Abstract
Rationale
Considerable research suggests that nicotine enhances cognitive control-related processes (e.g., attention, memory) among nicotine-deprived smokers, both in terms of behavior and neural indices (e.g., ERP, slow-wave EEG). Nicotine may also increase cognitive control among nonsmokers, and this may vary as a function of trait cognitive control. It is important to examine the effects of nicotine on cognitive control-related processes among nonsmokers as these effects may provide a path for the initiation of smoking.
Objectives
The objectives of the study were to examine in nonsmokers (1) the effect of nicotine on resting cortical activity, an indirect measure of cognitive control, and (2) trait cognitive control as a moderator of nicotine-induced cortical activity changes.
Method
Eighty participants were given placebo and 7-mg nicotine patches in separate sessions for this counter-balanced, double-blind, within-subject study. Resting cortical activity was measured with EEG for a 3-min period with eyes opened.
Results
Average alpha-1 band power density values in frontal and central regions were lower during the nicotine versus placebo condition, which provides evidence of nicotine-induced cortical activation. Furthermore, those with lower self-reported cognitive control exhibited greater nicotine-induced reductions in alpha-1 power density values.
Conclusions
These individual differences in nicotine-induced cortical activation are consistent with a model of nicotine self-medication whereby individuals with lower cognitive control may find smoking more reinforcing via amelioration of related cognitive deficits.
Keywords: Nicotine, Smoking, EEG, Personality, Cognitive control
The association between nicotine and cognitive control is well-established in heavy smokers. Cognitive control refers to a wide range of attention- and memory-related processes relevant to the performance of daily activities that require effort (Pontifex et al. 2011). Smokers deprived of nicotine (e.g., 12-h smoking abstinence) exhibit reduced cognitive-attentional functioning (Heishman et al. 1994; Leventhal et al. 2007). In particular, smokers’ behavioral performance on rapid visual information processing tasks and similar tasks of sustained/vigilant attention show consistent decrements following nicotine deprivation (Gilbert et al. 2005; Lawrence et al. 2002; Mancuso et al. 1999; Sacco et al. 2005; Zack et al. 2001).
In addition to substantial evidence regarding the restorative effect of nicotine on cognitive control deficits experienced during nicotine withdrawal among smokers, a growing consensus supports absolute facilitation of cognitive control-related processes by nicotine, even among nonsmoker (Heishman 1998; Heishman et al. 1994; Kleykamp et al. 2005). A recent meta-analysis concluded that nicotine facilitates multiple attention and memory processes independent of nicotine withdrawal reversal (Heishman et al. 2010).
Nicotine is generally an agonist to an array of major transmitter/modulator systems (e.g., Singh et al. 2004). Evans and Drobes (2009) suggested that each system differentially contributes to cognitive control-related processes which may result in diverse patterns of nicotine self-medication. In other words, nicotine may be ameliorative to an array of neurotransmitter deficits that play key roles in cognitive control. Thus, the specific processes “medicated” by nicotine may vary widely across smokers, but with the net effect being improved cognitive control.
The proposal that nicotine differentially affects individuals lower in cognitive control is frequently advanced (e.g., Evans et al. 2013; Newhouse et al. 2004; Potter and Newhouse, 2008), but the majority of supporting evidence is indirect (e.g., individuals with severe psychopathology) with few studies conducted in non-clinical populations. Nicotine-induced amelioration of cognitive control-related deficits (e.g., sensory gating; see Jessen et al. 2001) has been observed among individuals with schizophrenia (Adler et al. 1993). This effect has also been observed in the first degree relatives of individuals with schizophrenia (Adler et al. 1992), suggesting that nicotine may be reducing the cognitive deficit independent of psychological disorder status. Indeed, Evans et al. (2013) found that nicotine deprivation was associated with reduced event-related brain potential (ERP) indices of cognitive control-attentional function (i.e., orienting/novelty P300 or P3a) among smokers who report reduced levels of daily cognitive control.
