Abstract
Because E2F transcription factor 2 (E2F2) promoter polymorphisms have been implicated in carcinogenesis and prognosis, we investigated associations between genetic variants in five E2F2 promoter polymorphisms and recurrence risk of squamous cell carcinoma of the oropharynx (SCCOP) in 1,008 patients. A log-rank test and multivariable Cox models were used to assess the associations. Compared with patients with variant genotypes of E2F2-rs2742976 and E2F2-rs3218123, patients with common homozygous genotypes had better disease-free survival (both log-rank, p<0.001) and lower SCCOP recurrence risk (HR, 0.4, 95% CI, 0.3–0.6 and HR, 0.3, 95% CI, 0.2–0.5, respectively) after multivariable adjustment. Furthermore, among patients with HPV16–positive tumors, those with common homozygous genotypes of E2F2- rs2742976 and E2F2-rs3218123 had better disease-free survival rates (both log-rank, p<0.001) and lower recurrence risk (HR, 0.1, 95% CI, 0.1–0.4 and HR, 0.1, 95% CI, 0.0–0.2, respectively) than patients with variant genotypes. However, no significant differences were found for the other three polymorphisms. After combining the risk genotypes of the 5 polymorphisms and using the high-risk group (2–5 risk genotypes) as the reference group, we found that the low-risk groups (0 or 1 risk genotype) had significantly lower recurrence risk among all patients (HR, 0.4, 95% CI, 0.3–0.6) and among HPV16-positive patients (HR, 0.2, 95% CI, 0.1–0.5). Our findings suggest that E2F2 polymorphisms may individually or jointly modify SCCOP recurrence risk, particularly for SCCOP patients with HPV16-positive tumors.
Keywords: E2F2 variant genotypes, recurrence, predictive biomarkers, human papillomavirus, oropharyngeal cancer
Introduction
The rates of squamous cell carcinoma of the oropharynx (SCCOP) recurrence and of patient survival after definitive treatment differ among patients with similar lifestyles and clinical factors, which suggests that genetic factors may contribute to interindividual differences. Thus, understanding the effect of genetic variations on disease-free and overall survival may improve clinical and therapeutic decision-making and thereby improve patient survival and quality of life [1,2].
Between 25% and 60% of SCCOP cases are associated with an oncogenic human papillomavirus (HPV) infection, and HPV has a stronger effect on the prognosis of SCCOP than other known risk factors. Although epidemiologic, histologic, and clinical data from SCCOP patients have been used to predict survival outcomes, several genetic factors that interact with HPV have not been investigated. Adding to our previous reports of prognostic biomarkers from several molecular pathways for HPV type 16 (HPV16)–associated SCCOP, we investigated E2F transcription factor 2 (E2F2) as a potential predictive biomarker for recurrence risk in this study. E2F2 belongs to the activators of the family that controls the transition between G1 and S phase through various upstream signals [3]. E2F2 is critical to many cell processes, including the cell cycle, proliferation, differentiation, and cancer development [4–6]. Because many important checkpoints and regulatory pathways control the cell cycle and ensure the fidelity of DNA replication and chromosome segregation [7–10], dysregulation of the cell cycle may lead to carcinogenesis. Outcomes vary widely among patients [11], indicating that genetic alterations may affect these genes’ expression and SCCOP prognosis.
Genetic variants or polymorphisms in the promoter of E2F2 affect the expression of E2F2 [12,13]; such alterations of expression may also affect cell-cycle control and response to radiotherapy, thus leading to susceptibility to SCCOP recurrence. We and others have previously shown that E2F2 promoter polymorphisms, which affect the expression of E2F2 [12,13], are significantly associated with increased risk of many cancers [14–16] and that the combined risk genotypes of E2F1 and E2F2 may modify the risk of SCCOP, but not the risk of other subtypes of squamous cell carcinoma of the head and neck [17]. To our knowledge, no studies have assessed the associations of E2F2 promoter polymorphisms with risk of SCCOP recurrence. Given the important roles of E2F2 in cell-cycle regulation and the interaction between E2F2 and HPV, we hypothesized that E2F2 promoter polymorphisms may affect E2F2 expression and cause interindividual variations in responses to radiotherapy, leading to different risk of SCCOP recurrence. In the current study, we assessed the associations of common and variant genotypes of five E2F2 promoter polymorphisms, both individually and jointly, with the risk of SCOPP recurrence in a cohort of 1,008 patients with SCCOP and the subgroup of patients with HPV16(+) SCCOP (n=324).
