hospital readmissions J Palliat Med 2015. 18 956–961 [DOI] [PubMed] [Google Scholar]
- 24. May P Garrido MM Cassel JB et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect J Clin Oncol 2015. 33 2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanson LC Usher B Spragens L Bernard S Clinical and economic impact of palliative care consultation J Pain Symptom Manage 2008. 35 340–346 [DOI] [PubMed] [Google Scholar]
- 26. Ahronheim JC Morrison RS Morris J Baskin S Meier DE Palliative care in advanced dementia: a randomized controlled trial and descriptive analysis J Palliat Med 2000. 3 265–273 [DOI] [PubMed] [Google Scholar]
- 27. Volicer L Collard A Hurley A Bishop C Kern D Karon S Impact of special care unit for patients with advanced Alzheimer’s disease on patients’ discomfort and costs J Am Geriatr Soc 1994. 42 597–603 [DOI] [PubMed] [Google Scholar]
- 28. Greer JA Jackson VA Meier DE Temel JS Early integration of palliative care services with standard oncology care for patients with advanced cancer CA Cancer J Clin 2013. 63 349–363 [DOI] [PubMed] [Google Scholar]
- 29.Fu X, Batista-Navarro R, Rak R, Ananiadou S. Supporting the annotation of chronic obstructive pulmonary disease (COPD) phenotypes with text mining workflows. J Biomed Semantics. 2015;6:1. doi: 10.1186/s13326-015-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drawz PE Archdeacon P McDonald CJ et al. CKD as a model for improving chronic disease care through electronic health records Clin J Am Soc Nephrol 2015. 10 1488–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [Accessed September 28, 2017]; Available from http://www.pcornet.org/about-pcornet/
- 32. [Accessed September 28, 2017]; Available from http://www.clinicalleader.com/doc/duke-pcori-use-ehrs-to-transform-clinical-trials-0001.
- 33. Hirschberg J Manning CD Advanced in natural language processing Science 2015. 349 261–266 [DOI] [PubMed] [Google Scholar]
- 34. Barrett N Weber-Jahnke JH Thai V Engineering natural language processing solutions for structured information from clinical text: extracting sentinel events from palliative care consult letters Stud Health Technol Inform 2013. 192 594–598 [PubMed] [Google Scholar]