Electroencephalogram (EEG) activity collected while subjects are sitting quietly has been found to be highly related to individual differences in cognitive control. For example, individuals with attentional deficit disorder show higher power density values associated with slower wave EEG bands (Hermens et al. 2005). Furthermore, suppression of slower wave EEG is associated with performance on effortful cognitive tasks (Larson et al. 1998). Individuals lower in attentional control have also been found to exhibit higher ratios of slow wave to fast wave power density values, and this ratio EEG power effect correlated negatively with response inhibition on a go/no-go task (Putman et al. 2010). In summary, higher slow wave EEG is observed in individuals who exhibit chronic cognitive control deficits and is related to poorer performance on tasks requiring cognitive control.
Those with extreme, chronic cognitive control problems are often treated with stimulant medications. Consistent with the above findings, it has been observed that stimulant medications reduce slow wave EEG (Clarke et al. 2002; Loo et al. 1999). Furthermore, there are several studies showing that nicotine has a stimulant-like effect on resting EEG including the reduction of slow wave EEG (Fisher et al. 2012; Knott and Fisher 2007; Knott et al. 2005).
Interestingly, nicotine administered to smokers during withdrawal reduces slow wave EEG in a manner that is consistent with an increase in cognitive control. For example, Knott and Fisher (2007) found that nicotine reduced power density values in slow wave bands and increased power density values in the fast alpha (i.e., alpha-2) and beta band among smokers experiencing nicotine withdrawal. This general pattern has also been observed during cigarette smoking (Knott 1988). In contrast, Evans et al. (2015) recently found that nicotine withdrawal effects on the resting EEG spectrum are limited to theta and alpha-1 enhancement, with no evidence of effects on faster wave activity.
Nicotine-induced cortical activation is of interest because nicotine may reinforce the initial development of dependence (i.e., before tolerance and concomitant withdrawal are fully developed). A nonsmoker sample is therefore ideal for examining changes in putative neural substrates of cognitive control. Only two studies (Fisher et al. 2012; Foulds et al. 1994) have previously examined the effects of nicotine on the EEG of nonsmokers. The Foulds et al. study did not find nicotine effects for any EEG bands, but was underpowered (N=4). The Fisher et al. study (N=20) found trend level support for a nicotine × region (frontal vs. parietal) interaction, with some indications that nicotine increased frontal (primarily left) alpha-2 power density values.
The current study mimics Evans et al. (2015) where resting cortical activity was higher when heavy smokers received nicotine following abstinence relative to placebo (i.e., nicotine withdrawal). We examined nicotine effects on resting EEG among nonsmokers, using a larger sample and a denser EEG electrode array than the two prior studies. These secondary data were part of a study assessing the effect of nicotine on cognitive performance in nonsmokers (Evans et al. 2014). Furthermore, it has been suggested that nicotine acts differently on men and women (Perkins and Scott 2008), and some studies of nicotine effects on EEG among smokers have reported gender differences. Thus, the current study recruited equal numbers of men and women with sufficient power to detect medium effect sizes for gender.
Individual differences in trait cognitive control are important with respect to models of nicotine self-medication of cognitive control (Evans and Drobes 2009). Therefore, this study also explored the moderating effects of two well-established self-report measures (i.e., Cognitive Failures Questionnaire/CFQ and Adult Temperament Questionnaire-Attentional Control Scale/ATQ-ACS) on nicotine-induced changes in the EEG.
Specific a priori hypotheses were not advanced, but the literature cited above converges in the direction of nicotine-induced decreases in slow wave and increases in fast wave resting EEG, which may be indicative of enhanced cognitive control (i.e., attention- and memory-related processes). Specifically, the results of Evans et al. (2015) increased cortical activity in response to nicotine among smokers. Furthermore, based on the general model of nicotine self-medication of cognitive control deficits, nicotine is expected to produce greater EEG changes among individuals who self-report lower trait cognitive control.
Methods
Participants
Eighty-six nonsmokers recruited from the Tampa Bay area completed two experimental sessions of this double-blind, within-subject study of nicotine effects on neural indices of cognition. See Evans et al. (2014) for results of the primary study. Eligible participants met the following inclusion criteria: 18–45 years of age, had smoked no more than five cigarettes in their lifetime, had not smoked and/or used any nicotine-containing products within the past 5 years, and had expired air carbon monoxide levels <5 ppm. Participants were excluded who tested positive for psychoactive substance/drug use, were pregnant, self-reported using other medications that might affect physiological responding (e.g.,beta blockers), reported a significant neurological condition (e.g., past concussion, Parkinson’s, Huntington’s disease, epilepsy, dementia), reported a serious medical condition, or had vision problems that could not be corrected-to-normal. Two additional eligible participants were excluded because nausea, sickness, or vomiting interfered with their capacity to complete the study.