Patients and Methods
Study participants
Patients with SCCOP were prospectively enrolled for a molecular profiling study and were treated at The University of Texas MD Anderson Cancer Center (Houston, TX) from May 1995 through April 2010, as we described previously [2]. In the original study, all patients had newly diagnosed, histopathologically confirmed, previously untreated SCCOP and were enrolled regardless of age at diagnosis, sex, ethnicity, or clinical stage. Patients with distant metastases at presentation were excluded. Before participating in the original molecular profiling study, all patients agreed to sign a consent form. That study was approved by the Institutional Review Board of MD Anderson Cancer Center. That study achieved an approximately 95% enrollment rate by recruited patients. Details of the patients’ follow-up and clinical data were previously reported [1,2].
In the present study, we clearly distinguished SCCOP recurrence from second primary tumors to minimize the overestimation or underestimation of associations. Patients were typically followed and monitored through their treatment and post-treatment course with scheduled regular clinical and radiographic examinations. Patients were considered disease free if absence of disease was documented at the date of the last visit with the head and neck surgeon, head and neck radiation oncologist, or head and neck medical oncologist. There were no universal standards for imaging. Typically patients had either routine serial imaging, or follow-up imaging on the basis of symptoms or findings on physical examinations. Recurrent disease was defined as appearance of a new lesion of the same histology verified by biopsy (incisional, excisional, or needle biopsy), reappearance of any lesion that had disappeared, or development of tumor-related symptoms. Based on modified criteria of Warren and Gates, second primary malignancies (SPMs) were considered if the second lesions were different histopathologic type, or if they occurred more than 5 years following treatment for the index tumor, and/or clearly separated by normal epithelium based on clinical and radiographic assessment. Pulmonary lesions were considered as a SPM if they had a non-squamous histology; or if they were isolated squamous lesions greater than 5 years from initial SCCHN and felt to be SPM by the thoracic oncologist and thoracic surgeon. If there was discrepancy or differing of opinions regarding the origin of the tumor (i.e., SPM vs. recurrence), the second lesion were classified as a local recurrence rather than a SPM. We determined disease stage at presentation for all study patients using the sixth edition of the American Joint Committee on Cancer TNM staging system. Definitive radiotherapy was categorized as radiotherapy alone or radiotherapy in combination with other therapeutic modalities. Patients with comorbidities were categorized into two groups—none to mild and moderate to severe—using a modification of the Kaplan-Feinstein comorbidity index (Adult Comorbidity Evaluation 27) [18].
Genotyping
DNA was extracted from patient blood samples before treatment and was genotyped for five single nucleotide polymorphisms of E2F2 (E2F2-rs6667575, E2F2-rs3218121, E2F2- rs2742976, E2F2-rs3218123, and E2F2-rs3218148) as we previously described [17]. Both positive and negative controls were included for genotyping. For quality control, approximately 10% of samples were selected for retesting, and the repeated test results demonstrated 100% concordance with the original results. The patients were categorized into two risk groups. The high-risk group included patients with 2–5 risk genotypes of the two polymorphisms and the low- risk group included patients with 0–1 risk genotype of either polymorphism.
Tumor HPV16 status and E2F2 protein expression
As described previously, to determine tumor HPV16 status, we subjected DNA from paraffin-embedded tissue specimens to polymerase chain reaction and in situ hybridization [19]. We retested a subset of samples (5%) for tumor HPV16 status for quality control. The retested results were 100% concordant with the findings from the original assays. A total of additional 64 SCCOP patients were selected with tumor specimens available for E2F2 immunohistochemisrty (IHC). Paraffin blocks from SCCOP specimens were sectioned into 4-µM-think sections. For IHC, these sections were immunostained for E2F2 protein with primary antibodies against E2F2 (Epitomics, Burlingame, CA) and immunostained sections were evaluated according to previous study.
Statistical analysis
For this study, SCCOP recurrence was the primary endpoint. Time to recurrence was from the date of SCCOP diagnosis to the date of recurrence. Patients without known recurrence at the date of last follow-up, those lost to follow-up, and those who died of any cause were censored. A Student t-test was used to compare mean age at diagnosis and follow-up time between patients with and without SCCOP recurrence. The association of each demographic, epidemiologic, or clinical variable with time to SCCOP recurrence was evaluated using both univariate and multivariate Cox regression models. The estimates of association of each variable with disease-free survival (DFS)/recurrence status were assessed using a log-rank test. Associations of E2F2 polymorphisms with risk of SCCOP recurrence were estimated using hazard ratios (HRs) and 95% confidence intervals (CIs) among both the whole patients and patients with HPV16(+) tumors, while in this study we did not include similar analyses for patients with HPV16(–) tumors owing to the relatively small sample size and the rarity of SCCOP recurrence in this subgroup. The multivariable Cox model included variables adjusted for major confounders, including demographic variables (age at diagnosis, sex, and ethnicity); epidemiologic factors (smoking status and alcohol use status); and clinical factors (comorbidity group, tumor stage, and treatment). For all analyses, p values less than 0.05 were considered statistically significant, and all tests were two-sided. All statistical analyses were performed using SAS software (version 9.2.3; SAS Institute).