Procedures
Eligibility was determined using an initial phone screening and an in-person screening session. Eligible participants provided informed consent, and then completed demographic, personality, and current affective state measures at the screening session. For each experimental session, participants visited the laboratory 4–5 h prior for placement on the upper arm of a 7-mg Habitrol transdermal nicotine patch (nicotine condition) or a placebo patch (placebo condition). The Habitrol nicotine patch was used in an attempt to minimize the likelihood of nausea, headaches, etc. that may occur among nonsmokers because research suggests that it produces a slower rise in blood nicotine (Gupta et al. 1995). Approximately 0.05 cm3 of0.035 % capsaicin cream (CApzasin-HP7, Chattem, Inc.) was applied to the edge of each of the patches to control for the sensation sometimes associated with the nicotine patch. The patches were covered by a bandage to keep participant and experimenter blind to condition. Participants were told that they were able to remove the patch if they had any complaints, but in that event would not be able to continue the study.
During each 90-min experimental session (3–10 days apart), resting EEG was acquired approximately 10 min after the electrode cap was fitted at the start of the session. Participants also completed several questionnaires (affective state/craving) and cognitive tasks, including the 3-stimulus oddball and N-back tasks (not reported here). Compensation for participation was approximately $175, varying slightly by task performance.
Self-report measures
Self-report demographics collected during the initial screening session included gender, ethnicity/race, age, education level, income, and current occupation. Several trait measures were administered at the same session, with two being relevant to the current study. The Cognitive Failures Questionnaire (CFQ) (Broadbent et al. 1982) is a 25-item measure of absent-mindedness (e.g., “Do you daydream when you ought to be listening to something?”) that has been partially validated in relation to various ERP measures of attentional control (Roche et al. 2005). Higher scores on the CFQ represent more frequent cognitive failures and, in turn, reduced cognitive control (Broadbent et al. 1982). The Adult Temperament Questionnaire-Attentional Control Scale (ATQ-ACS) (Evans and Rothbart 2007) is a 12-item measure of attentional control (e.g., “When I am trying to focus my attention, I am easily distracted”). Higher scores on the ATQ-AC are indicative of greater cognitive-attentional control (Evans and Rothbart 2007). Due to experimenter error, three CFQ and five ATQ measures were not completed.
EEG recording
Research assistants who were blind to condition conducted the experimental sessions. The 64-channel EEG array with the 10–20 montages for electrode placement was used. EEG data was collected using the Neuroscan Synamps 2 system and its accompanying Neuroscan 4.3.2 software. EEG was sampled at 250 Hz with online low-pass filtering at 100 Hz and a reference electrode located near the vertex. Electrooculogram (EOG) was recorded from bipolar electrodes placed above and below the left eye and lateral to each eye for measuring vertical and horizontal eye movements, respectively. Impedance was monitored throughout the session and was generally kept below 10 kΩ at all channels.
Participants were instructed to sit in an upright position with eyes open for a single 3-min period. The participants were viewed on a video monitor to verify that they were following instructions.
EEG data processing
EEG was re-sampled offline at 256 Hz. EEG was re-referenced offline to the average of the mastoid electrodes then visually examined for artifact (e.g., eye blinks and movements, excessive EMG). Artifacts containing segments were removed at all electrode sites. One thousand twenty-four millisecond epochs that begin every 512 ms were computed across the 3-min session (up to 360 epochs). Seven sessions from six different participants were found to have less than 70 valid epochs. These six participants were excluded from data analyses. EEG from any single electrode with significant artifact (i.e., a faulty electrode) or no signal (broken electrode) from within a valid session was deleted prior to additional processing. Seventy-five percent of all good sessions had no broken or faulty electrodes, 19 % had 1–2, and 6 % had 3–6.