Results
The overall SCCOP recurrence rates, the 5-year recurrence rate, and the associations between DFS and age at diagnosis, sex, ethnicity, smoking, alcohol use, comorbidity group, index cancer stage, and treatment were previously reported [2]. The median follow-up time and mean age at diagnosis among all patients, patients with SCCOP recurrence, and patients without recurrence were also previously reported [2]. We included 1,008 patients in our final analysis [2], and Kaplan-Meier univariate analysis results showed that the 5-year rate of recurrence was significantly different between groups divided according to age at diagnosis, ethnicity, smoking status, alcohol use, comorbidity group, and treatment (all p<0.05) but was not significantly different between the groups for sex and index cancer stage (both p>0.05).
SCCOP patients with the common homozygous E2F2-rs2742976 GG genotype and E2F2-rs3218123 GG genotype had significantly better DFS than those with the other two corresponding genotypes (log-rank, p<0.001 for both polymorphisms; Fig. 1). We further estimated the recurrence rates of 324 patients with HPV16(+) SCCOP and found that DFS was significantly shorter in patients carrying the E2F2-rs2742976 GT/TT and E2F2-rs3218123 GT/TT genotypes than in those carrying the corresponding common homozygous GG genotypes (log-rank for both, p<0.001; Fig. 1). However, significant differences in DFS were not found for the other three polymorphisms: E2F2-rs6667575 (log-rank, p=0.594); E2F2-rs3218121 (log-rank, p=0.100); or E2F2-rs3218148 (log-rank, p=0.060).
Figure 1.
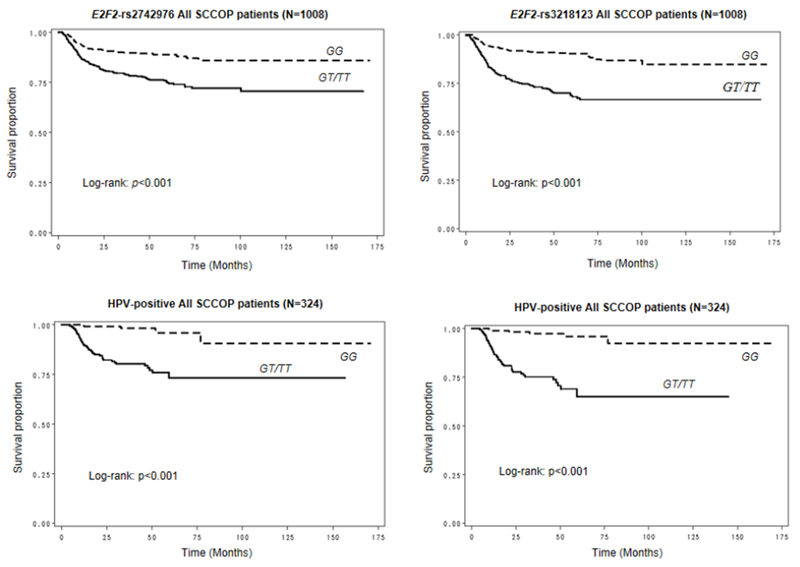
Kaplan-Meier estimates for DFS of patients according to the E2F2-rs2742976 and E2F2-rs3218123 genotypes among all patients and those with human papillomavirus (HPV)– positive SCCOP.
We performed a multivariable Cox proportional hazards regression analysis to assess the associations of genotypes of the five single nucleotide polymorphisms with recurrence risk in patients with SCCOP. As shown in Table 2, compared with patients with the E2F2-rs2742976 GT/TT and E2F2-rs3218123 GT/TT variant genotypes, patients with the E2F2-rs2742976 GG and E2F2-rs3218123 GG common homozygous genotypes had significantly lower risk of SCCOP recurrence (HR, 0.4,[95% CI, 0.3–0.6 and HR, 0.3, 95% CI, 0.2–0.5, respectively). However, no significant differences were observed for the other three polymorphisms: E2F2- rs6667575 (HR, 0.9, 95% CI, 0.7–1.2); E2F2-rs3218121 (HR, 1.3, 95% CI, 0.8–1.9); or E2F2- rs3218148 (HR, 1.3, 95% CI, 0.9–1.8).
Table 2.
Association between E2F2 genotypes and SCCOP recurrence in patients with SCCOP (N=1,008).
| Genotype | No. of recurrence/no. of patients | 5-year recurrence rate | Log-rank p values | aHR1, 95% CI |
|---|---|---|---|---|
| E2F2-rs6667575 | ||||
| GG(Ref.) | 88/474 | 0.17 | 0.594 | 1.0 |
| GA+AA | 93/534 | 0.18 | 0.9 (0.7-1.2) | |
| E2F2-rs3218121 | ||||
| GG(Ref.) | 152/885 | 0.18 | 0.100 | 1.0 |
| GA+AA | 29/123 | 0.24 | 1.3 (0.8-1.9) | |
| E2F2-rs2742976 | ||||
| GT/TT(Ref.) | 138/603 | 0.24 | <0.001 | 1.0 |
| GG | 43/405 | 0.11 | 0.4 (0.3-0.6) | |
| E2F2-rs3218123 | ||||
| GT/TT(Ref.) | 128/461 | 0.30 | <0.001 | 1.0 |
| GG | 53/547 | 0.09 | 0.3 (0.2-0.5) | |
| E2F2-rs3218148 | ||||
| AA(Ref.) | 63/415 | 0.16 | 0.060 | 1.0 |
| AG+GG | 118/593 | 0.21 | 1.3 (0.9-1.8) | |
aHR: adjusted hazard ratio.
Adjusted for age, sex, ethnicity, smoking status, alcohol use status, stage, comorbidity, and treatment.
Ref::Reference group.
Given the important role of HPV and E2F2 in regulating proliferation, cell-cycle progression, and tumorigenesis, we also evaluated the associations of genotypes of the five polymorphisms with risk of recurrence at 5 years among the subgroup of patients with HPV16(+) SCCOP. As shown in Table 3, patients with HPV16(+) tumors and the E2F2- rs2742976 GG and E2F2-rs3218123 GG common homozygous genotypes had significantly lower risk of recurrence (approximately 90%) than those with HPV16(+) tumors and the E2F2- rs2742976 GT/TT and E2F2-rs3218123 GT/TT variant genotypes (HR, 0.1, 95% CI, 0.1–0.4 for E2F2-rs2742976 and HR, 0.1, 95% CI, 0.0–0.2 for E2F2-rs3218123). However, no significant differences were observed for the other three polymorphisms: E2F2-rs6667575 (HR, 0.9, 95% CI, 0.5–1.7); E2F2-rs3218121 (HR, 1.6, 95% CI, 0.6–4.1); or E2F2-rs3218148 (HR, 1.3, 95% CI, 0.7–2.5).
Table 3.
Association between E2F2 genotypes and HPV-positive SCCOP recurrence in patients with SCCOP (N = 324).
| Genotype | No. of recurrence/no. of patients | 5-year recurrence rate | Log-rank p values | aHR1, 95% CI |
|---|---|---|---|---|
| E2F2-rs6667575 | ||||
| GG(Ref.) | 24/157 | 0.16 | 0.464 | 1.0 |
| GA+AA | 21/167 | 0.14 | 0.9 (0.5-1.7) | |
| E2F2-rs3218121 | ||||
| GG(Ref.) | 40/296 | 0.15 | 0.451 | 1.0 |
| GA+AA | 5/28 | 0.20 | 1.6 (0.6-4.1) | |
| E2F2-rs2742976 | ||||
| GT/TT(Ref.) | 41/196 | 0.21 | <0.001 | 1.0 |
| GG | 4/128 | 0.04 | 0.1 (0.1-0.4) | |
| E2F2-rs3218123 | ||||
| GT/TT(Ref.) | 39/146 | 0.31 | <0.001 | 1.0 |
| GG | 6/178 | 0.04 | 0.1 (0.0-0.2) | |
| E2F2-rs3218148 | ||||
| AA(Ref.) | 17/135 | 0.14 | 0.554 | 1.0 |
| AG/GG | 28/189 | 0.16 | 1.3 (0.7-2.5) | |
aHR, adjusted hazard ratio.
aHR, adjusted Hazard ratio for age, sex, ethnicity, smoking status, alcohol use status, stage, comorbidity, and treatment.
Ref: Reference group.
Finally, we explored the joint effect of risk group (based on number of risk genotypes) on risk of recurrence among all patients and the subgroup of those with HPV16(+) SCCOP. As shown in Figure 2, patients in the low-risk group had lower rates of recurrence in both the whole group and the HPV16(+) subgroup (all log-rank p<0.001).Furthermore, after combining risk genotypes of the five polymorphisms and using the high-risk group as the reference group, the low-risk groups were significantly associated with lower risk of recurrence among the whole patients (HR, 0.4, 95% CI, 0.3–0.6) and among the patients with HPV16(+) tumors only (HR, 0.2, 95% CI, 0.1–0.5; Table 4), respectively.
Figure 2.