Power densities μV2/Hz) for the theta (4–7 Hz), alpha-1 (8–10 Hz), alpha-2 (10–13 Hz), beta-1 (14–20 Hz), and beta-2 (21–30 Hz) EEG bands were computed from EEG at 14 homologous electrode pairs (Fp1/2, AF3/4, F3/4, F7/8, C3/4, T7/8, P3/4) and three midline electrodes (Fz, Cz, Pz). These sites from the traditional 10–20 montages were selected based on prior research examining nicotine effects on EEG among smokers (Evans et al. 2015). This montage allows for examination of EEG across most of the cortex: frontal pole, anterior frontal, midfrontal, lateral frontal, central, temporal, and parietal. A natural log transform was used to de-skew the distribution of power density values for each band at each site.
Data analyses overview
Separate mixed models with maximum likelihood estimation were used to examine the effects of nicotine on each EEG band for the seven homologous electrode pairs (e.g., midfrontal set, F3/F4) and for the midline set (i.e., Fz/Cz/Pz). This approach allowed for the maximum use of available data given faulty electrodes for some sessions. In addition to condition (nicotine vs. placebo), the model included session (first vs. second), hemisphere or region (left vs. right for homologous pairs or frontal vs. central vs. parietal for midline set), and all interaction terms including these variables with primary focus on the condition × hemisphere interaction. Separate models assessed gender and trait cognitive control (both CFQ and ATQ) as a potential moderator of nicotine effects (e.g., CFQ × condition). Alpha was set at 0.05 for all analyses.
Results
Demographics
There were 40 women and 40 men between the ages of 18 and 45 (M=27.2, SD=8.3). Mean years of education was 14.3 (SD=2.2). Self-reported race was 52 Whites, 21 Blacks, 3 Asians, 1 Pacific Islander, and 1 Native American with 2 not responding. Self-reported ethnicity was 57 non-Hispanics and 19 Hispanics with 4 not responding.
Trait cognitive control measures
CFQ values (M=28.7, SD=12.3) ranged from 1 to 59 for the 77 participants who completed the entire questionnaire. ATQ-ACS values (M=60.9, SD=13.1) ranged from 26 to 84 for the 75 participants who completed the entire questionnaire. Cronbach’s alpha for the two measures in this sample were 0.90 and 0.89, respectively, and their correlation was −0.669 (p<0.0001). There were no significant gender differences for these two measures (p’s>0.46).
Nicotine effects on EEG
Figure 1 presents three sets of average estimated power density values for the seven homologous pairs (combined) and the midline set (combined): one for the theta, alpha-1, and alpha2 band. Significant condition differences were consistently observed in alpha-1. Average power density values were significantly lower in the nicotine condition for the frontal pole (Fp1/2), anterior frontal (AF3/4), midfrontal (F3/4), lateral frontal (F7/8), central (C3/4), and midline (Fz/Cz/Pz) sets (p’s<0.042), but not for the temporal (T7/8) and parietal (P3/4) sets (p’s>0.14). There were no significant condition × hemisphere interactions (p’s>0.19). A post hoc test compared the nicotine-placebo difference for the midfrontal and parietal sites to assess prospective regional differences. The region × condition interaction was not significant (p>0.29).
Fig. 1.
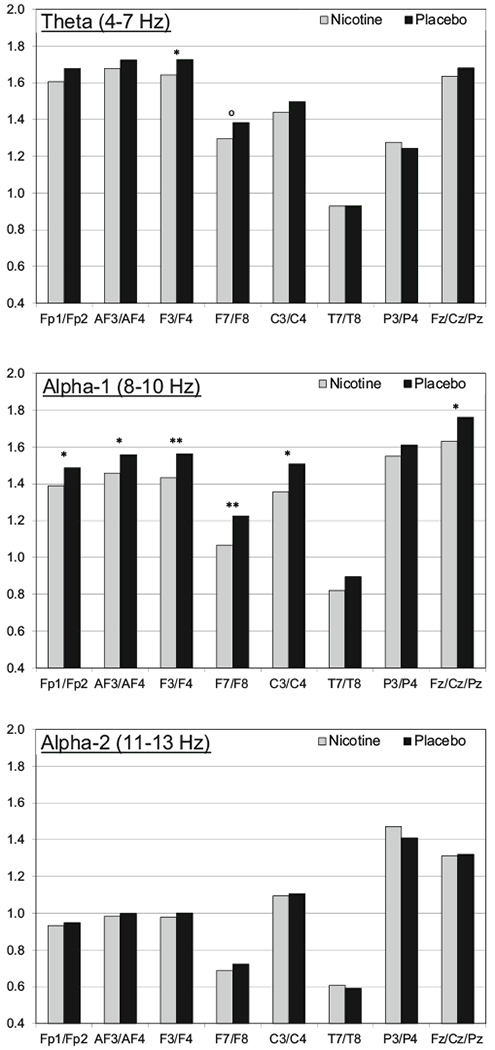
Adjusted means for the 7-mg nicotine patch (gray) and placebo (black) condition are presented for the theta (top), alpha-1 (middle), and alpha-2 (bottom) bands. The adjusted means are the average ln-transformed power density values across the homologous pair (e.g., Fp1/Fp2 for the frontal pole) controlling for the influence of session, hemisphere/region, and all interaction terms as presented in the primary mixed models. p values for the condition effect are noted as follows: °p<0.10; *p<0.05; **p<0.01; ***p<0.001