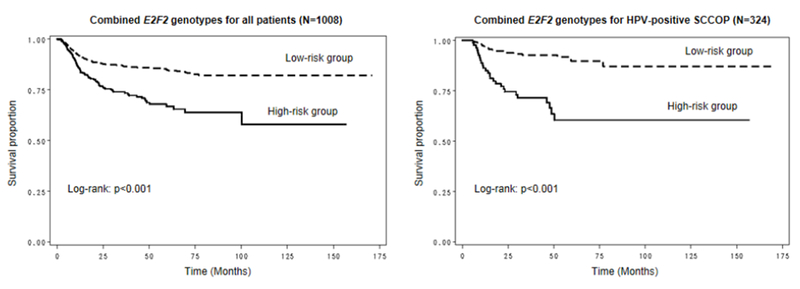
Kaplan-Meier estimates for the DFS of patients according to the combined risk genotypes of the 5 polymorphisms of E2F2 among all patients and those with human papillomavirus (HPV)–positive SCCOP. The high-risk group included patients with 2–5 risk genotypes, and the low-risk group included patients with 0–1 risk genotype.
Table 4.
Association between combined E2F2 genotypes and recurrence risk in overall and tumor HPV-positive patients with SCCOP.
| Combined genotypes | No. of recurrence/no. of patients | 5-year recurrence rate | Log-rank p values | aHR1, 95% CI |
|---|---|---|---|---|
| All patients (N=1008) | ||||
|
| ||||
| High risk group*(Ref.) | 73/247 | 0.32 | < 0.001 | 1.0 |
| Low risk group | 108/761 | 0.14 | 0.4 (0.3-0.6) | |
|
| ||||
| HPV-positive SCCOP patients (N=324) | ||||
|
| ||||
| High risk group(Ref.) | 26/82 | 0.40 | < 0.001 | 1.0 |
| Low risk group | 19/242 | 0.08 | 0.2 (0.1-0.5) | |
high-risk group: individuals carrying 2-5 genotypes; Low-risk group: individuals carrying 0-1 genotype.
aHR, adjusted hazard ratio for age, sex, ethnicity, smoking status, alcohol use status, stage, comorbidity, and treatment.
Ref: Reference group.
To further characterize the potentially functional relevance of E2F2 promoter polymorphisms, we performed correlation analyses between tumor E2F2 expression by E2F2 immunohistochemisrty (IHC) and genotypes of E2F2 polymorphisms among a subset of 64 SCCOP patients. Among 64 SCCOP tissue specimens, we found that 50 patients had HPV16- positive tumors and 14 patients had HPV16-negative tumors. In all patients, we found that the E2F2-rs2742976 GG genotype had a significantly higher E2F2 protein expression than E2F2- rs2742976 GT/TT genotypes (P = 0.028) and E2F2-rs3218123 GG genotype had a borderline significantly higher E2F2 protein expression than E2F2-rs3218123 GT/TT genotypes (P = 0.062), while no significant associations of other E2F2 SNPs with E2F2 protein expression (P = 0.345 for E2F2-rs6667575, P = 0.653 for E2F2-rs3218121, and P = 0.768 for E2F2-rs3218148, respectively). In HPV16-negative cases, the E2F2 protein expression was not significantly different between genotypes of any E2F2 SNPs.
Furthermore, in HPV-positive patients only, the patterns of above associations of these SNPs of E2F2 with E2F2 protein expression remained similar. E2F2-rs2742976 GG genotype had a significantly higher E2F2 protein expression than E2F2-rs2742976 GT/TT genotypes (P = 0.013) and E2F2-rs3218123 GG genotype had a borderline significantly higher E2F2 protein expression than E2F2-rs3218123 GT/TT genotypes (P = 0.057), while no significant associations of other E2F2 SNPs with E2F2 protein expression (P = 0.567 for E2F2-rs6667575, P = 0.687 for E2F2-rs3218121, and P = 0.877 for E2F2-rs3218148, respectively). In HPV16- negative cases, no any significant associations were found for any SNPs.
In this study, we did not perform the survival analysis by E2F2 protein expression because there were few outcome events (e.g., death, recurrence etc) among these 64 SCCOP patients. However, these findings will be validated in our future study with larger patients whose tumor specimens become available.
Discussion
We found that the E2F2-rs2742976 and E2F2-rs3218123 variants were significantly associated with the risk of SCCOP recurrence, particularly in patients with HPV16(+) tumors. Furthermore, we found that patients with more than 1 risk genotype among the five E2F2 polymorphisms had higher risk of SCCOP recurrence, especially in patients with HPV16(+) SCCOP tumors. These findings suggest that certain E2F2 polymorphisms may individually or jointly predict risk of SCCOP recurrence, particularly in patients with HPV16(+) tumors.