For theta, lower power density values in the nicotine condition were also observed, but the difference was significant only the midfrontal pair (p=0.047). The lateral frontal pair condition difference was marginally significant (p=0.079). There was a significant condition × hemisphere interaction for the anterior frontal pair (p=0.023) with a significant effect of nicotine (i.e., lower power density) only on the leftside (p=0.039). For alpha-2, there were no main effects for condition (p’s>30). There was a significant condition × hemisphere interaction for the lateral frontal pair (p=0.024) with a significant effect of condition only on the left side (p=0.048). There were no significant condition main effects or condition × hemisphere interactions for the beta-1 and beta-2 bands.
Moderation of nicotine effects
Gender and the two self-report measures of trait cognitive control were assessed as moderators of the nicotine effect. The interaction of gender and condition was not found to be significant for any band. In contrast, trait cognitive control as measured by the CFQ was a significant moderator. For the alpha-1 band, the CFQ × condition interaction was significant for the frontal pole, anterior frontal, central, parietal, and midline sets (p’s<0.035), marginally significant for the midfrontal and lateral frontal pairs (p’s<0.063), and not significant for the temporal pair (p=0.11). Figure 2 shows average placebo-nicotine differences for each set of electrodes for those below and those above the CFQ median. At all sites, higher CFQ scores (i.e., lower cognitive control) were indicative of greater nicotine-induced reduction in alpha-1 power density values. For the theta band, there were two significant CFQ × condition interactions with the same pattern as for alpha-1: anterior frontal pair (p=0.026) and midline set (p=0.033). There were no significant interactions for alpha-2 and beta-1. However, for beta-2, there were significant interactions at the frontal pole and midfrontal sites (p’s<0.024) with relatively higher power density values in the nicotine condition for those with higher CFQ scores.
Fig. 2.

Alpha-1 band nicotine minus placebo-adjusted means for low (white) and high (gray) CFQ groups (median split for presentation). The adjusted means are the average ln-transformed power density values across the homologous pair (e.g., Fp1/Fp2 for the frontal pole) controlling for the influence of session, hemisphere/region, and all interaction terms as presented in the primary mixed models. p values for the CFQ × condition interaction are noted as follows: °P<0.10; *p<0.05; **p<0.01; ***p<0.001
The ATQ-ACS measure of trait cognitive control was less likely to moderate the main effect of nicotine. There were no significant ATQ-ACS interactions for theta, alpha-1, alpha-2, or beta-1. For beta-2, there were significant interactions at the anterior frontal and central sites (p’s<0.048). Those with lower ATQ-ACS scores (lower trait levels) exhibited larger nicotine-placebo differences, with the nicotine condition resulting in greater beta-2 power.
Discussion
This study examined nicotine effects on resting EEG among nonsmokers using a large sample and a relatively broad EEG electrode array. Main effects were observed across anterior, central, and midline sites for the alpha-1 band, and for some frontal sites for the theta band. Power density values were significantly lower during the nicotine condition, which is consistent with nicotine-withdrawal reversal effects in smokers (Evans et al. 2015). These results are in contrast with previous research (Fisher et al. 2012; Foulds et al. 1994) that used smaller samples and fewer electrodes.