Although the exact mechanism by which these E2F2 promoter variants modify cancer recurrence remains unknown, it is likely that these variants could alter expression of E2F2 and thus might affect susceptibility of response to radiotherapy, leading to different clinical outcomes. Therefore, E2F2 variants could be potential predictive biomarkers for SCCOP recurrence and could help identify patients for individualized therapies. Binding of E7 to phosphorylated RB leads to activation of E2F2, which induces cell-cycle progression and p16 expression. Thus, it is biologically plausible that E2F2 promoter polymorphisms might affect E2F2 protein expression, which, in turn, could affect p16 expression level, thereby influencing cancer prognosis. So far, no investigations on functional relevance of these E2F2 promoter polymorphisms have been reported. Since E2F2 regulates expression of p16 through Rb- mediated pathways and these E2F2 polymorphisms are within the functional regions of the E2F2’s promoter, it is likely that these E2F2 polymorphisms may have potentially functional effect on expression levels of p16, thus leading to better survival. In fact, in this study, we found that E2F2-rs2742976 polymorphism significantly affected expression of E2F2 in SCCOP patients, particularly HPV16-positive patients but not in HPV16-negative cases in SCCOP tumor specimens. Although the functional relevance of the E2F2-rs2742976 polymorphism has not yet been elucidated, our results could partially suggest a functional correlation between this polymorphism and E2F2 expression, which may provide preliminary evidence of biological plausibility for the observed association in the current study. As we found from IHC staining, individuals with E2F2-rs2742976 GG genotype had significantly higher E2F2 expression than those with GT/TT genotypes in SCCOP patients, particularly HPV16-positive SCCOP patients, implying that binding of HPV16 E7 to Rb might lead to release of transcription factor E2F2 in HPV-associated SCCOP patients. Therefore, genetic variations, such as E2F2-rs2742976 polymorphism, may affect promotion of cell cycle progression and induction of p16 expression, thereby leading to better survival of SCCOP. However, all these findings need to be validated in future large and well-designed studies.
Several studies have shown the associations of E2F2 promoter polymorphisms with risk of various types of human cancer [20–22], but no studies have investigated the modifying effect of E2F2 polymorphisms on SCCOP recurrence risk, especially for HPV–associated SCCOP. Chen et al. reported that E2F2 was a potential marker for the prognosis of patients with non– small cell lung cancer [12]. Similarly, an association of ovarian cancer risk with E2F2-rs760607 and E2F2-rs3218203 polymorphisms was reported [20]. However, Lu et al. did not find a main effect of E2F2 variants or other variant genotypes on risk of squamous cell carcinoma of the head and neck [17], and Justenhoven et al. found that the E2F2_−5368_A>G (rs760607) promoter polymorphism was not significantly associated with risk of breast cancer [21]. In our study, we found a significant association between the E2F2-rs2742976 or E2F2-rs3218123 variant genotypes and an increased risk of SCCOP recurrence; however, we did not observe significant differences for the other three E2F2 polymorphisms.
The inconsistent results regarding the associations of E2F2 promoter polymorphisms with cancer risk and prognosis suggest that these effects might also depend on other factors, such as cancer type, genetic background, environmental factors, sample size, disease stage, treatment, adequacy of adjustment for other confounding factors, and different study populations [1]. The retinoblastoma-E2F pathway and other molecular pathways involved in cell-cycle control, DNA repair, and apoptosis [23,24] and/or interactions between functional genetic variants and therapeutic agents may also cause interindividual differences in prognosis. Tumor HPV status also significantly affected risk of SCCOP recurrence: patients with HPV16(+) SCCOP had a better prognosis than those with HPV(−) SCCOP. Patients with HPV(+) SCCOP, who have few somatic mutations, appear to have a better response to radiotherapy than those with HPV(−) SCCOP, whose disease is mainly driven by smoking and who have common somatic mutations, such as those in p53. Therefore, tumor HPV status significantly predicts SCCOP prognosis, and future studies of factors potentially related to SCCOP prognosis and recurrence should consider stratified analysis by tumor HPV status.
We also assessed the effect of E2F2 promoter variants on recurrence risk in patients with HPV16(+) SCCOP. The modifying effect of E2F2-rs2742976 and E2F2-rs3218123 variant genotypes on SCCOP recurrence risk was higher in SCCOP patients with HPV16(+) tumors than in those with common homozygous genotypes, suggesting that E2F2-rs2742976 and E2F2-rs3218123 variant genotypes interact with HPV status to affect SCCOP recurrence risk. Although the molecular mechanisms underlying these findings in patients with HPV16(+) SCCOP remain incompletely understood, the variant genotypes of these two polymorphisms may affect DNA repair capacity or apoptosis through controlling the cell cycle [22]. We also found that patients with more than one risk genotype had a significantly higher risk of SCCOP recurrence, particularly patients with HPV16(+) tumors, which supports the notion that SCCOP is mainly driven by HPV [25,26]. Thus, E2F2 variants may affect susceptibility to radiotherapy and lead to different recurrence outcomes. However, this hypothesis should be investigated in future studies.