The current study is the first to support cognitive control-related traits as moderators of nicotine-induced changes in a nonsmoker sample. The CFQ trait measure of cognitive control was found to moderate the effect of nicotine on alpha-1, and to lesser extents on theta and beta-2. These effects suggest that nicotine increases cortical activity selectively among individuals who report reduced cognitive control. The ATQ was not as strong of a moderator as the CFQ, and only moderated the influence of nicotine on beta-2, with the effect being the same as for the CFQ. These results suggest that individuals reporting poorer cognitive control display greater nicotine-induced cortical activation. These results are consistent with research examining individuals with attentional deficits, as well as research examining performance on cognitive tasks as a correlate of resting EEG (Hermens et al. 2005).
The present trait moderation findings may have important implications for understanding nicotine self-medication of cognitive control, as it may relate to the development of nicotine dependence among smokers. The nicotine effects observed in the current study could not be the result of nicotine withdrawal, as this was a nonsmoker sample. During the initial phases of smoking initiation, the effects of nicotine may reduce cognitive deficits via absolute facilitation of cognition independent of withdrawal effects. These earlier stages may result in accelerated development of dependence among individuals with relative deficits in cognitive control. Next, as dependence develops, nicotine becomes additionally reinforcing because of nicotine-induced reversal of cognitive deficits resulting from nicotine withdrawal, thereby resulting in both positive (independent of withdrawal) and negative (withdrawal reversal) reinforcement contributors to nicotine dependence (Evans and Drobes 2009).
The current study examining nonsmokers shows substantial support for the view that nicotine enhances cortical activation independent of withdrawal reversal as measured by slow wave EEG (primarily alpha-1) suppression. Unlike the two previous smaller studies examining resting EEG among nonsmokers, we did find support for nicotine-induced alpha (and more specifically alpha-1) suppression. In contrast, we did not replicate Fisher et al.’s (2012) finding supporting nicotine enhancement of left frontal alpha-2. However, the nicotine suppression of slow wave alpha-1 activity in the present study is consistent with nicotine suppression of alpha-1 activity in the context of withdrawal reversal via nicotine administration given to smokers following Evans et al. (2015). As discussed below, it is important to consider trait differences in cognitive functioning as a moderator of nicotine’s impact on cortical activation, and presumably concomitant cognitive control.
Limitation and future directions
One limitation of the current study is that resting EEG was only collected across a 3-min time period with eyes open. A more optimal measurement approach would be to include a full 8 min of resting EEG with alternating eyes open and closed (see Sutton & Davidson 1997). Further, resting EEG is not the most precise proxy of cognitive control. More specific hypotheses involving specific facets of cognitive control as measured during performance of cognitive tasks should be examined in future studies seeking to better understand the influence of nicotine on cognitive control.
A third limitation is the large number of statistical tests all performed with α=0.05 (i.e., multiple EEG bands, multiple EEG pairs). The results may reflect type I error. However, this seems unlikely given the pattern of statistically significant main effects and individual differences, as well as the results from other EEG studies. A replication and extension of the current study can readily address these methodological and statistical limitations.
Another limiting factor of the current study is that slow wave suppression (alpha-1 in particular) and fast wave enhancement are proxies for cognitive control, as opposed to actually measuring behavioral performance or the neural substrates during performance on a cognitive task. Although slow wave suppression and fast wave enhancement have independently been associated with increased cognitive control (Hermens et al. 2005; Ogrim et al. 2012), as well as slow/fast ratio (Putman et al. 2010), there are a number of reasons that various EEG frequency bands may be influenced independent of cognitive control.
An additional limitation is that this study did not directly test nicotine self-medication of cognitive control. That is, the population was a nonsmoking population with minimal exposure to nicotine, so it cannot be suggested that this sample uses nicotine to self-medicate. The current data may, however, suggest that if these nonsmokers were to begin smoking, and then these individuals might be more inclined to self-medicate via smoking for cognitive reasons. Despite these limitations, these findings are promising with respect to the potential impact of nicotine self-medication of cognitive control among individuals without ostensible forms of psychopathology (e.g., schizophrenia). Smokers who find smoking more reinforcing for cognitive reasons may be more likely to exhibit reduced levels of cognitive control following nicotine deprivation and/or greater enhancement of cognitive control independent of nicotine withdrawal reversal.
Preliminary resting state fMRI findings suggest that nicotine may shift processing from networks that process internal to networks that process external information (Tanabe et al. 2011). Simultaneous measurement of resting EEG and resting fMRI may build upon these preliminary findings. Research that leads to a better understanding of neural substrates of cognitive control impacted by nicotine may encourage the development of treatment strategies that target the characteristics of subgroups of smokers who find smoking more reinforcing because of nicotine self-medication of cognitive control.
Acknowledgments
This study was funded by the Florida Department of Health grant no. 09KN-02 (PI: David Evans) and by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). The authors would like to thank Renee Ornduff and Natasha Garcia for their work on the project.
Footnotes
Compliance with ethical standards
Conflict of interest David Drobes has served as an expert witness in litigation against tobacco companies. All other authors declare that they have no conflicts of interest.
References
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R (1992) Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 32:607–616. doi: 10.1016/0006-3223(92)90073-9 [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R (1993) Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150:1856–1861 [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR (1982) The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol 21:1–16 [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Bond D, McCarthy R, Selikowitz M (2002) Effects of stimulant medications on the EEG of children with attention-defcit/hyperactivity disorder. Psychopharmacology 164:277–284 [DOI] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ (2009) Nicotine self-medication of cognitive-attentional processing. Addict Biol 14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x [DOI] [PubMed] [Google Scholar]
- Evans DE, Rothbart MK (2007) Developing a model for adult temperament. J Res Pers 41:868–888. doi: 10.1016/j.jrp.2006.11.002 [DOI] [Google Scholar]
- Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ (2013) Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacology 38:2525–2531. doi: 10.1038/npp.2013.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Jentink KG, Sutton SK, Van Rensburg KJ, Drobes DJ (2014) 7 mg nicotine patch fails to enhance P300 neural indices of cognitive control among nonsmokers. Pharmacol Biochem Behav 126:77–82. doi: 10.1016/j.pbb.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Evans DE, Sutton SK, Oliver JA, Drobes DJ (2015) Cortical activity differs during nicotine deprivation versus satiation in heavy smokers. Psychopharmacology 232:1879–1885. doi: 10.1007/s00213-014-3821-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DJ, Daniels R, Jaworska N, Knobelsdorf A, Knott VJ (2012) Effects of acute nicotine administration on resting EEG in nonsmokers. Exp Clin Psychopharmacol 20:71–75. doi: 10.1037/a0025221 [DOI] [PubMed] [Google Scholar]
- Foulds J, McSorley K, Sneddon J, Feyerabend C, Jarvis MJ, Russell MA (1994) Effect of subcutaneous nicotine injections of EEG alpha frequency in non-smokers: a placebo-controlled pilot study. Psychopharmacology 115:163–166. doi: 10.1007/BF02244767 [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Izetelny A, Radtke R, Hammersley J, Rabinovich NE, Jameson TR, Huggenvik JI (2005) Dopamine receptor (DRD2) genotype-dependent effects of nicotine on attention and distraction during rapid visual information processing. Nicotine Tob Res 7: 361–379. doi: 10.1080/14622200500125245 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Okerholm RA, Eller M, Wei G, Rolf CN, Gorsline J (1995) Comparison of the pharmacokinetics of two nicotine transdermal systems: nicoderm and habitrol. J Clin Pharmacol 35:493–498. doi: 10.1002/j.1552-4604.1995.tb04093 [DOI] [PubMed] [Google Scholar]
- Heishman SJ (1998) What aspects of human performance are truly enhanced by nicotine? Addict 93:317–320 [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE (1994) Nicotine and smoking: a review of effects on human performance. Exp Clin Psychopharmacol 2:345–395 [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology 210:453–469. doi: 10.1007/s00213-010-1848-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Soei EX, Clarke SD, Kohn MR, Gordon E, Williams LM (2005) Resting EEG theta activity predicts cognitive performance in attention-deficit hyperactivity disorder. Pediatr Neurol 32:248–256. doi: 10.1016/j.pediatrneurol.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Jessen F, Kucharski C, Fries T, Papassotiropoulos A, Hoenig K, Maier W, Heun R (2001) Sensory gating deficit expressed by a disturbed suppression of the P50 event-related potential in patients with Alzheimer’s disease. Am J Psychiatry 158:1319–1321. doi: 10.1176/appi.aip.158.8.1319 [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Blank MD, Eissenberg T (2005) The effects of nicotine on attention and working memory in never-smokers. Psychol Addict Behav 19:433–438. doi: 10.1037/0893-164x.19.4.433 [DOI] [PubMed] [Google Scholar]