Our study has several strengths including its relatively large sample size, inclusion of tumor HPV status, and analysis of a homogeneous tumor site (SCCOP only). However, we did not have data about the exact dosage and duration of radiotherapy for our patients; detailed information on radiotherapy should be collected in future studies. Second, because there were relatively small numbers of patients with HPV16(+) tumors and recurrence, our results could have been due to chance. Third, most of our patients were non-Hispanic whites recruited at a single cancer center; thus, our results should be validated in other racial and ethnic populations. Lastly, SCCOP recurrence was identified retrospectively without a strictly defined screening or follow-up regimen, and our study likely had recall bias and selection bias.
Taken together, our findings suggest that the E2F2 promoter variants might individually or in combination modulate the risk of SCCOP recurrence, especially in patients with HPV16(+) tumors who have received definitive radiotherapy. However, the prognostic utility of these promoter variants should be validated, and further exploration of the molecular mechanisms underlying our findings is warranted.
Table 1.
Characteristics of patients with SCCOP (N = 1,008).
| Variable | No. of patients (n, %) | No of patients with recurrence | 5-year recurrence rate (%) | p1 value |
|---|---|---|---|---|
| No. of patients | 1008 (100) | 181 | 0.20 | |
| Age | ||||
| ≤ 57 years | 621 (61.6) | 85 | 0.15 | <0.0001 |
| > 57 years | 387 (38.4) | 96 | 0.27 | |
| Sex | ||||
| Male | 872 (86.5) | 161 | 0.20 | 0.3110 |
| Female | 136 (13.5) | 20 | 0.19 | |
| Ethnicity | ||||
| Non-Hispanic white | 913 (90.6) | 146 | 0.17 | <0.0001 |
| other | 95 (9.4) | 35 | 0.41 | |
| Smoking | ||||
| Never | 388 (38.5) | 51 | 0.14 | 0.0004 |
| Ever | 620 (61.5) | 130 | 0.23 | |
| Alcohol use | ||||
| Never | 247 (24.5) | 26 | 0.10 | 0.0005 |
| Ever | 761 (75.5) | 155 | 0.23 | |
| Comorbidity | ||||
| None or mild | 913 (90.6) | 157 | 0.19 | 0.0370 |
| Moderate to severe | 95 (9.4) | 24 | 0.27 | |
| Index cancer stage | ||||
| 1 or 2 | 72 (7.1) | 11 | 0.19 | 0.5280 |
| 3 or 4 | 936 (92.6) | 170 | 0.20 | |
| Treatment | ||||
| X/XC/SX | 947 (93.9) | 166 | 0.19 | 0.0030 |
| SXC | 61 (6.1) | 15 | 0.32 | |
X, radiotherapy; C, chemotherapy; and S, surgery.
p: Log-rank test for DFS between the two groups.
Novelty and impact statements.
E2F2 promoter variants individually or jointly modify the risk of SCCOP recurrence, and may be a predictive biomarker for recurrence risk of SCCOP, particularly in HPV-positive SCCOP patients.
Acknowledgments
The authors thank Margaret Lung, Kathryn L. Tipton, Liliana Mugartegui, and Angeli Fairly for their help with participant recruitment and Li-E Wang for laboratory management.
Funding support: This work was supported by The University of Texas MD Anderson Cancer Center start-up funds to E.M.S., the National Institutes of Health Head and Neck Specialized Program of Research Excellence Career Development Award (P50CA097007 to E.M.S.), The University of Texas MD Anderson Cancer Center Institutional Research Grant to E.M.S., the National Institutes of Health (ES 011740 and CA131274 to Q.W.), the Clinician Investigator Award (K12 CA88084 to E.M.S.), the National Institutes of Health Cancer Center Support, The University of Texas MD Anderson Cancer Center (CA16672), and National Institutes of Health grants (CA135679 to G.L., CA133099 to G.L., and CA186261–01A1).
Abbreviations
- CI
confidence interval
- DFS
disease-free survival
- E2F2
E2F transcription factor 2
- HPV
human papillomavirus
- HPV16
HPV type 16
- HPV16(+)
HPV–positive
- HR
hazard ratio
- SCCOP
squamous cell carcinoma of the oropharynx.
Footnotes
Conflict of Interest statement: None
References
- 1.Zhang C, Sturgis EM, Zheng H, et al. Genetic variants in TNF-α promoter are predictors of recurrence in patients with squamous cell carcinoma of oropharynx after definitive radiotherapy. Int J Cancer 2014;134:1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F, Sturgis EM, Sun Y, et al. Apoptotic variants as predictors of risk of oropharyngeal cancer recurrence after definitive radiotherapy. Int J Cancer 2015;137:2454–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherr CJ. Cancer cell cycles. Science 1996;274:1672–1677. [DOI] [PubMed] [Google Scholar]
- 4.Reimer D, Sadr S, Wiedemair A, et al. Expression of the E2F family of transcription factors and its clinical relevance in ovarian cancer. Ann N Y Acad Sci 2006;1091:270– 281. [DOI] [PubMed] [Google Scholar]
- 5.Reimer D, Sadr S, Wiedemair A, et al. Clinical relevance of E2F family members in ovarian cancer: an evaluation in a training set of 77 patients. Clin Cancer Res 2007;13:144–151. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki DE, Nakahata AM, Okamoto OK. Knockdown of E2F2 inhibits tumorigenicity but preserves stemness of human embryonic stem cells. Stem Cells Dev 2014;23:1266– 1274. [DOI] [PubMed] [Google Scholar]
- 7.Butt AJ, Caldon CE, McNeil CM, Swarbrick A, Musgrove EA, Sutherland RL. Cell-cycle machinery: links with genesis and treatment of breast cancer. Adv Exp Med Biol 2008;630:189–205. [DOI] [PubMed] [Google Scholar]
- 8.Nam EJ, Kim YT. Alteration of cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol Cancer 2008;18:1169–1182. [DOI] [PubMed] [Google Scholar]
- 9.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science 1996;274:1664–1672. [DOI] [PubMed] [Google Scholar]
- 10.Pharoah PD, Tyrer J, Dunning AM, Easton DF, Ponder BA. Association between common variation in 120 candidate genes and breast cancer risk. PLoS Genet 2007;3:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim MY, Dahlstrom KR, Sturgis EM, Li G. Human papillomavirus integration pattern and demographic, clinical, and survival characteristics of patients with oropharyngeal squamous cell carcinoma. Head Neck 2016;doi:10.1002/hed.24429. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Yu JH, Lu ZH, Zhang W. E2F2 induction related to cell proliferation and poor prognosis in non-small cell lung carcinoma. Int J Clin Exp Pathol 2015;8:10545–1054. [PMC free article] [PubMed] [Google Scholar]
- 13.Raju V Pusapati, Regina L Weaks, Robert J Rounbehler, Mark J McArthur, David G Johnson. E2F2 suppresses Myc-induced proliferation and tumorigenesis. Mol Carcinog 2010;49:152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Etzel CJ, Amos CI, et al. Genetic variants in the cell-cycle control pathways contribute to early onset colorectal cancer in Lynch syndrome. Cancer Causes Control 2009;20:1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Justenhoven C, Pierl CB, Haas S, et al. Polymorphic loci of E2F2, CCND1, and CCND3 are associated with HER2 status of breast tumors. Int J Cancer 2009;124:2077–2081. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Paranjape T, Stahlhut C, et al. Targeted resequencing of the microRNAome and 3’UTRome reveals functional germline DNA variants with altered prevalence in epithelial ovarian cancer. Oncogene 2015;4:2125–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M, Liu Z, Yu H, et al. Combined effects of E2F1 and E2F2 polymorphisms on risk and early onset of squamous cell carcinoma of the head and neck. Mol Carcinog 2012;51:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441–2447. [DOI] [PubMed] [Google Scholar]
- 19.Ji X, Sturgis EM, Zhao C, Etzel CJ, Wei Q, Li G. Association of p73 G4C14-to-A4T14 polymorphism with human papillomavirus type 16 status in squamous cell carcinoma of the head and neck in non-Hispanic whites. Cancer 2009;115:1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham JM, Vierkant RA, Sellers TA, et al. Cell-cycle genes and ovarian cancer susceptibility: a tagSNP analysis. Br J Cancer 2009;101:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justenhoven C, Pierl CB, Haas S, et al. Polymorphic loci of E2F2, CCND1, and CCND3 are associated with HER2 status of breast tumors. Int J Cancer 2009;124:2077–2081. [DOI] [PubMed] [Google Scholar]
- 22.Longworth MS, Wilson R, Laimins LA. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J 2005;24:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer 2009;9:738–748. [DOI] [PubMed] [Google Scholar]
- 24.DeGregori J, Johnson DG. Distinct and overlapping roles for E2F family members in transcription, proliferation, and apoptosis. Curr Mol Med 2006;6:739–748. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie MB, Rubinchik S, Hoel B, Sutkowski N. Human papillomavirus and oropharyngeal cancer: what you need to know in 2009. Curr Treat Options Oncol 2009;10:296–307. [DOI] [PubMed] [Google Scholar]
- 26.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 2007;13:1186–1191. [DOI] [PubMed] [Google Scholar]