- Knott VJ (1988) Dynamic EEG changes during cigarette smoking. Neuropsychobiology 19:54–60. doi: 10.1159/000118434 [DOI] [PubMed] [Google Scholar]
- Knott VJ, Fisher DJ (2007) Naltrexone alteration of the nicotine-induced EEG and mood activation response in tobacco-deprived cigarette smokers. Exp Clin Psychopharmacol 15:368–381. doi: 10.1037/1064-1297.15.4.368 [DOI] [PubMed] [Google Scholar]
- Knott VJ, Raegele M, Fisher D, Robertson N, Millar A, McIntosh J, Ilivitsky V (2005) Clonidine pre-treatment fails to block acute smoking-induced EEG arousal/mood in cigarette smokers. Pharmacol Biochem Behav 80:161–171. doi: 10.1016/j.pbb.2004.10.025 [DOI] [PubMed] [Google Scholar]
- Larson CL, Davidson RJ, Abercrombie HC, Ward RT, Schaefer SM, Jackson DC, Holden JE, Perlman SB (1998) Relations between PET-derived measures of thalamic glucose metabolism and EEG alpha power. Psychopharmacology 34:162–169. doi: 10.1111/1469-8986.3520162 [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA (2002) Cognitive mechanisms of nicotine on visual attention. Neuron 36:539–548. doi: 10.1016/S0896-6273(02)01004-8 [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB (2007) Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Exp Clin Psychopharmacol 15:21–36. doi: 10.1037/1064-1297.15.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Teale PD, Reite ML (1999) EEG correlates of methylphenidate response among children with ADHD: a preliminary report. Biol Psychiatry 45:1657–1660. doi: 10.1016/S0006-3223(98)00250-9 [DOI] [PubMed] [Google Scholar]
- Mancuso G, Andres P, Ansseau M, Tirelli E (1999) Effects of nicotine administered via a transdermal delivery system on vigilance: a repeated measure study. Psychopharmacology 142:18–23. doi: 10.1007/s002130050857 [DOI] [PubMed] [Google Scholar]
- Newhouse P, Singh A, Potter A (2004) Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem 4: 267–282. doi: 10.2174/1568026043451401 [DOI] [PubMed] [Google Scholar]
- Ogrim G, Kropotov J, Hestad K (2012) The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: sensitivity, specificity, and behavioral correlates. Psychiatry Res 198:482–488. doi: 10.1016/j.psychres.2011.12.041 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J (2008) Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res 10:1245–1250. doi: 10.1080/14622200802097506 [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF, Hillman CH (2011) Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cognitive Neurosci 23:1332–1345 [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA (2008) Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav 88:407–417. doi: 10.1016/j.pbb.2007.09.014 [DOI] [PubMed] [Google Scholar]
- Putman P, van Peer J, Maimari I, van der Werff S (2010) EEG theta/beta ratio in relation to fear-modulated response-inhibition, attentional control, and affective traits. Biol Psychiatry 83:73–78. doi: 10.1016/j.biopsycho.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Roche RA, Garavan H, Foxe JJ, O’Mara SM (2005) Individual differences discriminate event-related potentials but not performance during response inhibition. Exp Brain Res 160:60–70. doi: 10.1007/s00221-004-1985-z [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP (2005) Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry 62:649–659. doi: 10.1001/archpsyc.62.6.649 [DOI] [PubMed] [Google Scholar]
- Singh A, Potter A, Newhouse P (2004) Nicotinic acetylcholine receptor system and neuropsychiatric disorders. IDrugs 7:1096–1103 [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ (1997) Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychol Sci 8:204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x [DOI] [Google Scholar]
- Tanabe J, Nyberg E, Martin LF, Martin J, Cordes D, Kronberg E, Tregellas JR (2011) Nicotine effects on default mode network during resting state. Psychopharmacology 216:287–295. doi: 10.1007/s00213-011-2221-2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Belsito L, Scher R, Eissenberg T, Corrigall WA (2001) Effects of abstinence and smoking on information processing in adolescent smokers. Psychopharmacology 153:249–257. doi: 10.1007/s002130000552 [DOI] [PubMed] [Google Scholar]


